Abstract
During neural stimulation it is important to ensure charge transfer does not cause tissue damage. The safe range for stimulation is often defined by the oxidation/reduction of water. However, many biological molecules, such as ascorbic acid (AA), have lower oxidation potentials than water. Due to its low oxidation potential and high concentrations in the brain, we examined the role of AA oxidation during neural stimulation. By measuring the voltage transients during current-controlled stimulation we show significant AA oxidation occurs at stimulation levels typically deemed safe. These results highlight the importance of considering the effect of electrical stimulation on biological molecules.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Implanted electrodes used to electrically stimulate the nervous system have numerous clinical applications. Neural tissue is activated by charge delivered via current-controlled or voltage-controlled stimulation pulses. 1 Voltage-controlled stimulation has been more commonly used clinically, but recent improvements in implantable pulse generators and better stimulation reliability has increased the use of current-controlled stimulation. 2
During constant-current stimulation, the electrode is polarized to the potential needed to sustain the specified current amplitude. The potential limits for water reduction/oxidation (redox) reactions (−0.9 to 1.3 V (vs Ag/AgCl) for glassy carbon 3 ), termed the water window, are commonly used as safety boundaries for neural stimulation. 1 Ensuring the electrode remains within this potential range during stimulation avoids electrolysis; however, there are many biological molecules that are oxidized at potentials below this limit, such as ascorbic acid (AA) (Fig. 1C). The low oxidation potential of AA (E1/2 = 0.25 V vs Ag/AgCl) make it a strong antioxidant which is an important feature in many of its biological functions throughout the body. In addition to its anti-oxidative properties AA also serves as a neuromodulator in the brain, where the extracellular concentrations range from ∼200–500 μM and intracellular concentrations range from 1–10 mM. 4,5 Despite the high concentrations of AA in the brain and its low oxidation potential, the extent of AA oxidation during neural stimulation has not previously been explored.
Figure 1. Schematic of AA oxidation during a biphasic current pulse. (A) Anode leading biphasic current-controlled pulse applied to a (B) CFE electrode where AA is oxidized to DHA. (C) Voltage transient during the pulse showing the water window in blue and AA oxidation in orange. (D) Voltammograms from CV taken at 100 mVs−1 in PBS (black) and AA, where the oxidation peak at 0.25 V increases with AA concentration. Data shown as mean ± SME (N = 8).
Download figure:
Standard image High-resolution imageThis paper evaluates the role of AA oxidation during neural stimulation. We hypothesized that the presence of redox active molecules would decrease electrode polarization during current-controlled stimulation pulses. To test this hypothesis, we measured the voltage transients of carbon fiber electrodes (CFEs) during current controlled stimulation in physiological levels of AA.
Experimental
AA solutions were prepared daily using L-Ascorbic Acid (Sigma-Aldrich) and 1x phosphate buffered saline (PBS).
CFEs were prepared with 34.5 μm diameter glassy carbon monofilament (Specialty Materials Inc.) following a method similar to Swiergiel et al. 6 Carbon monofilament was thoroughly cleaned with acetone and distilled water then attached to stainless steel wire using electrically conductive epoxy (MG Chemicals) and allowed to cure for 24 h at 60 °C. The assembly was then placed within polyimide tubing and epoxy was applied to the carbon fiber side to create a seal. During epoxy application the assembly was slowly pulled into the tube, creating a smooth taper at the epoxy-carbon interface. The epoxy was allowed to cure at room temperature for one h. The carbon fiber was then cut to a length of ∼700 μm (706.6 ± 188 μm) with iridectomy scissors under a dissecting microscope, giving an average surface area of 0.077 ±.002 mm2.
All electrochemical measurements were performed with an Autolab PGSTAT128N (EcoChemie), equipped with an ADC10M module, using a Pt counter electrode and Ag/AgCl reference electrode (CH Instruments). A salt bridge (3% agar in 1M KCl) separated the Pt-counter electrode in PBS from the CFE (N = 8) and reference electrodes. Cyclic voltammetry (CV) measurements were performed by sweeping the CFE's potential from −0.8 to 0.8 V at 0.1 Vs−1. Current-controlled stimulation consisted of 20 biphasic pulses at 50 Hz having 125 μs phase durations and amplitudes from 5 to 500 μA. The CFE potential was recorded at 1 MHz throughout the testing. The polarization voltage (VP) was calculated by subtracting the ohmic voltage, calculated as the potential difference immediately before and 15 μs after the pulse onset, from the maximum voltage during the anodic phase.
Kruskal-Wallis analysis of variance followed by Bonferroni post hoc tests were used to compare VP between PBS and AA solutions, with p-values below 0.01 considered significant. Data are shown as mean ± standard mean error (SME) unless otherwise indicated.
Results and Discussion
Figure 1 presents a schematic of AA oxidation to dehydroascorbic acid (DHA) during the anodic phase of the biphasic current pulse. The potential range for AA oxidation is shaded orange on a representative voltage transient (Fig. 1C). This oxidation potential was confirmed with CV (Fig. 1D), where the current response at the oxidation potential increases with increasing AA concentration.
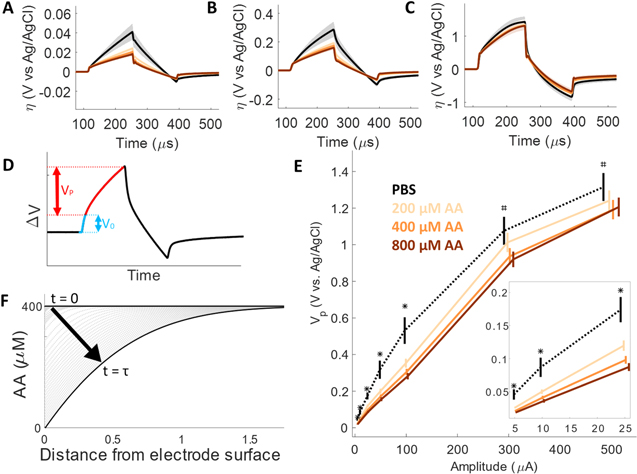
Increasing AA concentration significantly affected electrode polarization during current-controlled biphasic stimulation. The voltage transients during stimulation pulses of increasing amplitude in PBS and AA solutions are shown in Fig. 2 as the overvoltage, which is the potential difference from the zero-current potential. The effect of AA concentration and stimulation amplitude is shown further in Vp calculated from the voltage transients by subtracting the ohmic voltage drop (Fig. 2D). The Vp at increasing stimulation amplitudes in PBS and AA solutions are shown in Fig. 2E. At low stimulation amplitudes (5 to 100 μA) all AA concentrations had significantly lower VP compared to PBS (p < 0.01). At stimulation amplitudes greater than 300 μA only 400 and 800 μM AA had significantly lower VP than PBS (p < 0.01).
Figure 2. Effect of ascorbic acid (AA) oxidation during current-controlled pulses. Voltage of CFE electrodes during biphasic pulses having amplitudes of (A) 5 μA, (B) 50 μA, and (C) 500 μA, in PBS (black) and AA (oranges) solutions. (D) Calculation of the polarization voltage (Vp) calculated by subtracting the ohmic voltage from the maximum anodic potential. (E) Vp vs stimulation amplitude in PBS and AA solutions. *indicates all AA concentrations are significantly lower than PBS (p < 0.01), # indicates 400 and 800 μM AA are significantly lower than PBS (p < 0.01). (F) Schematic of AA concentration over the course of a current pulse, highlighting the transition time ( ), when the concentration reaches 0. All data shown as mean ± SME (N = 8).
), when the concentration reaches 0. All data shown as mean ± SME (N = 8).
Download figure:
Standard image High-resolution imageThe similarity of the AA voltage transients at high stimulation amplitudes (Fig. 2C) and the dependence of VP on concentration and stimulation amplitude (Fig. 2E) can be explained by the depletion of AA at the electrode surface over the course of the pulse. At low amplitudes the flux of AA to the electrode is sufficient to sustain the rate of oxidation needed to support the applied current. At high amplitudes the concentration of AA at the surface is rapidly depleted for all concentrations and the electrode rapidly polarizes to sustain the current. The time when the concentration reaches 0 is referred to as the transition time ( ) described by Sand's equation:
7
) described by Sand's equation:
7

where  is the concentration of the R in the bulk of the solution (mol cm−3), n is the number of electrons transferred, F is Faraday's constant (96,493 coulombs), A is the electrode area (cm2), D is the diffusion coefficient (cm2 s−1), and i is the current amplitude (A). This version of Sand's equation neglects capacitive charging and assumes semi-infinite linear diffusion. The depletion of AA at the electrode surface over time is illustrated in Fig. 2F. The modified equation for cylindrical diffusion is given in the supplemental, and
is the concentration of the R in the bulk of the solution (mol cm−3), n is the number of electrons transferred, F is Faraday's constant (96,493 coulombs), A is the electrode area (cm2), D is the diffusion coefficient (cm2 s−1), and i is the current amplitude (A). This version of Sand's equation neglects capacitive charging and assumes semi-infinite linear diffusion. The depletion of AA at the electrode surface over time is illustrated in Fig. 2F. The modified equation for cylindrical diffusion is given in the supplemental, and  is calculated for increasing stimulation amplitude and AA concentration (Fig S1 available online at stacks.iop.org/JES/168/085501/mmedia). Although the effects of double layer charging increase the
is calculated for increasing stimulation amplitude and AA concentration (Fig S1 available online at stacks.iop.org/JES/168/085501/mmedia). Although the effects of double layer charging increase the  -values, the relationship between AA concentration remain valid.
-values, the relationship between AA concentration remain valid.
Extracellular AA concentration dynamically controlled by both neurons and astrocytes and disrupting AA regulation can have negative consequences. Neurons take up AA where it serves as an antioxidant and is oxidized to DHA. DHA is then released and taken up by astrocytes where it is reduced back to AA and released into the extracellular space for neuronal uptake. The 200 and 400 μM AA used in this work represent steady state concentrations found throughout the brain. The 800 μM represents the increased AA concentrations that coincide with glutamate signaling, which is relevant because recent work has shown significant increases in glutamate signaling in close proximity (0 to 20 μm) to electrodes during stimulation. 8 The increased AA during glutamate signaling typically protects neurons against glutamate-induced oxidative damage. 9 However, if the extracellular AA is oxidized to DHA during stimulation, it will not be available to serve as an antioxidant (Fig. S2). The consequences of increasing extracellular AA oxidation to DHA have been shown in vivo, where decreasing AA concentrations by 50%–70% caused complete behavioral inhibition. 10 Furthermore, DHA concentrations of 200 μM have been shown to significantly decrease the viability of neuronal cells subjected to oxidative stress. 11 Since the environment around implanted electrodes has been shown to be oxidative, 12–14 stimulation-induced increases in DHA could contribute to decreased neuronal viability.
AA is just one of many extracellular molecules that have low oxidation potentials. Studies have examined the role of oxygen reduction during neural stimulation, 15,16 but the oxidation of other biological molecules remained unexplored. This work focused on AA due to its high extracellular concentration, but it is important to note the possible oxidation of other molecules such as dopamine (E1/2 = 0.145 to 0.295 V vs Ag/AgCl), noradrenaline (E1/2 = 0.145 to 0.295 vs Ag/AgCl), and uric acid (E1/2 = 0.345 to 0.445 vs Ag/AgCl). 17 Although these molecules typically have low extracellular concentrations device implantation can affect local tissue concentrations. For example, brain extracellular concentrations of uric acid are typically low (∼1 μM), but increase up to 50 μM after electrode implantation in a size-dependent manner. 18,19
The present study shows AA oxidation contributes to charge injection in vitro; However, the contribution to in vivo stimulation is unclear. The diffusion of AA is decreased in the brain compared to in vitro environments, 20 which will affect the flux of AA to the electrode. Furthermore, the data shown here were measured in PBS which has a different composition than in vivo cerebral spinal fluid. This discrepancy could impact in vivo stimulation AA oxidation. Further studies that better represent the in vivo environment will be needed to better understand the role of AA oxidation in vivo.
Considering the effects of electrical stimulation on biological molecules will become increasingly important as electrode sizes continue to decrease. For a given material, to deliver the same current-pulse smaller electrodes will require greater polarization than larger electrodes. 21 This increased polarization means smaller electrodes will reach the potential threshold for AA oxidation at lower stimulation amplitudes compared to larger electrodes. Thus, strategies to reduce electrode polarization should be considered when stimulating with small electrodes. These strategies can involve both modifications to the electrode (e.g. coatings to improve charge transfer 21 ) and the stimulation protocol (e.g. delivering charge through multiple sites on an electrode array 22 ).
Summary
This study evaluated the role of AA oxidation during charge injection by measuring electrode polarization during current-controlled stimulation in the presence of physiological levels of AA. All concentration of AA significantly reduced the anodic VP at stimulation amplitudes ranging from 5–100 μA. Above 100 μA VP was consistent with the relationship between transition time, concentration, and amplitude. While the role of AA oxidation during electrical stimulation in vivo remains to be elucidated, the present study suggests that AA oxidation can occur during stimulation even at low amplitudes.
Acknowledgments
This work was sponsored by the Defense Advanced Research Projects Agency Biological Technologies Office Targeted Neuraoplasticity Training program under the auspices of Drs. Doug Weber and Tristan McClure-Begley contract number HR0011-17-2-0019, and the National Institutes of Health grant number 1U01NS099700.