Abstract
Diagrams of the equilibrium potential E (relative to the standard chlorine electrode) against the activity of oxide ion ( expressed as its logarithm, pO2−) in systems of Ti-O-Cl-M (M=Li, Na, Mg, Ca, Sr and Ba) were plotted to investigate the electro-deoxidation of porous TiO2 pellets in different chloride molten salts, including LiCl, NaCl, MgCl2, CaCl2, SrCl2 and BaCl2. From the diagrams, the reduction pathways of TiO2 in different molten salts are similar; and the applied potential (vs Cl−/Cl2) on the cathode and O2− ion activity influence greatly on the reduction pathway of TiO2 and the deoxidation limit. To test and verify the results of the thermodynamic analysis, electro-deoxidation experiments in different molten salts were done to determine the actual pathways in the electrochemical reduction of TiO2 and the possible deoxidation limits in the molten salts. The results indicated that the thermodynamic analysis is helpful in predicting and understanding the electrochemical reduction process of titanium oxides though the experimental results differ slightly from the thermodynamic analysis in some systems. Low-oxygen Ti can be obtained only in molten CaCl2.
Export citation and abstract BibTeX RIS
FFC Cambridge process is a novel method for preparing metals and alloys from their oxides.1 In the process, compacted and sintered metal oxide pellets are used as the cathode, graphite or inert materials serve as the anode, and one or more alkali or alkaline earth metal chlorides (LiCl, NaCl, KCl, MgCl2, CaCl2, SrCl2, BaCl2 etc.) are the electrolyte medium, perhaps mixed with some corresponding alkali or alkaline earth metal oxides. Under a voltage which is lower than the thermodynamic decomposition voltage of the molten chloride used but higher than the decomposition voltage of the metal oxide serving as the cathode, the O2− ions are removed from the metal oxide to the molten electrolyte and transfer to the graphite anode to release CO and/or CO2, or to the inert anode to evolve O2 gas, and the metal stays at the cathode. The main mechanism of the process is the oxygen ionization of the metal oxides serving as the cathode in molten electrolyte under the driving of a voltage, which was firstly put forward by Chen et al.1 According to the mechanism, the molten chlorides serving as the electrolyte for FFC Cambridge process are required to have great solubility for O2− ions and wide electrochemical windows for the reduction of the metal oxides. From these, CaCl2, LiCl, SrCl2 and BaCl2 are qualified. Many metals and alloys had been prepared successfully in those molten salts.2–13 However the molten salts with a good solvent capacity for O2− ions would usually absorb CO2 gas, which always releases on graphite anode during electrolysis, to lead to the formation of CO32− ions. The CO32− ions will discharge on the cathode and convert to carbon powder, which not only reduces the current efficiency but also contaminates the reduced product. So MgCl2-based molten salts, which have low solubility for O2− ions, were tried.14 Single NaCl or KCl is rarely used as the electrolyte for electro-deoxidation of metal oxides. They are commonly used as supporting electrolyte to increase the conductivity or depress the electrolysis temperature. With the further research, it was found that the ability of alkali or alkaline earth metals to reduce the metal oxides serving as the cathode into corresponding metals played a more important role for the selection of electrolyte medium. Experiences indicate that only alkali or alkaline earth metals have the capability to reduce the metal oxides on cathode into the corresponding metals in thermodynamics, the corresponding alkali and alkaline earth metal chlorides can be used as the electrolyte for the electro-deoxidation to prepare corresponding metals in most cases. For the metals having large solubility and a strong affinity for oxygen, such as Ti, Zr, Hf and V, the reducing ability of alkali or alkaline earth metals determines greatly the deoxidation limits of the metals.
Until now, amounts of work have been done for the process, especially for the preparation of Ti metal through it. The process is considered to have the great potential to reduce the production cost of Ti metal and hence more widely utilised. In present paper, the electro-deoxidation of TiO2 in different molten salts was investigated through thermodynamic analysis and electro-deoxidation experiments. The reduction pathways of porous TiO2 pellets and the possible deoxidation limits in different molten salts were determined. We hope that it is helpful in the selection of the electrolyte used in the electro-deoxidation of TiO2 and understanding the electrochemical reduction process in different chloride molten salts.
Experimental
A commercial TiO2 powder (98%, 10022728, Sinopharm) was used as the starting material. Before preforming, about 10 wt% polyvinyl alcohol (PVA) solution (the PVA concentration is 25 wt%) was added into the powder to improve its formability. The damp powder was weighed and pressed into pellets under the axial pressure of 60 MPa. Then these pellets were sintered at 1050 °C for 4 h. The sintered pellets were sanded with sandpaper. Properties of the pellets prepared were: mass 2.5 g, diameter 28.4 mm, thickness 2.0 mm, and porosity about 45% which was measured by Mercury Porosimeter. The chloride salts used, including anhydrous LiCl (99%, L116328, aladdin), anhydrous NaCl (99.5%, 10019318, Sinopharm), anhydrous MgCl2 (99.9%, M140955, aladdin), anhydrous CaCl2 (96%, 10005860, Sinopharm) and SrCl2·6H2O (99.5%, S817918, Macklin), were firstly dried at 200 °C for 10 h in flowing argon (2 L min−1) and then at 300 °C for 4 h under vacuum before use. Electro-deoxidation experiments were carried out in a pressure-tight vertical tubular stainless steel reactor which was heated by silicon carbide rods. The upper end of the reactor was closed by a stainless steel cover, which was equipped with feedthroughs for the electrode leads and a thermocouple as well as gas inlet and outlet. A sintered pellet attached to a molybdenum wire (2 mm diameter, 700 mm long) was used as the cathode; a high-purity graphite rod (10 mm diameter, 80 mm long) was used as the anode which was connected with a stainless steel collector; and an alumina crucible (80 mm inner diameter and 120 mm high) severed as the container for the electrolyte. Before heated, the reactor was evacuated to 10 Pa and then was replenished with high-purity Ar gas (99.999%). This process was repeated twice. Then Ar gas passed continuously through the reactor vessel at 2 L/min until the end of each experiment. Because of the large differences in the melting points and the saturated vapor pressures of the chloride molten salts used, the electrolysis temperatures were different: 900 °C in NaCl and CaCl2 molten salts, 920 °C in SrCl2 molten salt and 830 °C in LiCl and MgCl2 molten salts. Similarly, because of the difference in the decomposition voltages of the chloride molten salts used, the applied voltage was also different but equal to or slightly lower than the decomposition voltage of the chloride molten salts used.
In order to investigate the actual reduction pathway of TiO2 in the molten salts, partially reduced samples were produced through interrupting the electrolysis after different reduction times ranging from 5 min to 48 h. After each experiment, the sample was moved out from molten salt and cooled down rapidly at argon atmosphere. The recovered samples were rinsed with flowing tap water to remove the solidified molten salt clinging to the surface of samples and then were placed in semi-concentrated acetic acid. After 24 h, acetic acid solution was replaced with distilled water. After another 24 h, the samples were removed out and dried in a vacuum oven at room temperature.
The phase compositions of the partially reduced samples were analysed by X-ray diffraction analyser (X'Pert Pro MPDDY2094, PANalytical B.V with CuKα radiation). A light optical microscopy (OLS4100, Olympus) was used to investigate the polished sections. Microstructure and chemical composition of the samples were investigated by means of scanning electron microscopy and energy-dispersive X-ray analysis (S-4800, Hitachi). The content of oxygen in final product was tested by oxygen/nitrogen analyser (TC500, LECO). The contents of alkali or alkaline earth metal oxides in the used salts were estimated by the acid-base titration method.
Thermodynamic analysis
Thermodynamic analysis is very helpful in estimating reaction pathways and conditions in some systems. Usually electro-deoxidation of metal oxides takes place in an oxygen-containing chloride molten salt system. The factors influencing the elecro-deoxidation of TiO2 involve the applied potential on cathode, temperature, O2− ions activity and properties of the used molten salt. In order to represent the systems well, the thermodynamic data was converted to diagrams of the equilibrium potential E (relative to the standard chlorine electrode) against the activity of oxide ion (expressed as its logarithm, pO2−) in the chloride molten salts, which has obvious advantages in predicting and understanding the electrochemical reduction process of TiO2 in different chloride molten salts.15 The equilibrium potentials (E) of the electrode reactions are calculated from the free energies as Eq. 1 (Nernst equation) and the potential zero is taken as the potential of a chloride electrode (1.013 × 105 Pa) in contact with a chloride molten salts at unit chloride activity. Pure component was used as the standard state of solid and liquid phases; and in case of gas phase, the gas of 1.013 × 105 Pa is the standard stare (Pθ). For O2− ions, the pure oxide of the cation of the chloride molten salt is chosen as unit activity of oxide ions. According to the above, the thermodynamic diagrams of E-pO2− in the systems of Ti-O-Cl-M (M=Li, Na, Mg, Ca, Sr and Ba) were plotted, as shown in Fig. 1.
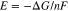
Figure 1. Diagrams of the equilibrium potential E (relative to the standard chlorine electrode) against the activity of oxide ion (expressed as its logarithm, pO2−) in the systems of Ti-Cl-O-M (M = Li (a), Na (b), Mg (c), Ca (d), Sr (e) and Ba (f)).
Download figure:
Standard image High-resolution imageFrom the E-pO2− diagrams, the deposition potentials of Li, Na, Mg, Ca, Sr and Ba metals of unit activity are −3.36 V, −3.17 V, −2.46 V, −3.23 V, −3.39 V and −3.49 V (vs Cl2/Cl−) respectively in corresponding alkali or alkaline earth metal chloride molten salts. At a lower O2− ion activity, TiO2 always can be reduced into Ti theoretically in all of the studied molten salts, which proceeds in a sequence from high-to-low order titanium oxides with the negative shift of the potential. However, so low O2− ion activity could not last for a long time. During the electro-deoxidation of titanium oxides, O2− ions will be released ceaselessly, which inevitably lead to the increase of the O2− ion activity around the titanium oxides particles. At a higher O2− ion activity, titanium oxides, including TiO2, Ti4O7, Ti3O5, Ti2O3 and even TiO, could react with alkali and alkaline earth metal ions (Li+, Na+, Mg2+, Ca2+, Sr2+ and Ba2+) and O2− ions to form the corresponding titanates before the formation potential of Ti metal except the cases of Ti2O3 and TiO in Ti-O-Cl-Mg system and TiO in Ti-O-Cl-Ca system. From Fig. 1c, when the O2− ion activity reaches unit activity in MgCl2 molten salt, Ti2O3 is stable at the potential of around −1.73 V ∼ −1.63 V and TiO is stable at around −2.22 V ∼ −1.73 V. In CaCl2 molten salt saturated with CaO, TiO is stable at the potential of around −2.81 V ∼ −2.59 V, as shown in Fig. 1d. With the decrease of the O2− ion activities, the above-mentioned potential ranges will shift negatively.
From the above, the electrochemical reduction of titanium oxides is inevitably accompanied by the formation of titanates. Titanates are stable at higher O2− ion activity and the reduction usually requires a more negative potential. During the reduction of titanates, amounts of O2− ions would be released, which could not diffuse promptly out of the pellets but deposit inside the pellets by the form of alkali or alkaline earth metal oxides. Thus the O2− ion activity inside the pellets would be very high during electro-deoxidation, even reaching unit activity. From the E-pO2− diagrams, at unit O2− ion activity, Li2TiO3, SrTiO3 and BaTiO3 can be directly reduced into Ti metal in corresponding chlorides molten salts and the equilibrium potentials are −3.28 V Cl2/Cl− (corresponding to +0.08 V vs Li+/Li), −3.17 V Cl2/Cl− (corresponding to +0.22 V vs Sr2+/Sr) and −3.47 V vs Cl2/Cl− (corresponding to +0.02 V vs Ba2+/Ba) respectively; MgTiO3 is firstly reduced into Ti2O3 at −1.63 V vs Cl2/Cl− (corresponding to +0.83 V vs Mg2+/Mg), and then the Ti2O3 is reduced into TiO and Ti metal successively at −1.73 V vs Cl2/Cl− (corresponding to +0.73 V vs Mg2+/Mg) and −2.22 V vs Cl2/Cl− (corresponding to +0.24 V vs Mg2+/Mg); CaTiO3 is firstly reduced into TiO at −2.59 V vs Cl2/Cl− (corresponding to +0.94 V vs Ca2+/Ca), and then the TiO is reduced into Ti metal at −2.81 V Cl2/Cl− (corresponding to +0.42 V vs Ca2+/Ca); with regard to Na2TiO3, it cannot be reduced even when the electrode potential reaches the deposition potential of Na in NaCl molten salt. Obviously at unit O2− ion activity, the formation potentials of Ti metal in the chloride molten salts are very close to the deposition potentials of the corresponding alkali or alkaline earth metal, especially in LiCl and BaCl2 molten salts where the formation potentials of Ti metal almost reach the deposition potentials of Li metal and Ba metal.
As is known to all, Ti metal has a large solvent capacity and a strong affinity for oxygen, and the solubility of oxygen in Ti metal is up to 13.7 wt% at 900 °C.16 Thus the Ti metal obtained at the above-mentioned equilibrium potentials of necessity contains amounts of oxygen. Usually the deoxidation of Ti-O solid solution is one of the main control step during the electro-deoxidation. The deoxidation limit is impacted by the applied potential, temperature, O2− ions activity and properties of the used molten salt. In order to estimate the deoxidation limits in different molten salts, the thermodynamic properties of Ti-O solid solution were required. Toru H. Okabe et al. measured the deoxidation limit of Ti-O solid solution at different temperatures by Ca metal, and gave the thermodynamic properties of Ti-O solid solution in Beta phase and the Gibbs free energy of reaction 2, as shown by Eq. 3.17,18
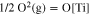
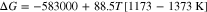
According to their experiment conditions, Eq. 3 is only valid for dilute Ti-O solid solution which contains oxygen below 0.2 wt%. Assuming that the applicable scope of Eq. 3 was expanded to Ti-O solid solution containing 1.0 wt% oxygen, the deoxidation limits of Ti-O solid solution and the corresponding conditions in different systems could be represented as the red lines in the E-pO2− diagrams. From these, for obtaining low-oxygen Ti, a low O2− ion activity and a very negative potential even reaching the deposition potentials of alkali or alkaline earth metals would be required. For example, in the system of Ti-O-Cl-Li at 900 °C, only when the O2− ion activity around the cathode is lower than  the titanium metal containing as low as 1 wt% oxygen can be obtained before the deposition potential of Li metal; and for obtaining the Ti metal containing as low as 0.2% and 0.01% oxygen, it is required that the O2− ion activities are lower than
the titanium metal containing as low as 1 wt% oxygen can be obtained before the deposition potential of Li metal; and for obtaining the Ti metal containing as low as 0.2% and 0.01% oxygen, it is required that the O2− ion activities are lower than  and
and  respectively. In other systems, the calculation results are similar with these in Ti-O-Cl-Li system, as shown in Table I. Here it should be noticed that for obtaining low-oxygen Ti (the oxygen content is below 1.0 wt%) before the deposition potentials of alkali or alkaline earth metals of unit activity, the O2− ion activities in corresponding systems must be lower than unit activity, except in Ti-O-Cl-Ca system. In Ti-O-Cl-Ca system, Ti metal containing 1.0% and 0.2% oxygen could be obtained at −3.06 V vs Cl2/Cl− (corresponding to +0.17 V vs Ca2+/Ca) and 3.14 V vs Cl2/Cl− (corresponding to +0.09 V vs Ca2+/Ca) at unit O2− ion activity; while for obtaining the Ti metal containing 0.01% oxygen before the deposition of Ca metal, the O2− ion activity must be below 0.26.
respectively. In other systems, the calculation results are similar with these in Ti-O-Cl-Li system, as shown in Table I. Here it should be noticed that for obtaining low-oxygen Ti (the oxygen content is below 1.0 wt%) before the deposition potentials of alkali or alkaline earth metals of unit activity, the O2− ion activities in corresponding systems must be lower than unit activity, except in Ti-O-Cl-Ca system. In Ti-O-Cl-Ca system, Ti metal containing 1.0% and 0.2% oxygen could be obtained at −3.06 V vs Cl2/Cl− (corresponding to +0.17 V vs Ca2+/Ca) and 3.14 V vs Cl2/Cl− (corresponding to +0.09 V vs Ca2+/Ca) at unit O2− ion activity; while for obtaining the Ti metal containing 0.01% oxygen before the deposition of Ca metal, the O2− ion activity must be below 0.26.
Table I. Oxygen contents of the reduced samples in different molten salts and the corresponding experimental conditions, and the thermodynamic conditions for obtaining low-oxygen and ultra-low-oxygen Ti.
| Thermodynamic conditions for Ti | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 wt% oxygen | 0.2 wt% oxygen | ||||||||
| Salt | T | t | P | CMO | CO | Potential | Activity | Potential | Activity |
| LiCl | 830 | 48 | 3.35 | 0.9 | 6.8 | −3.36 | 1.8 × 10−2 | −3.36 | 4.2 × 10−3 |
| NaCl | 900 | 10 | 3.2 | — | — | −3.17 | 1.0 × 10−10 | −3.17 | 1.9 × 10−11 |
| MgCl2 | 830 | 48 | 2.45 | 1.4 | 4.6 | −2.46 | 0.51 | −2.46 | 0.11 |
| CaCl2 | 900 | 48 | 3.15 | 1.1 | 0.3 | −3.06 | 1 | −3.14 | 1 |
| SrCl2 | 920 | 48 | 3.4 | 0.6 | 5.4 | −3.39 | 0.26 | −3.39 | 5.4 × 10−2 |
| BaCl2 | — | — | — | — | — | −3.49 | 1.7 × 10−2 | −3.49 | 3.0 × 10−3 |
Salt—Molten salt used; T—Electrolysis temperature, °C; t—Electrolysis time, h; P—the applied voltage, V; CMO—Concentration of corresponding alkali or alkaline earth metal oxides in the used molten, mol%; CO—Oxygen concentration in the reduced sample, wt%; Potential—Equilibrium potential required for obtaining the Ti metal, vs Cl2/Cl−; Activity—the value of the highest O2− ion activity for obtaining the Ti metal.
From the above, the electro-deoxidation of titanium oxides and titanates is greatly influenced by O2− ion activity in systems. Here the pure oxide of the cation of the chloride molten salt is chosen as unit activity of oxide ion, which means that the chloride molten salt saturated with the corresponding alkali or alkaline earth metal oxide is the standard state for O2− ions. Table II lists the solubility data of some alkali and alkaline earth metal oxides in corresponding chloride molten salts.19–22 Li2O, CaO, SrO and BaO have great solubility respectively in the corresponding chloride molten salts; the solubility of MgO in molten MgCl2 is very little; as for the solubility of Na2O in molten NaCl, no relevant data was found. According to the definition of the standard state for O2− ions, the activity coefficients of O2− ions in the chloride molten salts saturated with the corresponding alkali or alkaline earth metal oxides were calculated, as shown in Table II. Assuming that the activity coefficients of O2− ions in the chloride molten salts were independent on the O2− ions concentration at a certain temperature, the O2− ions activity in unsaturated chloride molten salts at the temperature could be estimated. However, activity coefficient is usually a function of the composition and temperature. For getting the accurate activity coefficient data, there is still a tremendous amount of work to be done.
Table II. Solubility data and calculated activity coefficients of alkali and alkaline earth metal oxides in corresponding chloride molten salts.
| Oxides | Li2Oat 650 °C | Na2O | MgOat 900 °C | CaOat 900 °C | SrOat 920 °C | BaOat 900 °C |
|---|---|---|---|---|---|---|
| Solutility/mol % | 11.5 | — | 1.4 | 19.5 | 10.5 | 12.5 |
| Activity coefficient | 8.67 | 71.4 | 5.13 | 9.53 | 8 |
Results and Discussion
From the thermodynamic analysis, TiO2 can be reduced into Ti metal in any of the studied molten salts above as long as the reduction conditions were satisfied. However during the practical electro-deoxidation experiments, some conditions are uncontrollable or hardly achieved. For example, in Ti-O-Cl-Na system, Ti metal can be obtained only when the O2− ion activity is below 10−8, which is almost impossible to be achieved and also uncontrollable during the electro-deoxidation. In addition, considering the kinetics factors and some unexpected mesophase, the actual reduction pathways and deoxidation limits of porous TiO2 pellets might differ greatly with the thermodynamic analysis. Thus electro-deoxidation experiments of porous TiO2 pellets were performed in the molten salts to determine the actual reduction pathways and the possible deoxidation limits of the porous TiO2 pellets. The applied voltage was equal to the decomposition voltage of the chloride molten salt used. Because of the strong toxicity of BaCl2, we have no condition to make the electrolysis in it. So the electro-deoxidation of TiO2 in molten BaCl2 was not discussed here.
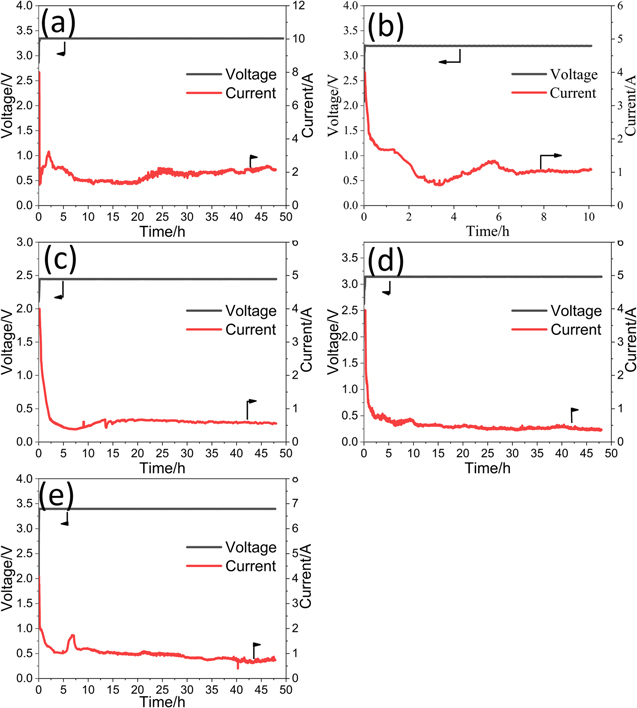
Figure 2 shows the I-t (current vs time) and E-t (voltage vs time) curves during the electro-deoxidation of TiO2 pellets in the molten salts. In order to avoid a too large initial current, a constant-current electrolysis was conducted at the initial stage of electrolysis. With the electrolysis time went on, the corresponding voltage increased progressively. Until the voltage reached the default value, the constant-voltage electrolysis began. From Fig. 2, the durations of the constant-current electrolysis were usually several minutes. Then the current began to decrease. After about 2 h of electrolysis, the current kept a low level until the electrolysis terminated. The oxygen content of some reduced samples in different molten salts and the corresponding experimental conditions were listed in Table I.
Figure 2. I-t (current vs time) and E-t (voltage vs time) curves during the electro-deoxidation of TiO2 pellets in different molten salts. Electrolytes: (a): 830 °C in molten LiCl, (b): 900 °C in molten NaCl, (c): 830 °C in molten MgCl2, (d): 900 °C in molten CaCl2 and (e): 920 °C in molten SrCl2; anode: graphite rod (10 mm diameter, 80 mm long); cathode: TiO2 pellets (2.5 g); anode-to-cathode distance: 6 cm; atmosphere: Ar gas.
Download figure:
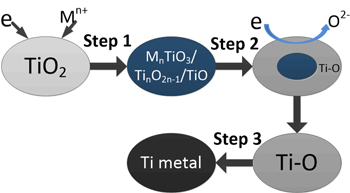
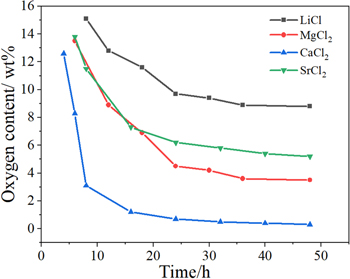
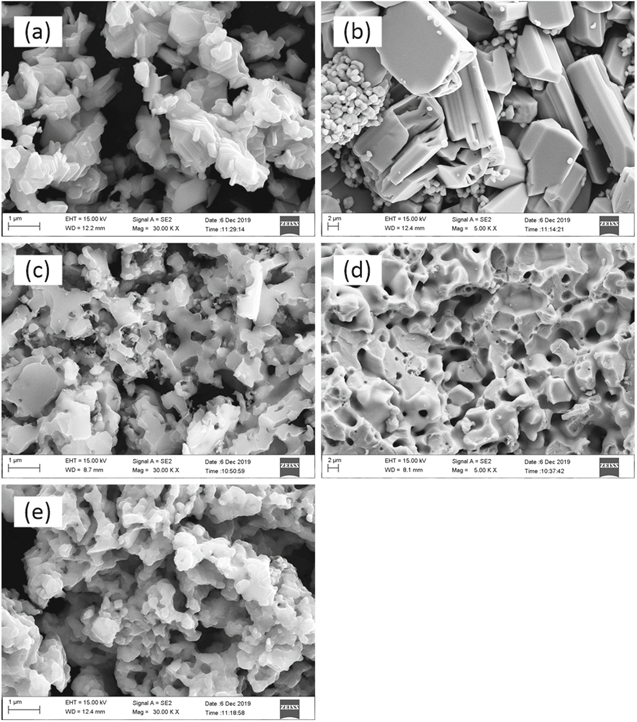
Standard image High-resolution imageThe detail discussion about the actual reduction pathways of porous TiO2 pellets in the molten salts was placed in the Supplementary Materials. Figures. S1–S8 (available online at stacks.iop.org/JES/167/082501/mmedia) show the XRD patterns and the optical micrographs of the polished sections of the samples the electrolysis treatment of which was terminated after different durations in the molten salts. From that, the actual reduction pathways of porous TiO2 pellets in different molten salts were determined and were listed in Table III. They are roughly similar and can be divided into three stages as shown in Fig. 3. In the first stage, alkali or alkaline earth metal ion (Mn+, n = 1 or 2) was incorporated into porous TiO2 pellets to form titanates and/or titanium sub-oxides (TinO2n−1, n = 1, 2, 3, 4 etc.) under the driving of the voltage. During this stage, no or very little oxygen was removed. In the second stage, the titanates and/or titanium sub-oxides were reduced into Ti-O solid solution. Meanwhile, amounts of O2− ions would be released intensively. In third step, Ti-O solid solution was further deoxidized into low-oxygen Ti (the oxygen content is lower than 1%). Still, some differences existed among the reduction pathways of porous TiO2 pellets in the molten salts, which mainly reflected in two aspects. The first is the reduction of the titanates: in LiCl, MgCl2 and CaCl2 molten salts, the corresponding titanates were firstly reduced into titanium sub-oxides (mainly TiO and Ti2O3) and then they were further reduced into Ti-O solid solution; while in SrCl2 molten salt, the SrTiO3 was directly reduced into Ti-O solid solution, and in NaCl molten salt, the sodium titanate could not further be reduced. The second is the possible deoxidation limits of TiO2 pellets in the molten salts. Figure 4 shows the changes of the residual O content in the samples obtained after various length of time of polarization in different molten salts. After 48 h of polarization, the changes of the residual O contents of the reduced samples were very small, which was considered to reach quasi-deoxidation limits. From the figure, only in CaCl2 molten salt, low-oxygen Ti can be prepared; while in other molten salts, just high-oxygen Ti (the oxygen content is more than 1 wt%) or TixO (x = 2, 3 or 6) was obtained. Figure 5 shows the SEM images of the samples obtained after 10 h of polarization in NaCl and after 48 h of polarization in LiCl, MgCl2, CaCl2 and SrCl2. Ti metal or TixO (x = 2, 3 or 6) obtained in molten LiCl, MgCl2, CaCl2 and SrCl2 showed varying degrees of sintering. The sample obtained in CaCl2 molten salt was the most compact.
Table III. Reduction pathways of porous TiO2 pellets in different molten salts.
| Molten salt | Reduction pathway of TiO2 |
| LiCl | TiO2 → LixTiO2/Ti2O3 → TiO → α-Ti |
| NaCl | TiO2 → NaxTiO2 |
| MgCl2 | TiO2 → Mg2TiO4/Ti2O3 → Ti2O3 → TiO →α-Ti |
| CaCl2 | TiO2 → CaTiO3/TiO → CaTi2O4 → TiO →α-Ti |
| SrCl2 | TiO2 → SrTiO3/TinO2n-1 → SrTiO3/Ti →α-Ti |
Note: According the Ti-O phase diagram, Ti2O, Ti3O, Ti6O and Ti phase detected in the reduced samples by XRD originate from α-Ti (Ti-O solid solution) at high temperature (830 °C–920 °C).
Figure 3. A schematic diagram of reduction pathways of porous TiO2 pellets in different molten salts.
Download figure:
Standard image High-resolution imageFigure 4. The changes of the residual O content in the samples obtained after various length of time of polarization in different molten salts.
Download figure:
Standard image High-resolution imageFigure 5. The SEM image of the samples obtained after 48 h of electrolysis in different molten salts: (a) in LiCl molten salt; (b) in MgCl2 molten salt; (c) in CaCl2 molten salt; (d) in SrCl2 molten salt.
Download figure:
Standard image High-resolution imageFrom the above, low-oxygen Ti was got only in molten CaCl2. While in LiCl, MgCl2 and SrCl2 molten salts, only high-oxygen Ti (the oxygen content is about 5 wt%) or TixO (x = 2, 3 or 6) were prepared, the oxygen contents in which were about 4–10 wt%. From the E-pO2− diagram, the main factors influencing the deoxidation limit of Ti metal are the applied potential and O2− ion activity. Obviously the applied voltages in all experiments satisfied the requirement for preparing low-oxygen Ti. The alkali metal or alkaline earth oxides concentrations in the used electrolytes after 48 h of electrolysis were tested by acid–base titration. The results were listed in Table I. Here the MgCl2 molten salt was saturated with MgO. Thus the O2− ion activity in MgCl2 molten salt was determined to be 1. But those in LiCl, CaCl2 and SrCl2 molten salts could not be determined because of the unknown activity coefficients. From the lower concentration of oxides in LiCl, CaCl2 and SrCl2 molten salts, it was surmised that the O2− ion activities might be far less than unit avtivity. Still, the product obtained in MgCl2 molten salt has a lower oxygen content than those in LiCl and SrCl2 molten salts. Thus O2− ion activity might be not the main factor influencing the deoxidation limit of Ti-O solid solution. The most predominant and fundamental factor might be the reducing ability of alkali or alkaline earth metals to titanium oxides, which might be the main reason lead to the great differences of electro-deoxidation conditions and limits for preparing Ti metal in different chloride molten salts.
For better comparison, the reducing ability needs to be quantised. The reducing ability behaves as follows: the activity of alkali or alkaline earth metals in corresponding chloride molten salt impacts on the deoxidation limit of Ti metal. Thus the activity of alkali or alkaline earth metals in corresponding chloride molten salt, balancing with a certain Ti-O solid solution (the thermodynamic property has been established), can be used to quantise the reducing ability. According to Nernst equation, the logarithm of the activity of alkali or alkaline earth metals (logam) in corresponding chloride molten salts is inversely proportional to the potential difference between the electrode potential and the standard electrode potential of reaction 4, as Eq. 5.
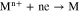

Where aM is the activity of alkali or alkaline earth metals, n is electron transfer number, F is Faraday constant, Eθ is the standard electrode potential of reaction 4, E is the electrode potential, R is gas constant and T is the temperature.
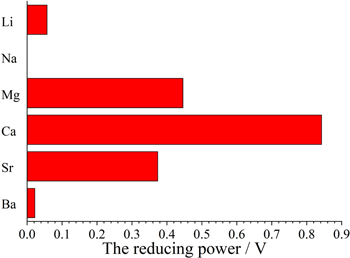
Assuming the thermodynamic properties of the Ti-O solid solution obtained at the equilibrium potential of Ti/TiO or titanates in all the chloride molten salts are identical, as shown in the E-pO2− diagrams, the reducing ability of alkali or alkaline earth metals can be expressed by the potential difference between the formation potential (E) of Ti metal at unit O2− ion activity and the deposition potential (Eθ) of alkali or alkaline earth metals of unit activity. In addition, considering the influence of electron transfer number n, the reducing ability of alkali or alkaline earth metals is expressed by n(E−Eθ). Here it should be noticed that Na metal cannot reduce titanium oxides into Ti at unit O2− ion activity in theory. Thus its reducing ability was expressed to be zero. Figure 6 shows the reducing ability of Li, Na, Mg, Ca, Sr and Ba metals. From that, the reduced ability of Ca metal is strongest, followed by Mg, Sr, Li and Ba. Meanwhile, experiments indicated that in molten CaCl2, the deoxidation limit of TiO2 pellets was lowest, followed by in molten MgCl2, SrCl2 and LiCl, which agreed well with the sequence of the reduced ability of the corresponding alkali or alkaline earth metals. Thus it can be concluded that the reducing ability of alkali or alkaline earth metals impacts greatly on the electro-deoxidation of TiO2 pellets in corresponding chloride molten salts. From the above, it can be surmised low-oxygen Ti was hardly obtained in BaCl2 molten salts.
Figure 6. The reducing ability of Li, Na, Mg, Ca, Sr and Ba metals.
Download figure:
Standard image High-resolution imageConclusions
From the above, E-pO2− diagrams are helpful in estimating and understanding the electro-deoxidation pathways and conditions of TiO2 pellets in different chloride molten salt though there are some differences between the experimental results and the thermodynamic analysis. Low-oxygen Ti was got only in molten CaCl2 after about 48 h of electrolysis. In LiCl, MgCl2 and SrCl2 molten salt, only Ti6O or high-oxygen Ti was obtained after 48 h of electrolysis, the oxygen content of which was about 4–7 wt%. While in molten NaCl, no Ti metal or Ti–O solid solution was obtained. The main factors influencing the electro-deoxidation of TiO2 pellets include the applied potential, temperature, O2− ion activity, polatization time etc But the most predominant and fundamental factor is the reducing ability of the metallic state of cation of the chloride molten salt to titanium oxides, which decides the electro-deoxidation limit of TiO2 in the chloride molten salts.
Acknowledgments
The authors sincerely acknowledge financial support from the National Natural Science Foundation of China (51674076) and the Fundamental Research Funds for the Central Universities (N162502002).