Abstract
Solid oxide fuel cells (SOFCs) with proton-conducting solid electrolyte, instead of the oxide-ion conducting solid electrolyte have attracted attentions because of their high potential to reduce operating temperatures and to enhance the electrical efficiencies of SOFCs. In addition, the proton-conducting SOFCs with multistage electrochemical oxidation configuration will be promising technology for critically-high electric efficiencies. However, it is known that there are non-negligible charge -carriers other than protons in typical proton-conducting solid oxide electrolytes at relatively high temperatures. The existence of the partial conductivities of holes and/or electrons will cause the internal leakage current that consumes fuel but never generates any electrical power output. The higher ratio of the leakage current to external current will more deteriorate the electrical efficiency. In this study, the effects of blocking -layers formed on the air side surface of base electrolyte layer consisting of BaZr0.8Y0.2O3−δ (BZY82) for suppressing the leakage current have been investigated by using electrochemical parameters of the partial conduction of the materials. The chemical potential profile and leakage current showed large dependence on the material of the blocking-layer. Lanthanum tungstate was found to play a role as unique and strong blocking-layer against the leakage current.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Proton-conducting solid oxide fuel cells (p-SOFCs) in which proton-conducting solid oxides are used as electrolytes instead of yttria-stabilized zirconia (YSZ) that is the most common electrolyte material for SOFCs have attracted much attention because of their high potential to reduce operating temperatures and to enhance the electrical efficiencies of SOFCs.1–8 Stability of SOFCs against cycle operation, such as heat cycles and redox cycles, is one of technical issues of SOFCs to broaden the applicability.9–13 Lower temperatures operation by using p-SOFCs would result in longer-term stability and higher stability against the cycle operations.
Since the proton-conducting electrolyte leads to the steam generation not on the anode side but on the cathode side, the fuel dilution around the downstream of the fuel-flow should be largely suppressed. Therefore, switching electrolyte from YSZ to an ideal proton-conducting solid electrolyte having a protonic transport number of unity will result in the enhancement of the cell voltage and the electrical efficiency thanks to the suppression of the fuel dilution.1
However, non-negligible charge-carriers other than protons are known to exist in typical proton-conducting solid electrolytes at relatively high temperatures.14–23 Figure 1 schematically illustrates the charge-carriers in the proton-conducting solid electrolytes. The existence of the partial conductions of oxide-ions, holes and electrons will decrease the protonic transport number and the electrical efficiency. Especially, the presence of conduction of holes in the electrolyte will cause internal leakage current that would result in serious decrease of electrical efficiency.23 Choudhury and Patterson established a theoretical treatment to evaluate the leakage current.24,25
Figure 1. Schematic representation of the partial conductivities in proton-conducting solid electrolytes. The presence of electronic conduction with holes and electrons in the electrolyte will cause leakage current.
Download figure:
Standard image High-resolution imageIn this study, the leakage current in bi-layered electrolyte with a base electrolyte layer consisting of Y2O3-doped BaZrO3 coated with a hole-blocking-layer have been investigated. Doped BaCeO3 and lanthanum tungsten oxide (La28−xW4+xO54+δ; LWO) were investigated as the hole-blocking layers, respectively. In previous study, we reported that Zr-, Y-, and Yb-doped BaCeO3 electrolyte showed higher energy efficiencies than YSZ electrolyte in the temperature range of lower than 600 °C.14 Recently, LWO has been developed as an electrolyte material of p-SOFCs. LWO has much lower conductivity of holes than typical proton-conducting solid oxides such as Y2O3-doped BaZrO3.26–28 In the estimation of the leakage current in the bi-layered electrolyte, the hole-blocking layer consisting of doped BaCeO3 or LWO was assumed to be formed on the air side surface of the base electrolyte layer consisting of Y2O3 doped-BaZrO3.
Materials and Methods
Materials of electrolytes
Y-doped BaZrO3 (Ba(Zr0.8Y0.2)O3−δ; BZY82) was used as a base proton-conducting electrolyte layer. Zr-, Y-, and Yb- doped BaCeO3 (BaZr0.1Ce0.7Y0.1Yb0.1O3−δ; BZCYYb) and La28−xW4+xO54+δ with a La/W ratio of 6.7 (LWO67) were investigated as hole-blocking layers formed on the air side surface of the base electrolyte layer, respectively. Table I indicates the physicochemical parameters, σion, σOh, σOe, of BZY82, BZCYYb, and LWO67, respectively, where σion is conductivity of ions. σOh and σOe are conductivities of holes,  and electrons, e−, respectively, at oxygen partial pressure of 1. These partial conductivities of ions, holes and electrons were taken from the literatures28–30 including our previous report.
and electrons, e−, respectively, at oxygen partial pressure of 1. These partial conductivities of ions, holes and electrons were taken from the literatures28–30 including our previous report.
Table I. Partial conductivities of BZY82, BZCYYb, and LWO67 at 600 °C. σion is conductivity of ions, and σOh, and σOe are conductivities of holes and electrons at oxygen partial pressure of 1.
| Material | Conductivity (S cm−1) | |
|---|---|---|
| BZY8229 | σion | 1.3 × 10–2 |
| σOh | 4.5 × 10–3 | |
| σOe | — | |
| BZCYYb30 | σion | 1.20 × 10–2 |
| σOh | 4.05 × 10–3 | |
| σOe | 3.60 × 10–11 | |
| LWO6728 | σion | 1.24 × 10–3 |
| σOh | 1.66 × 10–5 | |
| σOe | 1.18 × 10–10 | |
Defect chemistry-based reaction of holes formation was assumed to be as Eq. 1, where  and
and  are an oxygen vacancy and an oxygen atom existing at the oxygen site, respectively. The equilibrium constant of the reaction, Kh, can be expressed as Eq. 2. Under this equilibrium, the conductivity of holes, σh, is proportional to the 1/4 power of P(O2), because the concentrations of the oxygen vacancy and the oxygen atom are known to be independent to P(O2) within the operation condition of SOFCs.28–30 P(O2) dependences of the ionic transport numbers, ti,, of BZY82, BZCYYb, and LWO67 were calculated by using the parameters listed in Table I and Eqs. 3, 4, and were plotted in Fig. 2.
are an oxygen vacancy and an oxygen atom existing at the oxygen site, respectively. The equilibrium constant of the reaction, Kh, can be expressed as Eq. 2. Under this equilibrium, the conductivity of holes, σh, is proportional to the 1/4 power of P(O2), because the concentrations of the oxygen vacancy and the oxygen atom are known to be independent to P(O2) within the operation condition of SOFCs.28–30 P(O2) dependences of the ionic transport numbers, ti,, of BZY82, BZCYYb, and LWO67 were calculated by using the parameters listed in Table I and Eqs. 3, 4, and were plotted in Fig. 2.
Figure 2. P(O2) dependences of ionic transport numbers of BZY, BZCYYb, and LWO67 calculated by using the parameters listed in Table I.
Download figure:
Standard image High-resolution imageThe ionic transport numbers of BZY82 and BZCYYb showed almost the same P(O2) dependence except for P(O2) range of lower than around 10–20 atm, and decreased largely with increasing P(O2) in the high P(O2) range as a result of hole conduction. On the other hand, the ionic transport number of LWO67 showed unique P(O2) dependence. LWO67 can keep high ionic transport number even at high P(O2), but decreased with decreasing P(O2) in the P(O2) range of lower than around 10–15 atm.
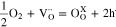
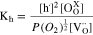
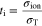
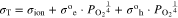
Estimation of the leakage current and potential profile
Leakage current should be caused by the existence of partial conductions of the holes and/or electrons in the electrolyte. Fuel will be consumed by both external- and leakage -currents. However, unlike the external current, the leakage current never generate any electrical power, so that the electrical efficiency will decrease with increasing a ratio of the leakage current to external current.23 The leakage current density, ileak, in a mono-layer electrolyte was estimated by using following equations as functions of electrical conductivity (σel) and ionic conductivity (σion).7,24,25,28–30


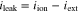
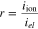
where σel consists of conductivities of electrons and holes and has PO2 dependence shown in Eq. 2, iext is the external current density, and iel is a current density induced by conductions of holes and/or electrons. r is a ratio of ionic current density to electronic current density and equals to −1 in a condition of open-circuit.
The oxygen potential profile in the electrolyte at open-circuit was estimated by using following equation with σel and σion:7,24,25

where L is a thickness of the electrolyte, x is a distance from the surface of electrolyte at fuel side toward air side.
σion in the typical proton-conducting solid oxides consists of conductivities of protons and oxide-ions. Electrochemical potential gradient of proton, which is driving force of proton diffusion, should be different from that of oxide-ion when the chemical potential of H2O has gradient across the electrolyte in terms of ionic current. Therefore, Eqs. 3–7 do not hold when potential gradient of H2O exists. Vøllestad et al.31 and Zhu et al.32,33 reported the analytical models that were more versatile in mixed conducting ceramics and should be able to address the effect of the H2O gradient. Considering the aim of this study to evaluate the effect of the hole-blocking layer, we used the condition with flat chemical potential of H2O across the electrolyte for simplicity and enabled Eqs. 3–7. Therefore, in this study, H2O partial pressures in both the cathode side and the anode side were assumed to be the same value of 10% to eliminate the H2O gradient resulting in same electrochemical potential gradient to protons and oxide-ions in terms of ionic current. In this condition, the leakage-current density and the oxygen potential profile in the electrolyte will be given by Eqs. 1–5 and 7, respectively as we previously reported.7
For the evaluation of integrals, P(O2) was defined as ey, so that lnP(O2) = y, where e is Napier number. For example, Eq. 3 can be expressed as Eq. 8 when r = −1 (i.e. open-circuit condition):
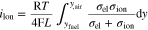
where σion is independent to y,  yair = lnP(O2)air = −1.666 for 10% humidified air, and yfuel = lnP(O2)fuel = −59.37 for equilibrium composition at 873.15 K in 10% humidified hydrogen. The calculation of the integral was performed by using a mathematical software, Maple 17.
yair = lnP(O2)air = −1.666 for 10% humidified air, and yfuel = lnP(O2)fuel = −59.37 for equilibrium composition at 873.15 K in 10% humidified hydrogen. The calculation of the integral was performed by using a mathematical software, Maple 17.
Determination of oxygen potential at interface of Bi-layered electrolyte
Figure 3 shows the schematic illustration of the cross-section of bi-layered electrolyte with constituent layers-a and -b. The leakage current in the layer- a depends on P(O2) at the interface between the constituent layers. The dependence of the leakage current in the layer-a on P(O2) at the interface differs from that in the layer- b. Under steady-state condition, the constituent layers-a and -b must have same leakage current. Therefore, the P(O2) at the interface should be determined to give the layers-a and -b same leakage current, respectively.
Figure 3. Schematics of the cross-section of the bi-layered electrolyte with constituent layers-a and -b. Broken line indicates oxygen potential across the bi-layered electrolyte. Under steady-state condition, the constituent layers-a and -b must have same leakage current.
Download figure:
Standard image High-resolution imageResults and Discussion
Mono-layer electrolyte of BZY82 with a thickness of 10 μm
Figure 4a shows the potential profile of oxygen calculated by Eq. 7 at open-circuit condition (r = −1) for the mono-layer BZY82 electrolyte having 10% humidified hydrogen and 10% humidified air as a fuel and an oxidant, respectively, at a temperature of 600 °C. The profile showed a sharper slope toward the fuel side due to lower electronic conductivity under lower oxygen partial pressures. Figure 4b indicates the dependence of the internal leakage current density in the BZY82 mono-layer electrolyte with a thickness of 10 μm on P(O2) in the oxidant at a temperature of 600 °C with fixed fuel of 10% humidified hydrogen. The leakage current density was calculated by Eqs. 3–6 at open-circuit condition (r = −1). When 10% humidified air was used as an oxidant, the leakage current density was found to be around 200 mA cm−2 as indicated by the right end of Fig. 4b.
Figure 4. Estimated chemical potential profile of oxygen (a) and internal leakage current density (b) in the mono-layer BZY82 electrolyte with a thickness of 10 μm having 10% humidified hydrogen and 10% humidified air as a fuel and an oxidant, respectively.
Download figure:
Standard image High-resolution imageBZY82 should be a promising electrolyte for p-SOFCs thanks to its high chemical stability and high proton-conductivity. However, the leakage current should be largely reduced to realize highly efficient power generation. As clearly suggested in Fig. 4b, the leakage current density can be significantly suppressed by reducing P(O2) at air side. Therefore, the reduction of P(O2) at the interface between the constituent layers- a and -b in bi-layered electrolyte will be effective for the decrease in the leakage current when having BZY82 layer at fuel side.
Bi-Layered electrolyte having BZY82 layer as a base electrolyte
In order to reduce the P(O2) at the interface of bi-layered electrolyte having BZY82 as base proton-conducting electrolyte layer, two types of electrolytes, BZCYYb and LWO67, were applied as hole-blocking layer formed on the air side surface of the base electrolyte layer.
Figure 5a indicates the potential profile of oxygen calculated by Eq. 7 for the bi-layered electrolyte consisting of 8 μm BZY82 and 2 μm BZCYYb with 10% humidified hydrogen and 10% humidified air as a fuel and an oxidant, respectively, at a temperature of 600 °C. In the case of the BZY82-BZCYYb bi-layered electrolyte, the potential profile was found to be almost the same as that in mono-layer BZY82 electrolyte due to the similar ionic transport number between BZY82 and BZCYYb as shown in Fig. 2, so that the significant effect on the reduction of the leakage current will not be expected. Figure 5b shows the potential profile of oxygen calculated by Eq. 7 for the bi-layered electrolyte consisting of 8 μm BZY82 and 2 μm LWO67 with 10% humidified hydrogen and 10% humidified air as a fuel and an oxidant, respectively, at a temperature of 600 °C. In the case of the BZY82-LWO67 bi-layered electrolyte, the potential profile largely changed from mono-layer BZY82 electrolyte, and P(O2) at interface was found to be reduced largely.
Figure 5. Chemical potential profiles of oxygen estimated by using electrochemical parameters listed in Table I at a temperature of 600 °C in the 10 μm bi-layered electrolyte having 8 μm BZY82 layer as a base electrolyte coated by (a) 2 μm BZCYYb and (b) 2 μm LWO67 on the cathode-side surface. 10% humidified hydrogen and 10% humidified air were assumed as a fuel and an oxidant, respectively.
Download figure:
Standard image High-resolution imageFigure 6a indicates the leakage current densities calculated by Eqs. 3–6 as a function of P(O2) at the interface for each layer of the bi-layered electrolyte, BZY82 (8 μm) and BZCYYb (2 μm), respectively, at open-circuit condition (r = −1). 10% humidified hydrogen and 10% humidified air were assumed as a fuel and an oxidant, respectively. The intersection indicates the leakage current and P(O2) at the interface at a steady-state condition. As predicted from the profile of chemical potential of oxygen as shown in Fig. 5a, the leakage current density was found to almost equal to that in the mono-layer BZY82 electrolyte. On the other hand, Fig. 6b indicates leakage current densities in each layer of the bi-layered electrolyte consisting of BZY82 (8 μm) and LWO67 (2 μm). As predicted from the profile of chemical potential of oxygen as shown in Fig. 5b, the leakage current density was found to be largely suppressed as compared with that in the mono-layer BZY82 electrolyte. The estimated leakage current density of the bi-layered electrolyte was found to be almost 200 times lower than that of mono-layer BZY82 electrolyte. These results suggest that LWO67 is promising material for hole-blocking layer combined with BZY82.
Figure 6. Leakage current densities in each layer estimated by using electrochemical parameters listed in Table I at a temperature of 600 °C in the 10 μm bi-layered electrolyte having 8 μm BZY82 layer as a base electrolyte coated by (a) 2 μm BZCYYb and (b) 2 μm LWO67 on the cathode-side surface. 10% humidified hydrogen and 10% humidified air were assumed as a fuel and an oxidant, respectively.
Download figure:
Standard image High-resolution imageIn the condition of power generation, the higher ratio of the leakage current to external current will more deteriorate the electrical efficiency. Fortunately, the ratio will decrease with increasing the external current density, which leads to higher Faradaic efficiencies. However, the high value of Faradaic efficiency should be kept even at turn-down operation for highly efficient power generation. LWO67 hole-blocking layer should enable high Faradaic efficiencies at all range of external current densities. In addition, the hole-locking effect will be more important in the condition of steam electrolysis operation.
Figure 7a shows the chemical potential profiles of oxygen in the bi-layered electrolyte having various thickness ratio of BZY82 to LWO67 at a temperature of 600 °C. 10% humidified hydrogen and 10% humidified air were assumed as a fuel and an oxidant, respectively. P(O2) at the interface decreased with increasing the thickness ratio of LWO67. Figure 7b shows the leakage current density and area specific resistance (ASR) of 10 μm bi-layered electrolyte as a function of thickness of BZY82 layer. By increasing the thickness ratio of LWO67, leakage current density could be reduced but ASR largely increased. However, 8 um BZY82 with 2 um LWO67 showed a compatibility with enough low leakage current density and ASR.
Figure 7. Effects of the thickness ratio of BZY82 to LWO67 in the bi-layered electrolyte on (a) chemical potential profiles of oxygen, and (b) leakage current density and ASR at a temperature of 600 °C. 10% humidified hydrogen and 10% humidified air were assumed as a fuel and an oxidant, respectively.
Download figure:
Standard image High-resolution imageTo enable our findings, electrochemical tests with the bi-layer electrolyte with LWO67 hole-blocking layer are very important. Therefore, we are now developing the bi-layer electrolyte. At this stage, there are some technical issues to be solved to obtain LWO67 thin layer having good compatibility with a base electrolyte in terms of chemical stabilities.34
Conclusions
BZY82 is expected to have both high chemical stability and high proton-conductivity. However, a large leakage current density of around 200 mA cm−2 was predicted for BZY82 mono-layer electrolyte with a thickness of 10 μm at 600 °C and open-circuit. Air side P(O2) dependence of the leakage current density of the BZY82 electrolyte with a fixed P(O2) in a fuel clearly showed that the leakage current density can be significantly suppressed with reducing P(O2) at air side surface of the BZY82 electrolyte. Therefore, bi-layered structure have been investigated to reduce P(O2) at air side surface of BZY82 electrolyte layer. Bi-layered electrolyte having BZY82 as base proton-conducting electrolyte layer combined with BZCYYb as hole-blocking layer was found to have almost the same P(O2) profile in the electrolyte as compared to mono-layer BZY82 electrolyte due to the similar ionic transport number between BZCYYb and BZY82. This indicates that no beneficial effect on the reduction of the leakage current will be expected in this combination. On the other hand, we have successfully obtained the promising results with bi-layered electrolyte consisting of LWO67 as hole-blocking layer formed on the air side surface of the base BZY82 electrolyte. In this combination, the potential profile largely changed from mono-layer BZY82 electrolyte, and P(O2) at interface was found to be largely reduced. The estimated leakage current of the bi-layered electrolyte was found to be almost 200 times lower than that of mono-layer BZY82 electrolyte under open-circuit condition. These results suggest that LWO67 is promising material for hole-blocking layer combined with BZY82.
Acknowledgments
This research is supported by The Japan Science and Technology Agency (JST) through its 'Center of Innovation Program (COI Program) JPMJCE1318.








