Abstract
Highly concentrated electrolytes (HCE) are intensively studied as electrolytes in energy storage devices, with a focus on lithium-metal batteries. Despite the numerous combinations of solvent and salt reported, the relationships between the HCE composition and their properties are not fully understood, which hinders the use of more systematic approaches to their development. In order to address this need, we present here a study of the impact of water on the properties of HCE composed of LiTFSI salt and acetonitrile solvent. The physicochemical properties (density, viscosity and ionic conductivity) and on the electrochemical windows were determined for three electrolytes of different concentrations (1, 3 and 4.1 M) of LiTFSI in acetonitrile with different water contents (20, 200 and 1000 ppm). While the physicochemical properties are only depend on the salt concentration and not the water content, the latter has a significant effect on the electrochemistry of the electrolyte as the electrochemical windows decreased by up to 1.25 V for the 4.1 M HCE with 1000 ppm of water. These results highlight the fact than physicochemical properties cannot be used to assess the water levels and that even 200 ppm decreases the electrochemical windows of the electrolyte.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: permissions@ioppublishing.org.
Currently, highly-concentrated electrolytes (HCEs) are intensively studied for applications in energy storage devices. These electrolytes are obtained using a specific combination of salts and solvents that allow the molar quantity of salt to achieve that of the solvent. The properties of electrolytes in such conditions differ from those in conventional diluted ones. The purported enhanced properties include better electrochemical stability,1 faster charge transfer kinetics,2 and a higher transport number3,4 for Li ions.
Many systems with different combinations of salt and solvent have been explored. The most frequently-studied salts are lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and lithium bis(fluorosulfonyl)imide (LiFSI). Both show a high ionic association, which mean lower viscosity and thus better conductivity. The more associated mixture will contain more free solvent, which may act as a lubricant for the solvates and thus reduce the solution's viscosity. The LiFSI salt shows the highest ionic association and therefore usually results in solutions with lower viscosity.5,6 The most commonly-used solvents in HCEs are acetonitrile (AN), carbonate (ethylene carbonate (EC)), dimethyl carbonate (DMC), propylene carbonate (PC) and water.1,7–9 These solvents are chosen because their high dielectric constant can easily dissolve electrolyte salts, allowing to reach high salt concentrations and exhibit higher ionic conductivity.1,7,9,10
Water is also a solvent of choice since it helps bypass issues related to moisture uptake, solvent toxicity and flammability.8 Water-in-salt (WiS) systems are therefore gaining considerable attention for energy storage applications in devices.8,11–13 Despite this high interest, they will not be discussed further here, since the current research focuses on the impact of water content on acetonitrile-based HCE properties.
The multiple combinations of solvents and salts listed above are widely reported in the literature, and are of particular interest for energy storage devices. This interest lies mainly in the possible use of solvents that are unstable under normal electrolyte conditions, which become more resistant towards electrochemical decomposition in highly-concentrated solutions. For instance, acetonitrile decomposes spontaneously on lithium metal, but has been applied as a HCE in a lithium metal battery.1 Early studies on HCEs were reported in the 1970s,14 but the application of such systems as electrolytes in energy storage devices was proposed by Yamada and collaborators in the early 2010s.1,5,9 Since then, HCEs have been studied for their potential use in Li-ion5,9,15–18 and Na-ion batteries,19 supercapacitors,20 Li-air10 and Li-S batteries3 among others.
Despite numerous reports on the behavior and benefits of HCEs in electrochemical systems, their properties are not yet fully assessed nor understood. The fact that different research groups report on different solvents, salts and salt concentrations further increases the difficulty of establishing structure-properties relationships.
The HCE preparation method varies from group to group. Several factors such as the time required to prepare the solution, its storage time and conditions, the product grade, and any salt pre-treatments6,7,21 are often disregarded or unspecified in reports. As will be shown here, such differences can cause significant variations in results, which could be attributed in part to different quantities of water. Although studies noting the HCE water content before and after testing in systems are available,6,7,15 to the best of our knowledge no systematic studies of the origin and impact of water content on HCE have been reported.
In order to tackle this issue, we present a study on the impact of water content on the physicochemical properties and electrochemical potential window of HCE for varying amounts of water and different concentrations of LiTFSI in acetonitrile. The LiTFSI concentrations selected are 1, 3 and 4.1 M, providing salt-to-solvent molar ratios of 1:16, 1:3.5 and 1:1.9, respectively. Those values were selected to represent a conventional electrolyte (1 M), the most often-used HCE ratio for LiTFSI-acetonitrile system (4.1 M) and an intermediate value.1,6,15,21 For each of these solutions, the water content was varied from 20 ppm to 1000 ppm. The density, viscosity, conductivity, and electrochemical windows of stability were determined for each solution. We demonstrate the most significant impact of water content is on electrochemistry at a platinum electrode. This work highlights the importance of using a standardized procedure to prepare and characterize HCEs.
Experimental
Material
Lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) was purchased from Sigma-Aldrich (99.95%) and from Solvionic (99.9%, extra-dry <20 ppm water). The acetonitrile (AN) was provided by Fischer-Scientific (99.8%+, anhydrous). All salts and solvents were transferred to an Ar-filled glovebox when received, except for the LiTFSI from Sigma, which was dried overnight in a vacuum oven at 80 °C. All solutions were prepared by dissolving the required amount of LiTFSI in AN under mild heating (60 °C) and stirring until complete dissolution of the salt. The deliberate contamination of HCE by water was achieved by adding the required amount of MilliQ water to reach the desired content in ppm (μg/g). KF titration was used to confirm the exact water content.
Physicochemical properties
Density and viscosity measurements were conducted simultaneously with an Anton-Paar DMA 5000 M and Lovis 2000ME, which use an oscillating U-tube principle for density and a rolling ball (falling sphere method) viscometer. The water content was determined using a Mettler-Toledo Q20 coulometric Karl–Fischer titration verified against a standard prior to each experimental run. The ionic conductivity was measured with a Jenway microvolume 120 mm reach glass conductivity probe (model 027815), made of two platinized platinum plates. The density, viscosity and ionic conductivity measurements were done at 30.0 ± 0.2 °C.
Electrochemical stability
To evaluate the electrochemical window, linear sweep voltammetry experiments were carried out using a SP300 potentiostat (BioLogic) in a three-electrode cell configuration. A platinum (Pt) microelectrode (65 μm radius) was used as the working electrode and two Pt wires as counter and quasi-reference electrodes (CE and QRE). The potential of the platinum QRE was calibrated for each solution (different salt and water concentrations) with the ferrocene/ferrocenium redox couple. Freshly-prepared electrolyte solutions were used for each measurement to avoid passivation of the working electrode. All three electrodes were cleaned before each experiment either using a flame (Pt wire) or by polishing with 0.05 μm alumina suspension (Pt microelectrode). All electrochemical measurements were carried out in an Ar-filled glovebox.
Results and Discussion
The objective of this work is to determine the impact of water content in LiTFSI-acetonitrile HCEs on their physicochemical and electrochemical properties. We began by examining the main source of water contamination, and the impact of preparation steps and storage on water content.
Water in starting materials
The primary factor that influences the water content of the HCE is the presence of water in the salt, due to the large weight used in their preparation, which often exceeds that of the solvent. For instance, the preparation of the 1:1.9 HCE requires 3.6 g of LiTFSI per g of acetonitrile. Table I presents the water content of HCEs at 1:1.9 LiTFSI-acetonitrile ratio, prepared with salt from three different suppliers. Only the Solvionic salt had a specified water content (<20 ppm). Using the Sigma salt as received resulted in an electrolyte with over 900 ppm of water, a significant amount (77 mM). As it will be shown later, 1000 ppm of water does not affect the properties of the electrolyte, except for a significant reduction in the electrochemical potential window of stability. The salt received from Acros provided an HCE with 4098 ppm of water, corresponding to 0.34 M of water in the electrolyte. This amount is sufficient to impact the physicochemical properties. Drying the same salt overnight at 80 °C before preparing the electrolyte decreased the water content enough to achieve solutions with 63 and 72 ppm (for the Acros and Sigma salts, respectively), illustrating the importance of the salt's moisture content on the final solution. Neither of these salts could result in HCE as dry as the one using an extra dry salt (Solvionic, 20 ppm). Note that the acetonitrile used to make the solution (99.8%+ anhydrous under inert atmosphere) was titrated to 14 ppm of water.
Table I. Water content in HCE of 1:1.9 LiTFSI-acetonitrile (4.1 M in salt) prepared using salts from three different suppliers. HCE were prepared in a glovebox using the salt as received and after drying the salt under vacuum.
| Supplier | Salt as received | Drieda) salt |
|---|---|---|
| Acros Organics (99%) | 4098 | 63 |
| Millipore Sigma (99.95% trace metal basis) | 931 | 72 |
| Solvionic (99.9% Extra Dry, packed under Ar) | 20 | n/ab) |
a)Drying under vacuum at 80 °C for 24 h. b)No further reduction in water content was obtained.
Water uptake by HCE
The main external factor likely to impact water content is atmospheric uptake. Obviously, any exposure to ambient atmosphere will result in a significant increase in moisture content. Dry salt in a sealed container exposed to air for a few days can uptake 8% in water weight (S5). After one week, the salt is now a solution of LiTFSI and water. However, it was not evident whether trace amounts of H2O from a glovebox atmosphere might contaminate the solution and if so, how long this process might take. Such information is important in determining the maximum storage time. HCE preparation usually requires a few hours of stirring to reach dissolution. Table II shows the water content increasing from 76 ppm to 185 ppm for the 1:16 electrolytes and from 99 to 267 ppm for the 1:1.9 electrolytes over a nine-day period. Regardless of the salt concentration, water uptake is possible after prolonged storage in a glovebox. The water content remains unchanged within the first 48 h of storage. Later, this work will show that 200 ppm of water has a significant effect on the electrochemical potential window of stability. Therefore, the delay between HCE preparation and analysis should be minimized, even when stored in a glovebox.
Table II. Water uptake (in ppm) of diluted and concentrated solutions of LiTFSI-acetonitrile during storage in an Ar-filled glovebox.
| Ratio (LiTFSI:AN) | 24 h | 48 h | 9 d |
|---|---|---|---|
| 1:16 | 76 | 84 | 185 |
| 1:1.9 | 99 | 96 | 267 |
Physicochemical properties
Solutions with different concentrations of water were prepared to study its impact on the physicochemical properties of HCEs. The concentrations were selected to reflect the possible conditions: one made with a very dry salt (20 ppm), a salt used "as is" (1000 ppm) and a solution stored in a glovebox for a week (200–400 ppm). A gas-tight syringe was used to transfer the sample between the glovebox and the measurement apparatus.
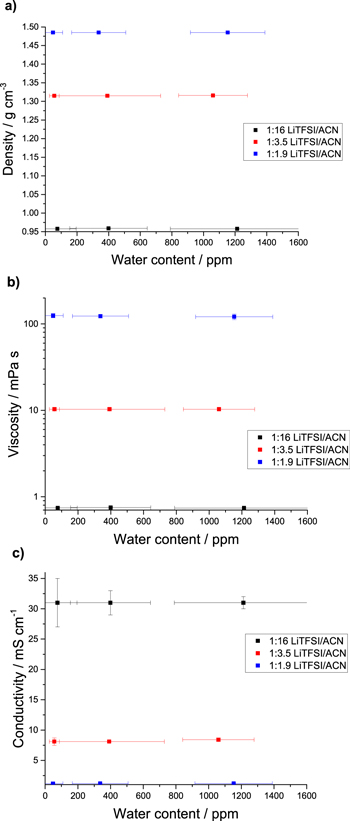
Figure 1 shows the viscosity, density, and conductivity of the three different LiTFSI-acetonitrile mixtures (1:16, 1:3.5 and 1:1.9 molar ratio) each at three different water contents. Figure 1 shows that for a given salt concentration, all measured properties remained identical from 50 ppm to 1200 ppm of water. This water content range represents a molar concentration of 2.5 mM up to 0.1 M of water, which is a significant value. Such results might have been expected for the diluted systems where the acetonitrile solvent is the dominant species and is mostly free in the solution. However, for the highly-concentrated solutions, the acetonitrile was mostly coordinated to the lithium ions, forming contact ion pairs (CIP) and aggregate (AGG) with the TFSI anion.1 It appears that water does not impact the formation of these assemblies, which are largely responsible for the properties of HCEs. A different behavior was noted for the HCE containing 4098 ppm of water (see Table I). With a calculated concentration of 0.34 M of water, this solution had a measurable yet small difference in density (1.48012 g cm−3 vs 1.48546 g cm−3 for the dryest, 20 ppm HCE). The viscosity however is significantly lower at 4098 ppm (94.341 mPa s vs 121.77 mPa s for the 20 ppm HCE).
Figure 1. Impact of water content on (a) density, (b) viscosity, and (c) ionic conductivity of electrolytes in 1:16 (≈1.0 M), 1:3.5 (≈3.0 M) and 1:1.9 (≈4.1 M) LiTFSI-acetonitrile of varying molar ratios. The error bars represent the standard deviation of three measurements (both on the water content and the property value). The detailed values are presented in Table SI of the Supporting Information.
Download figure:
Standard image High-resolution imageApart from this last extreme case, Fig. 1 shows that salt concentration is the major factor determining HCE properties. The presence of water does not influence the dependency of viscosity and conductivity with temperature (Figs. S1 and S2 is available online at stacks.iop.org/JES/167/120536/mmedia) neither for the dilute system (1:16) nor the HCE (1:1.9). As expected, the viscosity displays an Arrhenius behavior with temperature indicated by the linear trend in Fig. S2. The HCE on the other hand displays a curved trend, indicative of a complex structure (contact ion pairs and aggregates) that is affected by temperature. The difference in conductivity between the low and high water content of the HCE is within the uncertainty of the measurement. Solvent evaporation at higher temperatures made conductivity values irreproducible for the diluted solution.
These results differ from the observations made on water's impact on the properties of ionic liquids (ILs), another medium with high ion concentrations. For ILs, studies have shown a significant decrease in viscosity depending on water content.22–25 Viscosity decreased up to 13% between dry ILs and those containing 2000 ppm of water, an effect noted with several types of anions and cations.13,25 However, the impact on density was much less significant, with variation of roughly 1%, likely due to the large free volume in ILs.
Electrochemical window
The previous section's results suggest that water in LiTFSI-acetonitrile HCEs would be found as "free" water. When strongly associated to ions—such as in WiS systems—water reactivity decreases,26 and thus, the presence of "free" water will likely impact the HCE's electrochemical stability. Usually, either cyclic voltammetry or linear sweep voltammetry (LSV) is used to evaluate the potential window of electrochemical stability. The potential is swept until an abrupt current (or a given current density) associated with electrolyte oxidation or reduction is attained, providing a curve similar to Figs. 2a and 2c. The important aspects to consider are the effect of the working electrode material, the potential scan rate's impact on determination of the potential limit, the fact that some reactions yielding lower current densities may occur before the solvent reaction, and whether the reactions result in a deposit that affects determination of subsequent cycles. The cut-off current is often arbitrary and the potential windows may vary by several hundred mV.27 For the HCE, the difference between two studies is as high as 0.9 V for the LiTFSI-AN systems.1,15 This discrepancy is attributed to differences in experimental setup, potential sweep rate, and electrode material.1,15,21
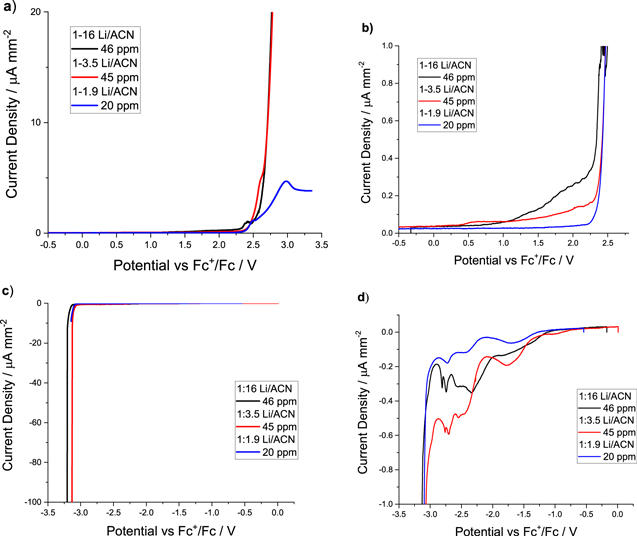
Figure 2. LSV curves at 1 mV s−1 presented at different scales (left- vs right-hand side) for each LiTFSI:acetonitrile molar ratio studied, showing the high and low potential regions. The figure compares the electrolytes with very low amounts of water (20 to 46 ppm). Each curve was obtained with cleaned electrodes and freshly-prepared electrolyte.
Download figure:
Standard image High-resolution imageWith this in mind, we studied the impact of water on the electrochemical stability of the LiTFSI-AN electrolytes using LSV with a Pt microelectrode. For each measurement, the scan was started at OCP and slowly swept in either the positive or the negative direction until a significant current increase was observed. The setup was reset after each LSV to ensure the freshly-prepared electrolyte and freshly-polished electrode were used only once. All curves are also presented with an enlarged scale to detect the presence of oxidation or reduction events prior to solvent decomposition.
Figures 2a and 2c show the large current increase associated with electrolyte oxidation and reduction, respectively. At the positive potentials, a steady increase in current was observed from about 1 V vs Fc+/Fc for the most dilute solution (1 M LiTFSI in AN) as seen in Fig. 2b. The two other concentrations show the same abrupt increase in current at 2.3 V vs Fc+/Fc. The oxidation potential of the AN corresponds to 2.35 V vs SHE.28 However, the 1:3.5 solution showed a small increase in current density at lower potentials. The oxidation of the 1:1.9 HCE was significantly different from the other molar ratios at higher potentials. The oxidation curve above 2.75 V shows a much lower rate and presents a peak. This behavior is attributed to the absence of free AN molecules and the lower reactivity of the AN bound in CIP and AGG.
Under reductive conditions (Figs. 2c and 2d), the three salts concentration show more complex curves (enlarged CVs) with similar peaks, with slightly higher currents for the 1:16 and 1:3.5 molar ratios. All three solutions show an abrupt increase in current between −3.15 and −3.25 V (vs Fc+/Fc) corresponding to the reduction of AN (Fig. 2c). The first observation is the much lower current densities observed at the highest salt concentration (HCE) which suggests greater stability. This phenomenon has already been reported for several systems on multiple occasions,1,7,15 and represents the primary rationale for using HCE in electrochemical systems. Based on a comparison of peak position from all three curves in Fig. 2d, it is noted that reactions are occurring at the electrode with the HCE (1:1.9 LiTFSI-acetonitrile), and these reactions may be the same observed with the more diluted solutions. However, the rate for these reactions is much lower in HCE, yielding smaller current density and likely impacting the system less.
To study the impact of water on these reactions and on the electrochemical stability window (ESW), each electrolyte composition was spiked with water to reach 200–400 ppm and 1000 ppm. Figure 3 shows the LSV towards the positive potentials for these nine samples. The curves obtained with the HCE are more affected by an increase in the amount of water than the diluted solutions (i.e. 1:16 and 1:3.5 ratios) and already show a significant current increase at 2.0 V vs Fc+/Fc with 1059 ppm of water (see blue curve on Fig. 3c). As expected, the 20 ppm provides a negligible current density up to 2.25 V vs Fc+/Fc, corresponding to 5.75 V vs Li+/Li.
Figure 3. LSV of oxidative condition of the electrolyte (a) 1:16 (1.0 M), (b) 1:3.5 (3.0 M), and (c) 1:1.9 (4.1 M) at 1 mVs−1 on a platinum microelectrode. The dashed line represents the equilibrium potential for the oxidation potential of water.
Download figure:
Standard image High-resolution imageThis result is higher than the potential reported in the works of Yamada and collaborators.1 They reported that the oxidative stability is retained over 5 V (about 5.4 V) vs Li+/Li, corresponding to the oxidation potential of free AN. For their part, Nilsson and collaborators reported an oxidation potential of 4.6 V vs Li+/Li.15 These differences in potential may come from the setup use or the presence of water in the electrolytes. Nilsson and collaborators used an aluminium electrode, and the study made no mention of the scan rate. The utilisation of an aluminum electrode may be the limiting factor in the determination of the oxidative stability of HCE. The oxidation potential of the aluminium is around 4.0 V vs Li+/Li.15 While Yamada and collaborators used the same scan rate and electrodes materials for the working electrodes as we did, but used a platinum plate instead of the microelectrode. The difference may come from the presence of water in the latter's electrolyte. The electrolyte with 1059 ppm of water shows an increase in current around 5.4 V vs Li+/Li, which is the potential Yamada and collaborators obtained. All those differences between our work and the results of Yamada and Nilsson may explain the difference in the report oxidation potential of the 1:1.9 electrolytes. It's difficult to compare potential limit result when various scan rates are used, because of the overpotential. A microelectrode allows to decrease the importance of the overpotential as the current is a magnitude lower. The fast diffusion rate of products away from the surface of microelectrode decreases the possible deposition or changes of the surface chemistry of the electrode. While determination of the products formed at the various potentials will be required to clarify the reactions, the potential of oxidation of the TFSI anion is around 2.1 V vs Fc+/Fc as determined through theoretical calculation.29 This is likely to be the reaction associated with a current increase between 1.5 and 2.25 V.
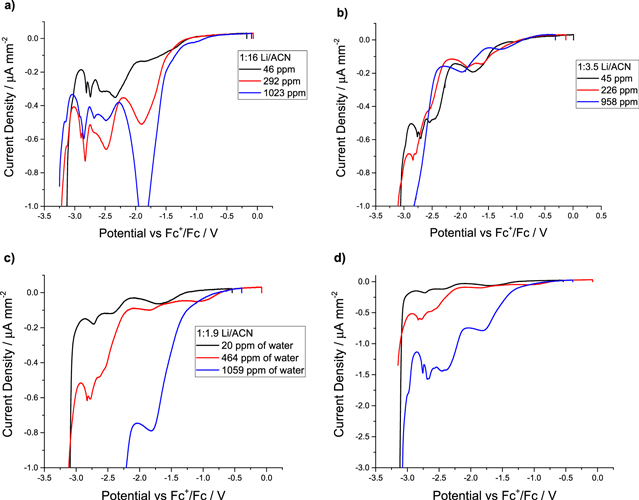
The LSV curves at negative potentials are far more complex, and show multiple peaks. The reactions most likely to occur at negative potentials are the reductions of water, of the TFSI anion, and of acetonitrile. The potential values for the reduction of these molecules in highly-concentrated media are not well-documented, which complicates assignment of the peaks. In addition, the potential for these reactions may very well shift from the standard potential measured in dilute solutions. The reduction of acetonitrile should occur around −3.0 V vs Fc+/Fc, as previously reported.1,15,21 Theoretical calculations place the reduction of the TFSI anion at about −2.5 V vs Fc+/Fc.1,30 Finally, the potential for the reduction of water is −1.4 V vs Fc+/Fc; however, this potential is for the reduction of bulk water. In addition to these, many other reactions may take place, including the reduction of products involved in the formation of a solid electrolyte interface (SEI), as noted in the studies found on the formation of SEI in HCE with LiTFSI salt.1,3,8,9,31,32 These studies show the main component of the SEI is LiF from reduction of the TFSI anion. However, many more decomposition products—which can also be electroactive—may be initiated by reduction of the TFSI anion, rendering identification of the multiple peaks in Fig. 4 difficult. The works of Dubouis and collaborators shows an effect of the water reduction on the reduction of the TFSI anion in Water in salt electrolytes a similar phenomena may happen in the HCE with 1000 ppm of water.32
Figure 4. LSV in reductive condition of the electrolyte (a) 1:16 (1.0 M), (b) 1:3.5 (3.0 M), (c) and (d) 1:1.9 (4.1 M) at 1 mV s−1 on a platinum microelectrode.
Download figure:
Standard image High-resolution imageFigure 4 shows a decrease in the number of peaks with the increase in LiTFSI concentration, and an increase of the current density with the water content for a given concentration. The more diluted system (1.0 M) presented more side reactions than in the highly-concentrated systems (4.1 M), suggesting that clusters of Li+, AN and TFSI− are less reactive than the species in the diluted solution. This observation aligns with the current understanding of HCE structure, thought to consist of 3D organization in CIP and AGG. In most cases, the current density increases with water concentration by a two to three-fold factor, from 50 to 1000 ppm.
As mentioned earlier, three possible reductions can be assigned. The first is the reduction of water, visible at each concentration of LiTFSI, with a peak around −1.75 V vs Fc+/Fc. This peak is likely assigned to the reduction of water because the current increases with water content. The second reduction peak can be assigned to the reduction of the TFSI anion. With the theoretical calculation in the highly-concentrated LiTFSI-AN systems, this reduction should happen at about −2.5 V vs Fc+/Fc. In this region, there is a series of peaks for each concentration of salts and water, and it is therefore difficult to precisely assign one peak to reduction of the TFSI anion. Finally, the last peak at about −3 V vs Fc+/Fc is assigned to reduction of AN solvent molecules, which corresponds to theoretical calculations and observations in other studies.1,15 This finding shows that the presence of water increases the intensity of the side reactions, thus decreasing the electrochemical window of HCE.
The most obvious impact is seen with the HCE (4.1 M, Fig. 4d). While the potential limit is similar for each water content, a significant reduction in current density occurs as early as −1.75 V for the 1059 ppm sample. While these reactions are often overlooked to the benefit of the −3.0 V limit, they may explain the limited cycling observed with HCE.
Even in HCE, the presence of small amounts of water (around 300–400 ppm) had a significant impact on the electrolytes' electrochemical stability. This level of water can be found in HCE when proper care is not taken to dry the components. Yet, even with 20 ppm of water, the reduction of water is visible and could lead to limited cycle life. The decrease in the stability window has an impact in the study of HCE in Li-ions batteries, as water and its decomposition products can react with the graphite or Li-metal anode. However, this could prove beneficial if the decomposition product forms a passivation layer. Further studies are required to understand and identify the interfacial product generated by the reduction of HCE on these materials.
Conclusions
This study sought to evaluate the impact of water on the various properties of an HCE based on LiTFSI and acetonitrile. Water is mostly incorporated in the HCE via LiTFSI salts, which are highly hygroscopic. We observed that for solutions with 1000 ppm and below, water content has no significant impact on the HCEs' physicochemical properties, measurements of viscosity, conductivity and density. In one case, an HCE with 4098 ppm was obtained and this water content was sufficient to observe a significant decrease in density and viscosity. Using "as is" salt is not recommended; rather, the salt should be dried in a vacuum oven for at least 24 h at a maximum temperature of 80 °C, unless the supplier guarantees a maximum moisture content at very low levels. Decomposition is possible at higher temperatures. The LSV measurement highlights the impact of water content on the HCE's electrochemical windows. Water content has a slight impact on oxidative stability but significantly affects reduction stability. The reduction potentials of the 1:1.9 electrolyte shift from −3.0 V to −2.0 V vs Fc+/Fc. Under the conditions used in this study, a the driest electrolyte of LiTFSI and acetonitrile has an ESW of 5.25 V starting at 0.45 V vs Li+/Li. The stability of such electrolyte may be lower when composite electrodes are used such as in batteries and supercapacitors, due to the reactivity of the active materials and electrode porosity. Obtaining a better understanding will require identification of the decomposition products associated with the various peaks present on the LSV. Our research group is currently focused on this question, in order to better understand the electrochemical stability and formation of the SEI.