Abstract
Production of ammonia through coupling renewable energy with electrolysis cells will undoubtedly aid in reducing carbon dioxide emissions from the ammonia production industry. However, if the cost for electrochemical routes does not reach a Haber-Bosch parity point, then it is unlikely that electrochemical ammonia synthesis will become industrially viable. This promotes a strong need for analyses that explore the economics of various system designs and production scales, to assess what systems and scales can attain Haber-Bosch price parity. Here, we aim to define the Haber-Bosch parity targets for various production scales. We then explore the economic considerations for two electrochemical systems for ammonia synthesis. The first system contains a single electrolysis cell where nitrogen and water are the sole reactants. The second system explores a two-staged electrolysis system. The first stage consists of a water electrolysis cell where water serves as the reactant and hydrogen and oxygen are the products. The second stage consists of a nitrogen electrolysis cell where the reactants are nitrogen and hydrogen and ammonia is the product. We emphasize the important role production scale plays in meeting Haber-Bosch price parity, and highlight the key challenges for electrochemical ammonia production.
Export citation and abstract BibTeX RIS
Technologies that aid in the industrialized production of ammonia are essential in order to meet the rising demand for nitrogen-based fertilizers. Today, one thermocatalytic process (Haber-Bosch) is responsible for the production of 150 million tons of ammonia per year,1 most of which (85%) is used as a feed-stock for the creation of fertilizers. The Haber-Bosch process requires the use of high temperatures (700 K) and pressures (100 bar) to achieve a desirable rate of production. The economical viability of the Haber-Bosch process; however, requires production scales that reach thousands of metric tons per day per chemical plant.2 This is because the infrastructure required to sustain high temperatures and pressures leads to large capital investments which favor large economies of scale. The Haber-Bosch process also heavily relies on the use of fossil fuels to produce hydrogen. This results in significant energy consumption (540 kJ/molNH3 to 800 kJ/molNH3) and carbon dioxide emissions (1.5  ).3–7 For this reason, the Haber-Bosch process accounts for approximately 2% of the total global energy consumption and 1.2% of the greenhouse gas emissions worldwide.3,8,9
).3–7 For this reason, the Haber-Bosch process accounts for approximately 2% of the total global energy consumption and 1.2% of the greenhouse gas emissions worldwide.3,8,9
Growing concerns regarding the environmental impact of the Haber-Bosch process have encouraged the development of alternative technologies aimed at producing renewable ammonia.10–12 Electrochemical production of ammonia from water and air at near ambient conditions using renewable energy is one heavily investigated approach, which may aid in reducing the CO2 footprint of ammonia production. According to the Department of Energy (DOE), electrochemical technologies for carbon-neutral fuel production have to achieve energy efficiencies higher than 60% and faradaic efficiencies (FEs) higher than 90% while operating at current densities above 300 mA cm−2 in order to meet economic viability requirements.13 These targets are based on the electrochemical production of chemical fuels with a fuel energy cost below $0.3 kWh−1. However, the current state of the art low-temperature electrochemical ammonia synthesis systems achieves energy efficiencies which approach ∼1% with FEs between 0.1% to 10% at less than 10 mA cm−2 (Reference experimental survey in SI). This results in a fuel energy cost greater than $10 kWh−1. There are a small number of investigations that report energy efficiencies greater than 10% with high FEs, but this often occurs at low current densities (<1 mA cm−2) or through the use of ionic liquid and non-aqueous based electrolytes which are capable of suppressing hydrogen evolution.14 Operating at low current density (<1 mA cm−2) is impractical from an economic point of view as the reactor size and capital cost exponentially increase with a decrease in the current density.15 Ionic liquid and non-aqueous electrolytes while effective at lab-scales, often have high cost and safety considerations and have yet to be investigated at larger scales.16,17 Therefore, independent of the route to attaining renewable ammonia, performance, cost, and safety must be considered holistically in order to design effective routes for electrochemical synthesis of ammonia.
While performance is most commonly determined through efficiency (energy, faradaic), catalyst selectivity, and production rate (current density), the most pressing economic consideration is the levelized cost of the ammonia (LCOA). The LCOA represents the average net present value of ammonia production over the lifetime of the production facility. Haber-Bosch plants can produce ammonia at incredibly low prices, sometimes as low as 160 dollars per ton of ammonia. This is primarily because of the high production capacities which often exceed 2,000 tons of ammonia per day.2 However, the production price of ammonia is often far lower than the market price due to the additional costs of transportation and storage.11 The market price of ammonia is around 600 dollars per ton of ammonia.18 There have been previous techno-economic analyses for the electrochemical production of ammonia. These analyses provide valuable insight into the viability of renewable alternatives to the Haber-Bosch process.2,19–22 However, none of these analyses have set performance targets for the FE and current density to achieve economic viability. Furthermore, most explore fixed production scales. One of the main advantages of electrochemical routes is the broad production range. Therefore, there is a need for techno-economic analyses that probe various production scales to explore the feasibility of decentralized and potentially farm-scale production of ammonia for various system designs.
Herein, we present a techno-economic model to evaluate the feasibility of large and small-scale electrochemical ammonia synthesis technologies. We discuss the relevant performance parameters that govern the production cost of an electrochemical system. We also discuss the benefits of electrochemical approaches by evaluating the social cost of carbon dioxide emissions associated with ammonia production practices. Lastly, we highlight challenges and opportunities for electrochemical ammonia production at low temperatures and pressures and set performance targets for future technologies.
Methods
Haber-Bosch parity
The Haber-Bosch parity point represents the ammonia production price comparable to the Haber-Bosch process at a given production scale. For the Haber-Bosch process, fifty-five percent (55%) of the cost comes from the Natural Gas used to produce hydrogen. The rest of the cost is attributed to capital expenditures (32%), and operation and maintenance costs (13%).2 However, this low cost is not maintained as the production capacity varies, and the capital and operation costs at different production volume are estimated by:
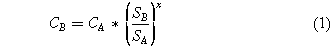
where CA is the capital and operation costs of a facility operating at a base production capacity (80 $/tonNH3), CB is the cost of a facility of arbitrary production capacity, SA is the base production capacity (2,000 tonNH3 day−1), SB is the desired production capacity of the facility of study, and x is the sizing exponent (0.65).2,23 Thus the Haber-Bosch parity point varies with production volume (Fig. S1 is available online at stacks.iop.org/JES/167/143504/mmedia). Variable production scales are growing in importance as there is a growing interest to create decentralized ammonia production plants that are located closer to the point-of-use to minimize the environmental impacts associated with fertilizers. This Haber-Bosch parity point will be used as a price reference to compare the cost produced by electrochemical routes for ammonia production.
Electrochemical modeling
The reversible energy demand for an electrochemical reaction corresponds to the change in enthalpy of the reaction (ΔH), which is a combination of the electrical energy demand or the change in the Gibbs free energy (ΔG) and the thermal energy demand which is the change in the product's temperature times the entropy of the system (TΔS).
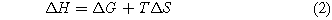
The open-circuit voltage (OCV) of the reaction is defined as the change in the Gibbs free energy (ΔG) divided by the number of electrons involved in the reaction (n = 6) and the Faraday's constant (F = 96,485 C mol−1).

The open-circuit voltage refers to the minimum voltage required for the reaction in an ideal system (Fig. S3). In real systems, losses are inevitable, and the performance cannot be quantified solely by the open-circuit voltage. The actual cell potential is determined by the reversible cell potential (V0) and the cell overpotential (ηtotal).

V0 refers to the reversible cell potential determined by the Nernst equation.
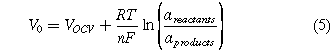
where ai corresponds to the activity of the reactants and products. Since the reactants and products are gases, their activity is approximated using the partial pressures. The activity of water is approximated to be one (a = 1) because water is the predominant solvent in the solution.
= 1) because water is the predominant solvent in the solution.

where Pi is the partial pressure of a component, yi is the molar fraction of a component, and PTotal is the total pressure of the system. The molar fraction of a component is the moles of a component in a mixture (ni) over the total number of moles in the mixture (nTotal).

The average molar fraction for each of the components relates to the single-pass conversion efficiency (X).
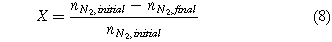
where  and
and  are the initial and final moles of nitrogen in the mixture. For the initial molar quantity, we used a 1 mole mixture with the appropriate stoichiometric ratio for nitrogen and hydrogen (
are the initial and final moles of nitrogen in the mixture. For the initial molar quantity, we used a 1 mole mixture with the appropriate stoichiometric ratio for nitrogen and hydrogen ( and
and  ).
).
The total cell overpotential is the combination of the activation overpotential (ηact), ohmic overpotential (ηohm), and concentration overpotential (ηconc).
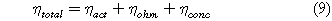
The activation overpotential is approximated using the Butler-Volmer equation with an assumed symmetric electron transfer coefficient (α = 0.5). This assumption is made to simplify the calculations.
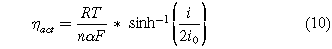
where R is the universal gas constant, F is the Faraday's constant, T is the reactor operating temperature, n is the number of electrons involved in the reaction (n = 6 for NRR, n = 4 for OER, and n = 2 for HER), α is the electron transfer coefficient, i is the operational current and i0 is the exchange current density.
The exchange current density of a reaction improves with temperature. The relationship between the exchange current density and temperature can be modeled using the following equation:
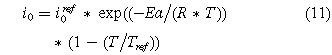
where  is the reference exchange current density at ambient temperature, Ea is the activation energy (1.03∗105 kJ∗kmol−1), R is the universal gas constant, T is the operating temperature, and Tref is the reference temperature (Tref = 298 K). The cathode reference exchange current density (
is the reference exchange current density at ambient temperature, Ea is the activation energy (1.03∗105 kJ∗kmol−1), R is the universal gas constant, T is the operating temperature, and Tref is the reference temperature (Tref = 298 K). The cathode reference exchange current density ( ) varies between 10−7 A cm−2 and 10−9 A cm−2. The anode reference exchange current density (
) varies between 10−7 A cm−2 and 10−9 A cm−2. The anode reference exchange current density ( ) is kept constant at 10−9 A cm−2 for OER and 10−2 A cm−2 for HER.
) is kept constant at 10−9 A cm−2 for OER and 10−2 A cm−2 for HER.
The ohmic overpotential can be described by Ohm's law
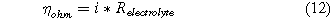
where Relectrolyte is the area-specific resistance of the electrolyte. We neglected the resistance due to the gas diffusion layer and the electrical components because these losses are negligible when compared to the losses in the electrolyte . The area-specific resistance of an electrolyte is
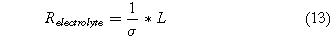
where σ is the electrolyte conductivity and L is the electrolyte thickness. For low-temperature electrosynthesis, we used the conductivity of a Nafion membrane as a model. For a fully humidified proton exchange membrane made of Nafion, the conductivity can be approximated by the following equation.24
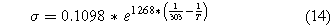
The concentration overpotential accounts for the losses due to mass transport.
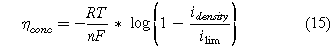
where the limiting current density (ilim) is the maximum current achievable with the mass transport properties of the system. The limiting current density depends on the effective diffusion coefficient of the reactant and the concentration of the reactant.
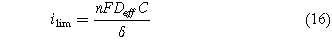
where Deff is the effective diffusion coefficient of the reactant, C is the reactant concentration, and δ is the thickness of the layer in which the mass transport takes place. For mass transport through a porous gas diffusion layer, δ corresponds to the thickness of the gas diffusion layer.
The effective diffusion coefficient on a porous gas diffusion layer can be approximated using (Eq. 17).25

where  is the porosity of the gas diffusion layer (
is the porosity of the gas diffusion layer ( = 0.8) and Dbulk is the bulk diffusion coefficient of the species.26,27
= 0.8) and Dbulk is the bulk diffusion coefficient of the species.26,27
Economic modeling
The levelized cost of ammonia (LCOA) is a measure of the normalized cost per unit mass of produced ammonia.

where CCap is the capital cost of the system, CRF is the capital recovery factor, CO&M is the operation and maintenance cost, CEnergy is the electrical cost, and MNH3 is the mass of the produced ammonia.
The capital recovery factor (CRF) transforms the total cost into a constant annual payment accounting for inflation.
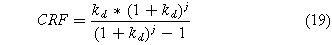
where kd corresponds to an inflation rate (6.5%) and j corresponds to a system lifetime of 30 years.22,28
The capital cost is a function of the sum of the cost of all the components in the electrolysis cell, including peripheral parts, and the balance of plant cost (BoP). The capital cost of the components of the electrolysis cell is based on areal prices at large production quantities (Table I).
Table I. Electrolysis cell component costs.
| Parameter | Price per Area | Source |
|---|---|---|
| Proton Exchange Membrane | $50 m−2 | 29 |
| Electrode | $96 m−2 | 29 |
| Bipolar Plate | $35 m−2 | 29 |
| Peripheral Parts | $3.46 m−2 | 29 |
| Catalyst (Pt) | $32,000 kg−1 | |
| Catalyst Loading | 1 g m−2 |
The balance of plant represents all the components that are essential to the function of the system but do not participate in the reaction (pumps, compressors, heat exchangers, storage tanks, electronic components, etc.). The balance of plant capital cost varies with the scale of the process (Table II).
Table II. Balance of plant capital cost.30
| Parameter | Current Distributed | Current Central |
|---|---|---|
| 3,560 kW | 118,000 kW | |
| BoP CapEx | $257 kW−1 | $118 kW−1 |
| Mechanical BoP Cost | $136 kW−1 | $36 kW−1 |
| Electrical BoP Cost | $121 kW−1 | $82 kW−1 |
We assumed that a power law could be used to approximate the balance of plant capital cost (BoPCapEx) for a system's power consumption (Si) (Eq. 20).
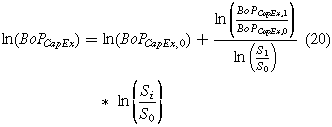
where BoPCapEx,0 and BoPCapEx,1 are the balance of plant capital costs at the distributed and central scales respectively. S0 and S1 are the system's power requirements for the distributed and central scales. Finally, the balance of plant cost described above does not include the cost of the air separation unit. The capital cost of the air separation unit depends on the production rate of nitrogen. The capital cost of the air separation unit is 171 M$/(tonNH3 h−1).31
The operational cost (CO&M) corresponds to 2% of the capital cost.22 Finally, the electrical cost corresponds to the total power required by the system times the levelized cost of electricity.32
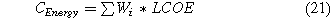
where ∑Wi is the total work required by the system and LCOE is the levelized cost of electricity. If H2 is used as the proton source in a two-step process with two electrolysis cells, the energy efficiency of the water electrolysis cell is 70% (vs. the lower heating value of hydrogen).33,34 Additionally, the capital cost of the water electrolysis cell is 600 $ kWh−1.33,34
System description
There are two different electrochemical routes in which to synthesize ammonia from air and water using renewable resources (Fig. 1). The first route toward renewable electrochemical ammonia is accomplished through the use of air and water as input feedstocks (Fig. 1a) where the whole cell, cathode, and anode reaction are listed below.
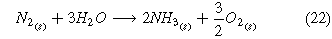
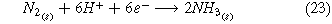
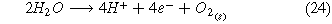
Figure 1. Schematic illustration of a single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants (a) and a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants (b).
Download figure:
Standard image High-resolution imageHere, liquid water is used as the source of hydrogen, and air is used as the source of nitrogen (Fig. 1a). The oxygen in the air is removed to form pure nitrogen through cryogenic separations. This consists of the liquefaction and distillation of the nitrogen, oxygen, and other gases in the air.35 Liquid water and gaseous nitrogen are compressed and heated to reach the desired operating conditions of the electrolysis cell. Nitrogen and water enter the electrolysis cell and produce ammonia and hydrogen. The water oxidation reaction takes place in the anode, in which water is converted to oxygen, protons, and electrons (Eq. 24). In the cathode, the two competing reactions are the nitrogen reduction reaction (ammonia is produced—(Eq. 23)) and the hydrogen evolution reaction. Ammonia is separated from nitrogen and hydrogen by compressing the outlet flow to 0.8 MPa and 20 °C. Under this temperature and pressure hydrogen and nitrogen remain in a gaseous state.36 Then, a distillation column separates the liquid ammonia from the gaseous nitrogen and hydrogen. The use of water as a feedstock for a renewable source of hydrogen instead of methane or other hydrocarbons is highly desirable to mitigate carbon-based emissions.
A second route toward renewable electrochemical ammonia is accomplished through the use of air and hydrogen as input feedstocks (Fig. 1b) where the whole cell, cathode, and anode reaction are listed below.
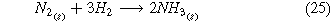
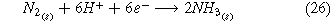
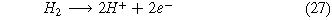
Similarly to the Haber-Bosch process, this reaction requires an external source of hydrogen. To achieve renewable ammonia, the production of hydrogen must occur through the use of a separate water electrolysis cell (Fig. 1b). Cryogenic air separation separates nitrogen from air, and compression and heating of hydrogen and nitrogen occur prior to the second electrolysis cell. The main by-product of this reaction is hydrogen. The hydrogen oxidation reaction takes place in the anode (Eq. 27). In the cathode, the two competing reactions are the nitrogen reduction reaction (ammonia is produced—(Eq. 23)) and the hydrogen evolution reaction. The hydrogen produced in the cathodic reaction when recycled serves as a reactant in the anode of the electrolysis cell. Finally, the procedure to separate ammonia from the outlet gases is the same in both systems.
Assumptions for the base case and optimized case
The base case scenario calculations were based on an electricity price of 0.06 $ kWh−1,37 a production scale of 0.03 tonNH3 day−1 (equivalent to the nutrients needed for a 100 hectare farm based on 100 kgN/ha∗yr11), and ambient operating conditions (T = 25 °C and P = 1 atm). The base case and optimized case values are specified in Table III.
Table III. Model parameters.
| Parameter | Base Case Value | Optimal Value |
|---|---|---|
| LCOE | 0.06 $ kWh−1 | 0.03 $ kWh−1 |
| i | 0.01 A m−2 | 1 A m−2 (single-stage) and 0.2 A m−2 (two-staged) |
| T | 25 °C | 100 °C |
| P | 1 atm | 5 atm |
| io | 10−9 A m−2 | 10−7 A m−2 |
| FE | 10% | 30% |
Results and Discussion
Sensitivity analysis
We first examine the potential LCOA through performing a sensitivity analysis around performance metrics (FE, current density, exchange current density) and operating conditions (temperature and pressure) which are achievable today. We only survey performance and operating conditions which are within 50% of these conditions (Fig. 2). Evaluating performance metrics using known values is important to determine the order of magnitude changes which are most important for electrochemical systems engineering.
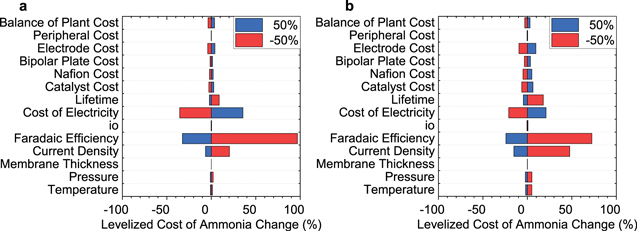
Figure 2. Sensitivity analysis of a single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants (a) and a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants (b). Relevant parameters for the base case are presented in Tables I–III. Each parameter was varied by ±50% during the sensitivity analysis.
Download figure:
Standard image High-resolution imageFor a single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants, the three most sensitive properties for the system's LCOA are the cost of electricity, the FE, and the current density (Fig. 2a). Altering the cost of electricity from 0.06 $ kWh−1 to 0.03 $ kWh−1 results in a reduction in the LCOA from ∼6,100 $/tonNH3 to ∼3,900 $/tonNH3 (∼36%). Altering the FE from 5% to 15% decreased the LCOA from ∼12,000 $/tonNH3 to ∼4,100 $/tonNH3. Finally, increasing the current density by 50% from 0.01 A cm−2 to 0.02 A cm−2 results in a marginal decrease in LCOA from ∼6,100 $/tonNH3 to ∼5,700 $/tonNH3.
It should be noted that nearly all the LCOAs surveyed in the sensitivity analyses are 10x that of the Haber-Bosch process. However, what is most notable is the clear sensitivity the cost of electricity and the FE have on the LCOA. Most experimental investigations of NRR in aqueous phase systems have exhibited low FE due primarily to the excess concentration of protons in an aqueous electrolyte. In these conditions, electrons are easily consumed by the hydrogen evolution reaction (HER). Ultimately designing a more selective catalyst (suppressing water adsorption) or operation in alkaline conditions may aid in mitigating the HER reaction. However, it is not clear if catalysts for low-temperature systems will be able to achieve FEs which approach 50%.
For a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants, the largest contributors to the LCOA are again the cost of electricity, FE, and current density (Fig. 2b). This system has a higher energy efficiency of 28% (vs. the lower heating value of ammonia) vs 7.2% (vs. the lower heating value of ammonia) for the single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants. This increase in energy efficiency is because the activation overpotential for hydrogen oxidation is typically less (50 mV) than that required for water oxidation (250 mV). In the two-stage system, altering the cost of electricity from 0.06 $ kWh−1 to 0.03 $ kWh−1 results in a 21% change in the LCOA from ∼2,600 $/tonNH3 to ∼2,100 $/tonNH3. In additional, altering the FE from 5% to 15% results in a decrease in the LCOA from ∼4,500 $/tonNH3 to ∼2,000 $/tonNH3. Altering the cost of the components changes the LCOA by 1% to 10%. The biggest contributor to the capital cost is the cost of manufacturing the electrodes, which leads to a 10% change in the LCOA. Here, the LCOA is still not near Haber-Bosch parity, yet it is only 3–5× beyond this point, indicating some potential advantages. Again, the FE is a significant challenge, yet may be more easily addressed in the absence of liquid water.
Pathway to the economic feasibility for electrochemical ammonia synthesis
Although a sensitivity analysis provided valuable insight into the relationships between the LCOA and the system's components and operating conditions, it is difficult to outline a sequential pathway toward economic feasibility. A waterfall analysis, however, is capable of defining a sequential pathway, as this analysis highlights a pathway of cumulative improvements that is informed from the sensitivity analysis (Fig. 3). We used the model parameters for the base case and optimized case as described in Table III.
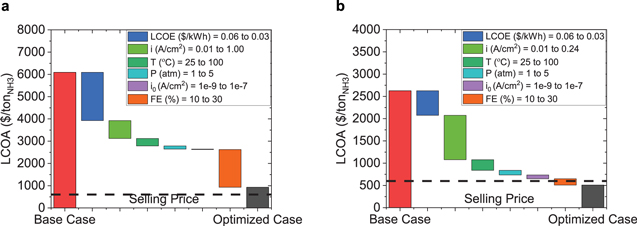
Figure 3. Waterfall analysis of a single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants (a) and a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants (b). Relevant parameters for the base case are presented in Tables I–III. Each parameter improves sequentially to the optimal values specified in Table III.
Download figure:
Standard image High-resolution imageFor a single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants, the system does not reach the target cost of ammonia of 600 $ ton−1 at a production scale of 0.03 tonNH3 day−1. This is set at a small production scale that can meet the needs of 100 hectares of farmland (based on 100 kgN/ha ∗ yr11). However, through sequential improvements, it is possible to reduce the LCOA for this system from ∼6,100 $ ton−1 to ∼930 $ ton−1 (Fig. 3a). These improvements include a decrease in the cost of electricity from 0.06 $ kWh−1 to 0.03 $ kWh−1 (LCOA decreases by ∼2,200 $ ton−1). Increasing the current density to 1 A cm−2 (decreases the LCOA by ∼800 $ ton−1). Increasing the temperature from 25 °C to 100 °C results in a cost decrease of ∼340 $ ton−1, and increasing the pressure from 1 atm to 5 atm results in a cost decrease of ∼140 $ ton−1. With improvements to catalyst properties, the exchange current density could increase from 10−9 A cm−2 to 10−7 A cm−2 leading to a cost decrease of ∼19 $ ton−1. This is fairly insignificant when compared to the cost of electricity and operational conditions (temp and pressure). Finally, increasing the FE from 10% to 30% leads to a cost decrease of ∼1,700 $ ton−1. Even though these improvements are not enough to reach the target cost of 600 $ ton−1, the improvements are able to reduce the LCOA by 85%. The improvement in the cost of electricity and the catalyst selectivity (FE) lead to the highest reduction in cost (36% and 65%). Beyond these calculations, it should be noted that most experimental investigations focused on aqueous phase systems, which have had significantly poor performance, and a number of published work have been debated for measurement accuracy. This is to say that there is significant doubt in the field regarding the viability of this approach. The safety considerations associated with not needing to store and handle hydrogen gas are also not included in these systems.
For a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants, the system reaches the target LCOA of 600 $ ton−1 at a production scale of 0.03 tonNH3 day−1. This is set at a small production scale that can meet the needs of 100 hectares of farmland. Through sequential improvements, it is possible to reduce the LCOA for this system from ∼2,600 $ ton−1 to ∼500 $ ton−1 (Fig. 3b). A decrease in the cost of electricity from 0.06 $ kWh−1 to 0.03 $ kWh−1 leads to a decrease in the LCOA by over 500 $ ton−1. Increasing the current density to 0.24 A cm−2 (the optimal current density) decreases the LCOA by ∼1,000 $ ton−1. Increasing the temperature from 25 °C to 100 °C results in a cost decrease of ∼240 $ ton−1 and increasing the pressure from 1 atm to 5 atm results in a cost reduction of ∼100 $ ton−1. Improving the exchange current density from 10−9 A cm−2 to 10−7 A cm−2 leads to a cost decrease of ∼86 $ ton−1. Finally, increasing the FE from 10% to 30% leads to a decrease in the LCOA of ∼140 $ ton−1. The improvement of the current density leads to the highest reduction in cost (48%).
Scaling electrochemical ammonia production
Thus far, we have only examined optimizing an electrochemical system for the fully decentralized production of ammonia at a scale of 0.03 tonNH3 day−1 (100 hectares of arable land). In reality, electrochemical technologies may be scaled to meet varied production needs. To put this production scale into perspective, current Haber-Bosch plants operate at scales of thousands of tons of ammonia per day. Thus, we next aim to examine the competitive cost of electrochemical routes as production volume scales.
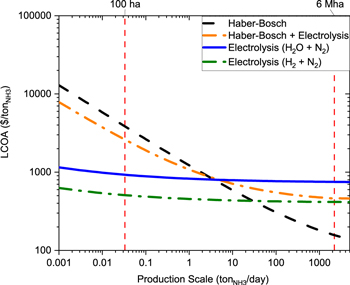
The price of the ammonia produced by the Haber-Bosch process (Haber-Bosch parity point) increases significantly as the plant size decreases (Fig. 4). For instance, a facility with an output of 2,000 tonNH3 day−1 can produce fertilizer for up to 6 million hectares of arable land (which is equivalent to ∼60,000 family farms). This facility is able to produce fertilizers at a cost of $159 ton−1 due to the economies of scale. However, a fully decentralized Haber-Bosch facility outputs 0.03 tonNH3 day−1 and produces fertilizer for 100 hectares (∼1 family farm) at a cost of ∼$4,000 ton−1.
A renewable alternative to the methane fed Haber-Bosch process is the Haber-Bosch process coupled with water electrolysis. With current electricity prices (0.06 $ kWh−1 37), this technology would not be economically feasible, as it cannot produce ammonia under the market value of 600 $ ton−1. However, if the electricity price decreases to the next decade target (0.03 $ kWh−137) the Haber-Bosch process coupled with water electrolysis achieves costs of ammonia under today's market price. The market price of $600 ton−1 is achieved at production rates higher than 40 tonNH3 day−1, which is equivalent to producing fertilizer for an arable area of approximately 120,000 hectares (∼1,200 family farms) (Fig. 4). However, a plant which outputs 0.03 tonNH3 day−1 and produces fertilizer for 100 hectares (∼1 family farm) cost ∼$5,500 ton−1. Thus, the benefit of coupling the Haber-Bosch process with a water electrolysis cell is primarily in the decarbonization of hydrogen production. Since the Haber-Bosch process coupled with water electrolysis still relies on economies of scale, there will still be challenges with scaling this technology to lower production values. Hence, this could be a viable approach to attain large scale renewable ammonia but may not be feasible for small-scale or farm-scale ammonia production.
Figure 4. Levelized cost of ammonia with variations in plant size. All the calculations were made with the optimal parameters specified in Table III.
Download figure:
Standard image High-resolution imageAnother renewable alternative to the Haber-Bosch process that might be able to compete at low production scales is the electrochemical production of ammonia. With the next decade's electricity price (0.03 $ kWh−1), the production cost from electrochemical approaches significantly exceeds the production cost from the Haber-Bosch process at large scales. At a production scale of 2,000 tonNH3 day−1, a single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants will produce ammonia at a cost of ∼750 $ ton−1. At this same scale, a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants produces ammonia at a cost of ∼420 $ ton−1. To put this into perspective, the Haber-Bosch process produces ammonia at a cost of 159 $ ton−1. In order to compete with the Haber-Bosch process, electrochemical approaches need to operate at smaller scales. For example, with a production scale of 0.03 tonNH3 day−1, a single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants will produce ammonia at a cost of ∼930 $ ton−1. At this same scale, a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants produces ammonia at a cost of ∼510 $ ton−1. At this same scale, the Haber-Bosch process will produce ammonia at a cost of ∼$4,000 ton−1. A single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants reaches Haber-Bosch parity at scales below 4 tonNH3 day−1. A two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants reaches Haber-Bosch parity at scales below 30 tonNH3 day−1.
The target LCOA for an electrochemical system depends on the scale of production. A centralized electrochemical system (2,000 tonNH3 day−1) will compete directly with the Haber-Bosch process due to elevated transportation and storage costs. Hence, the target LCOA at this scale is the production cost of the Haber-Bosch process ($159 ton−1). A fully decentralized electrochemical system (0.03 tonNH3 day−1) does not have to compete directly with the Haber-Bosch process due to the elimination of transportation and storage costs. A system operating at this scale has to achieve a LCOA equivalent to the market price of ammonia ($600 ton−1). To identify the operating space for electrochemical ammonia synthesis, we examined constant cost curves (Fig. 5). Each line represents a fixed electricity cost between 0.06 $ kWh−1 and 0.003 $ kWh−1 and a fixed LCOA. Here, we study the required FE and current densities to meet the economic viability requirement of 159 $ ton−1 for a production volume of 2,000 tonNH3 day−1 and 600 $ ton−1 for a production volume of 0.03 tonNH3 day−1. We consider the optimized case discussed in the waterfall analyses with the parameters from Table III. However, we vary the FE and current density in order to define targets for these parameters.
Figure 5. Design space for a single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants with a production volume of 2,000 tonNH3 day−1 (a) and with a production volume of 0.03 tonNH3 day−1 (b). Design space for a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants with a production volume of 2,000 tonNH3 day−1 (c) and with a production volume of 0.03 tonNH3 day−1 (d). We assumed the optimal temperature, pressure, and exchange current density shown in Table III for the calculations related to this figure. For a system with a production volume of 2,000 tonNH3 day−1 the lines represent a fixed cost of $159 ton−1. For a system with a production volume of 0.03 tonNH3 day−1 the lines represent a fixed cost of $600 ton-1.
Download figure:
Standard image High-resolution imageFor a single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants producing 2,000 tonNH3 day−1 (Fig. 5a), it is impossible to produce ammonia at the current electricity price (0.06 $ kWh−1) under 159 $ ton−1. In order to produce ammonia at that cost, the cost of electricity needs to decrease to at least 0.015 $ kWh−1. This electricity prices is half of next decade's target, indicating that it may not be a reasonable target. Furthermore, at an electricity price of 0.015 $ kWh−1 the minimum required FE is ∼80% at current densities above 0.2 A cm−2. If the electricity price is further halved (0.007 $ kWh−1), this system requires a FE of ∼40% to achieve the LCOA target of 159 $ ton−1.
For a fully decentralized single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants producing 0.03 tonNH3 day−1 (Fig. 5b), it is possible to produce ammonia at the current electricity price (0.06 $ kWh−1) at under 600 $ ton−1. In order to produce ammonia at that price point, the minimum FE required is ∼86% at a current density of over 0.5 A cm−2. Reaching a FE of 86% at a current density in the order of 0.5 A cm−2 is improbable. However, another alternative to reduce the LCOA is attained through reducing the cost of electricity. As the cost of electricity decreases, the required FE decreases as well. For example, if the electricity price is decreased to the next decade target (0.03 $ kWh−1) the required FE will decrease to 48%. However, as the electricity price decreases, the optimal current density increases because higher current densities lead to higher electrical costs and lower capital costs. At electricity costs below 0.03 $ kWh−1 the optimal current density is close to 1 A cm−2. However, most of the benefits of increasing the current density occur in current densities below 0.1 A cm−2. For example, the required FE at a current density of 0.1 A cm−2 is 49%. Thus, significant improvements in the catalyst selectivity (FE) and activity (exchange current density and activation energy) are necessary in order to meet these targets.
For a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants producing 2,000 tonNH3 day−1 (Fig. 5c), it is impossible to produce ammonia at current electricity prices under 159 $ ton−1. This system requires a maximum electricity price of 0.0075 $ kWh−1, 25% of next decade's target, to produce ammonia at 150 $ ton−1. To produce ammonia with an electricity price of 0.0075 $ kWh−1, the minimum FE is ∼60%. A FE of 60% at a current density of 0.5 A cm−2 is nearly impossible. However, if the electricity price is decreased, the required FE decreases sharply. For example, if the electricity price is halved (∼0.003 $ kWh−1) the required FE will decrease to 7.9%.
For a fully decentralized two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants producing 0.03 tonNH3 day−1 (Fig. 5d), it is impossible to produce ammonia at current electricity prices at under 600 $ ton−1. An electricity price lower than 0.044 $ kWh−1 is necessary to produce ammonia at 600 $ ton−1. To produce ammonia with an electricity price of 0.044 $ kWh−1, the minimum FE is ∼75%. A FE of 75% at a current density of 0.2 A cm−2 is nearly impossible. However, if the electricity price is decreased, the required FE decreases sharply. For example, if the electricity price is decreased to the next decade target (0.03 $ kWh−1) the required FE will decrease to ∼13%. Reaching a target of 13% FE will be less challenging than reaching a target of 48% FE (which is the target of the other system at an electricity cost of 0.03 $ kWh−1). Existing catalysts exhibit FEs around this range (∼10%). Most of the improvement in the LCOA occur at current densities below 0.1 A cm−2. We can conclude that the minimum required current density should be 0.1 A cm−2. However, it will be challenging to maintain these FEs while operating at high current densities (>0.1 A cm−2).
Studying the design space of electrochemical ammonia synthesis helps illustrate the importance of improving the different parameters and setting realistic targets for technology viability. For example, we can set targets in the current density (0.1 A cm−2) and the FE. Combining these with the electricity cost targets set by the DOE we can estimate the future targets for this technology. We conclude that by 2030, with an expected electricity cost of 0.03 $ kWh−1, the performance targets set for fully decentralized low-temperature ammonia electrosynthesis are 48% for a single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants and 13% for a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants. Additionally for electrochemical approaches to operate in a centralized production scheme, electricity prices as low as 0.003 $ kWh−1 might be needed. Hence, it is important to develop systems that operate with N2 and H2 as reactants and use a separate water electrolysis cell to provide hydrogen to the ammonia electrochemical reactor. Additionally, these analyses help us prioritize our efforts on technology development. For a single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants it is equally important to reduce the electricity cost and to increase the FE at high current densities because even at a low electricity price, the required FE is still high. However, for a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants, it is more important to reduce the electricity price because the required FE with low electric prices approaches the FE of existing systems. Even though a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants is not viable with the current electricity prices, as the electricity price decreases over the next decade it will be easier to develop and meet the target ammonia production costs.
These analyses help illustrate pathways to renewable electrochemical ammonia production and indicate that low-cost electrochemical ammonia is feasible. However, a significant amount of work is needed before these systems can be implemented. Based on the analysis presented, developing selective catalysts that can operate at current densities above 0.1 A cm−2 remains of paramount importance. However, advancements in renewable energy production that might help reduce the cost of electricity are equally important.
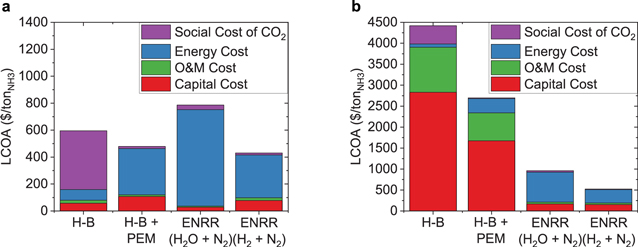
The calculations thus far do not include the social cost of carbon dioxide emissions. Due to the large advantage the Haber-Bosch process shows over other systems at large production scales, it is important to study how the social cost of carbon dioxide closes the LCOA gap between the Haber-Bosch process andrenewable approaches. We compare the LCOA produced by the methane-fed Haber-Bosch process, the Haber-Bosch process coupled with water electrolysis, and electrochemical approaches. First, we studied a centralized system with a production volume of 2,000 tonNH3 day−1 (Fig. 6a). At this production volume, the Haber-Bosch process has the lowest LCOA ($159 ton−1). However, after taking into consideration the social cost of carbon dioxide emissions, the system with the lowest LCOA is the two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants (∼$430 ton−1). The Haber-Bosch process coupled with water electrolysis has the second lowest LCOA when including the social cost of carbon dioxide (∼$480 ton−1). To put this into perspective, the social cost of carbon dioxide brings the LCOA from the Haber-Bosch process to ∼$600 ton−1. At these production scales, there are additional economic and safety considerations due to the transportation and storage of ammonia. Hence, we are interested in alternatives that can be deployed in a decentralized manner, leading to benefits in the safety and cost of ammonia synthesis. So, we studied a fully decentralized system with a production volume of 0.03 tonNH3 day−1 (Fig. 6b). From the systems operating at a production scale of 0.03 tonNH3 day−1, the two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants exhibits the lowest LCOA (∼$520 ton−1). The single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants has a LCOA of ∼$960 ton−1. The Haber-Bosch process and the Haber-Bosch coupled with water electrolysis suffer from elevated costs at a fully decentralized scale (∼$4,500 ton−1 and ∼$2,700 ton−1). This analysis suggests that policies that leverage the social cost of carbon dioxide to drive the adoption of renewable alternatives to the Haber-Bosch process are equally important as the technological advances of these systems. Ultimately, competing with the Haber-Bosch process will be a challenging problem, and solutions from both fronts (policy and technology) will be necessary. Additionally, it suggests that fully centralized Gen 2 approaches, namely the Haber-Bosch coupled with water electrolysis, might be the best immediate solution to mitigate the emissions from the Haber-Bosch process because Gen 3 approaches (electrochemical ammonia synthesis) are untested technologies at a commercial scale.
Figure 6. Cost of ammonia including the social cost of CO2 at a production scale of 2,000 tonNH3 day−1 (a) and at a production scale of 0.03 tonNH3 day−1 (b) All the calculations were made with the optimal parameters specified in Table III. The social cost of carbon dioxide is $291/tCO2.38 For the Haber-Bosch process we used the equivalent emissions of 1.5  .7 For renewable energy we assumed carbon dioxide emissions of 10
.7 For renewable energy we assumed carbon dioxide emissions of 10  kWh−1.
kWh−1.
Download figure:
Standard image High-resolution imageConclusions
Here we developed a techno-economic model to evaluate the economic viability of low-temperature electrochemical ammonia synthesis technologies. We find that the LCOA is most sensitive to variations in the cost of electricity and the FE, and least sensitive to variations in the capital cost of the system. In order to achieve economic feasibility, sequential improvements are needed to improve the catalyst and system operations. We found that for a single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants, it is possible to approach economic viability by optimizing the operating conditions. However, this system requires FEs higher than 30% in order to produce ammonia at the target costs. For a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants, it is possible to achieve LCOA as low at ∼500 $ ton−1 by optimizing the operating conditions and improving the FE to 30% and the exchange current density to 10−7 A cm−2. Using design maps, we found that a single-stage electrochemical ammonia synthesis system with N2 and H2O as reactants can achieve costs below 600 $ ton−1 at the current electricity prices, yet FEs greater than 86% and current densities between 0.1 to 0.5 A cm−2 are required. If the electricity prices are decreased to 0.03 $ kWh−1, the required FE would be reduced however to 48%. For a two-staged electrochemical ammonia synthesis system with N2 and H2 as reactants, it is impossible to meet viability requirements with the current electricity prices. If the electricity prices are decreased to 0.03 $ kWh−1, this system can produce ammonia below 600 $ ton−1 with FEs as low as 13%. However, achieving the FEs defined in the design maps while keeping the current density above 0.1 A cm−2 will require improvements of orders of magnitudes in the electrolyzer performance, as the existing catalysts operate at current densities around 0.001 A cm−2 and FEs below 10%.