Abstract
Despite significant improvements of polymer electrolyte properties, the interfaces towards the electrodes often yield high interfacial resistances due to poor contacts, which are bottlenecks for application of newly developed polymer, ceramic or composite electrolytes in lithium metal batteries (LMBs). Herein, the impact of processing as well as slurry composition of LiNi1/3Co1/3Mn1/3O2 (NMC111) based composite cathodes on the achievable electrochemical C-rate performance of LMBs based on quasi-solid single ion conducting polymer electrolytes (SIPE) is demonstrated. Composite cathodes with varying types and amounts of lithiated species are fabricated and systematically compared. Among all considered electrodes, cathodes with an addition of 5 wt% lithiated terephthalic acid (TA Li) yield the highest discharge capacity of 91 mAhg−1 at 1 C for Li metal∣SIPE∣NMC111 cells. Furthermore, similar cells operated with cathodes whose pores are impregnated with 5 wt% SIPE via drop/spin coating even provide a specific discharge capacity of 113 mAhg−1 at 1 C, thereby clearly highlighting the benefit of the selected processing strategy to realize cathodes with substantially improved charge carrier transport networks.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Lithium metal constitutes a promising anode material for future rechargeable batteries due to its superior energy density compared to common anode materials such as graphite or silicon composite.1,2 Nonetheless, the major challenge that still restricts the application of LMBs comprises the inhomogeneous Li metal deposition upon cycling, eventually resulting in highly reactive high surface area Li deposits that may lose contact to the bulk Li anode ("dead" Li formation) or cause short circuits due to penetration of porous separators.3–6 Polymer, ceramic or composite electrolytes are currently proposed as alternative to their liquid counterparts, since their mechanical stiffness in principle facilitates more homogenous Li deposition, thereby significantly suppressing or even avoiding inhomogeneous Li deposits (including "Li dendrites").7,8 Nonetheless, the application of such electrolytes has additional challenges and necessitates adaption of internal interfaces within the cells. Despite significant improvements considering achievable Li ion conductivities of polymer, ceramic or composite electrolytes, in some cases even approaching that of typical liquid electrolytes8–10 particularly at elevated temperatures, the often insufficient rate performance obtained in cell set-ups utilizing traditional composite cathodes (composed of active material, inactive binder and carbon-based electron conducting agent) was associated with charge transfer restrictions and contact losses at electrolyte∣electrode interfaces.11 While liquid electrolytes readily penetrate porous electrode networks, thus providing sufficient contacts between the active materials particles and the electrolyte, such contacts are limited to electrode surfaces, at which both the polymer, ceramic or composite electrolytes (at least initially) have intimate contact with electrode active materials ("conformal interfaces"), though this may not be valid within the pores of the composite electrode thereby leading to high interfacial resistances and quite low rate performances.12,13 Therefore, electrode and electrolyte processing and implementation of "smooth" electrolyte∣cathode interfaces is not only challenging but also crucial for achieving excellent electrochemical performance and longevity of the cells. To date, merely a few studies have reported strategies for optimizing polymer∣cathode interfaces, among which the production of rather thin composite electrodes (mass loading < 3.0 mg cm−2)10,14–16 constitutes the major approach to encounter insufficient ion transport through composite electrodes, thereby reducing the length of pathways that Li ions have to travel between active particles and the electrolyte. In case of polymer electrolytes often no further treatment of the electrodes was performed,14,17–19 though a recent study highlighted the benefits of incorporating lithiated species into the electrode paste, particularly suggesting that increasing the volume fraction of triple-phase contact boundaries where the three phases (active material, electronic conductor and Li ion conductor) are in contact should be maximized to reduce the overpotential generated by insufficient charge carrier transport within the composite electrode as well as through the polymer∣cathode interface.11 Several other works mainly considering LiFePO4 (LFP) based electrodes utilized incorporation of 2.5 wt% up to 20 wt% lithiated species (comprising either monomers or the conducting polymer itself,15,20 or even another lithiated polymer21) into electrode pastes. Nonetheless, no systematic approach for optimizing the necessary amounts of such species was provided. In this study, it is demonstrated, how the composition of composite cathodes containing two different lithiated species, specifically a single ion conducting polymer (SIPE) and lithiated terephthalic acid (TA Li) (presented in Fig. 1), as well as different contents of a lithiated species define the achievable rate performance of NMC111 based electrodes when applied as additional compound within the electrode paste. It is also shown that embedding of SIPE into the pores of the composite electrodes via drop/spin coating creates transport pathways that are advantageous compared to the mere addition of conductive species to the electrode paste, hence yielding further improvement of the rate performance.
Figure 1. Chemical structure of the single ion conducting polymer (SIPE) and lithiated terephthalic acid (TA Li).
Download figure:
Standard image High-resolution imageExperimental
Synthesis
Synthesis of the single ion conducting polymer and membrane fabrication (PVdF-HFP to single ion conducting polymer ratio 1:3, 140 wt% solvent (EC:PC, 1:1, v:v) uptake were done based on the synthesis route and materials developed in.10 Lithiation of terephthalic acid (TA, Sigma Aldrich, Germany) was performed in aqueous medium. 500 mg of TA was added to 100 ml of deionized H2O (yielding a suspension) and 144 mg of LiOH (Sigma Aldrich, Germany) were added to the solution at room temperature so that TA was dissolved. The reaction was stirred overnight; the solvent was then removed by rotary evaporation and the obtained white product (TA Li) was further dried under reduced pressure (10−3 mbar) at 100 °C for 48 h.
Cathode preparation
NMC111 (Shanshan, China) based composite cathodes with a mass loading of ∼2.8 mg cm−2 were prepared based on the compound compositions in wt% listed in Table I. Volume fractions (vol%) of the compounds were estimated and are additionally listed in Table I based on the determined true densities of the compounds (NMC111 ρ = 4.70 g cm−3, CB ρ = 1.95 g cm−3, PVdF ρ = 1.77 g cm−3, TA Li ρ = 1.95 g cm−3, SIPE ρ = 1.47 g cm−3).
Table I. Composition of the prepared electrodes.
| NMC (wt%) | CB (wt%) | PVdF (wt%) | Li Add. (wt%) | NMC (vol%) | CB (vol%) | PVdF (vol%) | Li Add. (vol%) | |
|---|---|---|---|---|---|---|---|---|
| NMC ref | 80.0 | 10.0 | 10.0 | 0.0 TA | 61.2 | 18.4 | 20.3 | 0.0 TA |
| NMC 3 wt% TA Li | 80.0 | 8.5 | 8.5 | 3.0 TA | 61.4 | 15.7 | 17.3 | 5.6 TA |
| NMC 5 wt% TA Li | 80.0 | 7.5 | 7.5 | 5.0 TA | 61.5 | 13.9 | 15.3 | 9.3 TA |
| NMC 5 wt% SIPE | 80.0 | 7.5 | 7.5 | 5.0 SIPE | 59.7 | 13.5 | 14.9 | 11.9 SIPE |
| NMC 15 wt% TA Li | 75.0 | 5.0 | 5.0 | 15.0 TA | 55.0 | 8.8 | 9.7 | 26.5 TA |
| NMC SIPE SC | 80.0 | 10.0 | 10.0 | +5.0 SIPE | 54.6 | 16.4 | 18.1 | 10.9 SIPE |
For the preparation of the cathode, firstly PVdF (Solef 5130, from Solvay, Belgium) was dissolved in NMP (anhydrous, 99.8%, Sigma Aldrich, Germany) yielding a 2 wt% solution that was stirred overnight. Subsequently, the additive (TA Li or SIPE) was added to the solution and stirred for another 3 h prior to addition of carbon black (Super C65, Imerys Graphite) and NMC111. The electrode paste was stirred for another 30 min and the viscous solution was transferred to a swing mill (MM 400 (Retsch Technology, Haan, Germany) to be stirred for 30 min at a frequency of 30 Hz. Aluminum foil (20 μm, Evonik Industries, used as current collector) was wiped with ethanol to remove likely surface contaminations. Then, the electrode paste was cast onto the Al foil using doctor blade technique with a gap width of 50 μm. The obtained coating was dried at 80 °C overnight. The electrodes were calendared to minimal thickness achievable (13 μm coating thickness). After punching of the electrodes (12 mm disk), they were further dried at 80 °C under vacuum for 48 h.
Drop/spin coating of the electrodes was performed in a dry room (H2O < 20 ppm, dew point below −65 °C) using 30 μl of a solution of 10 wt% SIPE in dimethylacetamide (anhydrous, 99.8%, Sigma Aldrich, Germany) for every electrode (12 mm diameter). The applied spin coating program contained two steps. In the first step the rotation speed was increased from 0 to 120 rounds per second (rps) within 120 s while the SIPE solution was applied dropwise onto the electrode starting at 0 rps followed by one drop every 10 s. Then, the rotation speed was kept at 120 rps for another 120 s. Subsequently, the cathodes were placed inside a vacuum oven at 80 °C for 24 h prior to cell assembly.
Coin cell assembly in two electrode configuration22 was performed in a dry room using roll pressed Li metal foil (Albemarle Corporation, initial thickness 500 μm) of 300 μm thickness as anode material.23 Ethylene carbonate (EC), ethyl methyl carbonate (EMC) and lithium hexafluorophosphate (LiPF6) (all compounds battery grade purity) were purchased from BASF SE. Cells containing liquid electrolyte were assembled utilizing 80 μl of LP57 (1 M LiPF6 in EC: EMC (3:7 by weight)) and one layer of a propylene separator (Celgard 2500).Cycling investigations were performed at a Maccor 4000 battery analysis system (USA) in the voltage range of 3.0 V to 4.3 V at 60 °C. For analysis of the rate performance, an asymmetric procedure was applied where the charging rate was varied (from 0.05 C to 2 C) and the discharge rate was kept constant at 0.1 C.
Scanning electron microscopy (SEM) was performed with a Zeiss Auriga electron microscope (Carl Zeiss Microscopy GmbH) using an accelerating voltage of 3 kV. Energy-dispersive X-ray spectroscopy (EDX) was carried out applying an acceleration voltage of 10 kV using an energy dispersive X-ray detector (X-MaxN 80 mm2, by Oxford instruments).
Results and Discussion
For this study, SIPE membranes developed in previous work10 were utilized as example polymer electrolyte to derive feasible strategies for proper processing thereof for improvement of charge transport at cathode∣polymer interfaces. Similar to recent studies demonstrating the benefits of the addition of lithiated species (either SIPE or lithiated monomer11) to electrode slurries of LFP based electrodes, two selected lithiated species (Fig. 1) were compared as slurry additives. Compound (1) is the single ion conducting polymer and compound (2) is lithiated terephthalic acid, which was selected based on its higher Li content compared to SIPE and its insolubility in the swelling solution (in this case a mixture of EC: PC) as otherwise the considered lithiated species might be washed out from the composite cathode upon cycling (which particularly in case of single-ion conducting polymer electrolytes would be disadvantageous as in case of (2), anionic mobile species would be released into the SIPE). In previous works on similar polymer electrolyte systems utilizing LFP based electrodes an addition of 5–20 wt% lithiated species was proposed,11,20,21,24 but no systematic study concerning the "ideal" composition was provided. Rather, varying cathode compositions were realized and combined with different polymer electrolytes in different studies, rendering a straightforward comparison and unambiguous recognition of likely trends difficult. Likewise, preferred compositions eventually identified in case of LFP may not be representative for NMC-based cathodes materials, particularly due to their different characteristics such as particle shape or size distribution, distinctly different volume changes upon cycling or merely the presence or absence of protective coatings. Hence, for all the NMC-based electrodes investigated here, systematically varied cathode compositions were realized (see Table I), incorporating either 3 wt%, 5 wt% or 15 wt% of lithiated species into electrode pastes. In addition to the weight fractions, Table I includes estimated corresponding volume fractions (vol%) of all compounds, reflecting the influence of the cathode additives (TA Li and SIPE) on the volumetric composition of composite cathodes since both quantities are considerably important for the development of battery systems, where energy density (Wh/l) as well as specific energy (Wh/kg) are considered.2,13 The wt% fractions of 3 wt%, 5 wt% and 15 wt% of TA Li correspond to volume fractions of 5.5 vol%, 9.3 vol% and 26.5 vol%, respectively, while 5 wt% SIPE represents a volume fraction of 11.9 vol%, which is significantly higher than in case of 5 wt% TA Li due to a lower density of SIPE.
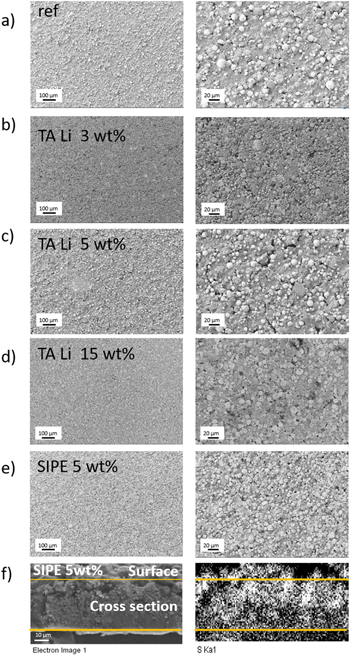
SEM images of the surface of the prepared composite electrodes are displayed in Figs. 2a–2e. For all samples, comparable porous microstructures and homogenous distribution of the constituents is observed, clearly indicating that the Li+ containing additive does not induce undesirable agglomeration of the compounds. In case of SIPE containing composite electrodes an additional SEM image of the cross section of the electrode and a corresponding distribution of sulfur (detected by EDX) were recorded (Fig. 2f), indicating a homogenous distribution of SIPE within the structure. Note, that in case of TA Li containing electrodes, specific elements which may be detected by EDX to identify its presence comprise carbon and oxygen, which however constitute atoms that may also be related to other electrode compounds (carbon black, NMC). Thus, EDX cannot be invoked to unambiguously analyze the distribution of TA Li in these samples.
Figure 2. SEM images of (a)–(e) the surfaces of the investigated composite cathodes (2 different resolutions per sample) and SEM and EDX of the cross section of the cathode containing 5 wt% SIPE in the electrode paste.
Download figure:
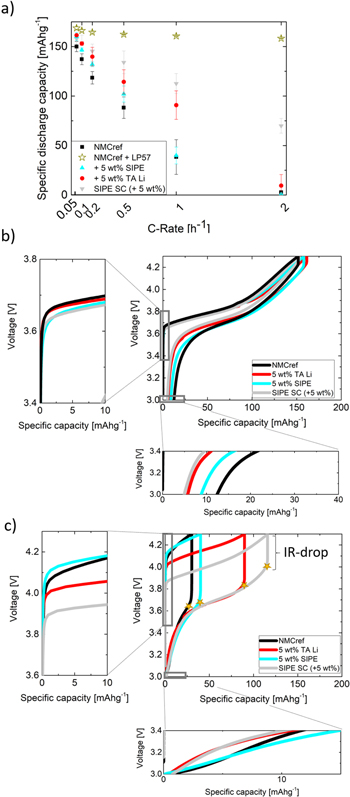
Standard image High-resolution imageFigure 3 demonstrates the impact of different weight contents (wt%) of lithiated additive (TA Li) within the composite cathodes on the rate performance. To estimate the maximum achievable specific capacity of the produced cathodes, in addition to analysis of SIPE containing cells, cycling of NMC reference electrodes (not containing the additive (TA Li)) was also done with liquid electrolyte (LP57) containing cells. As shown in Fig. 3a, a content of 15 wt% (TA Li) significantly reduces the obtained specific discharge capacity (Fig. 3a) compared to reference electrodes, resulting in low Coulombic efficiency as indicated by the irreversibility of the voltage profile at 0.1 C (Fig. 3b,ii). The strong increase in voltage at the beginning of cell charging (Fig. 3c,i) clearly reflects the presence of high overvoltages related to higher interfacial resistances among the polymer electrolyte membrane and the composite cathode. Most likely, the larger content (in wt% and in vol%) a of additive (TA Li) results in a higher amount (see Table I) of inactive material in the cathode as well as larger distances between the active particles, hence elongating the available diffusion pathways, which indeed would be unfavorable for effective Li ion transport. In addition, these electrodes contain a comparatively low content of carbon black (at least with respect to the high amount of inactive cathode material) which may reduce the electronic conductivity of this composite cathode, thus rendering the available volume fraction (vol%) providing contacts among electron conducting agent, Li+ conducting species and active material quite low and disadvantageous for charge carrier transport.11,25,26 In contrast, the composite electrodes with 3 wt% and 5 wt% of additive (TA Li) both yield higher discharge capacities and increased Coulombic efficiencies (Fig. 3b,iii) compared to reference electrodes, evidently affording better interfacial contacts and lower voltage losses (as indicated by the stars in Fig. 2c,ii). Based on the respectable increase in specific discharge capacity from 38 mAhg−1 to 91 mAhg−1 at a rate of 1 C, the additive content of 5 wt% (TA Li) reflects an optimal composition (affording enhanced Li ion transport and sufficient electron transport) among the considered electrodes. Nonetheless, even in this composition the remaining overvoltage reveals the presence of significant interfacial resistances that reduce the achievable discharge capacity compared to the specific discharge capacity of ≈161 mAhg−1 at 1 C available in case of liquid electrolyte containing cells. In Fig. 6 the rate performances (6a) and voltage profiles at 0.1 C (6b) and 1 C (6c) of the cathodes containing 5 wt% of additive (TA Li) and 5 wt% of SIPE compared to reference electrodes are presented. Both additives enhance the achievable rate performance, as evidenced by larger specific discharge capacities for all selected C-rates. While an admixture of SIPE as additive to the cathode paste merely affords slightly improved rate performances, the addition of TA Li significantly boosts the specific discharge capacity at 1 C (91 mAhg−1), likely due to the overall higher Li ion content of (TA Li) compared to SIPE, as intended by the choice of this compound.
Figure 3. (a) Rate performance (b) Voltage profiles at 0.1 C and (c) Voltage profiles at 1 C of NMC∣SIPE∣Li cells based on composite cathodes containing different amounts of lithiated terephthalic acid (TA Li) and NMCref∣LP57∣Li containing cells at 60 °C.
Download figure:
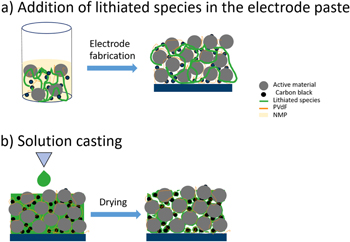
Standard image High-resolution imageIn addition to the application of lithiated species as additives within the electrode paste, a different processing approach was considered. As schematically shown in Fig. 4b, drop/spin coating of a SIPE solution onto the reference electrodes was performed, in this way allowing the dissolved polymer to deeply penetrate the porous cathode while simultaneously enabling a homogenous polymer deposition. Note that for this coating procedure, SIPE is strongly preferred over the additive (TA Li), mainly due to the poor solubility of (TA Li) in common organic solvents which may be beneficially exploited in case of cathode additive, but restricting its applicability for solution processing. Via drop/spin coating of polymer solution, the inner pore volume ("empty" space or voids) is reduced while bringing conductive polymer into intimate contact with the electrode active materials particles. Due to solvent evaporation of the coated SIPE solution, the resulting cathode remains porous, which is considered essential since the quasi-solid SIPE transports solvated Li species that require exchange and penetration of the pores for achieving an efficient charge transport within the composite cathode. Note that the overall weight increase after drop/spin coating was 5 wt%, hence allowing for a comparison of electrochemical performance among all the prepared cathodes, including those containing 5 wt% of SIPE or (TA Li) as additives within the electrode paste. Sulfur detection by EDX of the surface (Fig. 5a) and cross sections (Fig. 5b) of the modified electrodes indicate a homogenous and continuous distribution of SIPE throughout the electrode. While the specific discharge capacity of ≈161 mAhg−1 (1 C) of the liquid electrolyte containing cells is not achieved27,28 for a polymer electrolyte, the rate performance of the as prepared electrodes (SIPE SC) is significantly improved (113 mAhg−1 at 1 C) compared to the ones in which 5 wt% SIPE or 5 wt% (TA Li) were directly added to the electrode paste, where the increase in Coulombic efficiency (Fig. 6b,iii) at 0.1 C and the decrease in overvoltage/IR-drop at elevated charging rates (Figs. 6c, i and 6c,ii for 1C) unambiguously demonstrate the benefit of the drop/spin coating procedure. As a result of the advanced preparation technique cathodes with substantially improved ion transport network (and sufficient electron transport network) are obtained. In future studies, further approaches might be considered to improve the rate performance of polymer containing LMBs to approach the specific discharge capacities achievable for liquid electrolyte based cell systems, including coatings of lithiated flexible polymers to replace the binder of the cathode slurry, thus reducing contents of inactive material within the cathode while potentially boosting the usable energy density. Also, coating the active material particles with lithiated species prior to slurry preparation might constitute an option to optimally cover the surface of the active material thereby significantly increasing the contact area between both compounds. Moreover, further decreasing the electrode porosity by applying larger amounts of polymer solution for pore impregnation or preparation/fabrication of the polymer electrolyte membrane directly on top of the electrode might be beneficial to achieve improved interfacial properties and afford faster charging capabilities (e.g., C-rates higher than 0.5 C in case of polymer electrolytes). Generally, for all attempts, a rather optimal cathode composition reflecting the presence of both an efficient electron and ion transport network must be achieved. In summary, this work illustrates that polymer processing strategies and the optimization of polymer electrolyte∣electrode interfaces reflect a complex endeavor with multiple adjustable parameters, but also offer many possibilities for tailored and systematic improvement of the achievable cell performance, hence in principle endowing future longevity of electrochemical cells.
Figure 4. Schematics representing the strategies investigated. (a) Incorporation of a lithiated species in the electrode paste and (b) Coating of the electrode with a solution containing a lithiated species.
Download figure:
Standard image High-resolution imageFigure 5. SEM and detection of sulfur by EDX of (a) the surface and (b) the cross section of the coated NMC ref electrode.
Download figure:
Standard image High-resolution imageFigure 6. (a) Rate performance of NMC∣SIPE∣Li and NMCref∣LP57∣Li containing cells, (b) Voltage profiles at 0.1 C and (c) Voltage profiles at 1 C of NMC∣SIPE∣Li cells at 60 °C based on composite cathodes containing no(ref), TA Li or SIPE as lithiated species.
Download figure:
Standard image High-resolution imageConclusions
In this study it is demonstrated how the properties of NMC111 electrodes define the achievable rate performance in LMBs operated with polymer electrolytes. Different composite cathodes with mass loadings of 2.8 mg cm−2 containing variable contents of additive (TA Li) or SIPE were fabricated to enhance charge carrier transport through polymer∣NMC particle interfaces. The addition of (TA Li) results in a more significant increase of the specific discharge capacity compared to SIPE, reflecting higher Li content of (TA Li). Among the considered compositions, the addition of 5 wt% (TA Li) to the composite cathode paste represents an optimum, affording a respectable increase in achievable specific discharge capacity from 38 mAhg−1 to 91 mAhg−1 at 1 C. Beyond that, drop/spin coating of the cathodes incorporating up to 5 wt% SIPE into the pores of the porous electrodes results in even larger specific discharge capacity of 113 mAhg−1 at 1 C, clearly highlighting the benefit of the selected processing strategy to realize cathodes with substantially improved ion transport networks and better electrochemical performances.
Acknowledgments
All authors gratefully acknowledge generous support provided by the German Federal Ministry of Education and Research (BMBF) projects "Festbatt" (grant 13XP0175A) and "Benchbatt" (03XP0047B).