Abstract
Anion exchange membranes (AEMs) consisting of quaternary ammonium poly(vinyl alcohol) (QPVA) and poly(diallyldimethylammonium chloride) (PDDA) were prepared by a solution casting method. The influence of the concentration of the chemical crosslinker on the properties and performance of AEMs was investigated. Morphology, chemical structures, thermal and mechanical properties of AEMs were characterized by SEM, FTIR, TGA, and UTM. The performance of AEMs was evaluated by water uptake, swelling degree, ion exchange capacity, and OH− conductivity measurement. The tensile strength, water uptake, and OH− conductivity of AEMs were enhanced with the increase of the crosslinker concentration. By introducing 12.5% glutaraldehyde (GA), the QPVA/PDDA AEMs achieved the highest tensile strength, water uptake, and OH− conductivity of 46.21 MPa, 90.6% and 53.09 ms cm−1 at ambient condition, respectively. The investigations show that crosslinked QPVA/PDDA AEMs are a potential candidate for anion exchange membrane fuel cells.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Fuel cells are regarded as a potential electrical energy generation system to meet the global demand for efficient, clean, and sustainable energy. 1 Polymer electrolyte fuel cells, also known as proton exchange membrane fuel cells (PEMFCs), have been extensively studied because of their remarkable performance and potency compared to other fuel cell types. Nevertheless, there are still technical challenges that need to be overcome for market introduction, e.g. the replacement of platinum group metal catalysts in the electrode layer, the slow reaction kinetics in the acidic state, the complex water management and the permeation of the fuel from the anode to the cathode (fuel crossover). 2,3
The development of anion exchange membrane fuel cells (AEMFCs) has recently received increasing interest. Fuel cells of this type utilize OH− conducting anion exchange membranes (AEMs) as opposed to H+ conducting proton exchange membranes in PEMFCs. This has several advantages over PEMFCs, including a high oxygen reduction rate in the cathode, the possibility to use low-cost non-noble catalysts, low fuel permeability due to the opposite direction of the hydroxide flow and exceptional corrosion resistance in the alkaline conditions. 4,5
Anion exchange membranes contribute considerably to enhancing the performance and efficiency of AEMFCs due to their important roles as hydroxide conductor, electron insulator and gas barrier. 6 In general, AEMs consist of a polymer backbone, immobilized ion functionalized charge groups, and mobile counter-ions. 7
Various AEMs preparation methods have been proposed, including solution casting, 8,9 pore-filling, 10 sol-gel, 11 hot pressing, 12 radiation-induced graft polymerization, 13 and electrospinning. 14 Solution casting is the most widely used membrane manufacturing method. This is because the compatibility of solution casting with many polymers, easy and straightforward to use. 15
In recent years, quaternized polymer-based AEMs have been made from prominent aromatic polymer backbones including poly(sulfone), 16 poly(ether sulfone), 17 poly(ether ether ketone), 18 poly (2,6-dimethyl-1,4-phenylene oxide). AEMs are frequently synthesized by introducing an ion-conducting functional group (e.g., quaternary ammonium, imidazolium, guanidium) with chloromethylation on aromatic rings or bromination on the benzylic methyl group of polymers, followed by quaternization reaction with a cation source (e.g., trimethylamine, 19 1-methylimidazole, 20 guanidinium hydrochloride 21 ). However, AEMs fabricated by the aforementioned method have negative environment effects due to the use of carcinogenic chemicals, e.g. chloromethyl ether for chloromethylation and bromine for bromination, in their synthesis. 22,23 In addition, AEMs based on aromatic polymers-based tend to lose their alkaline stability over time due to the loss of quaternary ammonium groups via various degradation pathways (e.g., nucleophilic displacement (SN2), ylide‐intermediated rearrangements, and Hofmann (E2) elimination), in conjunction with the degradation of the polymer backbone (e.g., Aryl ether bond cleavage of BTMA‐functionalized poly(sulfone) under high‐pH conditions). 24,25 In the interest of avoiding use of carcinogenic chemicals in the synthesis and achieving excellent alkaline stability under alkaline conditions, AEMs produced entirely without chloromethylation or bromination by quaternization directly to the aliphatic polymers chain are a favorable alternative. 22
Poly(vinyl alcohol) (PVA) is a low-cost polyhydroxy-type polymer that possesses exceptional film-forming properties, is hydrophilic, and non-toxic. 26 PVA has a high density of reactive chemical functional groups that are favorable for crosslinking and chemical modification. 27 Furthermore, PVA has a low methanol permeability due to the high selectivity of water over alcohol and has the potential for application in alkaline fuel cells. 28 Hari Gopi and Bhat 22 synthesized AEMs by direct quaternization of PVA using flexible alkyl spacer as a charge carrier. Samsudin and Hacker 29 reported composite AEMs from PVA/PDDA/nano-ZrO2 with blending and solution casting. PDDA was used as an ion-conducting source and nano-ZrO2 as an inorganic additive to improve the mechanical properties of the membrane. Jiang et al. 30 evaluated the introduction of molybdenum disulfide (MoS2) into quaternized poly(vinyl alcohol)/chitosan composite AEMs. The mechanical strength of the membrane was enhanced, and the methanol permeability decreased with the addition of MoS2.
Pristine PVA membranes without modification swell in water and are unstable, which affects the integrity of the films and reduces their performance. Therefore, crosslinking treatment of the PVA chain is required to decrease the hydrophilicity of membrane and to form interpenetrating polymer networks that may entrap biomolecules, including other polymers or ionomers. 31 In general, crosslinking techniques can be divided into physical and chemical crosslinking. Heat treatment can be used as physical crosslinking. When PVA is heat-treated, the energy supplied is used to modify the spatial structure of the chains and to create stronger hydrogen bonding between hydroxyl groups, resulting in higher crystallinity. In contrast, chemical crosslinking relies on the reaction between the crosslinker and the hydroxyl groups of the PVA, which form strong covalent bonds. 31 Various chemicals including dialdehydes, 31,32 dicarboxylic acids, 33 dianhydride, 34 citric acid, 35 succinic acid, 36 sulfosuccinic acid, 37 trisodium trimetaphosphate 38 have been studied as crosslinkers for PVA modification. Among them, glutaraldehyde has been widely used as a chemical crosslinker due to its ability to create a covalent bond between the hydroxide groups of PVA. 31,39
In this work, the AEMs based on QPVA and poly(diallyldimethylammonium chloride)(PDDA) blends were successfully prepared and characterized. Quaternary ammonium from QPVA and PDDA was utilized as anion conducting sources. PDDA can provide quaternary ammonium functional groups that are able to offer OH− as charge carriers in its structure. 40 In addition, the degradation of the quaternary ammonium through SN2 nucleophilic substitution in alkaline environments can be hampered due to the high steric hindrance caused by the cyclic structures of PDDA. 41 PDDA is also not harmful to the environment and soluble in water. Du et al. fabricated semi-interpenetrating network (SPIN) anion exchange membranes (AEMs) based on quaternary ammonium polyvinyl alcohol (QPVA) and PDDA by direct chemical crosslinking. 42 In this method, glutaraldehyde was added as a crosslinker to the QPVA-PDDA polymers before casting the membrane. The produced membranes have relatively good conductivity and excellent mechanical properties, but the swelling capacity is quite high at over 100%. Samsudin et al. introduced a combination of thermal crosslinking indirect crosslinking of QPVA-PDDA membrane, resulting in AEM with better ionic conductivity and swelling properties. 8 In this study, we continued the previous work of QPVA/PDDA anion exchange membranes. Due to the low stability of PVA in water and to enhance the mechanical strength of AEMs, a combination of thermal and chemical crosslinking of the polymer chains of the membranes was established. The effect of the concentration of the chemical crosslinker for QPVA/PDDA AEMs was investigated.
Experimental
Materials
Poly (vinyl alcohol) (PVA, Mw = Mw 89,000–98,000 g mol−1, 99% hydrolyzed) was obtained from Sigma-Aldrich. Poly(diallyldimethylammonium chloride) (PDDA, Mw = 400,000–500,000, 20 wt.% in H2O) was purchased from Sigma-Aldrich. Glycidyltrimethylammonium chloride (GTMAC, ≥90%) was supplied by Sigma-Aldrich. Glutaraldehyde (GA, 25 wt.% in H2O) was acquired from Sigma-Aldrich. Potassium hydroxide (KOH, Titrisol®) was acquired from Merck. Ultra-pure water with a resistivity of 18 MΩ-cm was produced with the Barnstead E-PURE 4-Module system.
Preparation of quaternized-PVA (QPVA)
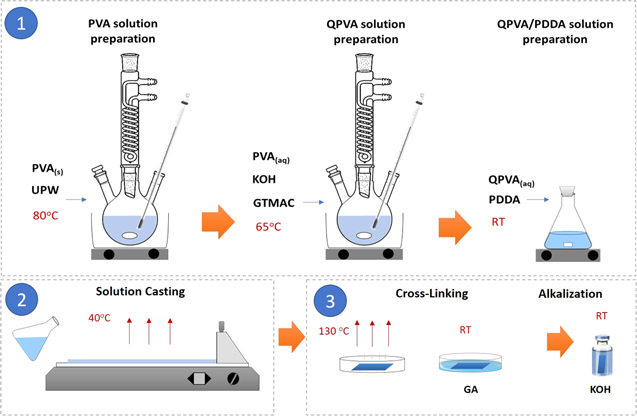
QPVA was prepared according to Ref. 30. First, 5 g PVA was dissolved in 95 ml distilled water to obtain a homogeneous viscous solution. Then 10 ml of KOH solution (2 M) and 10 g GTMAC were added to the viscous solution under continuous stirring for 4 h at 65 °C for the quaternization of PVA chains. The resulting viscous mixture was washed with anhydrous ethanol to obtain the precipitate and dried at 40 °C for 24 h. Finally, the white QPVA particles were obtained. Figure 1 shows the quaternization reaction of PVA.
Figure 1. Quaternization reaction of PVA.
Download figure:
Standard image High-resolution imageMembrane preparation
AEMs were prepared using a solution casting method. QPVA was dissolved in ultra-pure water at 80 °C–90 °C while continuously stirring to acquire 10 wt.% QPVA. Then 10 wt.% PDDA was mixed with the QPVA, in a weight ratio of 1:0.5 with continuous stirring for 4 h. The resulting solution was cast using an Elcometer 4340 automatic film applicator with an opening casting knife of 50 μm and then dried for 24 h at 40 °C. Subsequently, the dried membranes were peeled from the glass substrate and stored in a sealed compartment. The membranes were annealed at 130 °C for one hour to initiate thermal crosslinking, followed by immersion in crosslinking solutions of varying concentrations to induce chemical crosslinking. Different concentrations of the crosslinker solutions were obtained by diluting commercial GA 25 wt.% (in H2O) with acetone and adding a small quantity of HCl as a catalyst. To observe the change in membrane dimension due to the crosslinking treatment, the degree of swelling through-plane (SDtCL) and in-plane (SDiCL) after crosslinking was calculated using Eqs. 1 and 2, respectively.
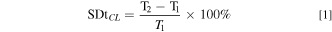
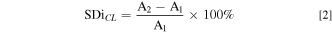
where T1 and A1 are the thickness and surface area of the membrane before crosslinking. T2 and A2 are the thickness and surface area of the membrane after crosslinking. Table I shows the content of the GA-based crosslinker solution. Figure 2 illustrates the procedure of QPVA/PDDA AEMs preparation.
Table I. Crosslinker solution composition.
| Cross-linker | Glutaraldehyde (wt.%) | Acetone (wt.%) | Water (wt.%) |
|---|---|---|---|
| GA 2.5 | 2.5 | 90.0 | 7.5 |
| GA 5.0 | 5.0 | 80.0 | 15.0 |
| GA 7.5 | 7.5 | 70.0 | 22.5 |
| GA 10.0 | 10.0 | 60.0 | 30.0 |
| GA 12.5 | 12.5 | 50.0 | 35.5 |
Figure 2. The procedure for QPVA/PDDA AEMs preparation.
Download figure:
Standard image High-resolution imageStructure characterization
The Fourier transform infrared spectroscopy (FTIR) analysis was performed with an IR-spectrometer (Bruker ALPHA, USA) to identify the functional structure of the AEMs. The IR-spectra were recorded in the wavenumber range 500−4000 cm−1 with a resolution of 4 cm−1. The surface morphology of membranes was imaged using an SEM measurement (Zeiss Supra 55VP) with a voltage of 15 kV. The thermal stability of AEMs was assessed by thermogravimetric analysis (TGA) using STA 449 C apparatus (Netsch). The TGA analysis was conducted at a heating rate of 10 °C min−1 from 25 °C to 600 °C under an N2 gas flow of 40 ml min−1. The mechanical properties of the AEMs were measured using a ZwickRoell Z010 universal testing machine. The samples with a size of 10 × 20 mm were elongated at the strain rate of 5 mm min−1 at ambient conditions.
Swelling properties
The water uptake (WU), swelling degree through-plane (SDt) and the swelling degree in-plane (SDi) of the membranes were calculated by measuring the change in weight and volume of the AEMs before and after water immersion, respectively. The membrane samples were weighted (Wd). Furthermore, their thicknesses (Td) and surface areas (Ad) were measured. The samples were then immersed in ultrapure water at ambient conditions for 24 h. The weight (Ww), thickness (Tw), and surface area (Aw) of the swelling membranes were measured immediately after the gentle elimination of the surface water. The WU, SDt, and SDi of the membranes were calculated using Eqs. 3–5, respectively.
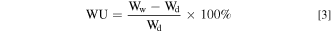
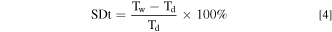
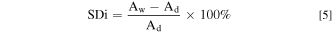
Ion exchange capacity (IEC)
The IEC was evaluated using a back-titration method. First, membrane samples were weighted (Wd). Furthermore, an alkalization was performed by immersing the membrane samples in 1.0 M KOH solution for 24 h to convert the Cl− into the OH− form of the AEMs. The membrane was then further immersed in ultra-pure water for 24 h to wash out the remaining KOH residue. The membrane was then equilibrated with a 0.1 M HCl solution (CHCl) for 24 h. The titration was performed with 0.1 M NaOH titrant solution. The consumed volumes of NaOH from the blank (Vb) and AEMs(Vm) samples were recorded. The IEC value was determined by Eq. 5:
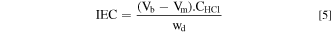
OH− conductivity
The hydroxide conductivity was determined by the method of electrochemical impedance spectroscopy. The AEMs samples were alkalized in a 1.0 M KOH solution for 24 h and then immersed in ultra-pure water for 24 h to remove excess KOH. The 2.5 × 1.0 cm samples were placed in the four-electrode configuration of the conductivity clamp (Bekktech BT110 lLC, Scribner Associates, USA) in ultra-pure water at room temperature. The impedance was measured using Gamry Reference 600 potentiostat (USA) over a frequency range of 0.1 Hz–10 kHz with a 50 mV amplitude. The membrane resistance (Rm) was obtained from the intercept of the Nyquist curve with the real axis Zreal. The OH− conductivity was calculated according to Eq. 6.
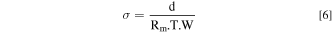
Where d is the membrane length between electrodes, T is the thickness, and W is the width of the membrane.
Results
Morphology
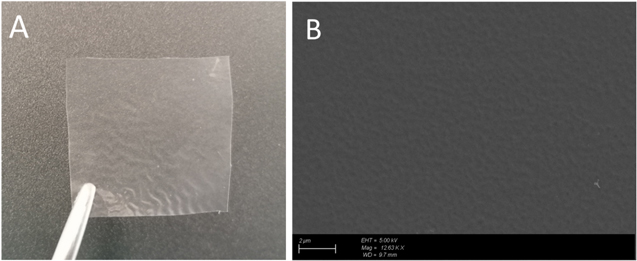
Figure 3 depicts the image and surface morphologies of the QPVA/PDDA anion exchange membrane. The appearance of the transparent membrane can be seen in Fig. 3a. From the SEM images (Fig. 3b), it can be observed that the QPVA/PDDA AEMs shows a smooth, uniform and defect-free surface, thus indicating that QPVA and PDDA are compatible each other.
Figure 3. (A) QPVA/PDDA AEMs., (B) SEM image of QPVA/PDDA AEMs.
Download figure:
Standard image High-resolution imageChemical structure
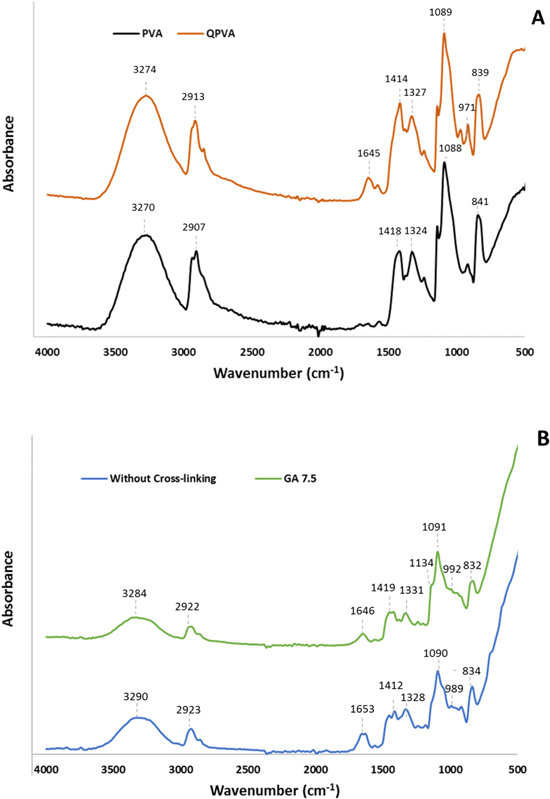
FTIR analysis was performed to identify the most important functional groups in the structures of the AEMs. This analysis is beneficial to confirm the change in the structural composition of PVA after quaternization and crosslinking reaction of PVA polymer membranes. 42 Figure 4A depicts the typical FTIR spectra of pristine PVA and QPVA. The main peaks of PVA were observed at 3270 cm−1, 2906 cm−1, 1418 cm−1, 1324 cm−1, 1088 cm−1, and 842 cm−1. These peaks are recognized as the -OH stretching vibration, C-H stretching vibration, CH2 stretching vibration, C-O carbonyl stretching vibration, another -OH stretching vibration, and C-H bending vibration. On the other hand, similar peaks were found for the QPVA polymer in addition to new peaks located at 1645 cm−1 and 971 cm−1, which refer to amine and typical C-N stretching vibration, respectively. 43,44 In addition, physically, pristine PVA that dissolves in hot water becomes more stable after crosslinking with GA solution resulting in swelling properties less than 31% (Table II). This indicates that quaternary ammonium groups are successfully attached to the polymer chain of the PVA.
Figure 4. FTIR spectra. (A) PVA and QPVA polymer, (B) QPVA/PDDA Membrane with and without crosslinking.
Download figure:
Standard image High-resolution imageTable II. Physicochemical parameters of QPVA/PDDA membranes.
| Cross-linker | IEC (mmol·g−1) | WU (%) | SD-t (%) | SD-I (%) | TS (MPa) | Eb (%) |
 (mS·cm−1) (mS·cm−1) |
|---|---|---|---|---|---|---|---|
| — | — | — | — | — | 26.56 | 269.40 | — |
| GA 2.5 | 2.01 | 38.0 | 12.4 | 20.5 | 36.25 | 6.55 | 20.78 |
| GA 5.0 | 2.19 | 42.9 | 14.8 | 20.7 | 39.10 | 6.60 | 28.47 |
| GA 7.5 | 2.37 | 60.1 | 18.5 | 21.5 | 44.89 | 13.80 | 42.17 |
| GA 10.0 | 2.13 | 72.7 | 27.5 | 21.7 | 45.57 | 23.00 | 50.77 |
| GA 12.5 | 2.12 | 90.6 | 30.3 | 22.2 | 46.21 | 18.05 | 53.09 |
Figure 4B represents the FTIR spectra of the QPVAPDDA membrane without and with GA 7.5% treatment. The main peaks of non-cross-linked QPVAPDDA were detected at 3290 cm−1, 2923 cm−1, 1653 cm−1, 1412 cm−1, 1328 cm−1, 1090 cm−1, 989 cm−1 and 842 cm−1. These peaks are identified as the –OH stretching, C–H stretching, Amine, CH2 stretching, C–O stretching, another –OH stretching, C–N stretching, and C-H bending vibration, respectively. In comparison to non-cross-linked QPVAPDDA, similar peaks were observed for the crosslinked QPVA polymer. The decreasing of –OH groups intensity at 3284 cm−1 and the appearance of a small peak at 1134 cm−1 in accordance with C–O–C groups indicate the chemical crosslinking reaction between the –H groups of PVA and the –CHO groups of GA was successfully formed. 30,40,45
Thermal analysis
The thermal behavior of QPVA/PDDA AEMs with different concentrations of GA is described in Fig. 5. It can be observed that all thermogravigrams display a similar trend with three major weight loss stages. The first stage emerges between 25 °C and 65 °C, with the total mass loss about 5%, indicates the discharge of water molecules in the membranes and moisture absorbed from the atmosphere. The second stage occurs between 200 °C and 300 °C. The total mass loss at this stage in TGA was about 12%. This can be due potentially to the degradation of quaternary ammonium cationic groups, the cleavage of crosslinking bonds, and the breaking of some C–O and C–C bonds from QPVA and PDDA, respectively. 42,44,46 The third stage arises between 310 °C and 470 °C with a total mass loss about 69% and is related to the degradation of QPVA and PDDA polymer backbones. 46 It can be observed that the introduction of different concentrations of GA as crosslinking did not significantly affect the thermal stability of QPVA/PDDA AEMs. Furthermore, according to the thermogravimetric analysis results, QPVA/PDDA AEMs possess sufficient thermal stability for the application in low temperature fuel cells.
Figure 5. Thermogravigrams profiles of QPVA/PDDA AEMs with different concentrations of GA.
Download figure:
Standard image High-resolution imageMechanical properties
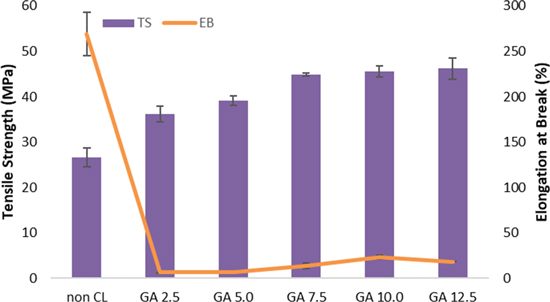
An important feature that must be taken into account when preparing the membrane electrode assembly is the mechanical property of the AEMs. Sufficient mechanical strength is required for the membrane to withstand the pressing in the assembly process and the operation of fuel cells. The mechanical properties of QPVA/PDDA membranes with different concentrations of GA crosslinker have been evaluated with respect to tensile strength and elongation at break. The results are depicted in Fig. 6, and the data in Table II. The membrane without crosslinking showed a tensile strength of 26.56 MPa and an elongation at break of 269.40%. With the introduction of GA crosslinking from 2.5 wt.% to 12.5 wt.%, the tensile strength of QPVA/PDDA membranes improved from 36.25 MPa to 46.21 MPa. It was concluded that GA crosslinking could enhance the tensile strength of QPVA/PDDA membranes. Interestingly, with the addition of GA 2.5 wt.%, the elongation at break of membrane decreased sharply from 269.40% to 6.55%. Subsequently, on an increase of the GA from 2.5 wt.% to 12.5 wt.%, the elongation at break of membrane increases from 6.55% to 23%. With a further increase of the GA concentration to 12.5 wt.%, the elongation at break of the membrane decreased to 18.05%. The improvement in tensile strength and reduction in elongation at break was attributed to the crosslinked networks formed within QPVA, which restricted the mobility of the polymer chains in membranes. 39
Figure 6. Tensile strength and elongation break of QPVA/PDDA AEMs.
Download figure:
Standard image High-resolution imageWater uptake and swelling degree
In order to achieve high OH− conductivity, the presence of water in the AEMs is required. The increase in the conductivity of the AEMs is strongly influenced by the rise in WU. Water clusters can increase the ionic conductivity by forming transport passages for anions within the AEMs. 40 In any case, exaggerated water uptake can cause intensive swelling of the membranes, which leads to a deterioration of the dimensional stability and possibly complicates the production of the membrane electrode assembly as the contact between the active layer, the electrodes and the AEM is reduced. 9 The excessive swelling might dilute the concentration of charge carriers to such an extent that the conductivity of the membrane decreases. 47
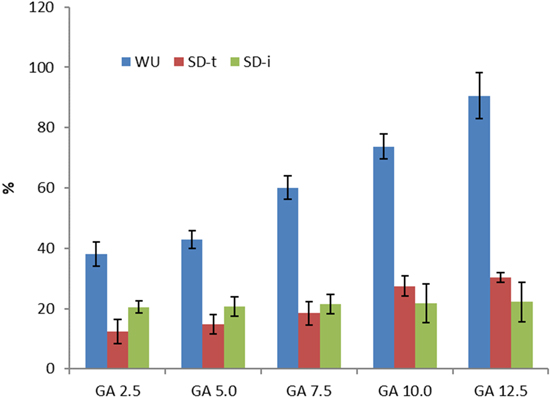
Table III and Fig. 7 demonstrate the water uptake (WU), swelling degree through-plane (SD-t), and swelling degree in-plane of the QPVA/PDDA AEMs. The membrane with the addition GA 2.5 crosslinking showed a WU of 38%. The WU then increases slightly with the addition of GA 5.0 to 42.9%. Subsequently, through the addition of GA 7.5, GA 10.0, and GA 12.5, WU enhances quite dramatically to 60.1%, 72.7%, and 90.6%, respectively. The membrane with the addition of GA 2.5, exhibits the SDt and SDi of 12.4% and 20.5%, respectively. Eventually, SDt increases with the addition of GA 5.0, GA 7.5, GA 10.0, and GA 12.5, resulting in SDt of 14.8%, 18.5%, 27.5%, and 30.3%, respectively. On the other hand, the SDi increases only slightly to 20.7%, 21.5%, 21.7%, 22.2%, with the addition of GA 5.0, 7.5, GA 10.0, and GA 12.5, respectively. This means that swelling is more likely to occur vertically.
Table III. IEC, WU, SD and OH− conductivity reported in the literature for PVA-based AEMs.
| Materials | IEC (mmol g−1) | WU (%) | SD (%) | Conductivity (mS cm−1) | References |
|---|---|---|---|---|---|
| QPVA/HDT | 0.73 | 64.0 | 25.0 | 4.8 (RT) | 22 |
| PVA/PDDA/ZrO2 | 0.54 | 73.0 | 29.0 | 31.6 (RT) | 29 |
| PVA/PDDA/MWCNT | 0.89 | 125.0 | — | 30.4 (RT) | 44 |
| PVA-PY-DL4 | 2.30 | 110.0 | 130.0 | 2.5 (RT) | 48 |
| QPVA/PDDA1-GA | 1.12 | 128.0 | 115.0 | 29.1 (RT) | 42 |
| PVA-Im/PC | 1.30 | 0.7 | 0 | 7.8 (RT) | 49 |
| PVA/PQ-10 | 1.35 | 59.2 | 8.5 | 40.0 (40 °C) | 50 |
| QPVA/PDDA/GA12.5 | 2.12 | 90.6 | 30.3 | 53.1 (RT) | This work |
Figure 7. Water uptake and swelling degree of QPVA/PDDA AEMs.
Download figure:
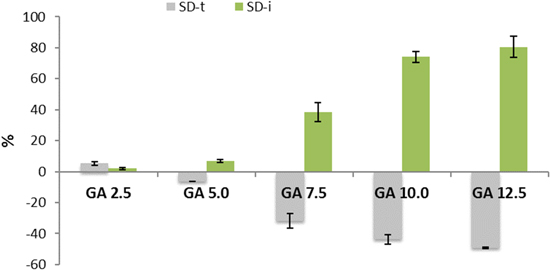
Standard image High-resolution imageThe rise of WU and SD can also be explained by the change of membrane dimension during the crosslinking process (Fig. 8). After treatment with GA 2.5, the membrane has an SDtCL and an SDiCL of 19.2% and 2.3%, respectively. Subsequently, the SDtCL decreases to 2.6%, −5.1%, −33.3%, −42.9% when treated with GA 5.0, GA 7.5, GA 10.0, and GA 12.5 respectively. By contrast, the SDiCL increases to 15.1%, 15.6%, 41.7%, 66.5% when treated with GA 5.0, GA 7.5, GA 10.0, and GA 12.5, respectively. This implies that the membrane becomes wider and thinner and the surface area (bases) increases. The surface area growth (bases) induces an increased contact of the membrane with water and the rise in free volume between the polymer chains, which leads to an increased capacity of the water in the membrane. The comparison of WU and SD in this work and other previous work can be seen in Table III.
Figure 8. Swelling degree of QPVA/PDDA AEMs during crosslinking.
Download figure:
Standard image High-resolution imageIon exchange capacity and ion conductivity
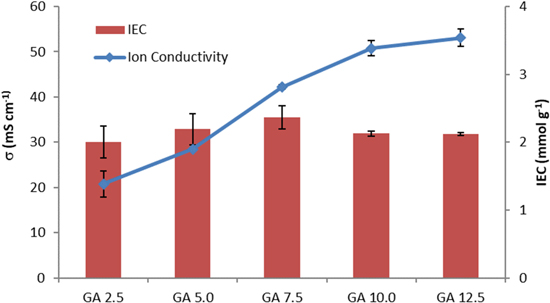
Ion exchange capacity (IEC), an essential parameter of the AEMs, reveals information about the number of ion-exchangeable groups in the AEMs, which is relevant for the OH− conductivity. 41 Table III and Fig. 9 illustrate the ion exchange capacity and OH− conductivity of QPVA/PDDA AEMs at room temperature. The IEC values of AEMs are within the narrow range of 2.01–2.37 mmol·g−1, which implies that the treatment with GA crosslinker has not significantly affected the IEC of the AEMs. The IEC values might be substantially influenced by the ion-conducting substance from QPVA and PDDA, which has a constant concentration and ratio for each membrane.
Figure 9. Ion exchange capacity and OH− conductivity QPVA/PDDA AEMs (ambient condition).
Download figure:
Standard image High-resolution imageThe OH− conductivity is one of the most critical properties of AEMs and corresponds to the performance of fuel cells. According to Fig. 9 and Table III, the membrane GA 2.5 showed the OH− conductivity of 20.78 mS·cm−1. Finally, the OH− conductivity increases to 28.47, 42.17, 50.77, and 53.09 mS·cm−1 when treated with GA 5.0, GA 7.5, GA 10.0, and GA 12.5, respectively. The increase of OH− conductivity could be due to the increase in water content present in the membrane (water uptake). In accordance with the Grotthuss mechanism, water clusters are able to provide transport channels for anions within the AEMs, resulting in enhancement of the OH− conductivity. 51
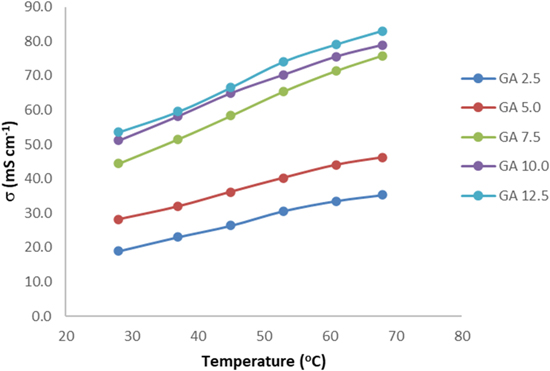
Figure 10 displays the temperature dependence of the OH− conductivity of the QPVA/PDDA AEMs at different concentrations of GA crosslinking treatment. The OH− conductivity of the AEMs reveals a positive increase with the elevation of temperature in the range of 30 °C–80 °C. The rise of temperature can also enhance the mobility of OH− in the AEMs. Furthermore, the increase in temperature can also induce the expansion of free space in the membrane, which is beneficial for ion transport. 52 The highest OH− conductivity of 82.9 mS·cm−1 was achieved for the QPVA/PDDA membrane with GA 12.5 treatment at 80 °C.
Figure 10. Temperature dependences of OH− conductivity for QPVA/PDDA AEMs.
Download figure:
Standard image High-resolution imageDue to the contact of the membranes with ambient air during the measurement of the conductivity of AEMs, carbonation might partially occur. This process arises in which OH− as a counter-anion of quaternary ammonium functional groups at QPVA and PDDA react with CO2 present in the air resulting in CO3 2− and HCO3− based on reactions 1 and 2, respectively. 53 The foremost outcome of this carbonation process is a significant decline in the effective OH− conductivity in the AEM. 54
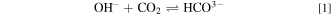
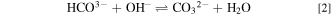
The presence of CO3 2− and HCO3− have a negative effect by reducing the effective conductivity of the membrane. This phenomenon occurs due to the larger ionic radius of these anions than OH−, which leads to lower diffusion coefficients and mobility. 53 This carbonation process reduces the OH− conductivity, but comparing the OH− conductivity with the published results of PVA-based AEMs (Table III), the conductivity is still relatively higher, indicating the potential of this AEM.
Alkaline stability
The issue in membrane stability, especially alkaline stability, is identified as the main obstacle affecting the performance of fuel cells. 55 The primary cause for the poor stability of AEMFCs performance is the continuous chemical degradation that occurs in the anion conducting polymer due to the alkaline environment of the fuel cell during the operation. Under the harsh pH environment of AEMFCs, the molecular structure of the anion conducting polymers (both in the membranes and ionomers) breaks down due to high reactivity of the OH− with the quaternary ammonium functional groups. This degradation, which leads to an unfavorable decrease in the ion exchange capacity of AEM, results in a reduction of OH− conductivity, an increase in cell resistance and an abrupt decline in performance. 56
In this work, the alkaline stability of the QPVA/PDDA AEMs with GA 7.5 crosslinking process was investigated by immersing the membranes in 5.0 KOH solutions at room temperature for a specific time. Finally, the OH− conductivity of the AEMs was measured after repeated washing with ultrapure water at 30 °C to completely remove the excess KOH on the surface. Figure 11 shows that the OH− conductivity of the QPVA/PDDA membrane rises for the first 24 h, which might be caused by the increased ion-conducting units as a part of the KOH enters the membrane at high concentrations of KOH in an alkaline environment. 44 Figure 11 shows that the OH− conductivity of the QPVA/PDDA membrane rises for the first 24 h, which might be caused by the increased ion-conducting units as a part of the KOH enters the membrane at high concentrations of KOH in an alkaline environment. 44 This could also be due to the fact that during activation with 1 M KOH before the initial conductivity test, not all of the counter ion Cl– in AEMs was exchanged for OH–. When immersed for 24 h with 5 M KOH, the remaining Cl– was exchanged with OH– due to the higher concentration than 1 M KOH solution, so that the conductivity increased. These results are also similar to those reported by Zhou et al. 44
Figure 11. The alkaline stability of QPVA/PDDA AEMs with GA 7.5 treatment.
Download figure:
Standard image High-resolution imageThe OH− conductivity then declines for the next 48 h. Subsequently, the OH− conductivity decreases slightly with time. The decreases of OH− conductivity result from the well-known E2 Hoffman degradation and SN2 nucleophilic substitution reaction, which lead to the decomposition of some functional quaternary ammonium groups. 57,58 After treatment in the solution for 180 h, the OH− conductivity of the AEMs remains at 49.51 mS·cm−1, showing that QPVA/PDDA AEMs have good stability. The thermal and chemical crosslinking modification possibly accounts for the improvement of OH− conductivity stability. 27
However, it should be noted that at pH 5, the hydration level is still considered relatively high (λ = 11). 59 When operating AEMFCs, low water content around the cathode causes the formation of hydroxides with low hydration levels. Lower hydration levels lead to increased OH− nucleophilicity, which increases the kinetics of degradation, even for ionomers, which are considered stable highly alkaline solutions. 56,60 Therefore, further studies are needed for the application of this membrane in fuel cells under real conditions.
Conclusions
Anion exchange membranes (AEMs) consisting of quaternary ammonium poly(vinyl alcohol) (QPVA) and poly(diallyldimethylammonium chloride) (PDDA) were prepared by solution casting technique. Thermal and chemical crosslinking was introduced into the membranes as a post treatment. FTIR spectra show that quaternary ammonium groups are successfully bound to the polymer chain of the PVA and the crosslinking bridge between the –OH groups of PVA and the –CHO groups of the glutaraldehyde was formed. The three major weight loss steps from the thermogravigrams verify the AEMs have sufficient thermal stability for low temperature fuel cell applications. By introducing 12.5% glutaraldehyde (GA), the QPVA/PDDA AEMs obtained the highest tensile strength, water uptake, and OH− conductivity of 46.21 MPa, 90.6% and 53.09 ms cm−1, at ambient condition, respectively. This study demonstrates that the crosslinked QPVA/PDDA AEMs are a potential candidate for use in AEMFCs.
Acknowledgments
The authors thank the Austrian Science Fund (FWF) for the financial support of this research under the project number I 3871-N37, Kemdikbud (Indonesia) and OeAD (Austria) for the IASP scholarship and Graz University of Technology for the Open Access funding.