Abstract
The design and synthesis of complex multi-component heterostructures is an effective strategy to fabricate cost-efficient catalysts for electrochemical water splitting. Herein, one-dimensional porous Fe3S4-Fe7Se8 heterostructured nanowires confined in carbon (Fe3S4-Fe7Se8@C) were synthesized via the selenization of Fe-based organic-inorganic nanowires. Benefiting from the merits of morphology, composition and surface structure characteristics, i.e., the high structural void porosity, the direct electrical pathways of nanowire topology and the conductive carbon layer coating, the titled catalyst not only offered a larger accessible electrocatalytic interface but also facilitated diffusion of the electrolyte and gas. Moreover, the electron redistribution at the Fe3S4-Fe7Se8 heterojunction interfaces reduced the adsorption free-energy barriers on the active sites, endowing the catalysts with faster reaction kinetics and improved electrocatalytic activity. Accordingly, the optimal Fe3S4-Fe7Se8@C produced a low hydrogen evolution reaction overpotential of 124 mV at 10 mA cm−2 with a Tafel slope of 111.2 mV dec−1, and an ultralow oxygen evolution reactions overpotential of 219 mV at 20 mA cm−2, respectively. When applied as both anode and cathode for overall water splitting, a low battery voltage of 1.67 V was achieved along with excellent stability for at least 12 h. The work presented here offered a feasible scheme to fabricate non-noble metal-based electrocatalysts for water splitting.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
Electrochemical water splitting has been recognized as a clean and sustainable technology to produce pure H2 for energy storage and conversion.1–3 Nowadays, the industrial electrolysis of water is mainly conducted in the basic electrolytes. The poor-proton electrolyte limits the supply of H intermediates, resulting in a high energy barrier of water dissociation and sluggish reaction kinetics. Thus, it's highly-desired to prepare efficient electrocatalysts that can simultaneously reduce overpotentials and accelerate the reaction rate. Up to now, Pt-based materials are the most efficient catalysts for cathodic hydrogen evolution reactions (HER), and Ru-/Ir-based oxides are the most efficient catalysts toward anodic oxygen evolution reactions (OER).4 However, their scarcity and high price largely impede their broad commercial applications. In recent years, many efforts have been paid to the design and synthesis of various non-precious catalysts toward HER or OER. Particularly, transition-metal-based materials involving phosphides,5,6 selenides,7,8 sulfides,9–11 etc., have been widely studied as an alternative HER and/or OER electrocatalyst owing to their highly active and low-cost features.12,13 Among them, Fe-based chalcogenides showed promising capabilities as highly efficient HER or OER catalysts due to the high earth abundance, fast charge transfer kinetics and high chemical stability. For example, the disk-like FeS2 nanoplates synthesized by Jasion et al. manifested comparable HER activity with Pt in neutral pH conditions.14 Guan et al.15 found that the FeSe2 nanomesh arrays on titanium foil exhibited a low overpotential and good stability in 0.5 M H2SO4 solution. Li et al.16 found that the FeS2/C nanoparticles on Ni foam (NF) could be served as a bifunctional catalyst for overall water splitting, delivering a current density of 10 mA cm−2 at overpotentials of 202 mV for HER and 240 mV for OER, respectively. Based on these facts, iron-based chalcogenide is a promising earth-abundant alternative for the commercial application of electrochemical water splitting, and is definitely worth exploring.
Although great success has been gained for Fe-based chalcogenide catalysts, pure iron sulfides or iron selenides still exhibited unsatisfactory catalytic performance. Recent research demonstrated that the construction of heterostructured electrocatalysts by assembling two or more materials into one system was an effective approach to obtain better HER or OER catalytic performance due to a variety of advantages.17,18 Firstly, the heterostructured catalysts possessed refined nanostructures with substantially exposed edges, which provided enough adsorption sites for the intermediates. Secondly, the redistribution of electrons at the interface of heterojunctions could regulate the electronic structures or the band structures of the hybrids, which was beneficial for the optimization of the hydrogen (H*) and oxygen-containing intermediates (OH*, O* and OOH*) adsorption free energy. Thirdly, the introduction of conductive components into heterostructures enabled fast mass diffusion. These merits made the multicomponent heterostructures as efficient bifunctional catalysts for electrochemical water splitting. For example, Zeng et al.19 reported that the strong coupling interactions between Ni2P and Ni3S2 in the Ni2P/Ni3S2 hetero-nanoflake arrays optimized the electronic structure and then tuned the hydrogen adsorption energy, significantly enhancing the overall electrochemical waters splitting activity. Zhang et al.20 reported that the synergistic effect between MoS2 and Ni2S3 endowed the catalyst with excellent catalytic activity and robust stability, displaying an overpotential as low as 110 mV for HER and 218 mV for OER at 10 mA cm–2. Additionally, it is necessary to expose more surface through the modulation of morphology to provide more active sites. One-dimensional (1D) porous micro-/nanostructure play a critical role in catalysis due to its direct electron-transfer pathways, and highly-efficient active sites on the exposed surfaces.21 Based on the discussion mentioned above, the elaborate design and fabrication of Fe-based chalcogenide catalysts with 1D porous heterostructures is a prospective area of research for the development of efficient and economically water splitting, but remains a challenge.
Herein, we reported the controlled synthesis of carbon-coated Fe3S4-Fe7Se8 heterostructured nanowires (Fe3S4-Fe7Se8@C) through the selenization of Fe-based organic-inorganic nanowires precursors. The unique carbon layer decorated 1D porous hetero-nanowires possessed large accessible electrocatalytic interface to contact catalyst with electrolyte, resulting in rapid electron transfer rate and efficient electrochemical water splitting. Most importantly, the electron redistribution at the Fe3S4-Fe7Se8 heterojunction interfaces reduced the adsorption free-energy barriers on the active sites, endowing the hybrids with faster reaction kinetics and improved electrocatalytic behaviors. As the result, the Fe3S4-Fe7Se8@C exhibited excellent electrochemical catalytic performance, which showed an overpotential of 124 mV at 10 mA cm−2 with a Tafel slope of 111.2 mV dec−1 for HER, and an overpotential of 219 mV at 20 mA cm−2 with a Tafel slope of 45.4 mV dec−1 for OER, respectively. Additionally, the assembled electrolyzer delivers a current density of 10 mA cm−2 at a low voltage of 1.67 V with excellent stability for at least 12 h. The strategy presented here can be extended for synthesizing other 1D porous heterostructures, which may be used in the fields of solar cells, elelctroreduction of CO2, gas sensor and so on.
Experimental
Synthesis of S-ethylenediamine/Fe-ethylenediamine (SEFE) precursors
The SEFE precursors were prepared according to Zhang's work with few modifications.22 Typically, 1.5 mmol of FeCl2·4H2O and 2.1 g of PVP (polyvinylpyrrolidone, M ≈ 40000) were mixed with 5 ml deionized water and 10 ml ethylene glycol with magnetic stirring for 1 h to form a clear solution of A. Meanwhile, 6 mmol of elemental sulfur powder was dissolved in 10 mL ethylenediamine to form solution B. The solution B was then introduced into solution A. After stirring for 10 h, the obtained mixture was transferred into a Teflon-lined stainless autoclave and kept at the temperature of 200 °C for 24 h. Finally, the resultant precipitate was collected through centrifugation, and washed by ethanol and deionized water thoroughly three times, and finally dried in a vacuum oven at 80 °C for 12 h.
Synthesis of Fe3S4-Fe7Se8@C hetero-nanowires
The Fe3S4-Fe7Se8@C hetero-nanowires were synthesized through a facile chemical vapor deposition method. Briefly, 30 mg of SEFE precursors and 300 mg of selenium (Se) powder were placed at two separate positions in a quartz crucible. Then, the crucible was heated to 350 °C for 2 h with a heating rate of 2 °C min−1 under the protection of N2. After being cooled to room temperature, high-quality porous Fe3S4-Fe7Se8@C hetero-nanowires were obtained. For comparison, pristine Fe3S4@C nanowires were also prepared using the same procedure without Se power.
Electrochemical measurements
The electrocatalytic performance of the as-prepared samples toward OER and HER was performed in a conventional three-electrode cell using a CHI660E electrochemical workstation (CH Instruments Inc., Shanghai, China), where catalyst-coated nickel foam, a KOH-saturated Hg/HgO and a graphite rod were employed as the working electrode, reference and counter electrode, respectively. To fabricate the working electrode, a catalyst powder of 6 mg was dispersed into a solution containing 165 μl of deionized water, 110 μl of ethanol and 40 μl of Nafion solution (5 wt%) to form a homogeneous ink. Then 800 μl of ink was extracted and coated onto a Ni foam (NF) electrode (1.0 × 1.0 cm2), and left to dry naturally. The potentials measured were referenced to a reversible hydrogen electrode (RHE) according to the Nernst equation E(RHE) = E(Hg/HgO) + 0.098 + 0.059 pH. The HER and OER tests were performed in 1.0 M KOH solution, respectively. Linear sweep voltammetry (LSV) measurements were conducted, if not specified, at a scan rate of 5 mV s−1 for both HER and OER in 1.0 M KOH solution by using IR compensation with a degree of 90%. The same parameters were applied to the long-term stability performance for HER and OER by repeating the LSV for 1000 cycles. The Tafel slope was obtained from the corresponding LSV curves according to the Tafel equation. The geometric double layer capacitance (Cdl) was estimated by non-Faradaic cyclic voltammetry (CV) tests, where the CV curves were recorded in 1.0 M KOH solution at the scan rates of 20 mV s−1, 40 mV s−1, 60 mV s−1, 80 mV s−1 and 100 mV s−1, respectively.
Results and Discussion
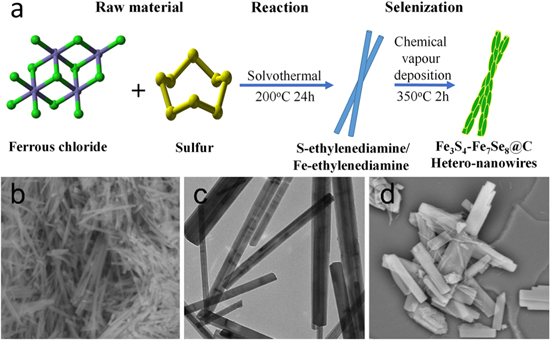
The strategy for the preparation of carbon layer decorated porous Fe3S4-Fe7Se8 hetero-nanowires was illustrated in Fig. 1. Firstly, a well-defined SEFE nanowire precursor was prepared through a facile solvothermal method, in which ethylenediamine and PVP served as ligands to induce the anisotropic growth of crystals by forming 1D nanostructure. Then, a chemical vapor deposition method was performed as the second step to transform the SEFE precursors into carbon layer coated Fe3S4-Fe7Se8 heterostructured nanowires. In the current case, we found the amounts of sulfur power played an irreplaceable role in controlling the morphology of SEFE nanowire precursors, which further affected the shapes and surface properties of Fe3S4-Fe7Se8 hetero-nanowires. To get high-quality nanowire catalysts with abundant catalytic active sites, various SEFE precursors with different molar of sulfur were prepared (Fig. S1 is available online at stacks.iop.org/JES/167/086501/mmedia). As shown in the SEM image of Fig. 1b, when 6 mmol sulfur was used in the synthesis, uniform SEFE nanowires were obtained with high yield. Careful observations showed that the diameter of the SEFE nanowires was in the range of 100 to 200 nanometers, and the lengths were up to several tens of micrometers. The corresponding TEM image clearly revealed the 1D morphology of individual SEFE nanowire with smooth surface (Fig. 1c). However, increasing the amount of sulfur power to 24 mmol while keeping other reaction parameters similar, irregular nanorods with smaller ratio of length to diameter were obtained (Fig. 1d).
Figure 1. Schematic illustration for the fabrication of Fe3S4-Fe7Se8@C hetero-nanowires (a). SEM (b), (d) and TEM (c) images of the as-synthesized SEFE precursors with 6 mmol sulfur (b), (c) and 24 mmol sulfur (d), respectively.
Download figure:
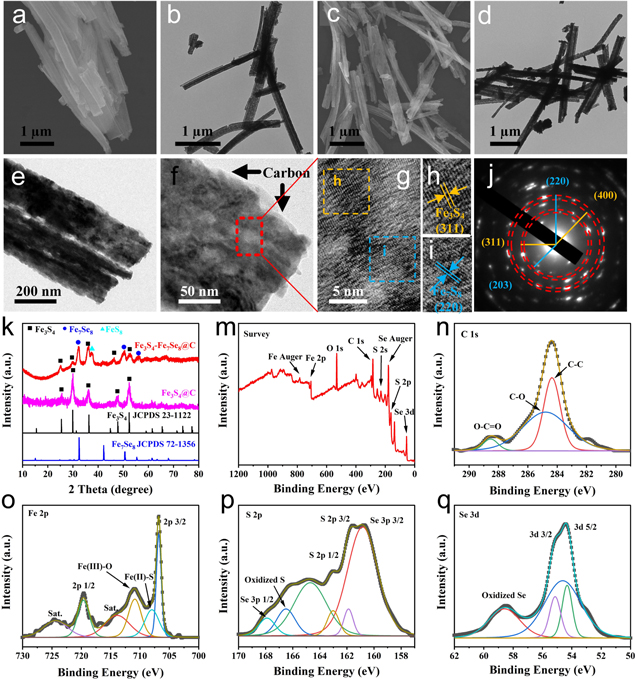
Standard image High-resolution imageThe microstructure and morphology details of the as-synthesized Fe3S4@C and Fe3S4-Fe7Se8@C heterostructured nanowires were then examined by TEM and SEM techniques. As shown in Figs. 2a–2d, the 1D structure of the Fe3S4@C and Fe3S4-Fe7Se8@C was basically maintained after the selenization process. However, the surface of the nanowires become rather rough. Numerous porous structures were generated due to the decomposition and carbonization of organic surfactants during the high-temperature selenization process (Figs. 2b, 2d and 2e). Obviously, the mesoporous structures and abundant exposed surfaces not only could provide a good deal of catalytic active sites for the electrocatalytic reactions but also could accelerate the diffusion of electrolytes. Figure 2f presented a typical TEM image of Fe3S4-Fe7Se8@C hetero-nanowires. The amorphous carbon layer could be easily found, indicating the Fe3S4-Fe7Se8 hetero-nanowires were embedded in a uniform thin amorphous carbon layer. Additionally, the lattice fringes with an interplanar distance of 0.298 nm were attributable to the (311) plane of Fe3S4, whereas the one with interplanar distance of 0.181 nm could be attributed to the (220) plane of Fe7S8, respectively (Figs. 2g–2i). The intimate contact between Fe3S4 and Fe7S8 suggested the formation of heterojunctions in the sample. The selected area electron diffraction (SAED) of Fe3S4-Fe7Se8@C clearly showed two different diffraction patterns which could be indexed into the overlap of Fe3S4 and Fe7Se8 because of their close crystal plane distances. The polycrystalline rings were matched well with (311), (400) planes of Fe3S4, and (203), (220) plane of Fe7Se8 (Fig. 2j), which was accordance with the XRD results as bellow.
Figure 2. SEM (a), (c) and TEM (b), (d), (e), (f) images of the as-obtained Fe3S4@C (a), (b) and Fe3S4-Fe7Se8@C samples (c)–(f). HRTEM images (g)–(i) and SAED pattern (j) of the Fe3S4-Fe7Se8@C catalyst. XRD pattern (k) of the as-obtained Fe3S4@C and Fe3S4-Fe7Se8@C hetero-nanowires. Typical XPS survey spectrum (m) and corresponding C 1s (n), Fe 2p (o), S 2p (p), and Se 3d (q) XPS spectra of the Fe3S4-Fe7Se8@C hetero-nanowires.
Download figure:
Standard image High-resolution imageFigure 2k showed the X-ray diffraction (XRD) patterns of pure Fe3S4@C and Fe3S4-Fe7Se8@C hetero-nanowires. The diffraction peaks emerged at 2 theta angle of 25.6°, 30.1°, 36.4°, 47.8°, and 52.3° could be well identified as the (220), (311), (400), (511), and (440) planes of Fe3S4 (JCPDS 23-1122), respectively. For the Fe3S4-Fe7Se8@C hetero-nanowires, the characteristic diffraction peaks of Fe3S4 could still be observed. However, some new diffraction peaks appeared at 2 theta angle of 32.4°, 50.3°, and 56.2°, which could be indexed as the (203), (220), and (029) crystal planes of iron selenide Fe7Se8 (JCPDS 72-1356). Besides, a minor peak at 2 theta angle of 37.6° corresponding to orthorhombic FeS2 phase (JCPDS 74-1051) was also found, indicating the dissimilar growth processes and reaction rates of sulfuration on Fe species. The X-ray photoelectron spectra (XPS) measurement was performed to confirm the chemical compositions of the Fe3S4-Fe7Se8 heterostructured nanowires. The survey spectrum in Fig. 2m revealed the co-existence of Fe, S, Se and C in the sample. Figure 2n displayed three main peaks at 284.3, 284.8, and 288.5 eV corresponding to C–C, C–O, O–C=O in the high-resolution XPS spectrum of C 1s.23,24 The high resolution XPS spectrum of Fe 2p in Fig. 2o presented the main peaks of Fe 2p1/2 and Fe 2p3/2 with the binding energy located at 706.8 and 719.7 eV, exhibiting characteristic of divalent iron.25 The peak at 710.9 are the 2p3/2 of Fe3+ species, suggesting the formation of iron-oxygen species owing to the exposure of sample in air.26,27 The high resolution XPS spectrum of S 2p was shown in Fig. 2p, the peaks at 163.4 eV corresponded to S 2p1/2 binding energies, the peaks at 161.9 eV corresponded to S 2p3/2 binding energies, the peak at 165.4 eV is typical of a metal-sulfur (M-S) bond in the material.28,29 The high resolution XPS spectrum of Se 3d in Fig. 2q presented two peaks at 54.3 and 55.1 eV, which could be attributed to Se 3d5/2 and Se 3d3/2, respectively. Similarly, the minor peak at 58.5 eV revealed the surface oxidation state of selenium species.30–32
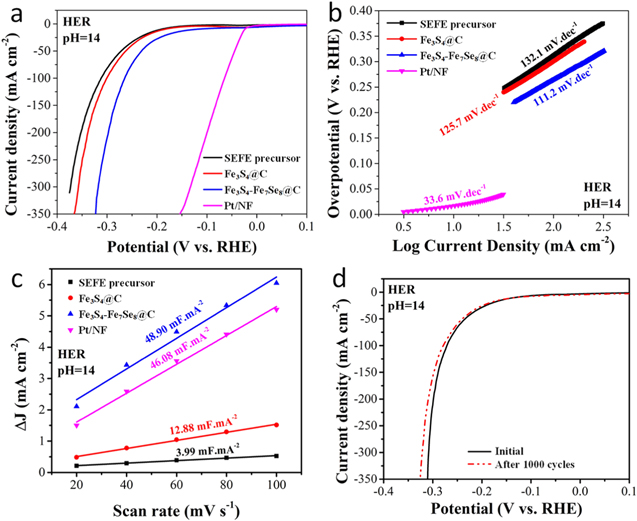
The electrocatalytic HER activities of the as-synthesized samples were evaluated by using linear sweep voltammetry (LSV) at a sweep rate of 5 mV s−1 in 1.0 M KOH solution. For comparison, the performance of the commercial 20 wt% Pt/C with the same loading was also measured. It's well accepted that the overpotentials (η10) at the current density of 10 mA cm–2 is a criterion to compare the electrocatalytic activity of different HER catalysts. Generally, a smaller η10 means better electrocatalytic activity. As shown in Fig. 3a, the SEFE electrode exhibited poor HER catalytic activity with a η10 of 193 mV due to its poor conductivity. In contrast, the Fe3S4@C manifested an impressive η10 of 186 mV, but lower than that of 124 mV for Fe3S4-Fe7Se8@C. Additionally, to optimize the catalytic activity of Fe3S4-Fe7Se8@C, different amounts of Se power were employed to synthesize various Fe3S4-Fe7Se8 hetero-nanowires (Fig. S2). As we expected, all the Fe3S4-Fe7Se8@C catalyst exhibited much better HER catalytic activity than Fe3S4@C (Fig. S3 and Table SI), confirming that the formation of multicomponent heterostructures could effectively improve the HER electrocatalytic reactivity.33,34 Although the optimized catalytic activity of Fe3S4-Fe7Se8@C was still inferior to the commercial 20 wt% Pt/C (37 mV), it was comparable to or better than many other iron-based chalcogenide catalysts in alkaline media, such as FeS2 nanoparticles/C hybrids (η10 = 202 mV),16 FeS2@C core–shell nanochains (η10 = 195 mV),35 Fe4.5Ni4.5S8 nanosheets (η10 = 146 mV),36 cobalt-doped FeSe2/RGO (η10 = 166 mV),37 etc (Table SII). The Tafel plots were then analyzed to give insights into the HER kinetic mechanism of the above-mentioned catalysts.38,39 As shown in Fig. 3b and Fig. S3b, the extracted Tafel slope of Fe3S4-Fe7Se8@C was 111.2 mV dec−1, comparable to those of Fe3S4@C (125.7 mV dec−1), but lower than SEFE precursor (132.1 mV dec−1). These results suggested that the HER on these catalysts was dominated by Volmer-Heyrovsky reaction mechanism, in which the discharge reaction (Volmer step) was fast and H2 was evolved by a rate-determining combination reaction (Heyrovsky step).40
Figure 3. HER properties of fabricated electrocatalysts: LSV curves (a) and the corresponding Tafel plots of different catalysts for HER (b). Double-layer capacitance (Cdl) determined by plotting capacitive currents as function of scan rate (c). Stabilities of Fe3S4-Fe7Se8@C after 1000 lSV cycles.
Download figure:
Standard image High-resolution imageIn order to gain more information about the exceptionally high HER efficiency of the heterostructured catalysts, the electrochemical surface area (ECSA) was examined by extracting the double-layer capacitance (Cdl) through cyclic voltammetry measurements in a potential range without Faradaic current (Fig. S4).41 The calculated Cdl value of Fe3S4-Fe7Se8@C was 48.90 mF cm−2, much higher than the Cdl values of SEFE (3.99 mF cm−2) and Fe3S4@C (12.88 mF cm−2). The larger value of Cdl for Fe3S4-Fe7Se8@C indicated that it possessed a larger effective surface area, which was responsible for its excellent HER catalytic behavior. In addition to catalytic activity, stability was another critical factor to evaluate the practical applications of electrocatalysts. The HER stability of Fe3S4-Fe7Se8@C was investigated via 1000 cyclic voltammetry cycles at a scan rate of 10 mV s−1. As shown in Fig. 3d, the LSV curve was basically the same as the initial curve after 1000 cycles, with the relative increment of overpotential not exceeding 2.5% at the current density of 100 mA cm–2, implying that the Fe3S4-Fe7Se8@C was rather stable in alkaline solution. The increase of overpotential during the durability test could be attributed to the embedding of protons into the Fe3S4-Fe7Se8@C structure, thus affecting electrical conductance and reducing hydrogen adsorption free energy during the HER process. These encouraging results, i.e., low overpotential, low Tafel slope, large electrochemically active surface area and long-term durability, suggesting that the as-synthesized Fe3S4-Fe7Se8@C was a promising HER candidate to substitute the Pt/C catalyst for electrochemical water splitting.
Similar to the electrocatalytic HER activity tests, the electrocatalytic OER performance of the samples was also evaluated with the same three-electrode system. As shown in Fig. 4a, the Fe3S4-Fe7Se8@C exhibited an optimal OER activity with an overpotential (η20) of 219 mV at 20 mA cm−2, which remarkably exceeded that of the SEFE (265 mV), Fe3S4@C (258 mV) and RuO2 (245 mV). The overpotentials at 50, 100 and 200 mA cm−2 for Fe3S4-Fe7Se8@C were 241, 256 mV, and 269 mV, respectively, much lower than the other three catalysts (Fig. S5 and Table SIII). These results revealed that the synergistic interactions between Fe3S4 and Fe7Se8 could modify the electronic structure of the catalyst to provide excellent OER catalytic activity. Additionally, Fe3S4-Fe7Se8@C presented the lowest onset potential of 1.47 V, implying smaller energy to drive the OER. Figure 5b further showed that the Fe3S4-Fe7Se8@C possessed a low Tafel slope of 45.4 mV dec−1, comparable to SEFE (49.0 mV dec−1), but lower than Fe3S4@C (51.4 mV dec−1) and RuO2 (113.4 mV dec−1). In contrast to the other Fe-based electrocatalysts reported previously, it is a remarkable fact that the Fe3S4-Fe7Se8@C electrode has excellent OER performances (Table SIV). The electrochemically active surface area for OER was also calculated by double-layer capacitance (Fig. S6). The Cdl value of Fe3S4-Fe7Se8@C for OER was 5.48 mF cm−2, higher than the Cdl values of SEFE (3.19 mF cm−2), Fe3S4@C (3.82 mF cm−2), and other Fe3S4-Fe7Se8@C catalysts, suggesting the existence of more active sites in the Fe3S4-Fe7Se8@C samples.42 The long-term electrochemical stability of the catalyst was tested with amperometric (i-t) measurements (Fig. 4d). The current density at the overpotential of 230 mV was almost constant after 12 h of continuing operation, demonstrating its remarkable stability toward OER in alkaline solution.
Figure 4. OER properties of fabricated electrocatalysts: LSV curves (a), and the corresponding Tafel plots of different catalysts for OER (b). Double-layer capacitance (Cdl) determined by plotting capacitive currents as function of scan rate (c). Durability test of the obtained catalyst at the corresponding at the overpotential of 230 mV (d).
Download figure:
Standard image High-resolution imageFigure 5. Overall water-splitting properties of fabricated electrocatalysts: LSV curves (a). Durability test of the obtained catalyst at a corresponding overpotential of 10 mA cm−2 (b).
Download figure:
Standard image High-resolution imageBased on the outstanding catalytic activity for both hydrogen and oxygen evolution reactions in alkaline solution, a two-electrode system had been constructed to present an overall water-splitting process in 1.0 M KOH solution, where the Fe3S4-Fe7Se8@C was used as both a cathode and an anode. As shown in Fig. 5a, the Fe3S4-Fe7Se8@C required a low cell voltage of 1.67 V and 1.87 V to drive the current density of 10 and 100 mA cm–2, respectively. The current density was very stable and remained unchanged as the applied overpotential decreased, demonstrating that the Fe3S4-Fe7Se8@C nanowires possessed favorable mass transfer and durability during overall water-splitting.43 The long-term stability of the water-splitting device was also evaluated. As shown in Fig. 5b, the obtained catalyst had superior overall water-splitting durability with negligible current density loss.
To further illustrate the advantages of the Fe3S4-Fe7Se8 heterostructure in catalyzing the HER and OER, the work function of Fe3S4 and Fe7Se8 were calculated to analyze the electronic behaviors of the sample via density functional theory (DFT) The detailed theoretical computation method was listed in the Supporting Information. The Fe3S4 (311) surface and the Fe7Se8 (220) surface are chosen to create slab modules for the calculations (Fig. 6). Both of the slabs consisted of a 15 Å vacuum layer and the surface cleaved for bulk material. As shown in Fig. 6, the work functions of Fe3S4 (311) is 4.967 eV, larger than that of 4.737 eV for Fe7Se8 (220), thus electrons will transfer from Fe7Se8 to Fe3S4 to equilibrate the Fermi levels when they form a contact.44 As a result, the Fe3S4 is positively charged and the Fe7Se8 becomes negatively charged near the heterojunction interface due to the formed electric field. In this regard, the accumulated electrons in Fe7Se8 will cause the adsorption of hydrogen species from the electrolyte, and the Fe7Se8 is favorable for the adsorption of hydroxyl species, leading to the acceleration of whole catalytic reactions. Therefore, the Fe3S4-Fe7Se8@C displays faster reaction kinetics and better catalytic performance than that of Fe3S4@C.
Figure 6. Average potential profile along c-axis of (a) Fe3S4 (311) and (b) Fe7Se8 (220). Evac is the vacuum level, EF are the Fermi levels, and EWF is the work function. The calculated slab modules of the energy change of the slab cell for Fe3S4 (c) and Fe7Se8 (d).
Download figure:
Standard image High-resolution imageBased on the experimental results discussed above, the Fe3S4-Fe7Se8@C performed an excellent catalytic efficiency for water splitting in an alkaline solution. The outstanding catalytic performance can be attributed to the following factors: Firstly, the porous 1D structure maximized the accessibility of interfaces, and as a consequence provided more active sites to catalyze the HER and OER. Moreover, the porous nanowire structure enlarged the contact area between Fe3S4-Fe7Se8@C and the electrolyte, beneficial for the release of H2 and O2 bubbles. Secondly, the synergistic effects facilitated the migration of charge carriers between Fe3S4 and Fe7Se8, promoting the initial H2O adsorption and reducing the energy barrier of water dissociation, resulting in rapid electron transfer rate and efficient electrochemical water splitting. Thirdly, the carbon coating improved the conductivity of the catalysts, providing a fast electron transfer rate. As a result, the as-synthesized porous nanowires enable enhancement of the hydrogen and oxygen evolution reactions, manifesting as a highly efficient bifunctional electrocatalyst for overall water splitting.
Conclusions
In summary, carbon-coated porous Fe3S4-Fe7Se8 heterostructured nanowires were synthesized via the controlled selenization of Fe-based organic-inorganic nanowires. This unique catalyst offered a larger accessible electrocatalytic interface and better conductivity. Moreover, the electron redistribution at the Fe3S4-Fe7Se8 heterojunctions interface reduced the adsorption free-energy barriers on the active sites. All these advantages endowed the hybrids with faster reaction kinetics and improved electrocatalytic activity. As the result, the Fe3S4-Fe7Se8@C exhibited excellent electrochemical catalytic performance, which showed an overpotential of 124 mV at 10 mA cm−2 with a Tafel slope of 111.2 mV dec−1 for HER, and an overpotential of 219 mV at 20 mA cm−2 with a Tafel slope of 45.4 mV dec−1 for OER, respectively. Additionally, the assembled electrolyzer delivers a current density of 10 mA cm−2 at a low voltage of 1.67 V with excellent stability for at least 12 h. This work may provide guidance in the design of other highly active Fe-based catalysts for future energy applications.
Acknowledgments
The work was financially supported by the National Natural Science Foundation of China (21601120, 21771124, 21805181 and 41877373), China Postdoctoral Science Foundation (2017M611529) and the Science and Technology Commission of Shanghai Municipality (17ZR1410500 and 19ZR1418100). We also acknowledged the High-Performance Computing Center of Shanghai University for providing the VASP software.