Abstract
The surface hydrophilicity of a gas-diffusion-type biocathode, in which bilirubin oxidase was immobilized, was regulated by varying the polymer binder hygroscopicity and UV-ozone treatment time of carbon composite electrodes. The surface hydrophilicity was found to influence its oxygen reduction activity by affecting the penetration of the electrolyte and O2 gas into the electrode layer. The oxygen reduction activity was maximized at an intermediate surface hydrophilicity because of the trade-off between enzyme utilization and oxygen diffusion. A glucose/O2 biofuel cell was fabricated by combining the optimal biocathode with a suitable bioanode fabricated with carboxylic acid-functionalized carbon nanotubes. We demonstrated that the glucose/O2 gas-diffusion-type biofuel cell exhibited a maximum current density of 3.0 mA cm−2 and a maximum power density of 0.66 mW cm−2.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: permissions@ioppublishing.org.
Biofuel cells and biosensors rely on bioelectrocatalytic reactions, as exemplified by the bilirubin oxidase (BOD)-catalyzed oxygen reduction reaction in biofuel cell cathodes. 1–7 Given that the concentration of O2 dissolved in aqueous media is limited to 0.25 mmol dm−3 under ambient condition and is, therefore, far lower than that in air, biofuel cells and other fuel cells (e.g., polymer electrolyte ones) are preferentially fabricated with gas-diffusion-type cathodes and not with sink-type cathodes, as the latter can only utilize oxygen dissolved in the electrolyte. 8–12 The formation of a three-phase electrolyte-electrode-gas interface is very important for realizing efficient biocathodic reactions. To construct an efficient biocathode, one should immobilize the enzyme while maintaining its activity and block electrolyte leakage and ensure that the electrode is gas-permeable. 8 Because of the significant hydrophobicity of carbon surfaces, we have previously employed water-soluble polymer binders used in composite electrodes of lithium-, sodium-, and potassium-ion batteries 13–17 to construct gas-diffusion biocathodes with increased surface hydrophilicity. 18,19 For example, we have demonstrated that activity of biocatalyzed oxygen reduction can be increased by mixing hydrophobic (Nafion) and water-soluble hydrophilic (sodium polyglutamate) ionomers; however, the reason for this increase remains unclear. 19 To address this and other knowledge gaps, in this study, we investigated the effects of polymer binder hygroscopicity and electrode hydrophilization (UV-ozone treatment) time of carbon composite electrode on the oxygen reduction activity of gas-diffusion biocathodes to enhance oxygen reduction activity and O2/glucose biofuel cell performance.
Experimental
Materials
BOD from Myrothecium verrucaria (2.45 U mg−1, Amano enzyme), flavin-adenine dinucleotide–dependent glucose dehydrogenase from Aspergillus sp. (FAD-GDH; 625 U mg−1, Funakoshi), glutaraldehyde (GA; 50% solution in water, Kanto chemical), 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS; Aldrich), 9,10-phenanthrenequinone (PQ; Aldrich), Ketjen black (KB; EC600JD, Lion Specialty Chemicals), carboxylic acid-functionalized multiwalled carbon nanotubes (MWCNT-COOH, >8% carboxylic acid functionalized, 9.5 nm × 1.5 μm, Aldrich), Nafion (5 wt% dispersion in water, Aldrich), sodium poly-γ-glutamate (PGluNa; Wako Chemical), sodium polyacrylate (PANa; n = 22000–66000, Kishida Chemical), sodium alginate (AlgNa; 80–120 cP, Wako Chemical), styrene-butadiene-rubber (SBR; 48.4 wt% Latex form, TRD2001, JSR), Triton-X100 (surfactant, Nacalai Tesque), carbon paper (CP; TGP-H-120, Toray), carbon felt (GF-20–2t, TMIL), D(+)-glucose (Kanto Chemical), and N,N-dimethylformamide (DMF; Wako Chemical) were used as received. Phosphate buffer solutions (PBS; 0.04–1.5 M) were prepared by dissolving KH2PO4 (Nacalai Tesque) and Na2HPO4 (Nacalai Tesque) in water, and their pH was checked with a pH meter. Based on the optimized concentrations reported previously, a relatively high concentration of 1.5 M was used for the biocathode while a low concentration of 0.04 M was used for bioanode. 20–22 The full cell was operated with 0.04 M PBS as the rate-limiting electrode is on the bioanode side judging from their current density. Deionized (DI) water with a conductivity of <1.0 μS cm−1 was obtained using a purification system (Purelite, PRA-0015, Organo) and used throughout all experiments.
Electrode Preparation
Gas-diffusion biocathodes
The employed procedure for the electrode preparation is schematically illustrated in Fig. 1. The electrode slurry was prepared by thoroughly mixing KB powder, ABTS, 5 wt% Nafion dispersion, and 1 wt% aqueous polymer binder (PANa, AlgNa, or PGluNa) in a weight ratio of KB: ABTS: Nafion: binder = 60:20:10:15 in a mortar. A carbon paper sheet (4 × 4 cm2, TGP-H-120, Toray) used as electrode substrate was hydrophilized by 10 min of UV-ozone pre-treatment (SSP16–110, Sen Light) prior to use, and then one side of the carbon paper was spray-coated with the slurry (KB loading = 30 mg) by using an air brush (PS-290, GSI Creos). The carbon surface was oxidized to form carboxyl groups by UV-ozone treatment. 23 The coated carbon paper was dried at 80 °C overnight, and hydrophilized again by UV-ozone treatment for a treatment time of 5, 10, or 15 min. A solution of BOD (1200 μl, 25 mg ml−1) in 0.04 M PBS with pH 7.0 was drop-cast onto the KB-coated CP sheet (2.4 × 2.4 cm2), and the obtained electrode was dried at room temperature (20 °C–30 °C) under reduced pressure.
Figure 1. Schematic illustration of the preparation process of gas-diffusion biocathodes.
Download figure:
Standard image High-resolution imageSurfactant-free bioanode
In our previous studies, surfactant was used to disperse the carbon nanotubes in aqueous solution. 20,24 In this study, we utilized MWCNT-COOH to prepare homogeneous dispersions without using surfactants. MWCNT-COOH and 48.4 wt% SBR latex were dispersed in DI water (MWCNT:SBR = 65:35, w/w) to achieve an MWCNT concentration of 2 g l−1, and the obtained dispersion was sonicated for 30 min to ensure its homogeneity. The carbon felt sheet (2.4 × 2.4 cm2) used as electrode substrate was hydrophilized through UV-ozone treatment (10 min on each side), drop-coated with the above dispersion (1000 μl), and dried at room temperature for 5 h. Drop casting and drying were repeated twice in total to increase the MWCNT loading. The obtained electrode was drop-coated with a solution of 50 mM PQ in DMF (1000 μl), dried in air overnight, drop-coated with 450 μl of [24 mg ml−1 FAD-GDH + 0.5 wt% GA] solution in 0.04 M PBS with pH 7.0, and dried under vacuum for 3 h. The drop-coating procedure for FAD-GDH was repeated twice in total to increase the loading.
Measurements
To examine hydrophilicity of binder polymers, polymer films for moisture content measurement were prepared by drop-casting of polymer solutions onto a Petri dish followed by drying at room temperature. Moisture content measurement was carried by storing the polymer films in a desiccator in which saturated salt solutions were placed to fix desired relative humidity (RH), and the amount of adsorbed moisture was recorded as the weight gain of the polymer film after storage inside the desiccator. The employed salt solutions and the obtained RH values 25 are listed in Table SI (available online at stacks.iop.org/JES/168/074506/mmedia). As equilibrium was reached within 8 h of storage in the desiccator (Fig. S1), the weight change of the polymer film after this time was recorded as the equilibrium moisture content at the RH determined by the corresponding saturated salt solution.
Biocathode surface morphology was observed with scanning electron microscopy (SEM; JCM-6000, JEOL). For specific surface area determination, the biocathode prepared without BOD drop-casting was cut into 3-mm-diameter pieces that were placed into a glass tube and subjected to N2 adsorption/desorption measurements at 77 K (BELSORP-mini II, MicrotracBEL). A sample weighing ∼0.8 g was loaded into the tube. The results were processed using the Brunauer-Emmett-Teller theory.
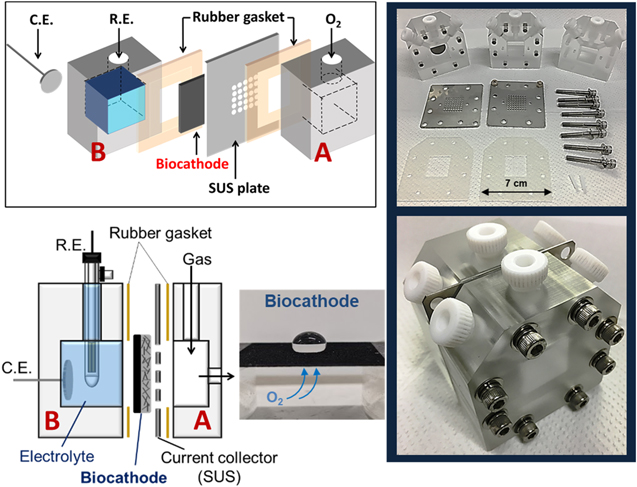
The gas-diffusion-type three-electrode cell (Fig. 2) comprised the working electrode (gas-diffusion biocathode supported by a stainless steel (SUS) plate with a hole), the reference electrode (Ag/AgCl in saturated aqueous KCl), and the counter electrode (Pt-coated Ti disk). N2 and O2 gases were supplied at 500 ml min−1 during measurements to the gas compartment adjacent to the biocathode, which was sandwiched between two rubber sheet gaskets with dimensions of 2.4 × 2.4 cm2. The cell setup used for full cell measurements is described in Fig. S2. Electrochemical measurements were conducted at room temperature (20 °C–30 °C) using a potentiostat (HZ-3000, Hokuto Denko).
Figure 2. Schematic diagram and photographs of the employed gas-diffusion-type three-electrode cell. R.E. and C.E. denote reference and counter electrodes, respectively.
Download figure:
Standard image High-resolution imageResults and Discussion
In this study, we selected four polymers for binders to form KB/CNT layers on carbon paper substrate to examine the effect of different polymer structure and physicochemical properties, especially hydrophilicity, on the enzymatic reaction of oxygen reduction on the composite electrode. Prior to electrode fabrication, the hygroscopicity of pure binder polymer films was evaluated using moisture sorption isotherms (Fig. 3). For all polymers, moisture content was very low after exposure to low-humidity and started to increase at higher humidities, namely at 30%, 40%, and 70% RH in the cases of PANa, PGluNa, and AlgNa, respectively. In contrast, the moisture content of Nafion, a well-known hydrophobic polymer, remained close to 0% over the entire humidity range. The equilibrium moisture content at RH = 98% were in the order of 348% of PANa > 268% of PGluNa > 119% of AlgNa > 6% of Nafion. The high hygroscopicity of PANa was ascribed to its numerous hydrophilic carboxyl groups. Although PGluNa and AlgNa also contained carboxyl groups, they were less hygroscopic than PANa, possibly because of their larger proportion of hydrophobic carbon chains. 26 We, therefore, utilized these binder polymers for fabricating composite electrodes of gas-diffusion biocathodes to study the influence of different hydrophilicity on biocathode performance.
Figure 3. Moisture sorption isotherms for binder films examined at room temperature.
Download figure:
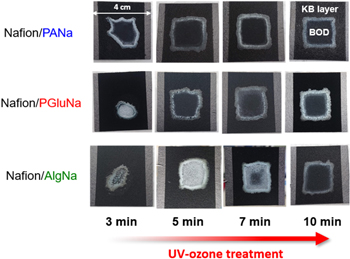
Standard image High-resolution imageFigure 4 shows photographs of KB-coated CP electrodes (KB loading = 30 mg) after BOD solution drop-casting and drying, on which, the KB coat, consisting of KB: ABTS: Nafion: binder = 60:20:10:15, was formed by spray-coating of the slurry. In the case of the least hygroscopic AlgNa, the BOD solution could not be properly drop-cast onto the entire surface of electrode subjected to a 3-min UV-ozone treatment, and BOD solution agglomeration was therefore observed. When the UV-ozone treatment time was increased to 7 min, the undesired agglomeration was suppressed, and after a 10-min treatment, the BOD solution could be successfully dispersed on the entire surface and did not turn cloudy. On the other hand, as the tendency of the BOD solution to aggregate decreased with increasing polymer hygroscopicity, the tendency of the above solution to be uniformly cast on the surface increased. For a treatment time of 5 min, the BOD solution was aggregated on the AlgNa electrode but was uniformly dispersed on PGluNa and PANa electrodes. Thus, both binder hygroscopicity and UV-ozone treatment time affected the uniformity of the aqueous BOD solution cast on the electrode surface.
Figure 4. Images of gas-diffusion biocathodes (BOD/ABTS/Nafion/binder/KB/CP) prepared using PANa, PGluNa, and AlgNa as polymer binders, and different UV-ozone treatment times.
Download figure:
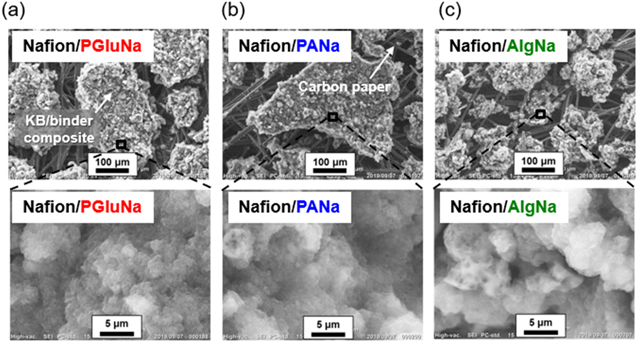
Standard image High-resolution imageThe effects of polymer binder on electrode surface morphology were probed by SEM imaging of KB electrodes before BOD solution casting (Fig. 5). The size of KB aggregates varied from ∼100 μm for AlgNa to ∼500 μm for PANa. However, as the dimensions of the enzyme and mediator molecules are much smaller (<10 nm), 27 we focused on the microscopic structure of the electrode surface. High-resolution imaging of KB particle surface (Fig. 5, bottom) revealed almost no change in their pore structures. As shown in Fig S3, all biocathodes had similar specific surface areas (<20 m2 g−1) much lower than that of KB (1270 m2 g−1), 28 which was ascribed to the presence of lower surface area CP and the coverage of the KB surface and its micropores within the polymer binders. As all of the polymer binders used in this study similarly covered the KB surface and showed similar surface morphology, we expected that changing polymer binder had no significant effect on the ability of the enzyme immobilization on the KB electrode. Thus, the variation of electrochemical properties could not be attributed to the difference in surface area and morphology.
Figure 5. SEM surface images of non-BOD-loaded non-UV-ozone-treated KB electrodes with (a) Nafion/PGluNa, (b) Nafion/PANa, and (c) Nafion/AlgNa binders formed on carbon paper.
Download figure:
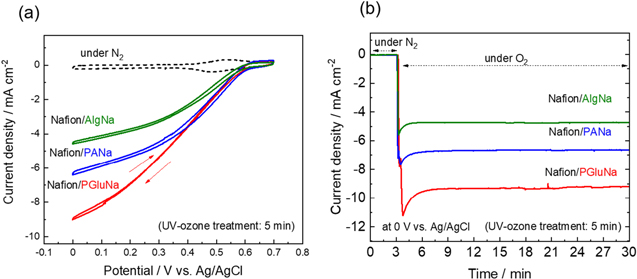
Standard image High-resolution imageFigure 6 shows the oxygen reduction activities measured in the three-electrode cell (Fig. 2) for gas-diffusion biocathodes prepared by using a fixed UV-ozone treatment time of 5 min, revealing that all electrodes could block the electrolyte and prevent its leakage. This observation indicated that the electrodes were sufficiently hydrophobic to separate the electrolyte solution from the gas phase. It should be noted that the electrolyte may leak into the gas layer if the electrode surface is overly hydrophilic; thus, appropriate hydrophilicity is required to obtain stable performance. Nafion was added to all water-soluble binders to maintain a certain degree of hydrophobicity, and a relatively stable oxygen reduction current was produced for at least several hours. 19 Figure 6a shows the cyclic voltammograms obtained under O2 and N2. The blank curve recorded in N2 featured a reversible redox peak of the mediator (ABTS) at ∼0.5 V vs Ag/AgCl. 29 In O2, the reduction current continued to increase from ∼0.6 to 0 V, and the reverse scan did not exhibit voltage hysteresis. This observation indicated that the enzyme-mediator reaction successfully proceeded, i.e., oxygen reduction by BOD, BOD reduction by ABTS, and electrochemical reduction of ABTS at the electrode. The maximum current density decreased in the order of PGluNa > PANa > AlgNa, which was different from the order of binder polymer hygroscopicity in Fig. 3. The reason for this discrepancy is discussed later. Figure 6b shows chronoamperograms recorded at 0 V under N2 and O2, revealing that the reduction observed upon switching from N2 to O2 was stable for more than 20 min, thus indicating stable oxygen reduction occurrence. The magnitude of this current decreased in the order of PGluNa > PANa > AlgNa, in line with the data in Fig. 6a.
Figure 6. (a) Cyclic voltammograms and (b) chronoamperograms of gas-diffusion biocathodes (BOD/ABTS/Nafion/binder/KB/CP) recorded under O2 in 1.5 M PBS at room temperature. Cyclic voltammograms were recorded at 2 mV s−1, and chronoamperograms were obtained at 0 V vs Ag/AgCl.
Download figure:
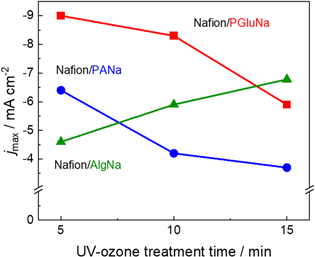
Standard image High-resolution imageTo understand why binder polymer hygroscopicity did not follow the same trend as the oxygen reduction current, we plotted the maximum oxygen reduction current vs UV-ozone treatment time (Fig. 7), with the corresponding cyclic voltammograms shown in Fig. S4. Reproducibility of the voltammograms were shown in Fig. S5. For the least hygroscopic AlgNa, the maximum current density increased with increasing treatment time, whereas the opposite trend was observed for PGluNa and PANa. As UV-ozone treatment results in hydrophilization, it is possible that the oxygen reduction activity of electrodes with slightly hydrophilic binders increased with treatment time, whereas that of electrodes with highly hydrophilic binders decreased with treatment time.
Figure 7. Maximum current density (obtained from cyclic voltammograms presented in the Supporting Information) as a function of polymer binder and UV-ozone treatment time.
Download figure:
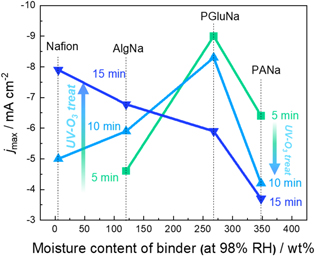
Standard image High-resolution imageFigure 8 shows oxygen reduction activity as a function of binder moisture content at 98% RH (obtained from Fig. 3) and UV-ozone treatment time. In the case of the Nafion-only electrode prepared for comparison, no data could be acquired for a UV-ozone treatment time of 5 min because of difficulty of the drop-casting due to the extremely low hydrophilicity of this electrode. In the cases of low-hygroscopicity (Nafion and AlgNa) binders, oxygen reduction activity increased with increasing UV-ozone treatment time, whereas the oxygen reduction activity decreased with increasing treatment time for the more hygroscopic PGluNa and PANa. Among the electrodes subjected to 5- and 10-min treatments, the highest oxygen reduction activity was observed for the ones with PGluNa, which showed medium hygroscopicity. This finding suggested that oxygen reduction activity was maximized at a certain optimal electrode hydrophilicity, as is frequently observed in the field of electrode catalysis of fuel cell ("volcano plots"). 30–32 The steady decrease in oxygen reduction activity with increasing binder hygroscopicity observed at a UV-ozone treatment of 15 min suggested that excessive hydrophilization decreased electrode performance, in line with the above hypothesis. Similar observations were reported for the gas-diffusion biocathode composed of polytetrafluoroethylene (PTFE) and KB. The electrode with PTFE content 50 wt% showed the highest current density, and further increasing and decreasing PTFE contents lead to activity decay. 33 As PTFE is known as a highly hydrophobic polymer, this observation also agrees with the idea that the surface hydrophilicity of the gas-diffusion bioelectrode governs oxygen reduction activity. Interestingly, the functionalized KB electrode showed shifted optimal PTFE content of 30 wt%, possibly due to hydrophilicity change of the carbon surface through functionalization. 34
Figure 8. Effects of binder moisture content and UV-ozone treatment time on maximum oxygen reduction current density, obtained from cyclic voltammograms at gas-diffusion-biocathode under O2.
Download figure:
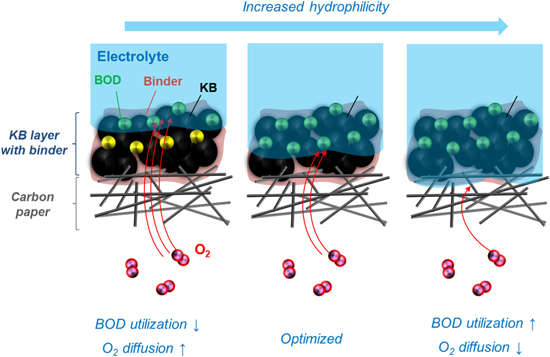
Standard image High-resolution imageA possible explanation of this behavior is provided in Fig. 9. Under the conditions of a less hygroscopic polymer binder and insufficient electrode hydrophilization, the electrolyte does not deeply penetrate the carbon layer, which limits the utilization of the BOD enzyme even though O2 diffusion is sufficient. On the other hand, for a highly hygroscopic binder and excessive hydrophilization, the electrolyte can deeply penetrate the electrode, and the immobilized enzyme can fully contribute to the reaction. However, in this case, the diffusion of O2 into the gas-diffusion layer is inhibited by the excessive electrolyte, and an efficient three-phase interface is not formed, which results in reduced oxygen reduction activity. Similar behavior has been reported for the gas-diffusion electrode for polymer electrolyte fuel cell, showing that too small of an amount of hydrophobic PTFE can lead to decreased performance due to electrolyte flooding at the gas-diffusion layer, whereas too much PTFE also deteriorates the performance because the extent of the three-phase boundary diminishes. 35 Therefore, it is important to provide a gas-diffusion biocathode with adequate hydrophilicity and thus maximize oxygen reduction activity by adjusting both of the hygroscopicity of the polymer binder and the UV-ozone treatment time. The obtained results in this study provide a clear and basic strategy for fabricating high-performance gas-diffusion electrodes.
Figure 9. Schematic of gas-diffusion biocathodes with different hydrophilicities.
Download figure:
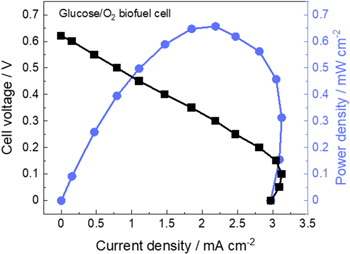
Standard image High-resolution imageThe best-performing gas-diffusion biocathode (binder = PGluNa, UV-ozone treatment time = 5 min) achieved a maximum current density of –9.0 mA cm−2 as seen in Fig. 8 and was used to fabricate a full glucose/O2 fuel cell. In our preliminary test, the employed surfactant (Triton-X100) which was used to disperse CNTs for glucose bioanode in our previous study 20 was found to be unsuitable for full cell demonstration, as it could partially dissolve from glucose bioanode into the electrolyte of full cell and migrate to the biocathode side during operation, decreasing cathode hydrophobicity and thus inducing electrolyte leakage through the carbon paper substrate. However, because of their ability to increase the hydrophilicity of carbon materials and disperse CNTs, surfactants are widely used for bioanode fabrication, 11,20,24 and the presence of surfactant is also considered undesirable in terms of the chemical and mechanical stability of enzyme electrodes. 36 Here, a surfactant-free bioanode was prepared using MWCNT-COOH, as described earlier. The electrochemical properties of the half-cell containing the electrode with FAD-GDH and the mediator (PQ) were evaluated by cyclic voltammetry (Fig. S6). In the blank (i.e., glucose-free) solution, only the redox peak of PQ was observed at ∼0 V; 37,38 however, when glucose was added in the solution, the current continued to increase with progressing oxidation sweeping in the positive direction, which indicated successful biocatalytic mediation and glucose oxidation. The maximum current density observed at the MWCNT-COOH bioanode equaled 3.0 mA cm−2. The results of the full cell polarization test performed with the MWCNT-COOH surfactant-free bioanode in combination with the optimal biocathode are shown in Fig. 10 (cell structure was shown in Fig. S2). The open-circuit voltage of the cell equaled 0.62 V. With increasing current, the cell polarization increased, whereas the cell voltage decreased. The maximal current and power densities were 3.0 mA cm−2 and 0.66 mW cm−2, respectively. These values are not the highest among existing publication, 5 which is possibly due to the limitations associated with our surfactant-free bioanode side. The current densities obtained for the MWCNT-COOH bioanode in the half-cell were smaller than those obtained for the gas-diffusion biocathode, suggesting that the performance of the full cell was limited by the surfactant-free anode side. Although further optimization is needed both for surfactant-free anode and gas-diffusion cathodes, this result suggests that tuning hydrophilicity of gas-diffusion biocathodes through polymer binder selection and UV-ozone treatment and utilizing surfactant-free counter electrodes are essential for improving biofuel cell performance.
Figure 10. Cell voltage vs current density plots of a glucose/O2 biofuel cell with a gas-diffusion biocathode operated at room temperature (20 °C–30 °C). Anode: FAD-GDH/PQ/MWCNT-COOH/CF, cathode: BOD/ABTS/KB/Nafion/PGluNa/CP with UV-ozone treatment for 5 min, and electrolyte: 0.04 M PBS (pH 7.0) with 0.1 M glucose. Cell voltage during constant current discharge was measured by chronoamperometry.
Download figure:
Standard image High-resolution imageConclusions
The effect of surface hydrophilicity on the oxygen reduction activity of gas-diffusion-type biocathodes with variable-hygroscopicity binders (PANa > PGluNa > AlgNa > Nafion) was systematically investigated, and a trade-off relationship was observed between this hydrophilicity (determined by binder hygroscopicity and the UV-ozone treatment time) and oxygen reduction activity. In the case of overly high hydrophilicity, the electrolyte could deeply penetrate the carbon electrode and inhibit O2 diffusion at the three-phase interface, whereas in the case of insufficient hydrophilicity, the enzyme utilization efficiency was low because of the lack of electrolyte penetration into the electrode. The PGluNa-based electrode prepared using a 5-min UV-ozone treatment exhibited the highest oxygen reduction activity (maximum current density of −9.0 mA cm−2 in half-cell configuration at room temperature) and was coupled with a surfactant-free bioanode (fabricated using MWCNT-COOH and glucose dehydrogenase). The resulting gas-diffusion-type glucose/O2 biofuel full cell achieved a maximum current density of 3.0 mA cm−2 and a maximum power density of 0.66 mW cm−2.