Abstract
Thermally treated caffeine doped active carbon (Caffeine-Norit) is studied as a catalyst for the oxygen reduction reaction (ORR) with application in metal-air systems with neutral aqueous electrolytes. Catalytic activity is characterized by polarization curves and Tafel plots and the results are compared with 5% Platinum (5% Pt-Norit) and 4% Silver (4% Ag-Norit) catalysts. The Tafel slopes of all three catalysts are equal, the activity of Ag-Norit being somewhat lower then both Caffeine-Norit and Pt-Norit. The polarization curves are also comparable, especially in the low current density region. The increase of overpotential for the Caffeine-Norit at current density higher than 50 mA cm−2 is due to accumulation of H2O2 in the catalyst layer. This was demonstrated by a simple method for detection of peroxides in neutral electrolytes based on indigo carmine indicator. The discharge curves of Caffeine-Norit in 4 M NaCl electrolyte at 10 mA cm−2 display a plateau at potential of −320 mV vs Ag/AgCl (1.23 V vs MA8M magnesium alloy).
Export citation and abstract BibTeX RIS
The worldwide growing energy needs, environmental pollution and health problems are the main motivation to develop and implement environmentally friendlier solutions for production and storage of energy. Ideally, energy should be stored during the periods of intensive power availability and consumed during the demand periods. In this respect, batteries are considered as a very important energy storage technology that can close the gap between electrical energy generation based on renewable energies, and consumption, e.g. in mobility. However, present battery systems, such as lead-acid, metal-hydride or lithium-ion, provide relatively low specific energy for the needs of the mobility and transportation market and are also based on environmentally harmful components. Therefore, the development of electrochemical systems with high specific energy using "green" technologies is of upmost priority.
Metal-air systems, as well as fuel cells, are among the most perspective ecological successors of lithium-ion technology, due to their high specific energy (several times higher than the present battery technologies can offer).1,2 Zinc-air primary cells are already commercialized as small coin cells for hearing devices or for some industrial applications. Other metal-air systems, such as lithium-air, magnesium-air, aluminium-air and iron-air, are in intensive development or pre-commercial stage.3–13 One of the challenges to the metal-air systems application in electrical vehicles and energy storage is the lack of cheap catalysts for the oxygen reduction (ORR) and oxygen evolution (OER) reactions.14 Another important requirement is an insensitivity towards catalytic poisons, existing in the ambient air, resulting in decrease in service life of the gas-diffusion electrode (GDE) and battery respectively.
ORR in aqueous electrolytes is a very complicated reaction, occurring only at high overpotentials and involving the exchange of 2 to 4 electrons:
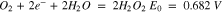
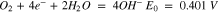
On carbon catalysts (active carbon, graphite), ORR is a 2 electrons process with hydrogen peroxide as main product (reaction 1). This case is undesirable because of the lower number of exchanged electrons per oxygen molecule and the highly oxidative properties of hydrogen peroxide. Generally, noble metal catalysts such as Pt, Au, Ag, Pd, Ru, Os but also some transition metals like Co, Ni, Mn and their oxides give rise to a 4 electrons exchange ORR (reaction 2).15–21 Because of the high price of noble metals at one hand, and the low electron conductivity of metal oxides at the other hand, these catalysts are frequently deposited on high surface area active carbons.22 Synergetic effect is observed during the synthesis of composite catalysts (metal/metal oxides—active carbon), resulting in 4 electron reaction on active carbon and minimization of noble metals or metal oxides loadings.
Catalytic activity of metal-organic chelate N4 complexes—phthalocyanines and porphyrins towards ORR has been reported for the first time by Jasinski et al. in 1964.23 Thereafter, enhanced investigations demonstrated high catalytic activity of pyrolyzed active carbon doped with phthalocyanines and porphyrins for ORR.24,25 Some of these catalysts, for example cobalt-tetra methoxyphenyl porphyrin, approach the catalytic activity of platinum.26,27 Beside their promising catalytic activity, these metal-organic complexes possess high stability against catalytic poisons existing in ambient air. Iliev et al.28 reported a pyrolyzed active carbon and CoTMPP based GDE for use in metal-air systems with neutral or alkaline electrolytes with service life of more than 8000 h at ambient conditions.
Although phthalocyanines and porphyrins are widespread in nature as basic components of life,29 they remain relatively expensive. In most cases, the preparation of highly effective catalysts requires artificially synthesized N4 chelate structures, which raises the production cost considerably.
Other highly effective non-noble metal ORR catalysts are carbon allotropic forms as graphene, carbon nanotubes or fullerenes doped with metalloids such as nitrogen, sulphur, and phosphor. During the last few years, a rising number of researchers are trying to develop catalysts based on natural derivatives.30 For example Guo et al.31 designed an effective 3D ternary doped ORR catalyst from thermally treated tea leaves doped with Fe ions.
The xanthine alkaloid caffeine (1,3,7-trimethyl xanthine) is widespread in nature and most commonly used as a psychotropic substance. The main caffeine sources are the leaves of tea and coffee fruits. Other plants contain smaller amounts of caffeine too. The structure of caffeine combines fused pyrimidine and imidazole rings. It is moderately solvable in water and ethanol at room temperature and has weakly alkaline (pKa = 14) character. Its melting temperature is 231 °C and it exists in two reversible crystallographic phases. The low temperature β-phase is stable up to 140 °C; above this temperature it changes to α-phase. After cooling, the α-phase very slowly turns back to β-phase.32 Degradation of the caffeine rings in presence of ozone was reported,33 but the substance proved to be very stable under electrochemical conditions.34,35
The aim of this work is to study the ORR catalytic activity of caffeine doped active carbon in aqueous electrolytes in the pH range from 7 (neutral) to 11 (alkaline) and to compare it with the ORR activity of noble metal catalysts (platinum and silver) on the same active carbon support.
Experimental
Materials
The catalyst Caffeine-Norit has been prepared with Norit NK (The Netherlands) active carbon as a support and 99.8 pure caffeine (194.19 g mol−1) from Chimspectar Ltd. (Bulgaria) The preparation procedure was as follows: 10 wt% of caffeine was dissolved in distilled water and mixed with 90 wt% of active carbon. The mixture was dried at 100 °C and pyrolyzed at 500 °C for 4 h under argon flow. The noble metal catalysts with 5 wt% Pt (Pt-Norit) and 4 wt% Ag (Ag-Norit) were synthesized by means of reduction of their salts on active carbon Norit NK with NaBH4.22
Each catalyst was used to prepare gas-diffusion electrodes with geometrical surface area of 10 cm2, according to the patented technology with teflonized acetylene black P1042 (XC 35).36 The average catalyst layer loading was 20 mg cm−2. The gas-diffusion layer was formed by pressing of copper mesh and teflonized P1042 and was approximately 0.8 mm thick. The two layers were sintered with pressing machine under heating at 280 °C, the final product being a mechanically stable electrically conductive hydrophobic tablet.
Methods
The catalysts studied were characterized by: transmitting electron microscopy (TEM) on HRTEM JEOL JEM 2100 at 200 kV accelerating voltage, scanning electron microscopy (SEM) on JEOL JEM 200CX at 80 kV accelerating voltage and powder X-ray diffraction spectroscopy (XRD) on Philips PW1030 X-ray Diffractometer with copper anode (Kα = 1.5406 Å) and Bragg–Brentano graphite monochromator. The diffractograms obtained were analysed with QualX 2 crystallographic software, with the latest database from June 2019.37 Inductive coupled plasma spectrometry (ICP-AES) was performed on Jobin Yvon Ultima 2 spectrometer with PMT high dynamic detectors in the 160–800 nm wavelength range.
Polarization curves, Tafel slopes and discharge curves were used for electrochemical characterization. The electrochemical measurements were carried out in a homemade 3 electrode electrochemical cell. 4M NaCl (analytic grade) solution in distilled water was used as electrolyte. The reference electrode was Ag/AgCl and the counter electrode was stainless steel plates for the GDE polarization curves. Magnesium alloy MA8M (Russia) was used as a counter electrode for the discharge curves in battery mode. All experiments were carried out at room temperature.
The electrochemical cell has a rectangular plastic body with 250 ml inner electrolyte volume. The working surface of the gas-diffusion electrode is 10 cm−2. Counter electrode is magnesium alloy type MA8M (in battery test methods) or stainless steel (in polarization curves and Tafel plots) with a surface of 56 cm−2. In all modes (polarization curves/Tafel plots methods and battery tests) ambient room air atmosphere is used.
Results and Discussion
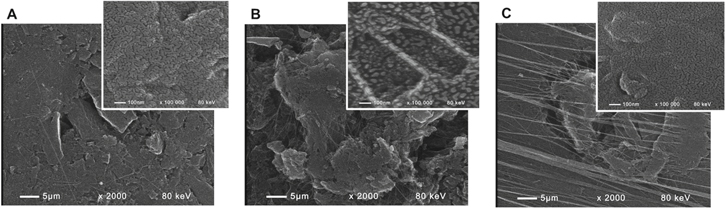
The surface topography of the three catalyst samples studied is shown in Fig. 1 by SEM images using secondary electron technique with 2000× magnification. In general, the morphology of Caffeine-Norit catalyst appears more complex compared to the noble metals' catalysts. After thermal treatment, the Norit NK particles appear rugged, eventually due to structural changes of caffeine (Fig. 1c). It was observed, however, that incorporated caffeine does not decompose during the thermal treatment, unlike the porphyrins and phthalocyanines. Most part of the adsorbed caffeine sublimates and condenses as a fine web of white needles in the furnace colder zones. This leaves the surface of the active carbon also rugged.
Figure 1. SEM images of Ag-Norit (a), Pt-Norit (b) and Caffeine-Norit (c) catalysts. In the latter case the catalyst is pyrolyzed at 490 °C.
Download figure:
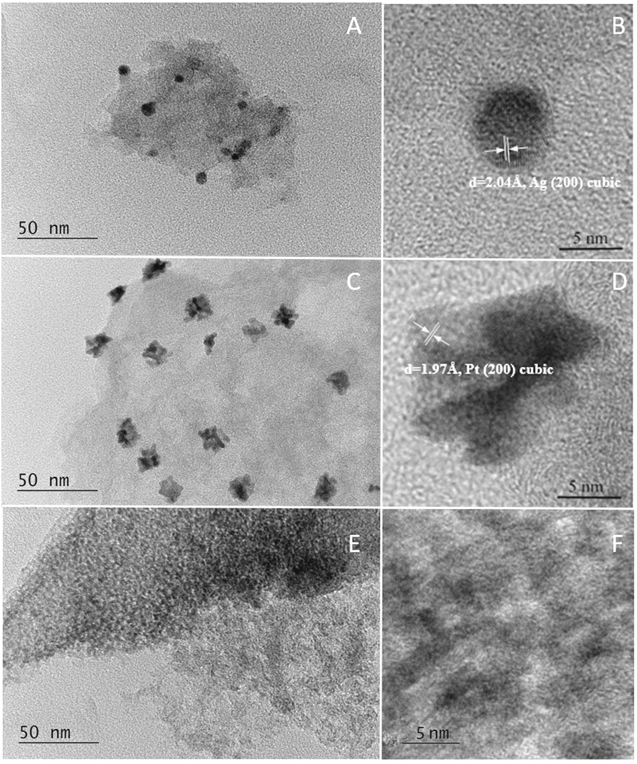
Standard image High-resolution imageTransmission electron microscopy was used to study the microstructure and phase composition of Caffeine-Norit catalytic materials, 5 wt% Pt-Norit and 4 wt% Ag-Norit. The bright field (BF) TEM image of the catalyst containing 4% Ag at 100,000× magnification and high resolution TEM at 600,000× magnification are shown in Figs. 2a, 2b. From the high separation lines, a phase was identified: Ag cubic, a = 4.0710, (# 96-150-9147); d = 2.0355, Int = 467 (hkl) = (200). Figures 2c, 2d show bright field TEM images of the catalyst containing 5 wt% Pt-Norit at 100,000× magnification, high resolution TEM at 600,000× magnification and the corresponding diffraction pattern. Phase identified: Pt cubic, a = 3.9440, (# 96-101-1104). Figures 2e, 2f shows bright field TEM images of the catalyst containing Caffeine-Norit at 100,000× and high resolution TEM at 600,000× magnification. The structure of the Caffeine–Norit catalytic material is amorphous.
Figure 2. TEM images of 4 wt% Ag-Norit (a), (b), 5 wt% Pt-Norit (c), (d) and Caffeine-Norit (e), (f).
Download figure:
Standard image High-resolution imageThe X-ray diffraction patterns of the noble catalysts are shown in Fig. 3. 5 wt% Pt -Norit catalyst shows peaks at 39.89°, 46.7° and 67.7°, revealing a cubic crystal structure of the platinum, Fm-3m space group (COD 00-101-1107), with lattice constant of 3.9110 Å and cell volume of 59.83 Å3 (COD 00-101-1107). The peaks in 4 wt% Ag-Norit sample at 38.11°, 44.29°, 64.44°, 77.40°, 81.54°, 110.51°, 114.92° and 138.44° correspond to cubic crystal structure of metal silver, Fm-3m space group, with lattice constant of 4.0862 Å and 68.23 Å3 cell volume (COD 00-900-8459). The remaining peaks in the noble metal catalyst diffractograms refer to Norit NK active carbon. The XRD patterns of pure and caffeine treated Norit indicate different structures. The peaks in the Caffeine-Norit spectra at ca. 28°, ca. 36° and 50° can be assigned to the caffeine.
Figure 3. XRD pattern of Active Carbon Norit NK, 5 wt% Pt-Norit NK, 4 wt% Ag-Norit NK and Caffeine-Norit NK.
Download figure:
Standard image High-resolution imageICP-AES is regarded as a most suitable technique of determining Pt in various specimens due to the broad linear dynamic range, low detection threshold and reasonable accuracy. The spectral lines of the ICP analysis of 5 wt% Pt-Norit NK appear at 203 nm and 214 nm and the calculated percentage is 4.93% platinum. The spectral lines of the ICP analysis of 4 wt% Ag-Norit NK appear at 328 nm and 338 nm and the calculated percentage is 4.05% silver.
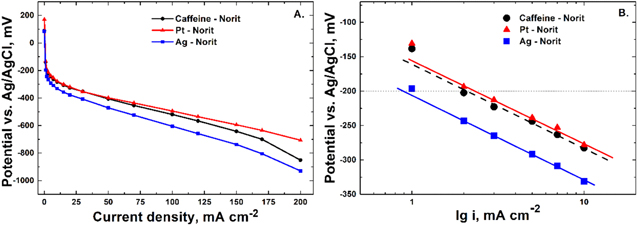
The electrochemical characterization of the catalysts studied was carried out by means of polarization curves, Tafel slopes and discharge curves. The polarization curves provide information about activation energy, ohmic drop and mass transport losses at different current densities. At small current densities, the main rate determining factor is charge transport and the overpotential depends only on the catalyst type. Low activation overpotential denotes high catalyst activity. Polarization curves for each of the three catalysts studied, averaged over 5 independent measurements, are shown in Fig. 4a. It is seen that the activity of Caffeine-Norit catalyst is very close to the activity of 5 wt% Pt-Norit catalyst at current densities below 70 mA cm−2, and outperforms the activity of 4 wt% Ag-Norit catalyst in the whole potential range of interest.
Figure 4. Polarization curves of different GDEs in 4 M NaCl with air supplied from the atmosphere at ambient conditions (a), Tafel plots of different GDEs in 4 M NaCl with air supplied from the atmosphere at ambient conditions (b).
Download figure:
Standard image High-resolution imageThe polarization data at low current densities can be plotted in Tafel coordinates (Fig. 4b) to provide information about the rate determining step of the ORR process and catalytic activity. As seen, the Tafel slopes are all equal—120 mV dec−1, which indicates that the electrochemical process of oxygen reduction for these catalysts at low current density is similar. The literature data about the Tafel slopes for ORR on platinum catalyst in neutral electrolyte vary in the range 120–180 mV dec−1.38–40 These slopes have been interpreted as indicating the first discharge step to be rate determining:
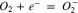
To compare the catalytic activity in the kinetic range, the current density at −200 mV vs Ag/AgCl has been chosen. Ag-Norit provides only 10 mA cm−2 at −200 mV, Caffeine–Norit succeeds at 100 mA cm−2, while Pt-Norit provides 200 mA cm−2.
At higher current densities (above 70 mA cm−2) the polarization of caffeine modified active carbon increases, and at 120 mA cm−2 transport hindrances in the catalyst layer become apparent. This is probably due to accumulation of hydrogen peroxide in the pores of the catalytic layer. A simple analytical procedure was developed to detect H2O2 with indigo carmine. Peroxides and superoxides oxidize indigo carmine to colourless isatin sulfonic acid, while hydroxides and chlorides do not affect indigo carmine up to pH 12.41 Therefore, we added a few drops of indigo carmine to the electrolyte, which resulted in a blue coloration. At open circuit potential and small current densities (up to 10 mA cm−2), no colour change of the electrolyte was observed for 24 h. However, at higher current densities (>30 mA cm−2) the solution gradually loses its colour. Eventually, the formation of H2O2 results in increase of the transport resistance for oxygen and decrease in the catalytic activity of Caffeine–Norit. Hydrogen peroxide may initialize oxidation of carbon to CO2, which may reduce the hydrophobicity of the pores. As a result, the gas-diffusion electrode is flooded by electrolyte and the gas transport hindrances increase.
Discharge curves are one of the main practical characteristics of each battery. Metal-air batteries are known for their almost flat discharge curves up to the total consumption of the anode active material. Therefore, the discharge curves of complete cells with the three different catalysts at 10 mA cm−2 during 1 h, were taken (Fig. 5). The open circuit voltage of a complete cell with platinum modified catalyst is ca. 100 mV higher than with caffeine or silver modified Norit. After higher initial polarization, Caffeine-Norit catalyst reveals similar discharge behaviour as Ag-Norit catalyst. The voltage loss due to polarization of the magnesium electrode (anode) is ca. 0.1 V. The average discharge voltages at 10 mA cm−2 current density of the three catalysts are 1.30 V, 1.23 V and 1.19 V for Pt—Norit, Caffeine-Norit and Ag—Norit respectively.
Figure 5. Discharge curves of the three catalysts at 10 mA cm−2 current density in 4M NaCl electrolyte: complete cell with MA8M magnesium alloy anode. Voltage is measured against magnesium electrode (complete cell). The cell operates with air at ambient conditions.
Download figure:
Standard image High-resolution imageThe stability of the Norit NK—Caffeine GDE was tested by 22 h discharge at 10 mA cm−2 (Fig. 6a). Magnesium alloy MA8M was used as anode in the complete cell and the electrolyte was 4M NaCl. After initial cell's voltage drop, due to the spontaneously created passive film on the magnesium surface, stable cathodic polarization is observed. The voltage plateau remains at approximately 1.23 V during the entire 22 h test. After the stability test, the experimental cell was disassembled and the gas-diffusion electrode was rinsed with distilled water to remove electrolyte residue. XRD diffractograms in catalyst layer of pristine and worked GDE are compared (Fig. 6b) to check for structural changes. Several additional peaks are observed in worked GDE. These peaks at 31.8°, 45.61°, 54.07°, 56.68°, 73.3°, 75.6° and 84.35° are associated to sodium chlorite with cubic crystal system, Fm-3m space group (COD 00-231-1042 ), remaining in the pores of the catalyst's layer. No other significant structural changes are observed. The test proves that the catalyst layer is stable with time.
Figure 6. Discharge curve at 10 mA cm−2 current density in 4 M NaCl electrolyte: 22 h service life test of a complete cell with caffeine modified Norit (a), XRD analysis of pristine and worked GDE (b).
Download figure:
Standard image High-resolution imageDue to accumulation of Mg (OH)2 in the electrolyte, resulting in pH increase up to 10–11, the cathode potential decreases with 20–30 mV. Refilling the cell with fresh electrolyte restores the cathodic performance. The voltage loss at 10 mA cm−2 due to polarization of the magnesium electrode is approximately 0.1 V and this value is constant wit time until the total depletion of magnesium.
Conclusions
Caffeine modified active carbon catalyst is introduced as a cheap and "green" alternative to modern high-performance ORR catalysts for use in metal-air systems with neutral aqueous electrolytes. The electrochemical behaviour of this novel catalyst is compared to noble metal modified catalysts, such as 5 wt% Pt-Norit and 4 wt% Ag-Norit. Its catalytic activity at small current densities (up to 50 mA cm−2) is similar to that of the platinum doped catalyst and outperforms the silver doped catalyst at all current densities. A possible explanation is that during thermal treating, the caffeine sublimates and makes the topological structure of active carbon more preferable for ORR than the silver catalyst. At higher current densities (more than 50 mA cm−2), formation of hydrogen peroxide at the cathode results in increase of transport hindrances in the catalyst layer causing higher polarization loss of Caffeine-Norit catalyst unlike the platinum supported Norit.
It is thus demonstrated that a possibility exists of employing biomaterials and biowastes from coffee-houses to produce efficient catalysts for the environmentally friendly metal-air systems.
Acknowledgments
The authors wish to place on regards their heartfelt thanks to Prof. Dr. Sasho Vassilev, Dr. Mariela Dimitrova and Ognyan Dimitrov for the P-XRD and SEM/HRSEM, to Assoc. Prof. Dr Daniela Karashanova for TEM/HRTEM analyses, to Assoc. Prof. Dr. Metodi Karadzhov for ICP measurements and to Dr. Willy Obretenov for article preparation assistance. This work was partially financially supported by the Bulgarian Ministry of Education and Science under the National Research Program "E Plus": Low Carbon Energy for the Transport and Households, grant agreement D01-214/2018 (period 2019–2021).