Abstract
Lead contamination in drinking water can pose serious health risks to humans, and can often go undetected as a result of corrosion of lead infrastructure installed in buildings constructed prior to 1986. Thus, there is an unmet need for timely, cost-effective, and onsite monitoring of lead in drinking water. Here, we have designed a four-electrode system to reliably respond to electrodeposited lead oxide that provides a near real-time indication of lead presence. To better understand this detection mechanism, we investigated the temporal and spatial electrochemical deposition of lead using potential response data, scanning electron microscopy (SEM), fractal dimension ( ), and COMSOL Multiphysics® finite element analysis. Our results suggest that the deposition of lead oxide on the sensor is diffusion limited. Such fundamental understanding of the detection mechanism is critical to improve and shorten the detection time of the sensor. We used this information to improve the detection time and reliability of the signal by reducing the electrode gap distance and agitating the solution. This study provides a path for further optimization of a continuous electrochemical sensor for onsite monitoring of lead in drinking water.
), and COMSOL Multiphysics® finite element analysis. Our results suggest that the deposition of lead oxide on the sensor is diffusion limited. Such fundamental understanding of the detection mechanism is critical to improve and shorten the detection time of the sensor. We used this information to improve the detection time and reliability of the signal by reducing the electrode gap distance and agitating the solution. This study provides a path for further optimization of a continuous electrochemical sensor for onsite monitoring of lead in drinking water.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Water infrastructure containing lead has the potential to cause great harm to humans. Lead is a neurotoxin that, when consumed, can slow growth and development of children and cause damage to the nervous system. 1,2 Prior to 1986, lead was widely used in residential plumbing including service lines, solder, and brass fittings. Changes to water chemistry can cause corrosion of a pipe's passivation layer, allowing lead and other heavy metals to leach into the drinking water source. 3,4 Corrosion events often occur within individual homes and contaminate clean water from the water utility source. Although no level of lead is safe to consume, the Environmental Protection Agency (EPA) and European Union (EU) have set a maximum action lead level of 15 part per billion (ppb) and 10 ppb, respectively. 5 In the United States, the EPA estimates that there are 6–10 million lead service lines still used to deliver water in homes. 6 Thus, a sensor capable of alerting the homeowner that lead is present within their home could accelerate the detection of a corrosion event, monitor the water quality, and determine the need for plumbing replacement.
Currently, traditional analytical methods are the gold standard for accurate and sensitive lead detection but are resource intensive and do not provide continuous monitoring. Analytical methods include inductively coupled plasma atomic emission spectroscopy (ICP-AES), 7 inductively coupled plasma mass spectroscopy (ICP-MS), 8 and atomic absorption spectroscopy (AAS). 9 Despite traditional analytical methods' accuracy and sensitivity, these methods are expensive, require highly trained operators, involve complicated and laborious pre-treatment processes, and take days or weeks to provide test results. Furthermore, water samples need to be collected by a homeowner and transported to a certified laboratory, requiring the diligence of homeowners to regularly test their water for lead contamination. Thus, a simple and inexpensive method is desirable for near real-time monitoring of a water sample.
Colorimetric and electrochemical sensors are being explored to address the need for lead detection and monitoring in the individual home. 9–15 As these sensors are miniature, portable, have fast detection times, and do not require trained operators, they are ideal for home use where contaminated lead infrastructure is often located. 5 Among these various detection technologies, colorimetric sensors are the most convenient methods due to their simplicity, but they are single use and often require the homeowner to add testing reagents to their water sample. Electrochemical sensors are accurate and detect dilute concentrations of 1–150 ppb lead, 9 but contain reference electrodes that can become unstable over time, making them unsuitable for long-term monitoring of lead contamination.
To create sensors that continuously operate at long time scales, Lin et al. developed a four-electrode system (positive anode, negative cathode, and two floating electrodes) to detect 15 ppb lead ions with no false responses in 3 days without the need for reference electrodes. 12 The anode and its neutral floating electrode create a locally acidic environment, while the cathode and its neutral floating electrode generate a locally basic environment. Lead detection takes place when the gap between the anode and the adjacent floating electrode is bridged by a lead oxide deposition. However, lead detection in that system could take several days to trigger a response.
In this study, we aim to understand and achieve more effective sensor detection strategies for identifying electrodeposited lead oxide on a microelectrode sensor based on the four-electrode system. 12 Initially, we designed a new four-electrode system and demonstrated successful lead oxide deposition. In order to shorten the lead sensor detection time and improve the stability of the detection signal, we investigated temporal and spatial behavior of lead oxide deposition and its underlying mechanism. Lead deposition was examined with respect to time, the gap between the anode/cathode and the adjacent electrodes (3–12 μm), and the absence or presence of agitation. Potential response data and scanning electron microscopy (SEM) images were obtained to further understand lead oxide deposition.
Materials and Methods
Sensor design and fabrication
To detect lead presence in drinking water, a 300/1000 Å Ti/Pt four-electrode sensor was designed and fabricated as shown in Fig. 1A. The four-electrode system consists of an anode (+), anode's adjacent floating electrode (F2), cathode's adjacent floating electrode (F3), and a cathode (−). Here, F2 and F3 serve as a reference point to determine if conductive materials are present. The gap between the two adjacent electrodes (e.g., anode and F2) was designed to be 3, 5, 8, 10 and 12 μm. Photolithography, electron beam evaporation, and lift-off were employed to fabricate the electrodes on borofloat wafers (Precision Glass & Optics) following published procedures. 12 The borofloat wafers were diced into 2.0 mm × 3.6 mm pieces. Each wafer produced approximately 500 sensors. Fabricated sensors were epoxied (Loctite) to custom printed circuit boards (PCBs) and were electrically connected by Gold ball wirebonding (Model 4124, Kulicke & Soffa) and protected by epoxy encapsulation (Loctite). Wires connecting the sensors to the LabVIEW test setup were soldered to the other end of the PCBs.
Figure 1. (A) The schematic diagram of the four-electrode system. (B) Sensor schematic with three different sensing configurations including: deposition mode ( ) between anode and cathode, lead measurement mode (
) between anode and cathode, lead measurement mode ( ) between anode and F2, and heavy metal measurement mode (
) between anode and F2, and heavy metal measurement mode ( ) between F3 and cathode.
) between F3 and cathode.
Download figure:
Standard image High-resolution imageChemicals and test solution preparation
Lead (II) chloride (PbCl2) and sodium chloride (NaCl, >98%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All experiments were conducted in 0.01 M NaCl with a conductivity of 1000 μS cm−1, the upper limit in drinking water. 16 The lead test solutions were adjusted to 15 ppb PbCl2 using deionized water. Control solutions consisted of 0.01 M NaCl and deionized water.
Experimental setup
The experimental setup uses LabVIEW Data Acquisition software to operate at three different modes: deposition mode (anode-cathode), lead measurement mode (anode-F2), and heavy metal measurement mode (F3-cathode). The sensor was connected in series with a 100 kΩ load resistor and a 3.2 V DC power supply (Fig. 1B). The sensor was submerged in 100 ml of test solution and ran in deposition mode for one hour. Next, the LabVIEW Data Acquisition software recorded potential measurements across the load resistor every hour for 3 min in deposition mode (∆V), lead measurement (∆V1) mode and heavy metal measurement mode (∆V2). A MATLAB program determined the average potential for each operating configuration by averaging five data points across the span of the 3-min recording. The sensor triggered after the load resistor in lead measurement mode (∆V1) reached a 1 V threshold potential. Tests have been conducted in stagnant and agitated solution. Agitated solutions were mixed by placing a 15.9 mm × 8.0 mm stir bar in solution on a stir plate.
Scanning electron microscopy
All SEM images were taken with a Field Emission SEM (Hitachi SU8000 In-Line) in the secondary electron detection mode. Samples were mounted on a 4'' wafer mount and loaded into the machine. A 2 KV acceleration voltage and a 7 μA emission current were used. It should be noted that the SEM images were taken following the electrical measurements, rinsing the sensor with deionized water, and preparing the sensor for SEM imaging. The presence of lead on the sensor was confirmed by Energy Dispersive X-ray Spectroscopy (EDS, Bruker Quantax 200) with a 5 KV acceleration voltage and a 7 μA emission current. The EDS spectra of the electrodeposits had oxygen and lead composition suggesting the formation of lead oxide (Figs. S1 and S2 in Supplementary Material (available online at stacks.iop.org/JES/169/017505/mmedia)).
Fractal dimension
Fractal box-counting method was employed to calculate fractal dimension ( ) of the electrodeposited structure between the anode and F2. The
) of the electrodeposited structure between the anode and F2. The  analysis was performed on the SEM images of six different lead sensors after applying potential for 6, 12, 18, 24, 48, and 72 h. Individual SEM images were analyzed with ImageJ® software and ImageJ® plugin FracLac V 2.5 software developed by Karperien.
17
Briefly, using ImageJ® software, a threshold was applied to the SEM images to distinctly separate pixels of the electrodeposited structures from those in the background. Next, the area of interest was selected by manually removing the anode and F2 from the SEM images. Using plugin FracLac V 2.5, the box-counting method was executed. SEM images were automatically converted into binary images and covered with grids of boxes. The software counts the number of boxes (
analysis was performed on the SEM images of six different lead sensors after applying potential for 6, 12, 18, 24, 48, and 72 h. Individual SEM images were analyzed with ImageJ® software and ImageJ® plugin FracLac V 2.5 software developed by Karperien.
17
Briefly, using ImageJ® software, a threshold was applied to the SEM images to distinctly separate pixels of the electrodeposited structures from those in the background. Next, the area of interest was selected by manually removing the anode and F2 from the SEM images. Using plugin FracLac V 2.5, the box-counting method was executed. SEM images were automatically converted into binary images and covered with grids of boxes. The software counts the number of boxes ( ) that covers the structures and contains the pixels of interest. The pixels of interest are considered to contain meaningful information while other pixels are background or noise. Finally, the
) that covers the structures and contains the pixels of interest. The pixels of interest are considered to contain meaningful information while other pixels are background or noise. Finally, the  is calculated as follows:
is calculated as follows:

where  is the length scale and is equal to the box size divided by the image size.
is the length scale and is equal to the box size divided by the image size.  is a unit-less measure and can be calculated by measuring the negative slope of
is a unit-less measure and can be calculated by measuring the negative slope of  vs
vs  When the
When the  value nears 2, the growth is more of a compact cluster, while an
value nears 2, the growth is more of a compact cluster, while an  value of 1 indicates the growth is closer to a one-dimensional structure.
18
value of 1 indicates the growth is closer to a one-dimensional structure.
18
Lead ion transport simulation
Lead ion concentration gradient and flux were numerically modeled with COMSOL Multiphysics® 5.5. The lead ion transportation was adapted from Lupo et al. 19,20 Due to the symmetry of the four-electrode system, the geometry was modeled and contained the electrolyte, anode, and F2. "Transport of Diluted Species" and "Level Set" were used to simulate the concentration gradient and ion flux. The transport module solved the diffusive term of the Nernst-Planck equation. 21 The initial concentration of the lead ion was defined as 0 mol m−3 at the anode, and 15 ppb (7.24 × 10−5 mol m−3) a distance of 15 μm away from the sensor in the z direction. The F2 and electrolyte regions between electrodes were assumed to have a no flux boundary condition. To save computation time, the mesh was defined to be coarser at a distance of 5 to 15 μm away from the electrode interface with a maximum element size and growth rate of 1.34 and 1.25μm, respectively. At the anode boundary, the mesh contained a maximum element size and growth rate of 0.43 and 1.1μm, respectively.
Results and Discussion
Lead sensor detection mechanism
We designed and fabricated an interdigitated four-electrode sensor capable of detecting lead in drinking water with no false positive results (Fig. 1). As previously described,
12
the four-electrode lead sensor operates by depositing lead oxide onto the anode. The sensor returns a positive signal for lead when the lead oxide bridges the gap between the anode and the adjacent floating electrode, F2. The presence of lead oxide in this gap lowers the resistance between the anode and F2 thus increasing the potential across the load resistor. Lin et al demonstrated the four-electrode sensor can also detect other heavy metals (i.e., Zn2+, Cu2+, and Fe2+) between the cathode and F3 and found that other heavy metals had minimal effect on the lead detection capability.
12
Figure 2A shows the electrical potential signal at three different operational modes: deposition mode, heavy metal measurement mode, and lead measurement mode. Lead solutions did not trigger a response during the deposition and heavy metal measurement modes. In the lead measurement mode, the initial potential ( ) was below the 1 V threshold potential before 18 h, oscillating below and above 1 V between 18 and 32 h, and above 1 V after 32 h. The rise in potential served as the detection signal for the presence of lead oxide on the sensor and, therefore, lead in the contacting solution. Control lead-free test solutions did not trigger a detection signal in the lead or heavy metal measurement modes (Fig. S3 in Supplementary Material).
) was below the 1 V threshold potential before 18 h, oscillating below and above 1 V between 18 and 32 h, and above 1 V after 32 h. The rise in potential served as the detection signal for the presence of lead oxide on the sensor and, therefore, lead in the contacting solution. Control lead-free test solutions did not trigger a detection signal in the lead or heavy metal measurement modes (Fig. S3 in Supplementary Material).
Figure 2. (A) Potential (∆V) signal vs time at three different operational mode: deposition mode, lead measurement mode, and heavy metal measurement mode. (B) The SEM images were taken and studied for six different sensors stopped after 6, 12, 18, 24, 48, and 72 h. SEM images show lead oxide growth over time at the tip side of the first anode digit. Sensors were rinsed with deionized water prior to imaging. (C) Deposited lead oxide length over time with a growth rate between 0.05 and 0.15 μm h−1. All devices had a 5 μm gap between anode and F2. Tests were conducted with lead solutions of 15 ppb PbCl2, 0.01 M NaCl, and deionized water.
Download figure:
Standard image High-resolution imageThe detection signal is based on depositing conductive lead oxide and bridging between the anode and F2 to trigger the sensor. To visually capture this bridging event, SEM images were studied over time as illustrated in Fig. 2B. Prior to 18 h, SEM images show fast linear growth of lead oxide deposition and correspond to the observed constant  in Fig. 2A. Following 18 h of lead oxide deposition, the deposited material starts to bridge the gap between the electrodes which coincides with the oscillating potential response. At this stage, lead oxide structures are similar to very thin wires and can easily break with the passage of current during the lead measurement mode. The interplay between connection and breakage appears to slow down the overall rate of lead oxide growth as observed in Fig. 2C. By 72 h, the electrical potential signal stabilizes as a thick layer of deposited lead oxide fills the electrode gap. The presence of sufficient material between the electrodes to carry the current can eliminate the stress caused by the measurement process. Note that the lead oxide deposition has a growth rate between 0.05 and 0.15 μm h−1 as indicated in Fig. 2C.
in Fig. 2A. Following 18 h of lead oxide deposition, the deposited material starts to bridge the gap between the electrodes which coincides with the oscillating potential response. At this stage, lead oxide structures are similar to very thin wires and can easily break with the passage of current during the lead measurement mode. The interplay between connection and breakage appears to slow down the overall rate of lead oxide growth as observed in Fig. 2C. By 72 h, the electrical potential signal stabilizes as a thick layer of deposited lead oxide fills the electrode gap. The presence of sufficient material between the electrodes to carry the current can eliminate the stress caused by the measurement process. Note that the lead oxide deposition has a growth rate between 0.05 and 0.15 μm h−1 as indicated in Fig. 2C.
The relatively slow process of oxide growth to bridge the electrodes is due to the limitation caused by ion diffusion and is supported by the observed morphology. As shown in Fig. 2B, the morphology agrees well with an open fractal pattern suggesting the ions are performing a random walk and aggregating one-by-one (i.e., a diffusion mechanism).
22,23
The open fractal patterns of lead oxide deposits are self-similar with a non-uniform growing front with respect to electric field lines and continuous tip splitting facilitating growth into a tree-like structure. The open fractal structures that emerge from this process can be described by the fractal dimension,  which is an index for quantifying open fractal pattern complexity as a ratio of the change in detail (
which is an index for quantifying open fractal pattern complexity as a ratio of the change in detail ( ) with respect to the change in scale (
) with respect to the change in scale ( ). As shown in Fig. 3, the
). As shown in Fig. 3, the  of the lead oxide formation varied between 1.65 to 1.74, which is approximately consistent with the diffusion limited value of 1.71 reported in the literature.
18,24
of the lead oxide formation varied between 1.65 to 1.74, which is approximately consistent with the diffusion limited value of 1.71 reported in the literature.
18,24
Figure 3. A plot of log N vs  using the box-counting method gives a fractal dimension
using the box-counting method gives a fractal dimension  = −slope. The
= −slope. The  of six different lead sensors were found to be 1.71, 1.74, 1.65, 1.71, 1.71, and 1.68 after 6, 12, 18, 24, 48, and 72 h, respectively. Devices had a gap distance of 5 μm. Lead sensors were tested in 15 ppb lead solutions.
of six different lead sensors were found to be 1.71, 1.74, 1.65, 1.71, 1.71, and 1.68 after 6, 12, 18, 24, 48, and 72 h, respectively. Devices had a gap distance of 5 μm. Lead sensors were tested in 15 ppb lead solutions.
Download figure:
Standard image High-resolution imageCOMSOL Multiphysics® simulation of lead ion diffusion
The diffusion-limited growth was modeled with COMSOL Multiphysics® simulations and agreed with the spatial observation of lead oxide deposition across the anode. Generally, in dilute systems, the ion flux,  is governed by diffusion, migration, and convection:
is governed by diffusion, migration, and convection:
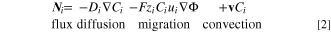
where  is ion diffusivity,
is ion diffusivity,  is the bulk ion concentration,
is the bulk ion concentration,  is Faraday's constant,
is Faraday's constant,  is the ion charge,
is the ion charge,  is the ion mobility,
is the ion mobility,  is the electric potential, and
is the electric potential, and  is the bulk velocity. In stagnant solutions that contain highly conductive supporting electrolytes, convection and migration can be assumed to be negligible.
25
Thus, Eq. 2 simplifies to:
is the bulk velocity. In stagnant solutions that contain highly conductive supporting electrolytes, convection and migration can be assumed to be negligible.
25
Thus, Eq. 2 simplifies to:

For a diffusion limited mechanism, the ion flux is primarily driven by the concentration gradient. Figure 4A shows the 2D cross-sectional plot of the concentration gradient in the x–z plane at y = 0. The anode tip (E1) and near-anode tip (E2) are identified as the area of interest across the 1st anode digit. After 12 h, a drastic drop in concentration occurs at the anode tip (E1) because this area receives a greater supply of ions giving rise to a larger concentration gradient. The large gradient at the anode tip is beneficial as the detection mechanism of the sensor relies on lead bridging across the anode and F2.
Figure 4. (A) Schematic of lead sensor geometry with deposition areas of interest. The ion concentration gradient is shown in the area closer to the anode tip generated with COMSOL®. (B) COMSOL® simulation of spatial dependence of Pb2+ ion flux vs location along the 1st anode digit was plotted at 0.2 μm above the surface in the z direction. The SEM images of (C) anode corner, (D) anode palm, and (E) anode tip after 12 h of lead deposition in 15 ppb lead solution. Sensor was gently washed with deionized water prior to imaging and had a 5 μm gap distance.
Download figure:
Standard image High-resolution imageTo correlate ion flux with the spatial observation of lead oxide deposition across the 1st anode digit, two additional area of interest were identified at the anode corner (C) and anode palm (D). Figure 4B shows the ion flux across the x direction at z = 0.2 μm. The ion flux is largest at the anode tip (E1) and corner (C), resulting in a greater possibility of forming electrodeposits at these locations. The simulated results agree with the increased deposition experimentally observed in SEM images, as shown in Figs. 4C and 4E. At the center of the anode (between D and E1), the ion flux decreases significantly resulting in less deposition displayed in Figs. 4D and 4E.
Shortening and improving the stability of the detection signal
The simplest way to decrease the detection time of the sensor is to reduce the gap distance between the anode and F2. As previously shown in Fig. 2, lead detection in the sensor geometry is a function of the time it takes for the lead oxide to form and bridge the gap between electrodes. To study the effect of gap distance on sensor detection time, sensors with different gap (3, 5, 8, 10, and 12 μm) were fabricated. Table S1 in the Supplementary Material summarizes the average actual gap distance, electrode width, and detection time of each studied device geometry. Figure 5A shows a representative curve for each gap distance. The oscillation period and detection time are significantly shorter with smaller gap distances of 3 and 5 μm, as smaller amounts of lead oxide deposits are needed to bridge the gap between electrodes.
Figure 5. (A) A representative potential signal curve for each gap distance in lead measurement mode. All experiments were conducted with 15 ppb lead solution. (B) The average detection time vs gap distance (n  3 sensors) between anode and F2 with a growth rate ranging between 0.05 and 0.15 μm h−1.
3 sensors) between anode and F2 with a growth rate ranging between 0.05 and 0.15 μm h−1.
Download figure:
Standard image High-resolution imageFigure 5B illustrates the average detection time vs gap distance. The dispersion of average detection time for 3 and 5 μm gap sensors is relatively low with the values of 19.8 ± 3.04 and 33.2 ± 5.44 h, respectively. The rate of lead oxide growth was found to be between 0.05 and 0.15 μm h−1, and agrees with the growth rate range calculated in Fig. 2C. Note that, for short times and distances in Figs. 2C and 5B, the growth rate appears to be linear with a constant value of 0.15 μm h−1. While this phenomenon is associated with the morphology of the growing lead oxide, further investigation is needed to determine the exact mechanism and to validate that the region truly involves linear growth. The growth rate, as determined by the relationship between gap distance and detection time, can also be used to envision an improved detection time with a smaller gap sensor (e.g., sub-micron made via nanopatterning) below what was possible in this work (i.e., 3 μm gap).
Electrolyte agitation was employed to facilitate transport of lead ions to the electrode surface and resulted in more stable electrical potential signals. The electrical response of three lead sensors are plotted for stagnant and 360 rpm agitated lead solutions in Figs. 6A and 6C, respectively. The electrical response of a stagnant lead solution exhibits noisy signals oscillating around the threshold potential, while electrical response of the agitated lead solution produces a smooth curve that reaches a steady potential. Additionally, two sensors (i.e., lead sensor 1 and 2 in Fig. 6C) show stronger signal response during agitation with a  larger than 2 V when triggered. Note that the agitated solution created a laminar flow regime, which demonstrates that the sensor can perform well in conditions consistent with the real world (e.g., laminar faucet flow). Further studies are needed to understand the effect of water flow in a turbulent regime on sensor performance.
larger than 2 V when triggered. Note that the agitated solution created a laminar flow regime, which demonstrates that the sensor can perform well in conditions consistent with the real world (e.g., laminar faucet flow). Further studies are needed to understand the effect of water flow in a turbulent regime on sensor performance.
Figure 6. Potential signal during lead measurement mode (∆V1) for (A) stagnant solutions and (C) 360 rpm agitated solutions. Each curve represents a unique device in isolated experiment. SEM images of sensor in (B) stagnant and (D) 150 rpm agitated solutions. Each SEM image was taken in different devices with 5 μm gap distance that both ran for ∼27 h in 15 ppb lead solution.
Download figure:
Standard image High-resolution imageThe improvement of signal stability could be due to the morphology of the electrodeposited material, which has been shown to be influenced by agitation. 26–28 Compared to the stagnant solutions (Fig. 6B), the SEM images of agitated solutions (Fig. 6D) were more densely packed, had a growing front that varied in direction, and were more uniformly distributed across the anode. In agitated solutions, the dense nature of the lead oxide structures could contribute to the stable signal observed during experiments. When agitating a solution, the diffusion layer thickness decreases 29 causing the rate of ion diffusion to increase. As more ions are closer to the surface of the electrode in agitated solutions, denser structures are more likely to form.
To study the lead oxide growth mechanism during agitation, the SEM images were horizontally divided at the center of the image into the top and the bottom regions, and the resulting segmented images were used to calculate  Despite increasing the ion transport to the surface of the electrode,
Despite increasing the ion transport to the surface of the electrode,  of the top region is similar to that of stagnant solutions. However, the
of the top region is similar to that of stagnant solutions. However, the  of the bottom region was found to be 1.82 ± 0.04 displaying deviation from the fractal patterns by forming denser structures. As stated previously, the formation of these dense structures greatly improves the sensor's potential signal stability.
of the bottom region was found to be 1.82 ± 0.04 displaying deviation from the fractal patterns by forming denser structures. As stated previously, the formation of these dense structures greatly improves the sensor's potential signal stability.
Conclusions
The lead sensor described in this work uses electrodeposition of lead oxide to reproducibly and reliably detect the presence of lead in aqueous solutions. The deposited oxide forms structures that bridge the gap between the anode and the adjacent floating electrode. The deposition is diffusion limited, and faster sensor response can be obtained by reducing the gap between the bridged electrodes, shortening detection times in our experiments by 40%. In agitated flow conditions, the sensor has been shown to have more stable electrical potential signals, and the laminar flow regimes at the end point of water service lines or in individual faucets would be similar to those conditions. Lead sensor performance can be further improved by masking the areas of the electrode (e.g., connecting wires, anode corner) that are not near a floating electrode to prevent deposition of lead in undetectable regions. With minor changes, the resulting miniature, sensitive, and user-friendly sensors can easily be deployed in a variety of water infrastructure and can generate actionable data to prevent consumption of lead during a corrosion event.
Acknowledgments
The authors would like to thank the T. C. Chang Professorship, Xylem Inc. and Helen of Troy Limited for partially funding this work. The authors would also like to thank Brian Johnson for his skill in developing the LabVIEW test setup, PCB wirebonding, packaging and soldering. The sensors were fabricated in the Lurie Nanofabrication Facility and packaged in the Chemical Engineering Cleanroom at the University of Michigan.







