Abstract
To understand the mechanism of repassivation, a key process that controls the performance of corrosion resistant alloys (CRAs), it is useful to obtain some fundamental parameters that describe the atomistic processes. Oxygen and chlorine adsorption energies were systematically calculated with density functional theory for Ni-22Cr alloys with seven different solute elements: Co, Cu, Fe, Mn, Mo, Ru and W. All elements other than Cu enhance both O and Cl adsorption to alloy surfaces. Cr has the strongest effect among these elements. Surface configurations that give stronger O adsorption also tend to give stronger Cl adsorption. While this makes it difficult to differentiate the effect of these solute elements on the oxidation/repassivation tendency of alloys, these fundamental properties provide the foundation for higher scale models that could be used to study the oxidation process during corrosion and potentially inform the design of CRAs. Bader charge analysis reveals a strong relationship between the adatom adsorption energy and the Bader charges of both O and Cl adatom. The work function of alloy surfaces is increased by O while decreased by Cl, suggesting that Cl adsorption can have an intrinsic negative impact on corrosion resistance by decreasing the barrier to electron transfer.
Export citation and abstract BibTeX RIS
Corrosion resistant alloys (CRAs) provide reliable performance in energy,1–3 aerospace,4 health,5,6 oil and gas7 and other applications. Nonetheless, alloys with higher corrosion resistance are always pursued by researchers and industries in order to reach longer lifetimes or survive more extreme environments. However, the design of CRAs has been mainly a trial-and-error process guided by intuition and past successes. Historically, one common method is adding a small amount of different solute elements to a base element, e.g. it is known that Cr, Mo and W increase the corrosion resistance of both Ni-based alloys and stainless steels.3,4,8,9,10 In recent years, high entropy alloys (HEAs) have been developed to potentially improve corrosion resistance of metallic materials.11–14 Whether it is the development of a major element-based alloy or high entropy alloy, the process has followed the path of picking/adjusting alloying element based on empirical experience and measuring the corrosion resistance of produced alloys with accelerated testing. Such a process is both time consuming and expensive. Integrated Computational Materials Engineering (ICME) approaches, which use models of the fundamental underlying science to predict material properties, have been applied in alloy design in recent years.15–18 These models allow high-throughput calculations to screen materials in a relatively short time compared to experiments, and therefore increase the efficiency of materials design tremendously. However, such models have not yet been applied in the design of CRAs. To move this field forward, one of the first steps is to understand the detailed mechanisms during corrosion and obtain the fundamental parameters.
Corrosion occurs through an interconnected system of processes that occur at the interface between alloys and aqueous solution. The adsorption of O and Cl onto alloy surface are key processes that can affect oxide formation, salt film formation as well as pit and crevice stability,19,20 all of which may become critical to the corrosion resistance of metallic materials. Since passivity for many CRAs is conveyed by a film that may be only a few nanometers thick, changes to the surface properties at the atomistic level may have a significant impact on their performance. Theoretically, density functional theory (DFT) has been used to study the effect of adsorption of atoms and molecules onto alloy surfaces.
Ni-based alloys represent an important class of CRAs.3,4,21–24 The adsorption of O atom onto pure Ni surface has been extensively studied by DFT, including the effect of surface coverage,25,26 different surface planes,27–29 effect of defects,30 and adsorption sites on O adsorption energies.29,31,32 It is found that fcc hollow site on Ni (111) surface is the most favorable site for O adsorption.29,31–33 Das et al. studied the effect of Cr on O adsorption in Ni-Cr binary alloys with doping method and found that Cr enhances the adsorption of O.26,27 Similarly, Alexandrov et al. studied effect of five different alloying elements including Al, Cu, Fe, Cr and Mn on adsorption of O on Ni-based binary alloys and found out that Cr and Mn exhibit the strongest binding to O, Al and Fe exhibit binding energies that are similar to Ni, and Cu tends to weaken the adsorption of O to alloy surface.29 Recently, Samin and Taylor studied the effect of Cr on O adsorption in Ni-22Cr alloys with special quasi-random structure (SQS) method,33 which can create supercells that possess atomic correlation functions that closely mimic those in random solid-solution alloys. They also dope the system with Mo to explore the effect of Mo in a Ni-Cr-Mo ternary system. While a few studies have been made on O adsorption to Ni-based alloys, from pristine Ni up to ternary system, few studies have been made on the adsorption of Cl to Ni-based alloys.
The objective of this work is to develop a scientifically-based descriptor to evaluate the repassivation tendency of CRAs by connecting calculatable fundamental parameters with environmental conditions, so that such a descriptor can be combined with other quantifiable descriptors contributing to corrosion to expedite the design of CRAs. This work consists of two parts. In Part I, a systematic study was implemented on the effect of solute elements on adsorption of both O and Cl for Ni-Cr-X ternary alloys using DFT. Besides Cr, a total of seven commonly used elements, namely Co, Cu, Mn, Mo, Fe, Ru and W, were examined. Bader charge of adatom and work function of alloy surfaces were also studied. In the next part,34 the adsorption energies obtained from DFT will be used in an isothermal model to calculate equilibrium surface coverage of Cl and O on an alloy surface by incorporating environmental conditions including temperature, applied potential, pH and Cl− concentration in solution, and a scientifically-based descriptor will be introduced to evaluate the repassivation tendency of CRAs.
Modeling Method
Spin polarized density functional theory calculations were used to solve the electronic structure problems using Vienna ab initio simulation package (VASP)35 with the implementation of the Projector-Augmented-Wave (PAW) pseudopotentials.36,37 A generalized gradient approximation (GGA) exchange correlation functional was used as parameterized by Perdew, Burke and Ernzerhof.38 The valence electron eigenfunctions were expanded in a plane-wave basis set with an energy cutoff of 450 eV and a Methfessel–Paxton smearing of the first order of width 0.1 eV was used in the simulations.39 Convergence with respect to the cutoff energy and the k-point mesh was checked and the convergence criterion for the electronic self-consistent calculation was set to 10−6 eV. Furthermore, the structure was allowed to relax until the total energy difference between two ionic steps was less than 2 × 10−4 eV. Reciprocal lattice integrations were performed using a (7 × 7 × 1) Monkhorst-Pack Γ-centered k-point grid.40 All calculations were performed on supercomputers at Ohio Supercomputer Center.41
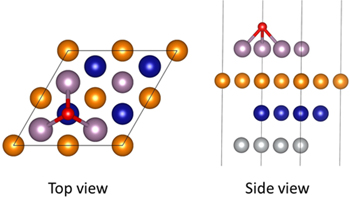
P(2×2) (111) surface slabs were created from Ni-22Cr bulk alloy structures generated with SQS method. More details regarding how these slabs were created were described in a previous paper.33 Each slab consists of eight layers and a total of 32 atoms with a vacuum of thickness of 18.43 Å to minimize interactions with slab images. The bottom five layers were fixed at bulk positions while the top three layers were allowed to relax. O and Cl adatoms were put on hollow-fcc adsorption sites with a coverage of 0.25 monolayer (ML) since it is shown that the hollow fcc site is the most stable adsorption site.29,31–33 Figure 1 shows a schematic plot of an adatom adsorbed at hollow-fcc site on a (111) surface plane. As can be seen from the figure, three atoms from the top surface are bonded with adatom. To study the effect of different solute elements, these three atoms were doped with different combinations of Ni, Cr and other solute elements. For a Ni-Cr-X ternary system, there is a total of 10 configurations for each solute element. Seven solute elements were studied: Co, Cu, Fe, Mn, Mo, Ru and W. Please note that, although other atoms on the top surface and right below the top surface may also affect the adsorption of adatoms, their effects can be neglected compared with the directly bonded atoms (shown in supplemental data, where results from different bulk configurations were given is available online at stacks.iop.org/JES/167/111502/mmedia).
Figure 1. Schematic view of hollow-fcc adsorption site on Ni-Cr (111) surface.
Download figure:
Standard image High-resolution imageAdsorption energy of O and Cl were calculated based on the dissociative adsorption of the parent molecules using
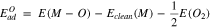
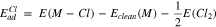
where Eclean(M) is the energy of clean metallic surface, E(O2) is the energy of O2 molecule, E(Cl2) is the energy of Cl2 molecule, and E(M-O) and E(M-Cl) are the energy of system with O and Cl bonded to surface, respectively. A lower adsorption energy means a higher adsorption strength.
Results
O adsorption energy
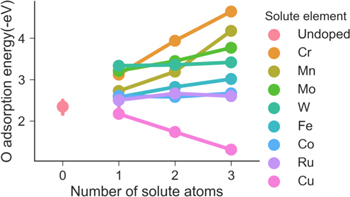
Figure 2 shows O adsorption energy as a function of the number of solute element atoms bonded with O adatom when Ni and only one other kind of solute element are bonded with the O adatom. When all the three atoms that are bonded with O adatom are Ni (Undoped), a few different bulk configurations are considered, and O adsorption energy is between −2.5 eV and −2.0 eV. This is consistent with what was calculated by Samin and Taylor.33 As the number of doping solute element atoms increases, O adsorption energy changes almost linearly for all the elements studied. Except for Cu, for which O adsorption energy increases with the number of Cu atoms bonded with O adatom (i.e. more positive, or weaker adsorption), for all other solute elements, the O adsorption energy decreases (stronger adsorption) with increasing number of solute atoms bonded with O adatom, although the effect of Co and Ru is low compared to other elements. The lowest adsorption energy occurs when all three atoms bonded with O adatom are replaced by Cr, which is −4.64 eV and almost double the adsorption energy than when all three atoms are Ni. The highest adsorption energy occurs when all three atoms are replaced by Cu atoms, which gives O adsorption energy of −1.31 eV. The order of effect of different solute element on enhancing O adsorption energy is in the order of Cr > Mn > Mo > W > Fe > Co ≈ Ru.
Figure 2. Effect of different solutes on O adsorption energy when Ni and X (Cu, Ru, Co, Fe, W, Mo, Mn and Cr) are bonded with O adatom. Undoped means all three atoms bonded with O adatoms are Ni.
Download figure:
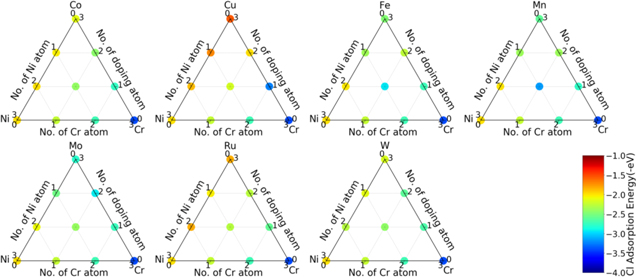
Standard image High-resolution imageFigure 3 shows the result of O adsorption energy for all configurations studied for Ni-Cr-X ternary systems. As can be seen from the figure, for most of the configurations studied, the adsorption energy with a configuration can be considered an interpolation of the adsorption energy of each solute element bonded with the adatom, which means no synergy was found among these elements with Ni and Cr other than a minor synergistic effect was found for the Ni-Cr-Mn system. This general trend is also consistent with the linearity of the plotted adsorption energies in Figure 2. The configuration of 1Ni1Cr1Mn gives a lower adsorption energy than configuration 1Ni2Cr, which means adding Mn to Ni-Cr system could potentially increase alloy O adsorption ability. At the same time, a slightly repulsive effect is also found when Mn and Cr are bonded with O adatom, e.g. O adsorption energy is higher when both Cr and Mn bonded with O than those cases that only Cr or Mn atoms are bonded with O adatom. While for other systems, decreasing the composition of Ni in a Ni-Cr-X system is always favorable regarding O adsorption, it is not the case for Ni-Cr-Mn system due to these synergies. Values of all these O adsorption energies are listed in supplemental data.
Figure 3. Oxygen adsorption energy for all configurations calculated for Ni-Cr-X ternary system. The plots were generated by modifying python package "python-ternary."42
Download figure:
Standard image High-resolution imageCl adsorption energy
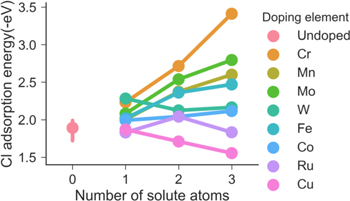
Figure 4 shows the adsorption energy of Cl on Ni-22Cr (111) surface with the effect of different solutes for configurations that include only Ni and one other solute element bonded with Cl adatom. When all three atoms bonded with adatom are Ni, the adsorption energy is between −1.99 eV and −1.72 eV. As can be seen from the figure, the effect of different solute elements on Cl adsorption energy is similar to that on O adsorption energy. As the number of solute element atoms increases, Cl adsorption energy changes linearly except for Fe and Ru. For Fe, the lowest Cl adsorption energy occurs when the combination of three atoms are 1Fe and 2Ni. For Ru, the lowest energy occurs when the combination of three atoms are 2Ru and 1Ni, although all these configurations containing Ru give Cl adsorption energy similar to that of all Ni configuration. Cu is still the only element that tends to increase Cl adsorption energy, and Cr is still the element that has the strongest effect deceasing adatom adsorption energy. When all three atoms are Cr, Cl adsorption energy is the lowest with a value of −3.41 eV. The order of effect of different solute element on enhancing Cl adsorption energy is Cr > Mo > Mn > W > Fe ≈ Co > Ru. Figure 5 shows the result of Cl adsorption energy for all configurations studied for Ni-Cr-X ternary systems. Synergies are found in Ni-Cr-X ternary system when X is Mn or Fe, where configuration of 1X1Ni1Cr gives lower Cl adsorption energy than configurations 2X1Cr and 1X2Cr. No synergies are found for other configurations.
Figure 4. Effect of different solutes on Cl adsorption energy when Ni and X (Cu, Ru, Co, Fe, W, Mo, Mn and Cr) are bonded with Cl adatom. Undoped means all three atoms bonded with Cl adatoms are Ni.
Download figure:
Standard image High-resolution imageFigure 5. Cl adsorption energy for all configurations calculated for Ni-Cr-X ternary system. The plots were generated by modifying python package "python-ternary."42
Download figure:
Standard image High-resolution imageDiscussion
O vs Cl adsorption energy
With a comprehensive database built for both O and Cl adsorption energies, relationships between these energies can now be considered. Figure 6 shows the relation between Cl and O adsorption energy for all configurations studied and highlights those configurations that all three atoms bonded with the adatom are of the same element type with square symbols. As can be seen from the figure, a strong relationship between Cl and O adsorption energy is found. Configurations that favor O adsorption also favor Cl adsorption, which makes it difficult to infer the exact effect of each element on the affinity for O vs Cl adsorption solely based on these adsorption energies. In other words, although Cr shows strongest O adsorption effect, it is not sufficient to explain the effect of Cr on corrosion resistance of alloys by O adsorption energy alone since Cr also enhances the adsorption of Cl at the same time.
Figure 6. Cl vs O adsorption energy in Ni-Cr-X alloys highlighting configurations that all three surface atoms bonded to adatom are of the same element type (square symbols).
Download figure:
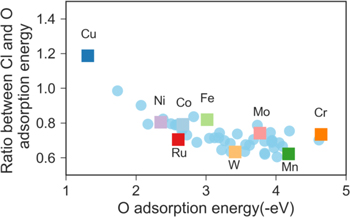
Standard image High-resolution imageFigure 7 shows the ratio between Cl and O adsorption energy as a function of O adsorption energy for all configurations studied and highlights configurations when all three atoms bonded with adatom are of the same element type. As can be seen from the figure, the ratio slightly decreases as O adsorption energy decreases. For most of the configurations, the ratio is around 0.6–0.8, except for Cu, which gives a very high ratio of 1.2 compared to other configurations.
Figure 7. Ratio between Cl and O adsorption energy in Ni-Cr-X alloys highlighting configurations that all three surface atoms bonded to adatom are of the same element type (square symbols).
Download figure:
Standard image High-resolution imageBader charge
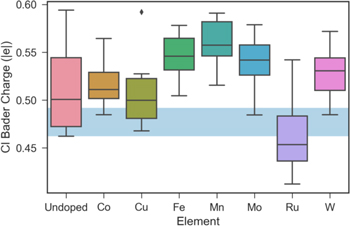
The Bader charge analysis43–45 reveals the electrostatic nature of the bonding between the surface atoms with adatoms. Figure 8 shows the box plot of O Bader charge for different doping elements. When all three atoms bonded with O adatom are Ni, Bader charge of O is about 0.855 ± 0.015 ∣e∣, which is shown as a blue horizontal band in the plot. Please note the wide range of undoped surface is due to the existence of Cr atoms, which tends to contribute more electrons to O adatom and lowers O adsorption energy. The addition of Cr increases the Bader charge of O up to 1.05 ∣e∣. For elements like Fe, Mn, Mo and W, the distribution of O Bader charge is wide for different surface configurations, but all moved to higher Bader charge range compared to the all Ni cases, which means all these elements tend to contribute to O Bader charge by increasing the extent of charge transfer. For Co and Cu, they do not affect O Bader charge much by staying close to all Ni cases. And it can be clearly seen that Ru tends to cause a lower O Bader charge by moving the entire box down.
Figure 8. Bader charge of O adatom for surfaces with different solute elements, blue horizontal band shows the Bader charge range when all three atoms bonded with O are Ni. Undoped means the surface do not contain any solute element other than Ni and Cr.
Download figure:
Standard image High-resolution imageFigure 9 shows the Cl Bader charge for different solute elements. Similar effect is found on Cl Bader charge as on O Bader charge for each element. Similar as for O, Ru moves the entire box down compared to undoped surfaces. Other elements give relatively higher Bader charge compared to all Ni cases, with Fe, Mn, Mo and W give relatively higher value, while Co and Cu give values closer to all Ni cases.
Figure 9. Bader charge of Cl adatom for surfaces with different solute elements, blue horizontal band shows the Bader charge range when all three atoms bonded with O are Ni. Undoped means the surface do not contain any solute element other than Ni and Cr.
Download figure:
Standard image High-resolution imagePrevious studies from the literature suggest that there might be a correlation between the adsorption energy and the amount of charge exchanged since it was found that the net charge transfer from the Ni–Cr surface to an oxygen atom is higher than from the Ni surface.31,33 Figure 10 shows the relationship between adsorption energy and Bader charge of adatom for all the configurations studied for both O adatom and Cl adatom. As can be seen from the figure, while there is some spread of the data points, a strong relationship between adsorption energy and Bader charge was found for both O and Cl adatom, with a R2 of 0.97 and 0.99, respectively. This further confirms that adsorption energy is significantly correlated with the electron exchange between adatom and surface atoms.
Figure 10. Relationship between adsorption energy and adatom Bader charge: (a) O adsorption; (b) Cl adsorption.
Download figure:
Standard image High-resolution imageWork function
Work function is a property that indicates the work required to remove an electron from the surface slab, and it could be a useful property to compute for CRAs as a function of the clean and adsorption states, since corrosion is an electrochemical process. Figure 11 shows the work function of the clean surface with different solute elements. All solute elements will expand the distribution of work functions, and the maximum work function obtained for each solute element is higher than that for undoped surfaces. Elements Ru and W raise the work function of the clean surface, therefore, they can be expected to enhance resistance to dissolution by stabilizing electrons in the metal (i.e. increasing nobility). Conversely, Mn, Cu and Fe (and Mo but there is a wider distribution) lower the work function, so they may be expected to decrease nobility.
Figure 11. Work function of clean surfaces with different solute elements. Undoped means the surface do not contain any solute element other than Ni and Cr.
Download figure:
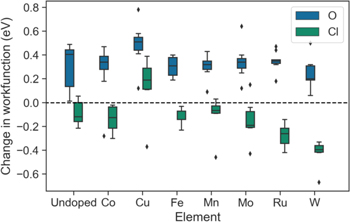
Standard image High-resolution imageFigure 12 shows the change in work function for surfaces with different solute elements after adsorption of O and Cl. It is found that O generally increases work function, stabilizing surfaces, whereas Cl seems to always decrease work function (except for Cu). These findings are consistent with previous studies of Cl adsorption on (111) Ni and Cu surface.46,47 Doping of Cu on Ni surface would modify the surface so that its electronic properties more similar to Cu, therefore Cl adsorption would increase the work function of some Cu doped surfaces. The result of atomic adsorption on surface work function is a combination of two counter effects: formation of interface dipole layer and polarization within adatom. The positively charged metal substrate and negatively charged adatoms forms an interface dipole with dipole moment pointing into bulk, which then increases the work function of the metal surface. For some metal surfaces and adatoms, there might be a polarization within the adatom that accumulates negative charge in the adatom-surface bonding region and depletes charge far from the surface. Such a dipole with dipole moment pointing away from the bulk would decrease the work function of metal surface. For Cl adsorption on Cu-doped surfaces and O adsorption, the formation of interface dipole layer dominates the change of work function. For Cl adsorption on other doped and undoped Ni–Cr alloy surfaces, the polarization effect is the dominant factor that changes the work function. These results are one indication that Cl adsorption generally has a negative impact on corrosion of Ni-based alloys.
Figure 12. Change in work function after adsorption of O and Cl for different solute elements. Undoped means the surface do not contain any solute element other than Ni and Cr.
Download figure:
Standard image High-resolution imageConclusions
By doing a series of systematic DFT calculations of O and Cl adsorption on Ni-Cr-X ternary alloy (111) surface, a comprehensive database of O and Cl adsorption energy on Ni-Cr-X (X=Co, Cu, Fe, Mn, Mo, Ru and W) ternary system was assembled. It is found that:
- (1)Except Cu, all other elements enhance the adsorption of O to alloy surface, and the order is Cr > Mn > Mo > W > Fe > Co ≈ Ru.
- (2)Similar to the effect on O adsorption, except Cu, all other solute elements also enhance the adsorption of Cl to alloy surface, and the order is Cr > Mo > Mn > W > Fe ≈ Co > Ru.
- (3)Surface configurations that favor O adsorption also tend to favor Cl adsorption, which makes it difficult to determine the final effect of different solutes on oxidation/repassivation of alloy surfaces solely based on DFT results.
- (4)Adatom adsorption energy to the surface is strongly related to net charge transfer between alloy surface and adatom for both O and Cl.
- (5)For all cases except Cu, adsorption of Cl decreases the work function while adsorption of O increases work function, which is one indication that Cl adsorption has a negative impact on corrosion of Ni-based alloys.
- (6)Cu tends to lower the corrosion resistance of alloys by lowering O adsorption energy as well as decreasing the alloy surface stability.
- (7)Ru on one hand tends to decrease the net charge transfer between alloy surface and O, and, on the other hand also increases alloy surface stability by increasing the work function.
Finally, this database lays the foundation for more advanced modeling of processes and mechanisms during corrosion. In the second part of this work, it will be used as input for a Langmuir isothermal model to study the competitive adsorption of Cl and O on alloy surfaces, a process which is significant to the repassivation of pits and crevices. Moreover, the database can also potentially be used to study salt film formation and pit stability.
Acknowledgments
This work was supported as part of the Center for Performance and Design of Nuclear Waste Forms and Containers, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award No. DE-SC0016584. DFT calculations were performed on supercomputers provided by Ohio Supercomputer Center.