Abstract
Herein, nanopore-embedded CoMn2O4 microspheres were successfully synthesized, and their electrochemical behavior as an anode for lithium-ion batteries (LIBs) and as a catalyst for oxygen-reduction/evolution reactions of lithium-air batteries (LABs) cathodes was investigated. The electrodes composed of specifically designed particles exhibited enhanced capacity retention with stable charge-transfer impedance change during the overall lithium conversion reactions as compared to the electrodes composed of conventional particulate nanoparticles. The LAB cathodes with the as-prepared porous compounds exhibit better reversibility with a ∼10% higher oxygen-evolution reactions efficiency and stable capacity retention than those of the Ketjen black-only electrodes. We believe that these novel performance is achieved due to the rational design of the pore-embedded/interconnected CoMn2O4 nanoparticles, which mitigate the detrimental volume change during the repetitive Li+  LiOx reaction and facilitate effective lithium ion (for LIBs) and oxygen diffusion (for LABs) in the porous electrode.
LiOx reaction and facilitate effective lithium ion (for LIBs) and oxygen diffusion (for LABs) in the porous electrode.
Export citation and abstract BibTeX RIS
Several researchers have mainly focused on finding electrochemically active, novel, metal oxide materials, including specifically designed particles, mixtures/composites, and hierarchically structured materials that fully sustain reversible, electrochemical reactions with lithium ions. In these studies, morphology and/or phase-controlled particles followed by formation of the hierarchical electrodes exhibited enhanced electrochemical performance in various rechargeable batteries.1–8 The as-prepared electrode materials are usually preapared by hydrolyzing the precursor solutions mixed with metal salts via controlled synthetic procedures followed by heat treatment.9–12 Additionally, the most preferred method of retaining efficient electrochemical reactions is the use of morphology-controlled particles with designated chemical compositions as electrodes as they exhibit a stable performance during the entire lithiation/delithiation process. Transition metals, such as Co and Mn, have been explored as promising electrode materials, lithium-storage materials and/or catalysts, for various lithium rechargeable batteries: lithium-ion batteries (LIBs) and the metal-air batteries (MABs).1,4,5,12–14 Due to its advantages such as natural abundance, environmental friendliness, cost-effectiveness, and proper oxidation/reduction voltage range, cobalt is favorable for use as a high-energy-density electrode material. Interestingly, transition metal ion-based ternary compounds such as CoMn2O4, MnCo2O4 and NiCo2O4, exhibit good charge retention during the entire charge-discharge process.15–18 For instance, Co ion has a higher oxidation potential than Mn ion; however, Mn ion can generate more electrons and create a higher charge capacity than other transition metal ions.
The cathodes for lithium-air batteries (LABs), similar to those of LIBs, are generally formed as a paste slurry with conductive carbons (e.g., Ketjen black and Super P). A polymer binder is employed as an adhesive to bind active materials to the current collector, and the resulting cathode finally leads to good oxygen-involved electrochemical reactions. However, the use of a polymer binder (e.g. polyvinylidene fluoride (PVDF) and polytetrafluoroethylene) causes undesirable parasitic reactions with the chemically generated LiOx during cycling, and/or the decomposition of the binder itself leads to an insulating property.19–22
Moreover, the fabrication of electrodes in assistance of polymeric species results in limited active sites with dead-volumes and clogging regions that are unfavorable for air permeation and reactant/product mass transport. The continuous evolution of gas, such as O2 and CO2, during the overall electrocatalytic reaction causes the peeling of the coated catalysts from the electrodes, which significantly impairs the catalytic activity and lifetime of these catalysts. Thus, two of the biggest challenges in developing efficient electrocatalytic electrodes are fabricating catalyst-incorporated air-permeable electrodes and finding novel catalysts that can efficiently and stably catalyze the overall electrochemical reaction.
Herein, taking the spinel CoMn2O4 compounds as a representative, multivalent, metal-oxide catalyst, we report a rational design to fabricate ant-cave-structured, porous, nano-spherical particles with controlled morphology and evaluate the effect of particle morphology on the electrochemical reaction with lithium ions, particularly on the oxygen-conversion efficiency of air cathodes. Nanocrystalline primary particles interconnected with pores were synthesized by simply varying the acid concentration and post-annealing conditions. The as-prepared microsphere particles, loosely packed with nanocrystalline CoMn2O4 particles, can be directly used as free-standing cathodes. The specified electrodes presented better capacity retention and cycling behavior during the Li-conversion reactions compared to those of the electrodes composed of particulate nanoparticles. We also systematically investigated the oxygen-conversion reaction for the proposed air cathode using a porous catalyst via X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and differential electrochemical mass spectroscopy (DEMS). In conclusion, it is very important to develop the facile and controllable synthetic procedures for obtaining lithium rechargeable electrodes with a well-dispersed nanoparticle metal-oxide catalyst for the lithiation/delithiation reaction.
Experimental
Spinel CoMn2O4 particles with porous/spherical microstructure were synthesized by co-precipitation followed by hydrothermal reactions. Reagent grade CoSO4·7H2O (99%, Sigma Aldrich), MnSO4·H2O (99%, Sigma Aldrich), and Na2CO3 (99%, High-Purity Chemicals) were used as the raw materials. Both sulphate chemicals were weighed in a stoichiometric ratio and then dissolved in distilled water. Na2CO3 was added to the given solution, resulting in precipitation. Then, the precursor solutions were poured into a teflon vessel, which was then placed in a microwave-assisted hydrothermal reactor (Model MARS6, CEM Corp., USA). The hydrothermal reaction proceeded at 180 °C for 30 min and the as-obtained powder was thoroughly washed. To obtain porous CoMn2O4 powders, the as-grown products were dispersed and stirred in dilute citric acid solution (0.15 M, 10 ml) followed by drying and post-annealing at 600 °C for 1 h. The phase and crystallinity of the as-synthesized powders were characterized by a powder X-ray diffractometer (XRD) (Rigaku Ultima IV Diffractometer). Additionally, a detailed structure of the components was studied using the synchrotron diffraction profile obtained via synchrotron diffraction at the Pohang Accelerator Laboratory in South Korea. The room temperature diffraction data were obtained in the 10.0° ≤ 2θ ≤ 130.5° range with a scanning step size of 0.02° using coherent synchrotron beam with wavelength calibrated as 1.4865 Å. The porous structure was confirmed by scanning electron microscopy (SEM, Tescan Mira 3 LMU FEG, 20 kV) and transmission electron microscopy (TEM, JEM-2100F, JEOL). X-ray photoelectron spectroscopy (XPS, K-Alpha Thermo SCIENTIFIC) was conducted to evaluate the chemical state of the elements.
A CR2032 type half coin cell with CoMn2O4 particles and lithium metal were used as the working and counter electrode, respectively, to demonstrate the LIB anode performance. The active material was mixed with carbon black and PVDF (Kureha KF-1100) dissolved in N-methyl-2-pyrrolidone (NMP, Sigma Aldrich, 99.5%) in a weight ratio of 80:10:10. The slurry was uniformly cast onto a copper foil and dried overnight in a 120 °C vacuum oven. The coin cell was assembled with the resulting working electrode, lithium metal, a separator (Celgard 2400), and an electrolyte (1 M LiPF6 in ethylene carbonate/dimethyl carbonate). The galvanostatic charge-discharge capacity was measured at the given current densities using the TOSCAT-3100 battery testing equipment (Toyo Co. Ltd.). Cyclic voltammetry (CV) was conducted at 0.1 mV s−1 scan rate.
Finally, the electrochemical behavior of the given powders as LAB air cathode catalyst was evaluated. The paste was generally prepared by mixing 80 wt% "conductive carbon mixed CoMn2O4 powder" with a 20 wt% PVDF binder followed by thorough mixing in NMP. In the case of cathodes without binders, we can prepare a slurry by thoroughly mixing only carbon (80 wt%) and CoMn2O4 powder (20 wt%) in acetone. The paste was cast on an Ni mesh and dried at 120 °C under vacuum for 12 h. The air-permeable 2032 coin cell was composed of lithium metal (anode) and the paste-overcoated Ni mesh (cathode), which were separated by glass fiber. The liquid electrolyte was tetra ethylene glycol dimethyl ether with 1 M Lithiumtrifluoromethane sulfonamide salt. The cell was tested using the galvanostatic WBCS-3000 battery cycler (WonATech) with an applied current density of 100 mA gtotal−1. The variation of the consumed/evolved O2 (g) partial pressure during the repetitive electrochemical reactions, was measured by in situ differential electrochemical mass spectroscopy (DEMS). The apparatus was custom designed with a setup similar to that reported in a previous work. A precision in-line pressure transducer measured pressure changes in real time, and a quadrupole mass spectrometer (UGA-200, Stanford Research Systems) monitored gas molecules within 200 atomic mass units that evolved in the cell during cycling. After discharge, O2 in the cell was removed and replaced with Ar. The pressure drop in the hermetically-sealed LAB cell during discharge was monitored to estimate the consumed O2. Gases that evolved in the isolated cell during charging were accumulated in programmed intervals, about 10 to 20 min, and transferred by argon (carrier gas) to the mass spectrometer for analysis
Results and Discussion
Materials characterization
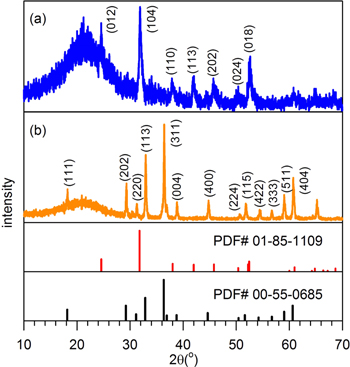
The degrees of phase formation and crystallography for the as-prepared Co-Mn compounds were characterized by powder XRD. As shown in Fig. 1a, the sample without post annealing exhibited phase-pure carbonate compounds: (Co,Mn)CO3 JCPDS 085-1109. The relatively broad diffraction peaks suggested that the nanoparticles were in an amorphous state in the as-grown samples. The sample was dispersed in a dilute citric acid solution and then heat-treated at 600 °C, as shown in Fig. 1b, in which all of the diffraction peaks were well matched to those of spinel structured CoMn2O4 (JCPDS 055-0685) compound. Thus, the Co/Mn intermixed spinel compounds with different molar ratios were successfully synthesized by the specified solvothermal reactions.
Figure 1. XRD patterns of solvothermally grown Co-Mn-O compounds (a) before and (b) after heat treatment.
Download figure:
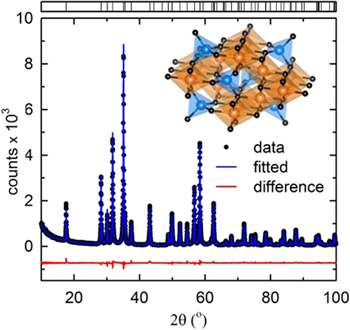
Standard image High-resolution imageRietveld refinement of the obtained diffraction profiles was performed using GSAS.23 The background was successfully fitted using a Chebyshev function with 12 coefficients, and a pseudo-Voigt function was used to fit the obtained diffraction profile. In the initial cycles, the lattice constants and profile coefficients of the selected function were refined. Subsequently, the atomic coordinates and thermal parameters were refined. The refinement parameters were converged such that reasonable goodness-of-fit parameters could be obtained. The structure crystallized in the tetragonal I41/amd space group with traces of impurity was identified as Co3O4. The weight percentage of the impurity phase was estimated to be less than 5 wt%. The unit cell parameters of CoMn2O4 were determined as a = b = 5.7197(0) Å, and c = 9.2570(1) Å. Figure 2 shows the results of the Rietveld refinement and the converged refinement parameters are summarized in Table SI (available online at stacks.iop.org/JES/167/080537/mmedia). Fractional coordinates and displacement parameters of the atoms in the unit cell are presented in Table SII.
Figure 2. Rietveld refinement results of the synchrotron diffraction profile of CoMn2O4. Dots represent the observed intensities, and the solid line is calculated ones. A difference (obs. − cal.) plot is shown beneath. Inset of figure shows the unit cell of the tetragonal structure with orange spheres representing octahedral 8d site and blue the tetragonal 4a site, shared by both Co and Mn, while black spheres represent 16 h site of O atom.
Download figure:
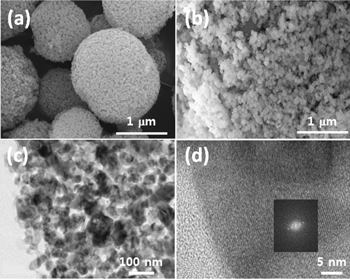
Standard image High-resolution imageWe further investigated the particle morphology and chemical composition of the as-synthesized CoMn2O4, which controlled the overall electrochemical behavior. Figure 3a show that the as-synthesized spinel compound has rather rough-surfaced spherical particles, preserving the morphology of the samples without heat treatment, as shown in Figs. S1a–S1c. The particles are in micron-size consisting of crystalline nanoparticles arranged as bunch of grapes and irregularly piled up to form a particulate electrode (Fig. 3b). The surface of the porous particles was quite rough, and we could clearly observe the primary nanoparticles interconnected with pores in high-resolution images. These unique inner-spacious morphologies of the resultant microspheres were induced by the uniformly emitting CO2 gas during post annealing at 600 °C. The surface area was measured by N2 adsorption/desorption analysis (Fig. S1d). From the results, we can realize that the as-prepared CoMn2O4 particles have a proper specific surface area with pores, which is expected to facilitate lithium-ion insertion/extraction during the overall electrochemical reaction with short pathways for rapid lithium-ion and electron conduction.
Figure 3. SEM images of (a) spherical and (b) nanoparticulate CoMn2O4 particles. (c), (d) Corresponding SAED patterns and high resolution TEM image of spherical CoMn2O4 particles.
Download figure:
Standard image High-resolution imageHigh-resolution TEM (HR-TEM) images clearly showed that the polygonal shaped CoMn2O4 nanoparticles (with size less than 50 nm) were loosely packed within the microspheres (Figs. 3c and 3d). Moreover, we could clearly observe the spacious voids that were formed within the interconnected nanoparticles entirely positioned within all spherical particles. We have already reported electrochemically reactive porous CoOx which is one of the promising spinel compounds.24 In that work, via both SEM and HR-TEM, we have found that the controlled particle morphology was stable enough even after cycling at a higher current density (above 100 cycles at 500 mA g−1). Thus, we can expect that the exceptional particle morphology of the ant-cave structured CoMn2O4 will improve the cycling properties by mitigating the stress induced by detrimental volume change. This has been discussed in a subsequent section with respect to the charge/discharge results. By dispersing the sample in the concentrated citric-acid solution followed by annealing (as shown in Fig. 3b), the overall primary particles were completely separated, while some particles were slightly agglomerated.
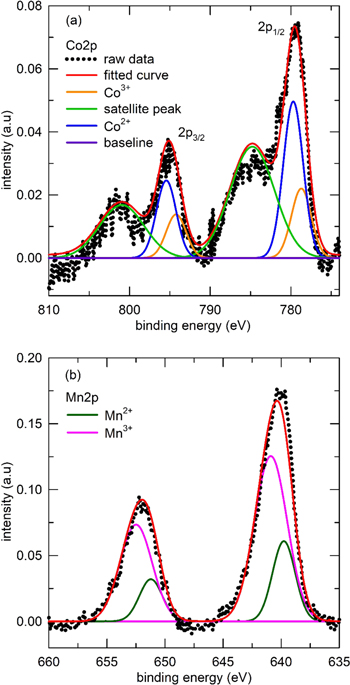
Finally, the chemical composition and metal oxidation state of the synthesized CoMn2O4 were verified by XPS. Figure 4 shows the core level spectra of Co2p and Mn2p. The Co2p spectra of CoMn2O4 are fitted by six peaks. The main peaks at 778.7 eV and 794.2 eV were assigned to Co(III), whereas the peak located at 779.7 eV and 795.4 eV were attributed to Co(II). The two additional satellite peaks at about 784.7 and 800.9 eV were assigned to Co2p3/2 and Co2p1/2, respectively. In the Mn2p spectra, the two Mn2p3/2 peaks and two Mn2p1/2 peaks observed at 639.7 and 651.2 eV and 640.7 and 652.4 eV were assigned to Mn(II) and Mn(III), respectively.25–28 According to the fitting area, the Co(II)/Co(III) and Mn(II)/Mn(III) atomic ratios were 2.2 and 0.33, respectively.
Figure 4. (a) Mn 2p and (b) Co 2p XPS spectra of spherical CoMn2O4 compounds.
Download figure:
Standard image High-resolution imageElectrochemical property of the LIB anode
The electrochemical behavior of the given microsphere particles was evaluated by galvanostatic charge/discharge profiles (Figs. 5 and S2). The first CV measurement (Fig. S2a) was performed for up to 50 cycles in a voltage range of 0.01–3.0 V (vs Li+/Li) at 0.1 mV s−1, to investigate the reversible Li+ insertion/desertion behavior of the given CoMn2O4 particles as a LIB anode. As shown in the CV curves, the CV profile for the first charge/discharge reaction is substantially different from that of the subsequent cycles. During the first lithiation reaction, the broad peak positioned at 1.26 V might be related to the reduction of Mn3+ to Mn2+; moreover, the prominent sharp peak at 0.46 V could be ascribed to the further reduction of the divalent transition ions, Mn2+ and Co2+ to Mn0 and Co0 metals, respectively, along with the growth of a solid-electrolyte interface (SEI) layer on the active materials surface. The two broad anodic peaks evolving the delithiation reaction were observed at 1.45 and 2.06 V and corresponded to the oxidation of Mn0 to Mn2+ and Co0 to Co2+. In the subsequent cycles, the cathodic peak moved to a slightly higher voltage region, ∼0.50 V, and became broader as compared to that in the first cycle, while the anodic peaks remained almost unchanged. The overall CV results are consistent with the previous related reports,29–32 and we observed that the controlled morphology with pore-embedded structures for the specified CoMn2O4 composite electrodes was electrochemically stable enough during the successive electrochemical reactions. From the second cycle onwards, the CV curves overlapped very well, revealing the good reversibility of the electrochemical reactions. Based on the CV results, the electrochemical reactions of the electrode comprising the porous CoMn2O4 particles can be expressed as follows:
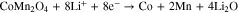
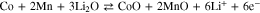
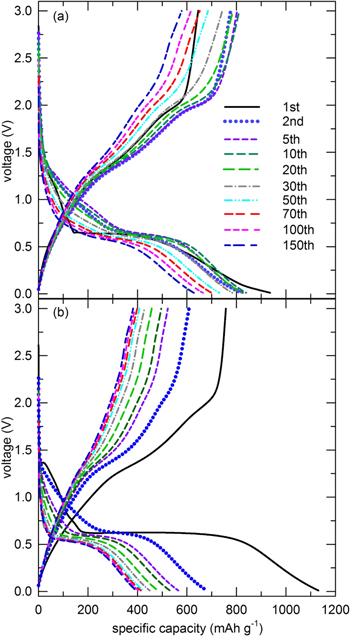
Representative charge/discharge curves from the first to the 150th cycle of the anodes comprising the as-prepared CoMn2O4 particles as well as nanoparticles at a current density of 100 mA g−1 are shown in Fig. 5. Although the 1st discharge capacities of porous CoMn2O4 electrode, ∼930 mA h g−1, were smaller than that of the electrode comprisng particulate nanoparticles, ∼1130 mA h g−1, the porous electrodes presented a rather stable capacity fading after 150 cycles. However, it is apparent that the electrodes simply composed of nanoparticles easily agglomerated, which finally lead to fast capacity fading and a large irreversible capacity right after the 1st discharge reaction. This is common for the conversion reaction preferred electrode materials; the reduction of metal ions to a metallic state with Li2O formation accompanied by the SEI layer formation.33–35 These results indicate that CoMn2O4 with an ant-cave-structured spherical shape exhibits a better electrochemical performance by accelerating the charge transport within the electrodes during repetitive cycling. This maximizes the overall electrochemical reactions and thus, a large capacity and good cycling stability are maintained throughout. Corresponding CV and charge/discharge profiles for carbonates, (Co,Mn)CO3, are presented in Figs. S2b, S2c.
Figure 5. Galvanostatic cycle charge/discharge profiles for (a) spherical and (b) nanoparticulate CoMn2O4 particles (@ 100 mA g−1 current density), respectively.
Download figure:
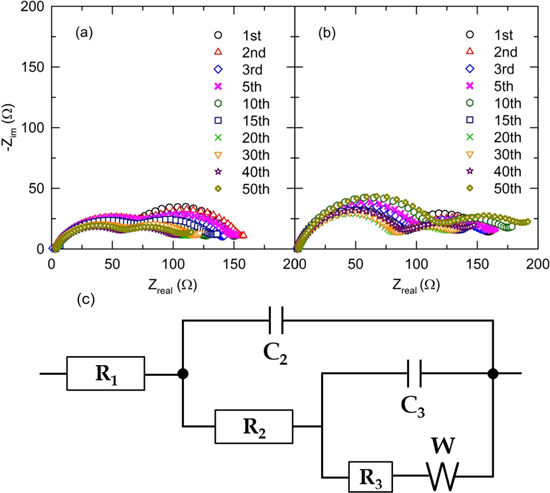
Standard image High-resolution imageTo confirm the characteristic cycling behavior of the as-prepared microsphere particles, we have performed a comparative analysis between the capacity retention and the lithium-ion kinetics during the cyclic electrochemical reaction via electrochemical impedance spectroscopy (EIS) measurements. From the supplemental EIS results, we can determine the constituent resistance components (charge transfer, SEI formation etc.) for the specified pore-embedded electrode materials. The EIS spectra shown in Fig. 6 exhibit two prominent semicircles positioned in a high frequency region; the former semicircle is related to the charge-transfer mobility (R2) and the other one is corresponding to the mass transfer region (R3). During subsequent cycles, up to 50th discharge reactions, completely different equivalent resistances were obtained for the two electrodes; after first discharge reaction, R2 decreasd from 60 Ω and stabilized at 50 Ω after 20th cycles for the electrode comprising microsphere CoMn2O4 particles; contrarily, R2 gradually increased for the electrode using particulate nanoparticles. The eventually decreased charge transfer resistance represents stimulating lithium-ion mobility induced by the excellent intimate and effective lithium ions and oxygen diffusion in the porous electrode. This is in good agreement with the fact that the structural relaxation favoring optimized electrochemical reactions for the pore-embedded electrode materials during repetitive cycling enhanced the electrochemical performance, as shown in Fig. 5.
Figure 6. Corresponding EIS curves after discharge in each cycle reactions for (a) spherical and (b) nanoparticulate CoMn2O4 particles. (c) Equivalent electrical circuits for the electrodes using CoMn2O4 compounds.
Download figure:
Standard image High-resolution imageElectrochemical property of the LAB cathode
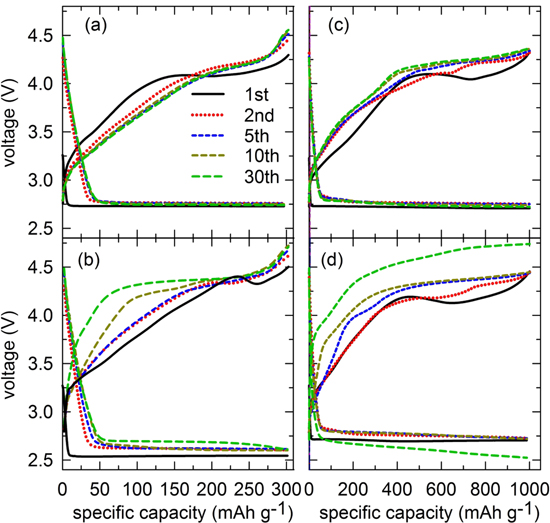
The air electrodes were assembled using the CoMn2O4 particles, and the catalytic effects of the LAB cathode were evaluated. Herein, we determine the capacity-limited condition to avoid electrolyte exhaustion and the deep discharge state of the oxygen electrode. Figure 7 presents the charge/discharge profiles of different air cathodes measured with the limited capacity at 300 and 1000 mA h gtotal−1. Note that the air cathode without catalysts is also presented as a reference sample. Typically, Li2O2 formation occurred following the oxygen-reduction reaction (ORR) and decomposition during the charge reaction is referred to as an oxygen-evolution reaction (OER).7,13,14
Figure 7. Corresponding galvanostatic charge/discharge profiles of the air cathodes using with ((a), (c)) and without ((b), (d)) spherical CoMn2O4 catalyst. Both measured at 300 and 1000 mA h g−1 current density, respectively.
Download figure:
Standard image High-resolution imageThe discharge plateau voltages of all the cells were similar to 2.75 V for the Li+ + O2(g) + e− → LiOx(s) equilibrium potential at 2.96 V. Obviously, the reaction voltages during the overall OERs were different enough for the two electrode compositions. The CoMn2O4–incorporated electrode exhibited a lower charge overpotential, stable enough with subsequent cycling with ΔV at approximately 1.22 V; however, it was ∼1.55 V for the cathode without catalysts. Moreover, there seemed to be stable catalytic reactions of the CoMn2O4–incorporated air cathode in charge process with repetitive cyclic reactions. The cathode presented a stable reversible cycling for up to 30 cycles without detrimental voltage during the repetitive charge/discharge electrochemical reactions. For further comparison, the galvanostatic charge and discharge curve of electrode incorporated with nanoparticulate CoMn2O4, which is conducted at the same limited capacities, were plotted in Figs. S3a, S3b. Accordingly, the nanoparticulate sample performs a significant capacity fading out via cycling number which further confirms the enhancement in catalytic behavior of ant-cave structured electrode is strongly affected by the specifically designed pore-embedded morphology. The similar phenomenon also was reported in our previous publication,24 which explains that the designed porous structure could accommodate the detrimental volume exchange during the reversible cyclic reactions as shown in this work.
Interestingly, we found that the charge/discharge profiles were more stable enough when the cathode without binder, PVDF, was used, as shown in Fig. S4, however, the effect of a "binder-free" in air cathode is still ambiguous. Thus, in-depth analysis and discussion considering different cathode conditions regarding the binder incorporation might be needed. Herein, we have only shown the empirical results and expect that they will stimulate the research on "binder-free" cathodes, by indicating new directions in developing "binder-free" cathode with good cycling ability.
The discharge and charge median voltages, which are a function of cycle numbers, are shown in Fig. S5 and summarized in Table SIII. We have found that the cyclic median voltages during discharging remain completely stable during charging at approximately 2.73 V while the median voltage is below 4.0 V within 30 cycles.
The substantially decreased charge overpotential with stable equilibrium charge voltage retention during the Li+ + O2(g) + e− → LiOx(s) electrochemical reaction might be due to the loose packing of the porous spherical particles with the reactive catalyst nanoparticles. This expedited the formation and decomposition of LiOx, prevented the pore- structured framework from clogging and finally led to a stable reversible redox reaction in the LAB. We believe that these rationally designed ant-cave-structured, pore-embedded, microsphere particles fully activate the nanoparticle catalysts and prevent agglomeration, which is necessary for high surface-area nanoparticles.
To further understand the catalytic effect of the proposed porous catalysts, we conducted supporting analyses using ex situ XRD/XPS to investigate the reaction products and in situ DEMS to quantify the oxygen-conversion efficiency. The ex situ XRD spectra (Fig. 8a) provide a the solid evidence for the reversible formation/decomposition of the by-product Li2O2 on the air cathode sides.7,13 The reaction product, the Li2O2 phase, was clearly observed even after 30 cycles of discharging, while the Li2O2 deposits on the cathode surface completely disappeared after full charging. The complete formation/decomposition of Li2O2 at the controlled catalyst-incorporated cathode after subsequent cycles proves its superior reversibility of this cathode in the ORR/OER processes. The significantly reduced over-potential with excellent stable cycle retention may be due to the more effective contact between semispherical spinel catalysts, which finally leads to a small charge transfer resistance.
Figure 8. Evaluation of the air-cathode performances using the given spherical CoMn2O4 catalyst; (a) XRD patterns of the repetitive cyclic reactions. (b) Li 1 s XPS spectrum of the fully discharged electrodes.
Download figure:
Standard image High-resolution imageXPS analysis also assists in determining the electrochemical reaction and reversibility of cathodes. We examined the CoMn2O4 incorporated air cathode after the 30th charge cycle, which showed only one band in the Li 1s region (Fig. 8b) that can be deconvoluted into two peaks positioned at the binding energies of 54.8 and 55.5 eV. In the spectra of the electrodes, a rather small peak attributed to Li2CO3 appeared at 55.5 eV along with that of the main product Li2O2 (54.8 eV). According to previous studies, Li2CO3 mainly originates from a side reaction between Li2O2 and CO2 in air cathodes.10,11 Thus, the actual concentration of Li2CO3 formed via subsidiary reactions such as the decomposition of the electrolyte, might be infinitesimal than that measured via XPS spectra analysis. Nevertheless, significant amounts of Li2CO3 were coated on the carbon-based cathode after repetitive cycling indicating that the formation of side products was not negligible even when the porous-structured catalysts were used.
Finally, the oxygen-evolution efficiency during the reversible electrochemical reaction for the specified air cathodes was measured using a real-time in situ DEMS apparatus (Fig. S6). The mutual relation of the DEMS data with the charge/discharge profiles during cycling can more quantitatively evaluate the stability of the air cathode and the flammable electrolytes; contrarily, most of the previously reported studies on carbon-based and/or binder-free cathodes have not quantitatively measured the actual oxygen consumption/evolution efficiency.3,36–39 We have clearly demonstrated the excellent performance and stability of the CoMn2O4–incorporated cathode. The CO2/O2 molar ratio and OER efficiency (ratios of the amount of oxygen released during the charge/the amount of oxygen consumed in the discharge)40 were 6.3% and 73% for the catalyst-incorporated air cathode, almost ∼10% higher than the OER efficiency for the Ketjen black-only cathode, (9.2% and 64%), respectively. Moreover, as shown in Fig. S6, the evolved O2 gas was constant during the overall recharge process, indicating that most of the electrochemical reactions were induced by the designated electrochemical reaction Li2O2 → 2Li + O2 with highly efficient oxygen evolution. Some CO2 (g) evolved towards the end of the charging for both air cathodes. Our previous work and other studies indicated the presence of residual CO2 for carbon-based cathodes due to parasitic oxidation reactions at high potentials (>4 V) during charging. This may originate from the decomposition of the electrolyte and/or carbonate byproducts on carbon during repetitive cycling.
Conclusions
In this study, we synthesized ant-cave-structured porous CoMn2O4 microspheres and characterized their electrochemical reactions. The LIB anode comprising the as-synthesized compound exhibited an excellent charge/discharge capacity retention of ∼610 mA h g−1 even after 150 cycles, which was 1.6 times higher than that of the electrodes using particulate nanoparticles, with a stable charge transfer resistance change during overall cycling. Moreover, the characteristic pore-embedded features of CoMn2O4 microspheres could be effective in the overall reversible electrochemical reaction of Li2O2 produced at a low-charge overpotential with a good cycle life; ΔVcharge/discharge was ∼22% lower than that for the Ketjen black only cathode. In particular, the proposed electrode had better reversibility for the ORR/OER and ∼10% higher oxygen efficiency, suppressing the side reactions related to the electrolyte. This unique three-dimensional nanostructure consisted of a crystalline CoMn2O4 nanoparticle interconnected compound that provided a large surface area for the catalytic sites for the ORR/OER. Furthermore, it created an effective and spacious buffer area to accommodate the morphological volume stress due to the formation/decomposition of Li-Ox during both the conversion reaction in LIBs and oxygen uptake in LABs. We believe that those novel performances are achieved due to the rational design of the pore-embedded/interconnected CoMn2O4 nanoparticles, which both effectively mitigate the detrimental volume change during repetitive cycling and facilitate the oxygen-conversion reaction with numerous reactive sites. The controlled synthesis of fully activating transition-metal-based catalysts incorporated electrode materials followed by systematic analysis can be applied to comparable high-energy-density electrode materials for post-lithium batteries.
Acknowledgments
This work was financially supported by the R&D Convergence Program (CAP-15-02-KBSI) of NST (National Research Council of Science & Technology) of Republic of Korea. This research was also supported by the Basic Science Research Program through National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (Project no. 2017R1A2B3011967).