Abstract
Ion transport membrane (ITM) technology is a key perspective for efficient oxygen separation. At the present time, semi-industrial modules based on ceramic ITMs produce oxygen of 98.9%–99.9% purity. In order to improve the oxygen purity, we suggest using newly developed liquid-oxide ITMs with a record oxygen selectivity (O2/N2 > 105). Along with the highest oxygen selectivity, these membranes exhibit competitive oxygen permeability and could be successfully used for ultrahigh purity oxygen separation. Here we review the advantages and future prospects of liquid-oxide ITMs for a potentially disruptive technology which will provide improvement in oxygen purity and energy efficiency.
Export citation and abstract BibTeX RIS
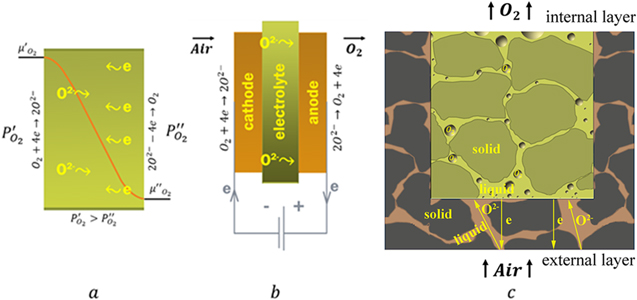
Oxygen is the second-largest volume industrial gas that has numerous applications in metallurgy, power engineering, environmental, medicine, etc.1–4 Ultrahigh purity oxygen (>99.999% purity) is in demand in the solar, semiconductor, chemical, and pharmaceutical industries.1,2,5,6 Recently, renewable sources of energy (sun, wind, biomass, etc.) are rapidly gaining in popularity. Clean energy, especially photovoltaics (PV), is a field of major growth and investments. Oxygen is one of the ultrahigh-purity process gases needed by the PV cell manufacturing.7 At the present time, ultrahigh purity oxygen is produced by water electrolysis or distillation method.8,9 However, these method-based oxygen production technologies are energy-intensive. In recent decades, energy-efficient ion transport membrane (ITM) technology is developing to produce pure oxygen.10,11 Conventional ITMs are the ceramic membranes with high oxygen ion conduction at elevated temperatures. There are two ITM processes. In the first, a mixed ionic electronic conducting (MIEC) membrane operates with a difference of the oxygen partial pressures, as illustrated in Fig. 1a. Under the oxygen electrochemical potential gradient, ambipolar conductivity of ions and electrons provides a sufficient oxygen permeation flux through the MIEC membrane. The MIEC membrane-based separation process is referred to as ITM oxygen. In the second process, an ion-conducting membrane (or an electrolyte) operates with a voltage. In contrast to the first process, electron transfer occurs in the outer circuit, as illustrated in Fig. 1b. Devices utilizing electrolytes are referred to as oxygen generators. The envisioned ITM applications vary from the generation of pure oxygen and partial oxidation of methane (membrane reactors for syngas production) to the capture of CO2 in oxy-fuel power plants.10–13
Figure 1. Schematic of (a) mixed ionic electronic conducting (MIEC) membrane, (b) ion conducting membrane (or electrolyte), and (c) mixed ionic electronic conducting—redox (MIEC-Redox) membrane.
Download figure:
Standard image High-resolution imageOxygen permeation flux through MIEC membrane is limited by diffusion. Therefore, the minimization of membrane thickness is necessary to achieve a high oxygen permeation flux. However, the brittle thin-film ceramic membrane material has a very low mechanical strength. As a rule, a thin membrane film is deposited on a porous support (it is so-called asymmetric membrane).14–17 To provide the thermochemical compatibility between membrane and support, they are usually made of the same material. The surface exchange reaction rates can limit the oxygen permeation flux through a thin MIEC membrane.18 An appropriate catalyst is deposited on the membrane to increase the rate of surface exchange reactions.19 Gas transport in a porous support can also control the oxygen permeation flux through asymmetric membranes.20 In order to ensure the sustainable production of oxygen, numerous asymmetric membranes with high oxygen permeability have been developed.17,20–22 Currently, asymmetric membrane-based semi-industrial modules produce oxygen of 98.9%–99.9% purity.10,11 However, such oxygen is insufficiently pure to be used in the solar, semiconductor, and pharmaceutical industries.
Before ITM technology can gain a considerable share of the gas market, important issues have to be addressed. These issues comprise the development of alternative ITM materials. Present ceramic ITM materials were selected over twenty years ago. Commercialization aspects, including cost, have revealed inadequacies in many of these materials. As an alternative, we suggest the newly developed liquid-oxide ITM materials.23–26 These liquid oxide-based membrane materials demonstrate a record oxygen selectivity (O2/N2 > 105) and competitive oxygen permeability and are easily made of affordable and low-cost metal oxides. The liquid-oxide ITM materials represent composites consisting of electron- or ion-conducting solid grains and ion- or mixed-conducting intergranular liquid layers. The intergranular liquid layers provide high ionic conductivity, gas tightness, and ductility. Three types of liquid-oxide ITM materials have been developed: (i) mixed ionic electronic conducting (MIEC),23 (ii) bilayer mixed ionic electronic conducting—redox (MIEC-Redox)24 and (iii) ionic conducting (electrolytes).25,26 In contrast to the MIEC and ionic conducting membrane materials (Figs. 1a and 1b), the bilayer MIEC-Redox membrane materials have a combined diffusion-bubbling oxygen mass transfer. The chemical diffusion of oxygen takes place in the external layer of the membrane material, while in the internal layer, the redox reactions and nucleation, growth and transport of oxygen gas bubbles occur, as illustrated in Fig. 1c.
The concept of highly selective liquid-oxide ITMs opens up ample opportunities for oxygen separation technology. However, in order to realize the potential of these membranes and successfully commercialize them, many scientific and technical challenges remain to be solved. In particular, it is necessary to understand how mass transport and thermodynamic factors combine to control the intergranular and membrane layer thicknesses. This understanding is important to be able to predict what combinations of solid and liquid oxides will form single- or two-layer membrane material with high oxygen mass transfer and sufficient mechanical strength. Similarly, to assess the technological potential of liquid-oxide membranes, it is necessary to understand their transport (ionic and electronic conductivities, oxygen permeation flux, permeability, solid/liquid interface contribution to overall ionic conductivity, etc.) and electrochemical (combined area-specific resistivity (ASR) of the cell components (electrolyte, anode and cathode), diffusion or surface exchange controlled kinetics, etc.) properties and how these are related to their composition and microstructure. The aim of this perspective is (i) to highlight the progress made in developing this understanding and (ii) to specify the areas of research which need further study.
Current Status
Oxygen generator electrolytes
The electrochemical oxygen generator operation principle is illustrated in Fig. 1b. The generator consists of two main components: electrodes (anode and cathode) separated by an electrolyte. Under the electric potential, oxygen ions migrate in the ceramic electrolyte from cathode to anode. The rare-earth stabilized bismuth oxide and rare-earth doped ceria are usually used as the oxygen generator electrolytes.27–34 Temperature dependences of conductivities of these ceramic electrolytes are presented in Fig. 2a. To achieve a sufficient ionic conductivity of these electrolytes, oxygen generators operate in the temperature range of 700 °C–800 °C. The purity of oxygen produced by the ceramic electrolyte-based generators significantly depends on the electrolyte density. At the present time, the product grades of 98%–99.99% oxygen are available.32–34
Figure 2. (a) Temperature dependence of oxygen ionic conductivity of δ-Bi2O3-0.2 wt% B2O3 composite with liquid GBs (temperature dependences of oxygen ionic conductivities of ceramic electrolytes for comparison), (b) current density dependence of oxygen generation rate of a cell based on this composite (0.6 mm thickness) at 750 °C, (c) flexibility of this composite:25 (1) ceramic Bi2O3-0.2 wt% B2O3 composite sintered at 600 °C and (2) liquid GB-containing Bi2O3-0.2 wt% B2O3 composite after deformation at 750 °C.
Download figure:
Standard image High-resolution imageMIEC membranes
Intensive study of the MIEC membrane materials was started in the mid-80 s of the last century when Teraoka et al.35 reported a high oxygen permeation flux of ceramic La1−xSrxCo1−yFeyO3−δ perovskite. The MIEC membrane operation principle is illustrated in Fig. 1a. Different perovskite-type La1−x(Sr, Ba, Ca)xCo1−y(Fe, Mn, Cu, Ni)yO3−δ and composite mixed-conducting ceramic membrane materials have been developed.17,21 Carolan and Dier36 (Air Products & Chemicals Inc.) patented an asymmetric membrane with a thin oxygen selective perovskite film on a porous support. A number of techniques such as tape casting, slip casting, dip coating, phase inversion, screen printing and spin coating have been developed to manufacture asymmetric membranes.21 At the present time, there are asymmetric membranes with the highest oxygen permeability.
Thus, the ITM technology based on ionic conducting and MIEC ceramic membranes does not allow the separation of ultrahigh purity oxygen (>99.999% purity).
Future Needs and Prospects
Taking into account the growing global demand for ultrahigh purity oxygen,1,2,5–7 we suggest using the ITM technology based on liquid-oxide membranes with record oxygen selectivity (O2/N2 > 105).23–25 Let us consider the most promising liquid-oxide membrane materials for ultrahigh purity oxygen separation.
Ion-conducting δ-Bi2O3-0.2 wt% B2O3 composite with liquid GBs as an ultrahigh purity oxygen generator electrolyte
The oxygen permeation flux ( ) through an oxygen generator electrolyte is expressed by Eq. 1
) through an oxygen generator electrolyte is expressed by Eq. 1
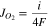
where i is the current density which is defined as
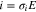
where  is the oxygen ionic conductivity and
is the oxygen ionic conductivity and  is the electric field strength (
is the electric field strength ( where
where  is the voltage and
is the voltage and  is the electrolyte thickness). In accordance with Eqs. 1 and 2, the electrolyte should have maximum ionic conductivity, since the oxygen generator voltage power losses are due to the electrolyte and electrode resistance as well as electrode polarization. Of the known electrolytes, ceramic δ-Bi2O3 electrolyte shows the highest oxygen ionic conductivity of 2.3 s·cm−1 at 780 °C (Fig. 2a).27 However, it cannot be used as an oxygen generator electrolyte, since the polymorphic transformation of monoclinic α-Bi2O3 into cubic δ-Bi2O3 at 730 °C is accompanied by a large volume change (∼12 vol%) leading to the cracking of the ceramic δ-Bi2O3. This cubic phase can be stabilized by the partial replacement of Bi3+ with Y3+, Er3+, Dy3+ and W6+ in the crystal lattice. However, ionic conductivity of the stabilized Bi0.75Y0.25O1.5, Bi0.8Er0.2O1.5 and Bi0.88Dy0.08W0.04O1.56 is substantially lower than that of δ-Bi2O3 (Fig. 2a). In order to improve the purity of industrial oxygen, we suggest to use containing liquid grain boundaries (GBs) δ-Bi2O3-0.2 wt% B2O3 composite as an oxygen generator electrolyte.25,38 The intergranular liquid layers predominantly consisting of molten δ-Bi2O3 provide ionic conductivity, gas tightness and ductility to the composite. This solid/liquid composite demonstrates the highest oxygen ionic conductivity (Fig. 2a), which is due to the high disordering of oxygen sublattice in fluorite structure of solid δ-Bi2O339 and short-range disorder of liquid Bi2O3.38 The highest oxygen selectivity of the solid/liquid δ-Bi2O3-0.2 wt% B2O3 composite is due to the fact that the liquid phase (molten Bi2O3-1.2 wt% B2O3) with almost zero solubility of nitrogen is an excellent high-temperature sealing component. Moreover, the intergranular liquid layers provide ductility to the composite and thus overcomes the problem of brittleness (Fig. 2c). Note that the volume fraction of the liquid cannot exceed 30%–35%.25 A further increase in the volume fraction of the liquid leads to the mechanical instability of the composite. GB wetting plays a critical role in the manufacturing of the solid/liquid composite (regarding the thermodynamics of GB wetting in ceramic oxide materials, the reader is referred to the comprehensive review40).
is the electrolyte thickness). In accordance with Eqs. 1 and 2, the electrolyte should have maximum ionic conductivity, since the oxygen generator voltage power losses are due to the electrolyte and electrode resistance as well as electrode polarization. Of the known electrolytes, ceramic δ-Bi2O3 electrolyte shows the highest oxygen ionic conductivity of 2.3 s·cm−1 at 780 °C (Fig. 2a).27 However, it cannot be used as an oxygen generator electrolyte, since the polymorphic transformation of monoclinic α-Bi2O3 into cubic δ-Bi2O3 at 730 °C is accompanied by a large volume change (∼12 vol%) leading to the cracking of the ceramic δ-Bi2O3. This cubic phase can be stabilized by the partial replacement of Bi3+ with Y3+, Er3+, Dy3+ and W6+ in the crystal lattice. However, ionic conductivity of the stabilized Bi0.75Y0.25O1.5, Bi0.8Er0.2O1.5 and Bi0.88Dy0.08W0.04O1.56 is substantially lower than that of δ-Bi2O3 (Fig. 2a). In order to improve the purity of industrial oxygen, we suggest to use containing liquid grain boundaries (GBs) δ-Bi2O3-0.2 wt% B2O3 composite as an oxygen generator electrolyte.25,38 The intergranular liquid layers predominantly consisting of molten δ-Bi2O3 provide ionic conductivity, gas tightness and ductility to the composite. This solid/liquid composite demonstrates the highest oxygen ionic conductivity (Fig. 2a), which is due to the high disordering of oxygen sublattice in fluorite structure of solid δ-Bi2O339 and short-range disorder of liquid Bi2O3.38 The highest oxygen selectivity of the solid/liquid δ-Bi2O3-0.2 wt% B2O3 composite is due to the fact that the liquid phase (molten Bi2O3-1.2 wt% B2O3) with almost zero solubility of nitrogen is an excellent high-temperature sealing component. Moreover, the intergranular liquid layers provide ductility to the composite and thus overcomes the problem of brittleness (Fig. 2c). Note that the volume fraction of the liquid cannot exceed 30%–35%.25 A further increase in the volume fraction of the liquid leads to the mechanical instability of the composite. GB wetting plays a critical role in the manufacturing of the solid/liquid composite (regarding the thermodynamics of GB wetting in ceramic oxide materials, the reader is referred to the comprehensive review40).
To assess the potential of the solid/liquid δ-Bi2O3-0.2 wt% B2O3 composite as an oxygen generator electrolyte, a symmetric cell based on this composite has been manufactured and tested. The electrochemical reactions took place within porous Bi2Ru2O7 + 1.2 wt% Pt electrodes deposited on the electrolyte. The electrodes were selected for their conductivity and catalytic activity.41 The oxygen generation rate as a function of applied current is presented in Fig. 2b. The purity of the generated oxygen has been more than 99.999%. The measured area specific resistance (ASR) of the cell was 0.55 Ω cm2 at 750 °C. The cell was operating for 5 h with electrolyte Faradaic efficiency of 97%. These initial results show that the δ-Bi2O3-0.2 wt% B2O3 composite with liquid GBs can be used as an oxygen generator electrolyte for production of ultrahigh purity oxygen.
Liquid oxide-based Co3O4-36 wt% Bi2O3 composite as a MIEC membrane material for ultrahigh purity oxygen separation from air
The gastight and mechanically stable (under a temperature gradient) solid/liquid Co3O4-36 wt% Bi2O3 composite MIEC membrane material with a record oxygen selectivity (O2/N2 > 105)23 and competitive oxygen permeability (Fig. 3f) has been developed. In this composite membrane material, the liquid phase predominantly consisting of molten Bi2O3 provides oxygen ionic conductivity, gas tightness and ductility, while the solid phase consisting of cobalt oxide ensures the electronic conductivity. The ionic conductivity of the composite is increased with the volume fraction of the liquid. However, due to mechanical requirements, the volume fraction of the liquid does not exceed 35%.23 In the membrane thickness range of 1–3 mm, the oxygen permeation flux through the solid/liquid Co3O4-36 wt% Bi2O3 composite membrane material is limited by diffusion of oxygen ions in the liquid phase and described by Eq. 342
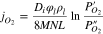
where  is the volume fraction of liquid,
is the volume fraction of liquid,  is the diffusion coefficient of oxygen ions in liquid,
is the diffusion coefficient of oxygen ions in liquid,  = 1 and
= 1 and  and
and  are the density and molar mass of liquid phase, respectively. The simplicity and cheapness of the manufacture of solid/liquid Co3O4-36 wt% Bi2O3 composite membrane material combined with the highest oxygen selectivity and competitive oxygen permeability makes it most suitable for the efficient separation of ultrahigh purity oxygen from air.
are the density and molar mass of liquid phase, respectively. The simplicity and cheapness of the manufacture of solid/liquid Co3O4-36 wt% Bi2O3 composite membrane material combined with the highest oxygen selectivity and competitive oxygen permeability makes it most suitable for the efficient separation of ultrahigh purity oxygen from air.
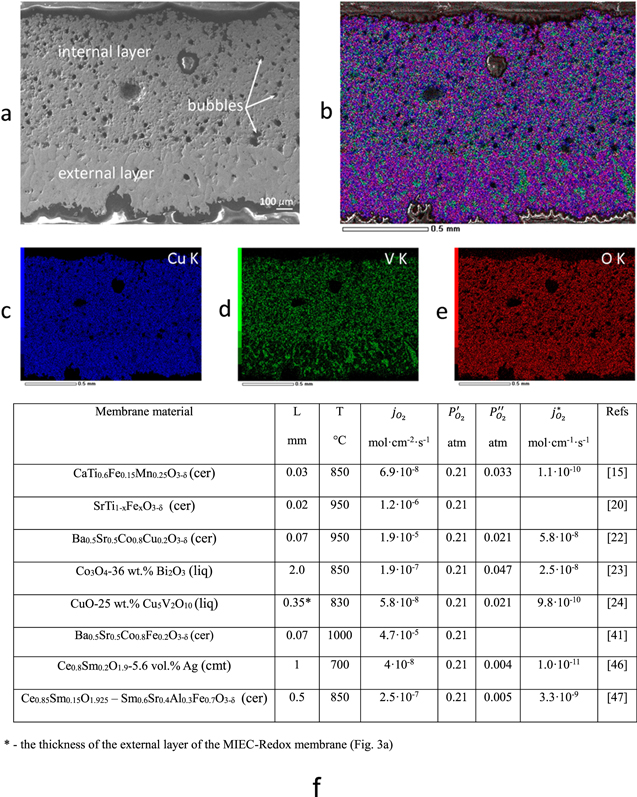
Figure 3. (a)–(e) Copper and vanadium oxide-based MIEC-Redox membrane microstructure:24 (a) SEM image of the MIEC-Redox membrane materials quenched after operating for 5 h at 830 °C and (b)–(e) EDX maps of different elements, where b—overlay Cu, V and O, c—Cu, d—V, and e—O, and (f) oxygen permeability ( ) calculated by formula
) calculated by formula  for ceramic (cer), cermet (cmt), and liquid-oxide MIEC and MIEC-Redox (liq) membranes.
for ceramic (cer), cermet (cmt), and liquid-oxide MIEC and MIEC-Redox (liq) membranes.
Download figure:
Standard image High-resolution imageLiquid oxide-based CuO-25 wt% Cu5V2O10 composite as a MIEC-Redox membrane material for ultrahigh purity oxygen separation from air
A promising candidate for ultrahigh purity oxygen separation from air is the copper and vanadium oxide-based bilayer MIEC-Redox solid/liquid composite membrane material with combined diffusion-bubbling oxygen mass transfer.24 This original membrane material is spontaneously formed during thermal treatment of ceramic CuO-25 wt% Cu5V2O10 composite in a "chemical field" (under an oxygen chemical potential gradient) above 816 °С. This temperature corresponds to the peritectic transformation of Cu5V2O10 compound by reaction: Cu5V2O10 → solid CuO + liquid (Cu2O + V2O5 melt) + gas O2.24 The result is a bilayer MIEC-Redox composite membrane material consisting of solid, liquid, and gas phases (Fig. 3). The oxygen permeation flux through this membrane material is limited by diffusion of oxygen ions in the V2O5-based liquid phase in the external layer of the membrane (Fig. 1c) and is described by Eq. 3. According to the dynamic polymer chain model,
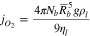
were  is the oxygen gas bubble density,
is the oxygen gas bubble density,  is the average radius of oxygen gas bubbles, g is the gravity, and
is the average radius of oxygen gas bubbles, g is the gravity, and  is the liquid phase viscosity. The calculated by Eq. 4 oxygen permeation flux value of 6.7·10−8 mol cm−2 s−1 at 830 °С (
is the liquid phase viscosity. The calculated by Eq. 4 oxygen permeation flux value of 6.7·10−8 mol cm−2 s−1 at 830 °С ( ≈ 1.4 10−5 m (Ref. 42),
≈ 1.4 10−5 m (Ref. 42),  ≈ 0.01 Pa s [Ref. 45],
≈ 0.01 Pa s [Ref. 45],  ≈ 4.0 1012 m−3 [Ref. 42], and
≈ 4.0 1012 m−3 [Ref. 42], and  ≈ 5.6 103 kg m−3) is in agreement with the experiment (Fig. 3f).
≈ 5.6 103 kg m−3) is in agreement with the experiment (Fig. 3f).
For a process to be cost-beneficial, it has been suggested37,46,47 that the oxygen permeation flux through MIEC membrane should be higher than 6.9·10−7 mol cm−2 s−1. However, the oxygen permeation flux through the CuO-25 wt% Cu5V2O10 MIEC-Redox membrane (Fig. 3f) is an order of magnitude lower than the targeted value. It was shown48 that the targeted value could be achieved by reducing the thickness of the external layer of the membrane or increasing the ionic conductivity of this layer. Currently, we cannot purposefully change the thickness of the external layer of the membrane, since a relationship between the gradient of the chemical potential of oxygen and the thickness of the external layer is not established. This is a subject for further research. The systems containing liquid Bi2O3 or TeO2 are also most suitable for further research, since the conductivity of oxygen ions in these liquid oxides is an order of magnitude higher than that of liquid V2O5.49,50 Other systems containing liquid oxides with high oxygen ionic conductivity are also attractive.51 Upon reducing the external layer thickness of the membrane, the surface exchange reactions can limit the oxygen permeation flux.18 This problem can be solved by choosing the appropriate catalyst.19
Outlook
Selectivity is a central aim for ultrahigh purity oxygen separation ITM technology, and liquid-oxide membranes are the main platform for addressing these challenges. However, the liquid-oxide ITMs have both advantages (record oxygen selectivity, high ionic conductivity, ease of manufacture, and low cost) and disadvantages (insufficient productivity of MIEC-Redox membranes and liquid evaporation at operating temperatures). Besides, the oxygen ion conduction mechanisms in liquid oxides and their structure are not well understood. This understanding is important to be able to increase the productivity of existing liquid-oxide ITMs (in particular MIEC-Redox membranes) and develop new liquid-oxide ITMs with lower operating temperatures. Low operating temperatures have the potential to improve durability, since degradation mechanisms (including liquid oxide evaporation) that affect liquid-oxide ITMs are aggravated by high temperatures.
The liquid-oxide ITMs contain a significant proportion of solid/liquid interfaces. It is known that the space charge regions with enhanced ionic conductivity can form near solid/liquid interfaces in composite materials.52,53 Therefore, an increase in the density of solid/liquid interfaces can lead to an enhancement in the oxygen ionic conductivity of the liquid-oxide ITMs. In the liquid oxide-based composite ITMs, the solid/liquid interfaces are usually formed by wetting the high-angle GBs, since their energy is much higher than that of the low-angle and so-called special GBs.40 Recently, the low-angle and special GB wetting has been established.54 It was also shown that the thermodynamic condition for low-angle GB wetting  (where
(where  and
and  are the low-angle GB and solid/liquid interface specific free energies, respectively) is only satisfied at
are the low-angle GB and solid/liquid interface specific free energies, respectively) is only satisfied at  < 75 mJ m−2.55 The search for systems meet this thermodynamic condition seems reasonable.
< 75 mJ m−2.55 The search for systems meet this thermodynamic condition seems reasonable.
To guarantee long-term stability, the liquid-oxide ITMs should have CO2-resistance and a low vapor pressure of liquid phase. CO2-resistance is an enabling property for the implementation of MIEC membranes in clean energy technologies.17,56,57 However, CO2-resistance and liquid oxide evaporation of liquid-oxide MIEC membranes have not been studied at operating temperatures. A study of these aspects is required. Nevertheless, we can assume that long-term stability of the liquid-oxide ITMs will not differ significantly from that of liquid-salt ITMs,58 since their vapor pressures are comparable in order of magnitude.45
Summary
Liquid oxide electrolytes are widely used in metallurgy,59,60 but they are practically not used in energy and separation technologies, with the exception of newly developed molten oxide fuel cells (MOFCs).26,34,51 This perspective demonstrates a promising way to separate ultrahigh purity oxygen from air by using highly selective and low-cost liquid oxide-based electrochemical membrane materials. The proposed concepts and materials have a great potential to be used in the next generation technology for ultrahigh purity oxygen production. However, the development of commercially viable liquid oxide-based ITM materials for ultrahigh purity oxygen separation depends on not only achieving the highest oxygen selectivity and permeability, but also long-term stability and operating temperature reduction. We hope that this perspective will stimulate additional efforts to solve these problems and will also accelerate the implementation of new membrane technology into solar, semiconductor, chemical, and pharmaceutical industries.
Acknowledgments
This work was supported by the Russian Science Foundation (Project No. 20-19-00514). We thank PhD graduate student M. Sedov for his assistance in preparing this manuscript.