Abstract
The use of biocathodes in bioelectrochemical systems (BES) for the removal of nitrate from waste water has become a vital field for research. However, the elucidation of the underlying extracellular electron transfer (EET) fundamentals of denitrifying biocathodes is rarely done, but it is required for a deeper BES understanding and engineering. This study shows the thermodynamics of microbial electron transfer for reduction of nitrate and nitrite using bacteria immobilized on a biofilm used in BES. Thiobacillus denetrificans are best species that demonstrate both direct extracellular electron transfer and the phenomenon of microbial denitrification. The Cyclic voltammetry and chronoamperometry confirm the reduction of the nitrate at a potential of −0.46 V and the nitrite at −0.68 V vs Ag/AgCl, however the electrochemical response changes according to both the concentration and the temperature.
Export citation and abstract BibTeX RIS
Nature has always been a mystery, as for its self-purifying power, indeed natural environments find their equilibrium whatever the disorder may occur. This equilibrium is due to the existence of microorganisms that have the capacity to metabolize chemical residues from pesticide residues or other industrial sectors. Nitrates and nitrites are increasingly important pollutants in surface waters. The microorganisms best known for their ability to denitrify nitrates and nitrites are Thiobacillus denitriricans, Shewanella oneidensis and Paracoccus denitrificans.1–3 The bacteria in the center of this studyis Thiobacillus denitrificans. an autotrophic gram-negative bacterium, i.e. living in an environment without any source of organic carbon).
Water pollution by nitrate (NO3−) is worldwide a concern.1 Conventional procedures based on biological heterotrophic denitrification (i.e. organic carbon as source of bacterial carbon) are limited by the lack of availability of electron donors.2 Alternatively, the cathodes of bioelectrochemical systems (BES) could be used as a safe and endless source of electrons for nitrate reduction but microbial extracellular electron transfer (EET) is still insufficiently exploited and, to promote potential applications, it is necessary to better understand the autotrophic microbial metabolism, and to evaluate for electron transfer reactions linked to denitrification of aquifer polluted by nitrate and nitrite when inorganic carbon is the only source of microbial carbon.3,4
The midpoint potentials (Em, formal potentials) of isolated denitrifying enzymes of specific microbial species were elucidated. Nitrate reductase GHI,5 Cu nitrite reductase6 and Cu nitrous oxide reductase7 have potentials of −0.19 V, −0.07 V and −0.14 V vs Ag/AgCl, respectively. However, little is known about EET of denitrifying biocathodes.
Within this publication, we report on the EET characteristics of denitrifying biocathodes using a biocomposite as an artificial biocathode, i.e. a simplified ecosystem using protamine to immobilize Thiobacillus denitrificans. Protamine is positively charged at neutral pH and allows the assembly of biocomposite elements together. This self-assembly has been evidences by dynamic light scattering and scanning electron microscopy (SEM).8 MWCNTs show exceptional electrical and mechanical properties due to their relatively high surface area, small dimensions and stable nature.9 They can improve the electronic performance of the biofilm and facilitate the electron transfer between bacteria and electrodes.10 MWCNT acts as a conductor in the bacterial biocomposite that is free of mediating metabolites that can interfere in the direct transfer of electron between the bacterial membrane and the electrode.11,12 Detailed analysis in cyclic voltammetry and chronoamperometry are provided.13,14 This study focuses on nitrate and nitrite reduction as a model process, and evaluate the effect of temperature and nitrite concentration on the electrochemical response.
Materials and Methods
Bacterial strains and growth conditions
Thiobacillus denetrificans from DSMZ laboratory, cultivated at mineral medium (N°113 DSMZ)15; preparing four solutions: A, B, C and D for 1 l. The four solutions are prepared separately: the solution of trace elements, the solution A (containing in addition to the base salts, the electron acceptor, NO3−), the solution B containing the thiosulfate (electron donor), solution C containing the carbon source (bicarbonate), and solution D. The composition of these different media is trace element solution SL-4: 50 g Na2–EDTA; 20 g FeSO4.7 H2O; 10 g ZnSO4.7H2O; 30 mg MnCl2.4 H2O; 0.3 g H3BO3; 0.2 g CoCl2.6H20; 10 mg CuCl2.2 H2O; 20 mg NiCl2.6H2O; 30 mg Na2MoO4.2H2O; 1000 ml water. Solution A16: 2 g KH2PO4; 2 g KNO3; 1 g NH4Cl;0.8 g MgSO4.7H2O; 2 ml elements trace solution SL-4 and 960 ml water. The pH adjusted at 7 with NaOH. Solution B17: 5 g Na2S2O3.5H2O, 20 ml water; Solution C18: 1 g NaHCO3, 20 ml water; Solution D19: 20 mg FeSO4.7H2O; 10 ml 0.1 N H2SO4.
Media A, B and D are boiled and degassed under filtered N2. Solution C is degassed under filtered N2/CO2 (80%/20%). Solutions A, B, C and D are sterilized separately by autoclaving at 121 °C for 15 min. The pH of solution A is checked after autoclaving with a drop taken aseptically and deposited on a piece of pH paper (it should remain around 7).20
Bio-composite preparation
Five bottles of 100 ml containing 96 ml of solution A were prepared as described before and completed with 2 ml of solution B, 2 ml of solution C and 0.1 ml of solution D taken by syringe 2 to 3 ml of Thiobacillus denitrificans suspension are added in four medium bottles.21 The 5th bottle is used as a control. The five bottles are incubated at 30 °C in a stove for one week.22,23 Bacteria growth was monitored by direct counting with an optical microscope of a drop put between two microscope glass slides at 100X magnification. At the last day of the growing period, pictures of bacteria were taken for epifluorescence analysis. After one week of incubation, DO600 = 1 it was equivalent of 6.4 * 108 cells ml−1 and it was conserved at +4 °C.24
The culture was harvested by centrifugation at 5000 g for 10 min at room temperature.25 The pellet was washed twice with 1 mM KCl, and then suspended in 1 mM KCl in order to reach a cell density between 2 × 109 and 5 × 109 cell ml−1 (determined by optical density measurement) as shown in Fig. S1 in the Supplementary Material (available online at stacks.iop.org/JES/167/135502/mmedia).26
The biocomposite was prepared according do protocol from the literature8 by mixing one volume of bacterial suspension (5 * 109 Cells ml−1), one volume of MWCNT-COOH suspension (5 mg ml−1 dispersed in water by sonication for 30 min), protamine (5 mg ml−1, dispersed in water by vertexing for 30 min), and two volumes of 1 mM KCl for 15 min until a sedimentation was observed (Fig. S2 in the Supplementary Material). After that biocomposite has sedimented in the suspension, it was deposited it on the graphite felt by vacuum filtration as shown in Fig. S3 in the Supplementary Material.
Microscopies
In epifluorescence microscopy, the LIVE ™ Bac Light™ kit (Thermo Fischer Scientific) was used.27 Briefly; samples were mixed with DNA dyes SYTO 9 (1.67 μM) and propidium iodide (1.67 μM) and incubated for 15 min before filtration (on 0.22 μm membrane filters).28 The filtrate was afterwards dropped on microscope glass slide and observed with an epifluorescence microscope (OLYMPUS BX51) with a magnification factor ×1000.29 A sample image is provided in Fig. S4 in the Supplementary Material. High magnification scanning electron microscopy (SEM) was performed with JSM-IT 500 HR from JEOL. Energy-dispersive X-ray spectroscopy (EDS) was performed with a table-top JEOL SEM equipped with a JEOL analyzer.30
Electrochemical measurements
The experiments were carried out in a conventional electrochemical three electrodes cell (Fig. S5 in Supplementary Material). with a graphite felt as working electrode having 17 mm diameter and 5 mm thickness (GFD4.6 EA from SGL, Germany), stainless steel as a counter electrode and Ag/AgCl as a reference electrode (sat. KCl, SE11 Sensortechnik Meinsberg, Germany). The cell was connected to a bath water thermostat at 30 °C. Experiments were performed in 20 ml of PBS at pH 7 under nitrogen flow and slow magnetic agitation If not stated otherwise, all potentials in this work are provided vs Ag/AgCl (sat. KCl; +0.197 V vs Standard hydrogen electrode SHE). Cyclic voltammetry experiments were done under nitrogen atmosphere at a scan rate 5 mV s−1, at room temperature and at 30 °C.31 Nitrate (20 or 40 mM) was added after the current response was stabilized; Amperometric measurements have been performed at constant potential at −0.46 V or −0.68 V vs Ag/AgCl. After decay of the capacitive current, nitrate or nitrite have been added in the solution.
Results and Discussion
Microscopic observations
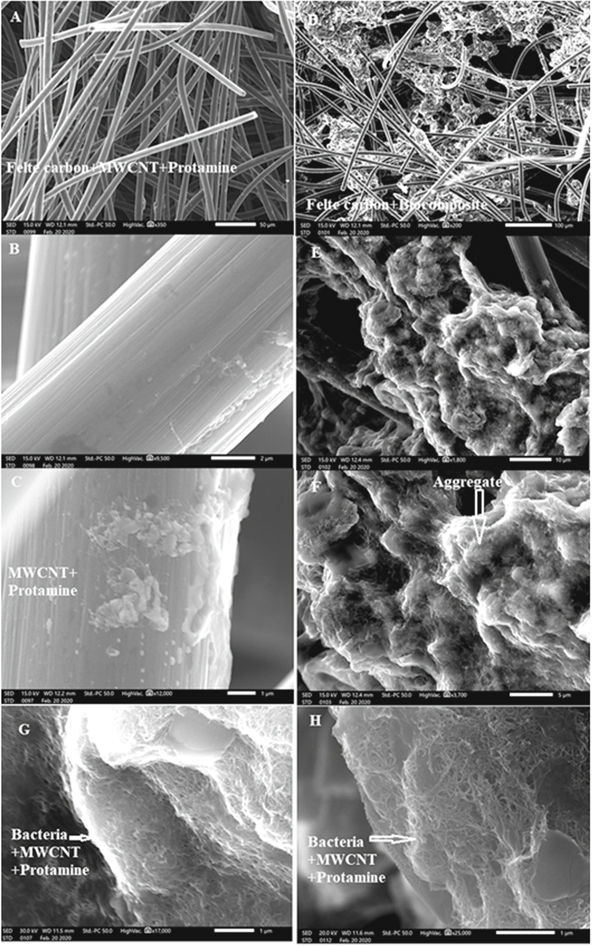
Figure S5 in Supplementary Material shows the Thiobacillus denitrificans observed in epifluorescence microscopy. With a Bac Light Live/Dead assay, the green staining shows that bacteria display low membrane permeation and are potentially viable.32 After formation of the biocomposite of Thiobacillus denitrificans cells and carbon nanotube, the material is deposited on graphite felt. Figure 1 reports details of this biomaterial when immobilized on the graphite fibers (Figs. 1D–1G) and the electrode obtained by following the same protocol but without bacteria (Figs. 1A–1C). Aggregates are visible in the presence of bacteria. High resolution imaging allows to get a detailed view of the interaction between MWCNT with bacteria when protamine is present. At pH 7, protamine is positively charged (isoelectric point of 9.5) whereas bacterial cells and MWCNT are negatively charged.33 Together, these elements self-assemble and MWCNTs surround the bacterial cell (see Figs. 1G and 1H).
Figure 1. Scanning electron micrographs of the graphite felt modified with a suspension of MWCNT and protamine (A)–(C) or with the bacterial composite prepared with Thiobacillus denitrificans, MWCNT and protamine (D)–(H).
Download figure:
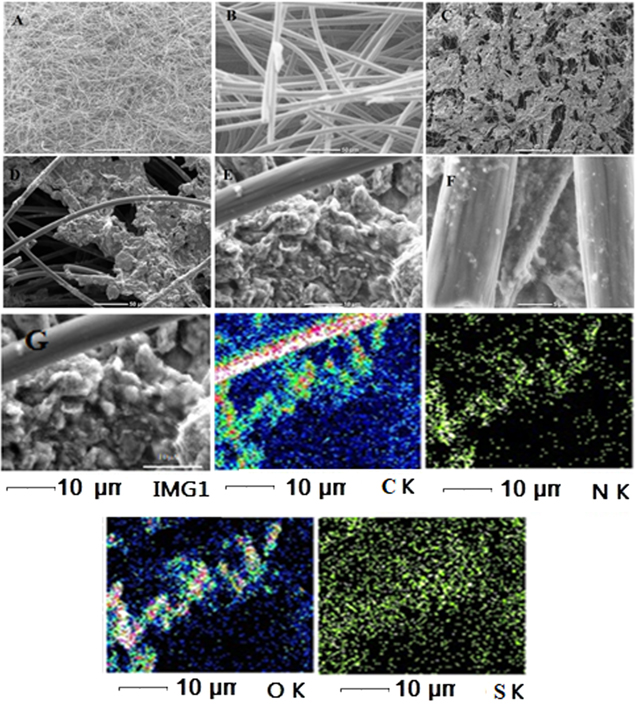
Standard image High-resolution imageFigures 2A, 2B show the morphology of the graphite felt with two different magnifications as a bare electrode.33 Figures 2C to 2D shows the MWCNT and protamine after deposition in graphite felt with different magnifications. Figure 2F shows complex of protamine and MWCNT-COOH on the carbon felt as white dots.34 Figure 2G shows the Mapping EDX which show the distribution of the elements. The percentage of all C, N, O and S are 76, 4, 12 and 8% ensuring the presence of both MWCNT and protamine.35
Figure 2. SEM images (A), (B) for graphite felt, (C), (F) for felt carbon with MWCNT and protamine. G: EDX Mapping of graphite felt with MWCNT and protamine.
Download figure:
Standard image High-resolution imageElectrochemical response to nitrate and nitrite
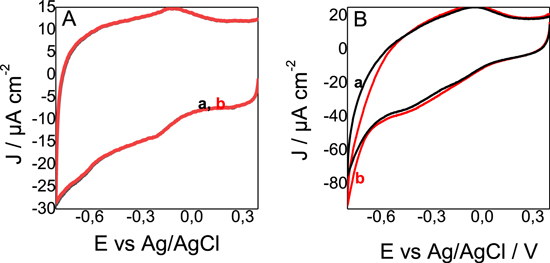
Preliminary experiments have been directed to evaluate the response to 20 mM nitrate of Thiobacillus denitrificans in a biocomposite electrode. First, a control experiment with an electrode prepared by following a similar protocol but in the absence of the bacterial cells do not show any response to the introduction of nitrate (Fig. 3A). The bacterial cells were stored in 1 mM KCl for 5 (Fig. 3B), 10 (Fig. 3C), and 15 d (Fig. 3D) before to be introduced in the biocomposite for electrochemical characterization. With the fresher culture (5 d), a current increase in observed upon the introduction of nitrate in the solution. The signal is broad with a peak around—0.35 V vs Ag/AgCl. After 10 d, a change is still noticed in the voltammogram in the same potential region and a higher current is noticed at higher potential, close to −0.6 V vs Ag/AgCl. After 15 d, no current change was observed upon addition of nitrate. These results suggest that Thiobacillus denitrificans possesses membrane proteins responsible for the extracellular electron transfer linked to nitrate reduction but also that electron transfer chain is degrading with time. The response to nitrite was also evaluated with a relatively fresh Thiobacillus denritificans culture (Fig. 4). No response of the electrode is observed in the absence of bacterial cells (Fig. 4A) and a clear current response to nitrite is observed at low potential, below −0.6 V Fig. 4B. A hypothesis is that the low potential response (close to −0.35 V) is linked to nitrate reduction while the current increase at a lower potential (close to −0.6 V) is linked to nitrite reduction. To control that idea, constant potential amperometry have been performed and discussed in the next section.
Figure 3. (A) Cyclic voltammetric response measured with graphite felt electrodes modified with (A) a suspension of protamine and MWCNT, (B)–(D) biocomposite made with protamine, MWCNT and Thiobacillus denitrificans from (B) a 5 d old culture, (C) a 10 d old culture and (D) a 15 d culture. (a) Response in the absence of nitrate and (b) response in the presence of 20 mM nitrate. Scan rate of the potential sweep was 5 mV s−1. All experiments are done under nitrogen atmosphere.
Download figure:
Standard image High-resolution imageFigure 4. Cyclic voltammetric response measured with graphite felt electrodes modified with the biocomposite made with protamine, MWCNT and Thiobacillus denitrificans from a 5 d old culture. (a) Response in the absence of nitrite and (b) response in the presence of 20 mM nitrite. Scan rate of the potential sweep was 5 mV s−1. All experiments are done under nitrogen atmosphere.
Download figure:
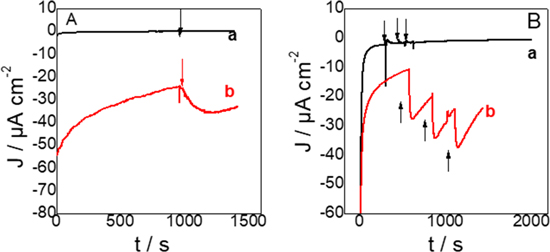
Standard image High-resolution imageFigure 5B A reports the amperometry measurement performed at −0.46 V vs Ag/AgCl, first in the absence of nitrate and then after spiking 20 mM NO3−. When the experiment was performed in the absence of bacterial cells (curve (a)), no current change was observed when nitrate was spiked but a clear response was visible in the presence of Thiobacillus denitrificans in the biocomposite electrode (curve b), and the current was increasing by about 1 μA cm−2. A similar experiment was performed with nitrite. (Fig. 5B). A lower potential was applied to the biocomposite electrode, −0.68 V vs Ag/AgCl. No response was observed in the absence of bacterial cells (curve a) and a significant current increase was observed upon successive additions of 20 mM nitrite. Interestingly, the current increase was about 10 times larger with nitrite that with nitrate, about 10 μA cm−2 increase after each addition of 20 mM nitrite.
Figure 5. Amperometric responses to (A) 20 mM NO3− and (B) 20 mM NO2−, measured with graphite electrodes modified with (a) a composite electrode made of protamine and MWCNT and (b) a biocomposite made with MWCNT, protamine and Thiobacillus denitrificans from a five days culture. All experiments are done under nitrogen atmosphere. (A) was done at −0.46 V and (B) at −0.68 V vs Ag/AgCl.
Download figure:
Standard image High-resolution imageEffect of temperature
The purpose of the following experiments is to evaluate the influence of temperature on the electrochemical reactions that were identified in the previous section. Figure 6 reports the electrochemical response of Thiobacillus denitrificans in the biocomposite to 20 mM nitrate at room temperature.36 The response is similar as the one observed before (compare it with Fig. 3B). Here, the next step was to increase the temperature of the electrochemical cell to 30 °C (curve c). In that conditions, the current response increases significantly and the catalytic signal becomes more visible. The peak potential was moved to a slightly higher value, from −0.35 V to −0.43 V vs Ag/AgCl. We explain this relation between the increase of the current and the temperature to the fact that the membrane proteins of the bacteria are more active at the temperature of 30 °C and as we have deprived the bacteria of all its extracellular metabolites.37
Figure 6. Cyclic voltammetric response measured with graphite felt electrodes modified with biocomposite made with protamine, MWCNT and Thiobacillus denitrificans, (a) at room temperature (21 °C) in the absence of nitrate, (b) at room temperature in the presence of 20 mM nitrate. and (c) at 30 °C, in the presence of 20 mM nitrate. Scan rate of the potential sweep was 5 mV s−1. All experiments are done under nitrogen atmosphere.
Download figure:
Standard image High-resolution imageEffect of nitrate concentration
The influence of concentration of nitrate on the response of Thiobacillus denitrificans biocomposites at 30 °C was evaluated.32 It is presented in Fig. 7. When relatively small amount of nitrate was added (from 10 to 20 mM) a limited current increase was observed at −0.46 V vs Ag/AgCl and a second signal can be noticed at −0.61 V vs Ag/AgCl. In the presence of 40 mM nitrate, the current at −0.46 V increased significantly and the presence of second redox signal at −0.61 V is confirmed. The largest concentration (60 mM) leads only to a limited increase in current.
Figure 7. (A) Cyclic voltammetric response measured with graphite felt electrodes modified with biocomposite made with protamine, MWCNT and Thiobacillus denitrificans at 30 °C, in the presence of increasing amount of nitrate: 0, 10, 20, 40 and 60 mM. Scan rate of the potential sweep was 5 mV s−1. All experiments are done under nitrogen atmosphere. Catalytic current at −0.46 V vs nitrate concentration. The catalytic current is estimated by subtracting the current measured in the absence of nitrate.
Download figure:
Standard image High-resolution imageFigure 8 shows the calibration curve as a linear relationship between different NO3− concentrations (10–60 mM) and the current density (absolute values).
Figure 8. Calibration curve for detection of NO3− by the Thiobacillus denitrificans biocomposite.
Download figure:
Standard image High-resolution imageThe linear equation38 was J/μA cm−2 = 0.32 C + 13.7 with a correlation coefficient of 0.94. This means that the steady state reduction current increases with gradual increase in NO3− concentration. The sensitivity of the proposed method was evaluated using both the limit of detection (LOD) and limit of quantification (LOQ) values. The LOD and LOQ were calculated using the following39:
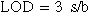
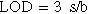
Where s is the standard deviation of the reduction peak current (three runs) and b is the slope (mA M−1) of the related calibration curves; they were found to be 6.3 μM and 21 μM, respectively. This is a good detection limit which means that we can use this system as a sensor to know the current of any nitrate concentration.40
Conclusions
This publication demonstrated for the first time the EET characteristics of denitrifying biocathode. Artificial biofilm in which Thiobacillus denitrificans was devoid of all the metabolites acting as electron mediators and in a hostile composite. It has been used successfully to study the thermodynamics of EET as well as CV analysis of a denitrifying biocathode has shown that the reduction of nitrate to nitrites occurs at −0.46 V and the reduction of nitrites at −0.68 V. The results presented allow to better understand the EET principles of denitrifying cathodes based on Thiobacillus sp. The engineering used in the preparation of the artificial biofilm can help put into operation the exploitation of denitrifying bioelectrochemical systems, e.g. rate of removal of nitrates as a function of the potential of the cathode. In addition, the strategy of using these artificial biofilms allowed to answer questions related to the effect of the temperature on the extracellular transfer and the denitrification activity of Thiobacillus denitrificans which gave a better increase of the current at 30 °C than that at the ambient temperature which was 21 °C. This question asked by Pous et al. about the proportional relationship between the concentration of nitrate and the increase of the current of microcosms exploited here may be more suitable, e.g. study the generation of other reduced nitrogen species (such as NO2) and heterogeneities in packed electrodes using microbial electrode reactions.41
The increase in current to negative values appears only when added nitrite in the solution. This can be explained by the simultaneous reduction of nitrite and hydrogen. so we can suggested this question, from which way hydrogen is produced, we can suppose two hypotheses: first by using NADH as an electron donor to reduce nitrite to ammonium, but without generating a proton energy gradient,42 the second we can think the existence of an NO2−/H+ symporter which is a transporter similar to the NarK1 protein named the symporter NO3−/H+; known in some bacteria.43 The challenge in future work is to assert or deny the existence of the transporteur named the NO2−/H+ symporter in Thiobacillus denetrificans.