Abstract
The present work reports the changes in CdTe quantum dots' size and optical properties (CdTe QDs) after interaction with hydroxyl radicals (•OH). Through characterization by UV–vis spectroscopy, fluorescence spectroscopy, and transmission electron microscopy, it was possible to observe the reduction in the size of the nanoparticles when exposed to Fenton's reagent (source of •OH radicals) under constant exposure to UV light. Furthermore, the results show shifts in the emission peak values at lower wavelengths, dependent on the initial size of the QD and the radical's concentration.
Export citation and abstract BibTeX RIS
Semiconductor nanoparticles, called quantum dots (QDs), are widely used in the fields of medicine, 1 chemistry, 2 electronics, 3 biology, 4 and others as a marker and species sensor of interest. 5 Specifically, the use of QDs as a sensor reveals a current interest in detecting and quantifying free radicals. The reduction of CdTe QDs sizes due to the interaction with hydroxyl free radicals (·OH) under an ultraviolet light (UV) source is reported in this brief communication. From CdTe QDs of different sizes and by controlling experimental conditions such as oxygen-free solvent, irradiation wavelength, pH, radical, and precursor concentration, 6 changes in sizes could be established depending on the radical concentration used.
Experimental
Five sizes of CdTe QDs with mercaptosuccinic acid (MSA) (Merck) were used as the capping agent. The nanoparticles in aqueous media were synthesized following a modified protocol: 7,8 In a three-neck flask, portions of 100 mM cadmium chloride (CdCl2) (Sigma-Aldrich) and 300 mM of MSA were mixed with 100 ml of the borax/citrate buffer solution (prepared at pH = 9.0) under vigorous stirring. 250 mM sodium tellurite (Na2TeO3) (Sigma-Aldrich) was added, followed by a portion of sodium borohydride (Merck) (TeO3 2−/BH4 − = 1:40 ratio). The reaction was kept under stirring until the evolution of the gas generated and subsequently brought to constant reflux. The molar ratio of the precursors ([Cd:Te:MSA] = [4:1:12]), pH of buffer solution and temperature (90 °C) were adjusted to fixed values, the reaction time being the only variable to control the size of the nanoparticles. All synthesized CdTe QDs were separated from the solution by ultracentrifugation in 2-propanol at 8000 rpm and re-dissolved in deionized water (Millipore, 18.3 MΩ). The synthesized QDs were characterized by UV–vis spectroscopy using a Shimadzu spectrophotometer, model UV-2600 (absorbance measurements) and fluorescence spectroscopy using a Thermo spectrofluorometer, model AMINCO-Bowman Series 2 for the obtention of the emission spectra. The size of the nanoparticles was estimated by high-resolution transmission electron microscopy (HR-TEM-Tecnai F20 STWIN operated to 200 kV) and calculated by Peng et al.'s method. 9 Our previous work 6 uses the Peng model and the maximum absorbance wavelengths (λabs ) for QD diameter estimation. The estimated results are shown in Table I.
Table I. Estimated diameter by HRTEM and Peng model.
| Sample | Reaction time (hr:min) | Diameter by HR-TEM (nm) | Diameter by Peng model (nm) |
|---|---|---|---|
| QD1 | 1:00 | 2.24 | 2.37 |
| QD2 | 3:00 | 2.50 | 2.81 |
| QD3 | 5:00 | 3.00 | 3.03 |
| QD4 | 7:00- | 3.07 | 3.11 |
| QD5 | 9:00 | 3.41 | 3.67 |
The hydroxyl radicals were obtained by Fenton's reaction, 10,11 which consists of mixing hydrogen peroxide (H2O2) (Merck) in the presence of Fe2+ ((NH4)2Fe(SO4)2, Merck) in a 1:10 ratio of Fe2+/H2O2, according to the reaction indicated in the supplementary material. To avoid interference from Fe3+ (sub-product of the Fenton reaction), EDTA chelating agent (Merck) was added.
For each QD size obtained, 5.00 mg of the total solid was measured, redissolved in 50 ml of deionized water, and stored in a cold chamber (4 °C) to avoid particle agglomerations. Absorbance and emission spectra were obtained from these suspensions. The QD/•OH radical reaction was performed by adding a 2.00 ml aliquot of CdTe QDs solution in a 1.00 cm long quartz cuvette, adding different volumes (in μl) of Fenton's solution to the cuvette, and adding deionized water to reach a final volume of 3.00 ml. Each mixture was exposed before and after the interaction with the •OH radical at a 365 nm wavelength beam.
Results and Discussion
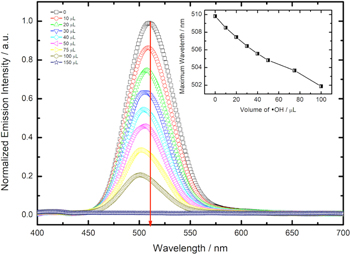
The analysis of the emission spectra shows a systematic quenching of the fluorescence for all sizes of CdTe nanoparticles, which was previously reported using stable nitroxyl free radicals. 8 In addition, a shift of the maximum emission wavelength was observed for each sample due to the photoreaction generated by the action of the irradiated UV light, evidencing an effect on the size compared to as-synthesized QD. Additionally, a shift in the absorbance wavelength (labs) as a function of exposure time was observed. Figure S2 (supplementary information available online at stacks.iop.org/JES/168/097503/mmedia) shows the changes in labs at lower wavelengths and reaching lower absorbances, i.e., decreasing QDs' concentration. Such effects become more significant when the QD is smaller before exposure to the hydroxyl radical, as described in Fig. 1.
Figure 1. Emission spectra for the QD1 sample after exposure to different volumes of •OH radical solution. Inset: Variation of maximum emission wavelength vs volume of hydroxyl radical added.
Download figure:
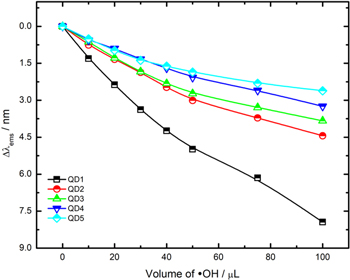
Standard image High-resolution imageFigure 1 shows the emission spectra of QD1 exposed to a different volume of hydroxyl radical solution. A shift of emission peak to lower wavelengths can be observed (λems ). The emission spectra for QD2, QD3, QD4, and QD5 are available in the supplementary material. The λems shift for all samples can be seen in Table II and Fig. 2.
Table II. Wavelength shift in the emission peak (Δλems ).
| Δλems (nm) | |||||
|---|---|---|---|---|---|
| Volume of •OH (μl) | QD1 | QD2 | QD3 | QD4 | QD5 |
| 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | 1.31 | 0.76 | 0.63 | 0.56 | 0.51 |
| 20 | 2.37 | 1.34 | 1.28 | 0.89 | 0.98 |
| 30 | 3.38 | 1.87 | 1.84 | 1.33 | 1.38 |
| 40 | 4.24 | 2.48 | 2.32 | 1.69 | 1.62 |
| 50 | 4.99 | 3.01 | 2.73 | 2.08 | 1.86 |
| 75 | 6.14 | 3.72 | 3.29 | 2.60 | 2.31 |
| 100 | 7.95 | 4.44 | 3.84 | 3.24 | 2.61 |
Figure 2. Graph of Δλems vs volume of hydroxyl radical solution added.
Download figure:
Standard image High-resolution imageThe change is directly related to the QD's size, i.e., more significant size reduction can be seen in smaller QDs (such as in the case of QD1). The high reducing power of the hydroxyl radical (E0 •OH/H2O = +2.32 V at pH 7.0) is responsible for snatching excited electrons from the singlet state (S1) in the excited QDs, generating hydroxyl ions (OH−) in the interface. The presence of OH− ions and positive vacancies (h+) in the QDs causes the photocorrosion of CdTe, which we called the attack by hydroxyl radicals. 12 Previous studies, have shown the possibility of electrochemical oxidation of CdTe QDs in the presence of OH− ions according to the total reaction. 13
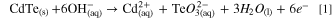
The electrochemical potential applied in that study for CdTe oxidation would be equivalent to the redox potential of the h+ vacancy, allowing the occurrence of reaction (1). Therefore, the redox potential of the hydroxyl radical is high enough to induce the thermodynamic transfer of electrons from the conduction band (BC) of QDs to the singly occupied molecular orbital (SOMO) of the hydroxyl radical (•OH + e− → OH−) as illustrated in the scheme of Fig. 3. In addition, both the precursors and the other by-products of the Fenton's reaction have bands of negligible absorption, i.e., non-existence of spectral overlap, ruling out possible energy transfer between those species and QDs. The HR-TEM image analysis shows the variation in the size of the QD1 sample, evidencing a diameter change from 2.24 nm to 1.75 nm (supplementary material).
Figure 3. Schematic representation for: (A) interaction QDs/•OH generated from Fenton's reagent; (B) Fluorescence shutdown mechanism by electron transfer from QDs—•OH radical.
Download figure:
Standard image High-resolution imageIn a similar way, Pérez-Prieto et al. 14 have observed the same effect when commercial CdSe QDs and CdSe/ZnS core/shell have been exposed to nπ* aromatic ketones, which are highly efficient at abstracting hydrogen from suitable hydrogen donors. The authors report a variation in the λems values when QDs are exposed to different ketones under ultraviolet light. Aromatic ketones, such as benzophenone, absorb the energy provided from the light source, going into an excited state in the anti-binding orbitals (π*).
Conclusions
In this brief communication, it was possible to show the size reduction of the CdTe QDs through an "attack" by •OH radical under constant UV–light irradiation. This effect is dependent on the initial size of the QD (before the interaction with the radical). A more significant size change was observed for smaller QDs. This "attack" was associated with the photocorrosion of the QDs while the irradiation of UV light is maintained to produce Cd(II) and Te(IV) in solution. It is expected that this will be verified by elementary analysis of the reaction medium. This demonstrates an alternative route for obtaining monodisperse and size-controlled nanoparticles by controlling the reaction variables, such as UV-light irradiation, the radical concentration, and the initial size of the QDs.
Acknowledgments
We thank FONDECYT (grant N° 1210408 and grant N° 3200216), VRIEA-PUCV (grant no. 039.438 NÚCLEOPUCV and 125.737/2021 DII-PUCV) and the Dirección de Investigación of the Universidad de la Frontera, Chile for financial support for this study. Ricardo Marotti also acknowledges the support received from CNPq (Brazil, Prosul Program, Project # 490580/2008-4), PEDECIBA-Física, ANII (Administración Nacional de Investigación e Innovación) and the CSIC (Comisión Sectorial de Investigación Científica) of the Universidad de la República in Montevideo, Uruguay.
Data availability statement
N.A