Abstract
All solid-state lithium batteries (ASSLBs) employing inorganic solid electrolytes or solid polymer electrolytes are attracting increasing interests for electrochemical energy storage devices due to their advantages of high energy density, high safety, wide operating temperature range and long cycle life. However, the large interfacial resistance originated from the insufficient solid-solid contact at electrolyte/electrode interface hinders the development of ASSLBs. In addition, the interfacial stability and compatibility also greatly affect the electrochemical performance of batteries. To realize the ASSLB's application requires significant research in solid electrolyte materials and solid electrolyte/electrode interfaces. This review summarizes the research and development in solid electrolyte materials and the interfaces of solid electrolyte/electrode, paying special attention to the challenges and progress for the studies of interface issues in ASSLBs. Based on the overview, we attempt to propose approaches to the issue by interface engineering and prospective developments of ASSLBs.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Since the introduction of secondary lithium ion batteries (LIBs) on the market first in the world by Sony in 1991, the LIBs have been applied to consumer products, such as mobile phones and laptops, electric vehicles, and stationary storage applications. Years of technology researches and efforts to improve LIBs performances enabled LIBs to play a leading role in the portable electronics market.1 The LIBs with LiCoO2 (LCO), NMC (LiNixMnyCozO2, x + y + z = 1) or NCA (LiNi0.8Co0.15Al0.05O2), and LiFePO4 cathode coupled with the carbon anode and an organic liquid electrolyte have been successful in many industries as the dominant energy storage source. Today the state-of-the-art LIBs have offered volumetric and gravimetric energy densities up to 770 Wh l−1 and 260 Wh kg−1, respectively. However, the energy density of conventional LIBs will soon reach its limit.2 However, there is an ever-increasing demand for batteries with even higher energy density as well as high power density in our modern life, such as electric vehicles. The LIBs with organic liquid electrolyte still suffer from safety issues and insufficient cycle time, due to its flammable charactoristic and leakage possibility of liquid electrolyte.
All solid-state lithium batteries (ASSLBs) have recently attracted great interest as potentially safe and stable high-energy storage systems. The ASSLBs, which use solid state electrolytes (SSEs) instead of flammable, hazardous liquid electrolyte, could offer high energy and improved safety.3 The ASSLBs is enable to use the lithium metal as the anode, with the theoretical gravimetric capacity (3860 mAh g−1) approximately 10 times that of currently used carbon (372 mAh g−1). As a next-generation energy storage technology, ASSLBs have shown great potential to provide higher energy density, better safety, longer cycle life, and a wider operating temperature range than current commercial LIBs.4
Despite fast growing interest in ASSLBs technology, many challenges remain in both fundamental understanding and manufacturing of the batteries. The high interfacial resistance at the electrolyte/electrode interface is a crucial problem in ASSLBs, limiting the power and rate performances of ASSLBs. The high interfacial resistance is attributed to poor interfacial contact, interfacial degradation due to mutual diffusion, the mechanical failure of the contacts, or the formation of a lithium-depleted space-charge layer.5–8 The influence of interfaces represents a critical factor affecting the use of ASSLBs in many practical applications. However, current understanding of this issue remains somewhat limited. From ASSLBs product development standpoint the vital factors that require considered are the cell energy density on volumetric and gravimetric basis, operable capabilities for given temperature, cell lifetime for useage and shelf life, safety, and the cost of the battery. The processing parameters can have significant effects on battery properties. It is necessary to take cell and product design considerations into account in creating tangible product. There are also a number of significant engineering challenges that require methodical effort to enable a robust product.9
In this review, the developments of solid-state electrolytes, including the sulfide-based, oxide-based and polymer-based solid electrolytes, were presented at first. The advanced characterization techniques associated with the electrolyte/electrode interfaces in ASSLBs were introduced. Interface engineering of lithium metal anode/solid electrolyte interfaces, cathode/solid electrolyte interfaces were elaborated. The surface coating on lithium metal anode and composition modification of the solid electrolyte are effective ways to reduce the impedance of the anode/solid electrolyte interfaces. Functional film coating on cathode active materials and multilayer design of cathode/electrolyte interface can be measured to solve the cathode/electrolyte interface problems. At the end, the research aspects to solve the interface issue of ASSLBs from understandings on fundamental scientific problems and interfacial engineering processes,as well as the commercial reality of ASSLBs are prospected.
Solid Electrolytes for All Solid-state Lithium Batteries
The solid state electrolytes (SSEs) have being explored in the last few decades as an alternative for liquid organic electrolyte systems. Unlike liquid organic electrolytes, SSEs are usually nonflammable, enable the use of Li-metal anode, and increase cycle life of lithium batteries.
To realize the all-solid-state lithium batteries, the SSEs should satisfy the following requirements: (1) high ionic conductivity, no less than that of current liquid electrolytes (10−3−10−2 S cm−1 at RT), (2) high chemical compatibility vs cathodes and anodes, (3) wide electrochemical window, (4) negligible electron conductivity, (5) the possibility of high energy density and power.2,10,11
The SSEs can be classified into two categories: inorganic ceramic electrolytes and polymer electrolytes. The inorganic ceramic electrolytes can be subdivided into sulfide-based, oxide-based, nitride-based materials and so on. Inorganic solid-state electrolytes used in lithium-ion battery have been researched since the 1990s, after a lithium phosphorus oxynitride (LiPON) material was fabricated as a thin film by Oak Ridge National Laboratory.12,13 Since then much effort has been made towards the development of inorganic lithium-ion conductive ceramic materials, such as garnet-type cubic Li7La3Zr2O12 (LLZO), sulfide-type materials, perovskite-type, and sodium superionic conductor (NaSICON)-type.
Among them, sulfide-based inorganic solid electrolyte materials provide relatively high ionic conductivities, for instance Li10GeP2S12 (LGPS) shows an ionic conductivity as high as 1.2 × 10−2 S cm−1, which is comparable to those of organic liquid electrolytes.14 Owing to the natural softness of sulfides, the sulfide-based solid electrolytes can intimately contact with electrode particles by pressure, providing low resistance at the interface. In addition, sulfide-based solid electrolytes particularly possess comparably high reduction stability, resulting in their wide electrochemical window. Consequently, the sulfide-based solid electrolytes have shown significant promise to be combined with electrode active materials, due to their high lithium ionic conductivity, flexibility and good mechanical strength, low grain boundary resistance, as well as a wide electrochemical window.15–17 The glass-ceramic Li2S-P2S5 has been reported to have a conductivity of exceeding 10 × 10−3 S cm−1.10,18 Sulfur-containing lithium argyrodites Li6PS5X (X = Cl, Br, I) are considered as a promising candidate for ASSLBs application because they possess a high ionic conductivity of 10−3 S cm−1 for X = Cl and Br at room temperature, and have the added advantage of utilizing cheap precursors for their synthesis. The sulfide-based SSEs have the potential to suppress polysulfide "shuttle" in lithium-sulfur (Li-S) battery.19 One of the deficiency of the sulfide-based SSEs is their instability in air condition.
Oxide-based solid electrolytes, like garnet-type Li7La3Zr2O12, have overall lithium-ion conductivity of 10−4 S cm−1 at room temperature, high electrochemical stability, and high chemical stability in ambient condition in contact with Li metal. Although the garnet-type electrolyte has created considerable interest in recent years for their potential application not only to all-solid-state lithium-ion batteries but also to Li-air batteries, decreasing the interfacial resistance is of significance in the oxide-based solid electrolytes. Many types of the oxide-based electrolytes have been reported so far, including NaSICON-type phosphates AM2(PO4)3 (A = alkali ion, and M = Ge, Zr, or Ti),20 perovskite type titanates Li3xLa2/3−xTiO3,21 lithium phosphorus oxynitride (LiPON),12 and garnet-type LLZO and their doped variants like Li6.25La3Zr2Al0.25O12.22
Benefiting from shape versatility, flexibility, low-weight and low processing costs, solid polymer electrolytes (SPEs) are being investigated as promising candidates to replace currently available organic liquid electrolytes in ASSLBs. A number of authors have provided reviews on the development of solid polymer electrolytes in recent years.23,24 They discuss the different molecular structures, ion-transport mechanisms of the solid polymer electrolytes, their interfacial compatibility with lithium, as well as the factors that govern the properties of the solid polymer electrolytes. However, it still remains a challenge to solve the problems of the inferior Li-ion conductivity and poor mechanical performance of these promising SPEs.
There have several comprehensive reviews on solid electrolytes for ASSLBs recently.2,3,25,26 The ion conductivity, structural effects, doping strategies, and research status of several types lithium ion conductors were summarized. The fundamental relationship between anion packing and ionic transport in fast Li-conducting materials and the desirable structural attributes of good Li-ion conductors were revealed.27 The ionic conductivities of inorganic solid electrolytes have been compared to those of liquid electrolytes and a typical polymer electrolyte.28 Highly conductive solid ionic materials have paved the way for the potentially breaking technology of high energy density true ASSLBs. However, most solid electrolytes are easily reduced at low potentials (for example, by lithium metal) and oxidized at intermediate potentials. Protecting interphases are therefore required to stabilize the electrolyte/electrode contact.9
Inorganic ceramic electrolytes
Sulfide-based solid electrolytes
Sulfide-based solid electrolytes are advantageous to ASSLBs owing to their high ionic conductivity and deformability. Because sulfide ions are larger than oxide ions, they leave wider channels in the structure acting as conduction paths. In addition, sulfide-based SSEs is relatively soft and deformable materials. They are deformed and packed densely only by cold pressing, and in the cold-pressed form the electrolyte particles connect tightly to make grain boundary resistances low.29 In fact, the highest ionic conductivities among sulfide-based SSEs had already reached 10−3 S cm−1 in the early 1980s in Li2S-P2S5-LiI glass.30 Years development in this period had been improving the performance of sulfide-based SSEs,31–33 the highest ionic conductivities have exceeded 10−2 S cm−1 in LGPS in 2011.14 The properties of typical sulfide-based solid electrolytes are listed in Table I.
Table I. Properties of typical sulfide-based solid electrolytes.
| Typical electrolyte | Composition | Ionic conductivity (S cm−1) | Activation energy Ea (eV) | Electrochemical stability vs Li/Li+ (V) | References |
|---|---|---|---|---|---|
| thio-LiSICON | Li7P3S11 | 4.1 × 10−3 (RT) | 0.15# | / | 31 |
| Li10GeP2S12 | 1.2 × 10−2 (27 °C) | 0.25# | exceed 5 | 14 | |
| 70Li2S–30P2S5(glass ceramic) | 3.2 × 10−3 (RT) | 0.19# | / | 32 | |
| 70Li2S–30P2S5 (heat-treated) | 1.7 × 10−2 (RT) | 0.18# | / | 33 | |
| 0.37Li2S–0.18P2S5–0.45LiI | 10−3 (RT) | 0.075# | / | 30 | |
| Li9.54Si1.74P1.44 S11.7Cl0.3 | 2.5 × 10−2 (RT) | 0.23 | / | 34 | |
| argyrodite | Li6PS5Cl | 1.7 × 10−3 (RT) | 0.35 | 5 | 35 |
| Li7P2.9Mn0.1S10.7I0.3 | 5.6 × 10−3 (RT) | 0.208 | 5 | 36 | |
| Li6PS4.7O0.3Br | 1.54 × 10−3 (RT) | 0.32 | / | 37 | |
| 1.13 × 10−3 (90 °C) | |||||
| Li6PS5Cl0.5Br0.5 | 3.63 × 10−3 (32 °C) | 0.31 | 4.12 | 38 |
Notice: RT—room temperature, /- not mentioned, #- In the references the unit of activation energy is kJ mol−1, here changed it to eV (1 kJ mol−1 = 1.036427 × 10−2 eV), LiSICON—lithium super ionic conductor.
The highest lithium ionic conductivity of the Li2S–GeS2–P2S5 crystalline system is 1.2 × 10−2 S cm−1 for Li10GeP2S12, while that of the Li2S–P2S5 glass-ceramic system remains in the order of 10−3 S cm−1. Y. Seino et al. reported that a heat-treated 70Li2S–30P2S5 glass-ceramic conductor has an extremely high ionic conductivity of 1.7 × 10−2 S cm−1 and the lowest conduction activation energy of 17 kJ mol−1 (0.18 eV, 1 kJ mol−1 = 1.036427 × 10−2 eV) at room temperature. In their opinion, the heat treatment reduce the grain boundary resistance and influence of voids, resulting in increasing the Li+ ionic conductivity of the solid electrolyte.33 The finding of LGPS with an outstanding ionic conductivity of 1.2 × 10−2 S cm−1 has made the use of sulfide-based SSEs an appealing option for ASSLBs. However, the high cost of Ge largely limits the practical use of LGPS. Creating isostructural analogues by substituting Ge with Si, Al, or Sn, has been suggested.34,39,40
Another promising sulfide-based SSEs, halogen-doped Li6PS5X (X = Cl, Br, I) argyrodites, have attracted intensive attention due to their relatively high ionic conductivity and moderate electrochemical stability window. They are synthesized by solid-state sintering, mechanical alloying, or liquid-phase synthesis processes.35–38,41,42 However, the reported ionic conductivity values of Li6PS5X are still rather scattered in the range of 10−2 to 10−5 S cm−1 at room temperature. The highest ionic conductivity of 5.6 × 10−3 S cm−1 is measured from Li7P2.9Mn0.1S10.7I0.3 at room temperature with a low activation energy of 0.208 eV.36
Oxide-based solid electrolytes
High Li-ion conductivity and excellent stability are essential properties for solid electrolytes used in ASSLBs. Various types of oxide-based solid electrolytes have been developed, including cubic garnet-type Li7La3Zr2O12 (LLZO),43,44 lithium phosphorus oxynitride (LiPON),45 NaSICON-type LiM2(PO4)3 (M = Ti, Ge, Zr, Hf),46,47 and perovskite-type Li3xLa2/3−xTiO3 (LLTO)48,49 etc. Among these solid electrolytes, cubic-phase LLZO has been widely studied because of its excellent stability against lithium metal anode and cathodes.50,51 The garnet-type cubic LLZO ceramics and their doped variants like Li6.25La3Zr2Al0.25O12 deliver high ionic conductivity of about 10−4 S cm−1. Generally, supervalent doping of Al3+, Ga3+ and Ge4+ 52,53 at the Li sites as well as Ta5+ and Nb5+ 54 at the Zr sites has been conducted to stabilize the cubic garnet and increase the ionic conductivity. It has been reported that supervalent cation Zr4+ substitution on the site can increase the vacancy concentration, which can improve Li-ion conductivity by lowering the activation energy of Li ions, and that the substitution of an element with a larger ion diameter can enlarge the size of the Li-ion migration pathway and increase the mobility of Li-ions.55,56 The ionic conductivity can be obtained with 4.78 × 10−4 S cm−1 at 20 °C, while the activation energy was only about 0.29 eV when substitution of Zr4+ sites by the same value of Ge4+ in the Li7La3Zr2O12. The results indicate that doping of Ge4+ in the LLZO was able to increase the density of the pellets as well as reduce the sintering temperature.53 J. Gai and coworkers substituted the Zr sites of a Li7La3Zr2O12 electrolyte with Nb and Y elements simultaneously to improve its Li-ion conductivity and air stability. The Li7La3ZrNb0.5Y0.5O12 pellets treated at 1230 °C for 15 h had a total conductivity of 8.29 × 10−4 S cm−1 at 30 °C, having much higher conductivities than undoped ones.54 Dual substitution strategy can farther enhance Li+ ionic conductivity in LLZO solid electrolyte. Substituted the Li+ sites by Ga3+and replaced of Zr 4+ by Sc cation, a total ionic conductivity has been achieved 1.8 × 10−3 S cm−1 for Li6.65Ga0.15La3Zr1.90Sc0.10O12 at 300 K, which is the highest ionic conductivity observed for the garnets so far.57 The properties of typical oxide-based solid electrolytes are listed in Table II.
Table II. Properties of typical oxide-based solid electrolytes.
| Typical electrolyte | Composition | Ionic conductivity (S cm−1) | Activation energy Ea(eV) | Electrochemical stability vs Li/Li+ (V) | References |
|---|---|---|---|---|---|
| Garnet type | Li7La3Zr2O12 | 3 × 10−4 (25 °C) | 0.32 | / | 43 |
| Li7La3ZrNb0.5Y0.5O12 | 8.29 × 10−4 (30 °C) | 0.3065 | / | 54 | |
| LLZO-0.3 B2O3 | 2.5 × 10−4 (RT) | 0.35 | 4.5 | 58 | |
| Li7La3Zr1.7Ge0.3O12 | 4.78 × 10−4 (20 °C) | 0.29 | 4.5 | 53 | |
| Li6.65Ga0.15La3Zr1.90Sc0.10O12 | 1.8 × 10−3 (300 K) | 0.29 | / | 57 | |
| LiPON | LiPON | 2.3(±0.7) × 10−6 (RT) | 0.55(±0.02) | 5.5 | 45 |
| NaSICON type-LAGP | Li1.70Al0.61Ge1.35P3.04O12(melt-quench) | 2.3 ± 0.2 × 10−4 (RT) | 0.32 ± 0.015 | / | 47 |
| Li1.5Al0.5Ge1.5 (PO4)3 | 3.1 × 10−4 (30 °C) | 0.37 | 6.0 | 46 | |
| (sol-gel) | 1 × 10−3 (60 °C) | ||||
| perovskite-type | Li0.34La0.51TiO2.94 | 1 × 10−3 (RT) | 0.40 | / | 48 |
| Li0.29La0.57TiO3 | 5.2 × 10−3 (300 K) | 0.45 | / | 59 | |
| Li3/8Sr7/16Ta3/4Zr1/4O3 | 2 × 10−4 (bulk 30 °C) | 0.36 | ∼1.0 | 60 |
Notice: RT—room temperature, /—not mentioned, LiPON—lithium phosphorus oxynitride, NaSICON—sodium super ionic conductor, LAGP—Li1+xAlxGe2−x(PO4)3.
Although amorphous lithium phosphorus oxynitride (LiPON) shows the relatively low Li ionic conductivity of 1–3 × 10−6 S cm−1 at 25 °C, it has the good chemical stability with the Li metallic anode, and the broad electrochemical potential window of 0–5.5 V (vs Li/Li+), making it a competitive electrolyte material.45,61 There were many studies focusing on the optimization of LiPON electrolyte layer in the past years, especially on the improvement of Li ionic conductivity, including the investigation on the deposition condition and the optimization of thickness. D. L. Xiao et al. had deposited Li-compensated LiPON thin film solid electrolyte by sputtering a sintered Li-rich Li3.3PO4 target instead of the normally Li3PO4 target. The Li ionic conductivity in Li-LiPON was improved to 3.2 × 10−6 S cm−1 from the 2.4 × 10−6 of LiPON.62
The name of NaSICON was initiated from sodium super ion conductor with formula NaM2(PO4)3, where M is a cation. NaSICON-type Li-ion solid electrolyte LiM2(PO4)3 can be prepared by replacing Na-ion in NaM2(PO4)3 by Li-ion. The NaSICON-type Li-ion solid state electrolyte LiM2(PO4)3 (M = Ti, Ge, Zr, Hf) has some advantages, such as excellent stability in ambient atmosphere, mass production and low cost. By partially replacing M4+ ions by trivalent ions (Al3+,Sc3+), Li1+xAlxGe2−x(PO4)3, Li1+xAlxTi2−x(PO4)3 and Li1+xScxTi2–x(PO4)3 were obtained with low porosities and enhanced ionic conductivities.63–65 Y. Liu et al. have prepared Li1.5Al0.5Ge1.5(PO4)3 (LAGP) electrolytes by a facile sol-gel method in aqueous solution. The ionic conductivity of LAGP at 30 °C was 3.1 × 10−4 S cm−1, with an activation energy value of 0.37 eV.46 E. Zhao and coworkers have prepared Li1.3Al0.3Ti1.7(PO4)3 (LATP)/Li1.3Al0.3Ge1.7(PO4)3 (LAGP) bi-layer structured solid state electrolyte via a simple dry pressing and post-calcination method. This electrolyte sample exhibited a high ionic conductivity of 3.4 × 10−4 S cm−1 and a negligible electronic conductivity of 9.6 × 10−9 S cm−1 at room temperature.63 R. Kahlaoui et al. have prepared Li1+xScxTi2–x(PO4)3 (0 ≤ x ≤ 0.5) using a conventional solid-state reaction. For x = 0.2, the Li1.2Sc0.2Ti1.8(PO4)3 exhibits high bulk ionic conductivity (2.5 × 10–3 S cm–1) and low activation energy (Ea = 0.25 eV) at room temperature.65 However, the LATP electrolyte tends to react with lithium anode due to the Ti4+/Ti3+ redox reaction. The problem can be solved by adopting a barrier layer introduced between electrolyte and lithium anode, which requires sufficient stability against lithium metal and block the contacts between them so as to prevent the undesired chemical reactions.
The perovskite-type (ABO3) solid electrolyte Li0.34(1)La0.51(1)TiO2.94(2) (LLTO), which showed bulk ionic conductivity of 1 × 10−3 S cm−1 and total ionic conductivity of higher than 2 × 10−5 S cm−1 at room temperature was firstly prepared by Y. Inaguma and coworkers.48 Y. Inaguma et al. also synthesized a perovskite-type lanthanum lithium titanate (LLTO) with the nominal composition of Li0.29La0.57TiO3 by a solid state reaction. The total lithium ion conductivity from Li3xLa2/3−xTiO3 (3x = 0.29) was calculated to be 5.2 × 10−3 S cm−1 at 300 K, with an activation energy of 0.45 eV.59 A common drawback of LLTO solid electrolytes is that they contain Ti element. The Ti is in tetravalent state and can be reduced easily by contact with low-potential anodes, such as Li3xLa2/3−xTiO3 with the reduction potentials of 1.6 V (vs Li+/Li). Thus to increase the electrochemical stability, it is necessary to substitute Ti by other elements. Substituted Ti4+ with Zr4+ and Ta5+, a new composition Li2x−ySr1−xTayZr1−yO3 were selected and synthesized. An optimized composition of Li3/8Sr7/16Ta3/4Zr1/4O3 (LSTZ) showed the ionic conductivity with bulk and grain boundary ones of 2 × 10−4 S cm−1 and 1.33 × 10−4 S cm−1 at 30 °C, respectively. This solid electrolyte was found to be stable above 1.0 V against metallic lithium.60
Solid polymer electrolytes and polymer-based composite electrolytes
Solid polymer electrolytes
Solid polymer electrolytes (SPEs) have several advantages, such as high flexibility, low-weight, safe, enhanced electrode/electrolyte interfacial compatibility, and low processing costs.66 Poly(ethylene oxide) (PEO)-based polymer electrolytes have been studied widely due to their outstanding film-forming ability and relatively better compatibility towards electrodes. The PEO is a semicrystalline polymer at room temperature which has the capacity to dissolve high concentration of metal salts. The major drawbacks of PEO-based SPEs are that they exhibit low ionic conductivity (10−6 to 10−8 S cm−1) at room temperature, poor mechanical strength, and inferior electrochemical window. Many strategies have been carried out to improve the performance of pristine PEO, like copolymerization,67 cross-linking,68 polymer blending69,70 and incorporating fillers such as SiO2, Al2O3, BaTiO3, and garnet oxide into the polymer matrix.71–75 Except for PEO-based system, various lithium-ion conductive polymer materials, such as (polymethacrylic acid) (PMAA),76 polycarbonate-based polyurethane,77 modified silyl-terminated polyether (MSTP),78 poly(propylene carbonate) (PPC),79 and Jeffamine®-based80,81 polymer have been exploited for polymer electrolyte matrix. P. Deng et al. have used lithium (fluorosulfonyl)(pentafluoroethylsulfonyl)imide (LiFPFSI) as a conducting salt for building LiFPFSI/PEO SPEs. The LiFPFSI/PEO electrolytes show a high ionic conductivity of 6.2 × 10−4 S cm−1 at 80 °C and a lower glass transition temperature.82 The development of solid polymer electrolytes in recent years has proved that the SPEs are promising candidates to replace currently available organic liquid electrolytes for ASSLBs. The performance of some typical solid polymer electrolytes is listed in Table III.
Table III. Properties of typical solid polymer electrolytes.
| Typical electrolyte | Composition | Ionic conductivity (S cm−1) | Activation energy Ea (eV) | Electrochemical stability vs Li/Li+ (V) | References |
|---|---|---|---|---|---|
| PEO based | PEO + S2TFSI + LiTFSI | 1.2 × 10−3 (25 °C) | / | 4.5 | 74 |
| 1.17 × 10−4 (0 °C) | |||||
| PEO based | PEO:LiClO4/PAA/PMAA | 9.87 × 10−4 (20 °C) | / | 3.7 | 76 |
| PEO based | LiFPFSI/PEO | 6.2 × 10−4 (80 °C) | / | 5.6 | 82 |
| PCPU based | polycarbonate-based polyurethanes (PCPU) +LiTFSI | 1.12 × 10−4 (80 °C) | / | 4.5 | 77 |
| MSTP based | Modified silyl-terminated polyether(MSTP)+LiTFSI | 3.6 × 10−4 (RT) | / | 5.0 | 78 |
| PPC based | PPC-LiFSI (80 wt%) | 6.26 × 10−4 (30 °C) | / | 4.5 | 79 |
| Jeffamine®-based | LiTFSI/Jeffamine-based | 5.3 × 10−4 (70 °C) | / | 4.0 | 80 |
| 4.5 × 10−5 (RT) |
Notice: RT—room temperaure, /—not mentioned, PEO—polyethylene oxide, PCPU—polycarbonate-based polyurethanes, MSTP—modified silyl—terminated polyether, PPC—poly(propylene carbonate).
Polymer-based composite electrolytes
To improve overall performance of SPEs, the polymers are being coordinated with other components to form composite polymer electrolytes (CPEs). The integration of polymer-based electrolytes and inorganic solid electrolyte filler to construct composite electrolytes to balance ionic conductivity, mechanical strength, flexibility, and interfacial stability is a promising strategy to improve electrolyte performances.
K. Karthik et al. had prepared lithium garnet composite polymer electrolyte membrane consisting of large molecular weight polyethylene oxide (PEO) complexed with lithium perchlorate (LiClO4) and lithium garnet oxide Li6.28Al0.24La3Zr2O12 (Al-LLZO) by solution-casting method. The maximized Li+ conductivity at 30 °C is 4.40 × 10−4 S cm−1 and the electrochemical window is 4.5 V for PEO/LiClO4 + 20 wt% Al-LLZO membrane.83 L. Chen and B. J. Goodenough et al. have fabricated composite electrolyte consisting of polyethylene-oxide and garnet (Li6.4La3Zr1.4Ta0.6O12) containing LiTFSI as the lithium salt. The composite electrolytes, from "ceramic-in-polymer" to "polymer-in-ceramic," are flexible and mechanically robust and have a Li+ conductivity σLi > 10−4 S cm−1 at 55 °C.84 X. Wang et al. have synthesized CPEs with a three-dimensional (3D) perovskite-type Li0.33La0.557TiO3 (LLTO) network as a nano-backbone in poly(ethylene oxide) matrix. The LLTO nanofiber network was fabricated by electrospinning and subsequent calcination processes. For the 3D-CPE, a small mount of the PEO-LiTFSI solution was cast on the LLTO nanofiber network, after drying at RT in a glove box the composite membranes were hot-pressed at 75 °C and quenched in liquid nitrogen. These 3D-CPE membranes had much better thermal stability and enhanced mechanical strength in comparison with solid polymer electrolytes. And an ionic conductivity as high as 1.8 × 10−4 S cm−1 was achieved at room temperature for PEO + 40 wt% LiTFSI. The electrochemical window of the 3D-CPEs was 4.5 V vs Li/Li+.85
Zhang et al. used Li6.75La3Zr1.75Ta0.25O12 (LLZTO) ceramics powders to modify poly (vinylidene fluoride) (PVDF) polymer electrolyte. The flexible PVDF/LLZTO composite electrolytes were prepared by a conventional solution-casting method in which LLZTO powders were dispersed into a PVDF matrix. The ionic conductivity of the PVDF/LLZTO CPE with 10 wt% LLZTO particles can reach as high as about 5 × 10−4 S cm−1 at 25 °C with the activation energy of 0.20 eV.86 Liu et al. fabricated a novel solid composite polymer electrolyte using the electrospinning method to disperse 15 wt% Li0.33La0.557TiO3 nanowires into polyacrylonitrile (PAN)-LiClO4 matrix. Because of the fast ion transport on the surfaces of ceramic nanowires acting as conductive network in the polymer matrix, an ionic conductivity of 2.4 × 10−4 S cm−1 at room temperature was obtained. In addition, the LLTO nanowire filled composite polymer electrolyte shows an enlarged electrochemical stability window in comparison to the one without fillers.87 The properties of some typical composite polymer electrolytes is listed in Table IV.
Table IV. Properties of typical polymer-based composite electrolytes.
| Typical electrolyte | Composition | Ionic conductivity (S cm−1) | Activation energy Ea (eV) | Electrochemical stability vs Li/Li+ (V) | References |
|---|---|---|---|---|---|
| PEO/LLZO composite | PEO/LiClO4 + 20 wt% LLZO | 4.4 × 10−4 (30 °C ) | / | 4.5 | 83 |
| PEO/garnet composite | PEO8/LiTFSI +10 wt% LLZTO | 1.17 × 10−4 (30 °C) | / | 5.0 | 84 |
| 1.58 × 10−4 (80 °C) | |||||
| PEO/LLTO composite | PEO/40 wt% LiTFSI + 3D-LLTO | 1.8 × 10−4 (RT) | 0.218 | 4.5 | 85 |
| PVDF/LLZTO composite | PVDF/10 wt% LLZTO | ∼ 5 × 10−4 (RT ) | 0.20 | 4.2 | 86 |
| PAN/LLTO composite | PAN/LiClO4 + 15 wt% LLZO nanowires | 2.4 × 10−4 (RT ) | 0.34 ± 0.02 | 4.7 | 87 |
Notice: RT—room temperaure, /—not mentioned, PEO—polyethylene oxide, LLZO—Li7La3Zr2O12, PVDF—poly (vinylidene fluoride), LLZTO—Li6.75La3Zr1.75Ta0.25O12, PAN—polyacrylonitrile.
The CPEs, incorporating fast ionic conductor as fillers in SPEs, combine synergistically the beneficial properties of high ionic conductivity and strength from inorganic solid electrolytes, as well as good interfacial properties and flexibility from SPEs. The developments of polymer-based composite electrolytes in recent years have proved that the CPEs are promising electrolyte candidates for flexible solid-state lithium-ion batteries.
Lithium Metal Anode/Solid Electrolyte Interfaces
Lithium metal is considered to be the ultimate anode because of the highest theoretical capacity (3860 mAh g−1), lowest electrode potential (−3.04 V vs standard hydrogen electrode), and low density (0.53 g cm−3).88 Owing to its high specific capacity, the use of a lithium metal anode can significantly increase the energy density of the battery,regarding as the key factor to achieving high energy density energy storage. However, Li metal is still regarded as "unsafe" and "unstable" for practical application because lithium metal is extremely reactive and it is easy to form uncontrolled dendritic Li due to highly inhomogeneous lithium metal deposition during charging. Due to the formation and growth of Li dendrites and subsequent degradation process during electrochemical cycling, the rechargeable lithium metal anode suffers from poor recharge ability and low safety in most of the liquid organic solvent-based electrolytes. Those dendrites not only cause waste of Li metal anodes by creating electrochemically inactive Li, but can also cause internal short-circuit and safety hazard. The short circuit of the cell may happen when the dendrites grow across the electrolyte to the cathode.89 Overcoming the safety issue toward lithium metal anode is still a great challenge.
In order to incorporate Li metal anode into practical application, efforts must be made to: (1) suppress the formation and growth of Li dendrite, (2) stabilize and optimize the interface of Li/electrolyte, and (3) accommodate and coordinate the volume change. Applying designed protective layers to the surface of Li anodes is a widely used strategy owing to its feasibility, controllability, and variety. The principal challenge for Li-based ASSLBs lies in gaining better understanding and control of the electrode/electrolyte interface. However, the main difficulty stems from a lack of available techniques to probe the evolution of the electrode/electrolyte interface. In order to address this issue, several interface structures that electrochemical stability against lithium metal is under extensive study theoretically50,90,91 and experimentally.92–96 W. D. Richards et al. have developed a computational methodology to examine the thermodynamics of formation of resistive interfacial phases in ASSLBs. The predicted interfacial phase formation was well correlated with experimental interfacial observations and battery performance.50 Y. Zhu et al. have performed the first principles calculations to evaluate the thermodynamics of the interfaces between solid electrolyte and electrode materials and to identify the chemical and electrochemical stabilities of these interfaces. Their computation results revealed that many solid electrolyte/electrode interfaces have limited chemical and electrochemical stability, and that the formation of interphase layers is thermodynamically favorable at these interfaces.90 P. Braun et al. presented one-dimensional model for ASSLBs based on a design concept which employs composite electrodes. The internal cell resistance was calculated by linking two-phase transmission line models representing the composite electrodes with an ohmic resistance representing the solid electrolyte. The model calculations were compared with experimental data, proving a very high agreement. This model can be used for the model-based development of cell architectures for ASSLBs.91
Actually, it is difficult to study the interface with surface sensitive techniques, such as photoemission, as the interface is buried between the solid-state electrodes and solid electrolytes. C. Ma et al. successfully revealed the Li/LLZO interfacial chemical and structural progression through an in situ aberration-corrected scanning transmission electron microscope.92 A. Kato et al. analyzed the solid electrolyte structure of Li/Li3PS4 interface by X-ray photoelectron spectroscopy (XPS) and SEM.93 N.-W. Li et al. investigated dynamic Li plating/stripping processes in Li-polyacrylic acid (LiPAA) interphase layer by self-adapting interface regulation, which is demonstrated by in situ atomic force microscopy (in situ AFM).94 C. Yu et al. used solid-state NMR to measure the spontaneous Li-ion exchange between different Li-ion containing materials, such as a mixture of electrode and electrolyte phases, to obtain unique selectivity for charge transport over phase boundaries for the Li6PS5Cl–Li2S solid electrolyte–electrode combination.95,96
The high interfacial resistance is recognized as the main factor limiting the performance of ASSLBs. Hence the key issue impeding the performance of ASSLBs is the interface, both in terms of the transport of Li-ions across the interface between the electrode and electrolyte as well as the chemical and electrochemical stability of the interface itself.97 The mechanism leading to a high resistance interface involves several aspects. First, poor contact has been recognized as one source of high resistance, originating from the mismatched lattice of the two different materials and/or the volume changes occurring during cycling. Second, the space charge layers developed along the interface, in which the solid electrolyte side of the interface can become short of lithium-ion concentrations in order to maintain the equilibrium of chemical potentials at the interface, resulting in a high resistance. Third, an interphase layer was also reported, which originated from the diffusion of non-lithium ions and/or the chemical reaction between the cathode and electrolyte.98,99 S. Wenzel et al. summarized that three different types of interfaces can be distinguished in the case of Li/solid electrolyte contacts100: A two-dimensional thermodynamically stable interface, a reactive and mixed conducting growing interphase (MCI) and a reactive and metastable solid electrolyte interphase (as schematically shown in Fig. 1). In the first case (Fig. 1a), the solid electrolyte does not react with the lithium metal at all and a sharp two-dimensional interface is formed. In the second case, materials form a three-dimensional interphase due to chemical reactions between the solid electrolyte and lithium metal, as shown in Fig. 1b. The formed reaction layer owing to sufficient partial electronic and ionic conductivity of the formed products, the interphase may steadily grow "into" the solid electrolyte and thereby alter the properties of the whole bulk material. The formation of such a mixed conducting interphase will eventually allow electron transport through the electrolyte and finally lead to the self-discharge of the battery. Thermodynamically driven MCI formation can only be avoided by introducing a protecting film. Thirdly, as shown in Fig. 1c, a stable interphase may form if the reaction products are electronically non-conductive or if the electronic conductivity is low enough to limit the growth of the interphase to a very thin film. This layer is then comparable to the SEI as known from batteries comprising liquid electrolytes. The performance of the battery will then critically depend on the ion conducting properties of the interphase.
Figure 1. Types of interfaces between a solid lithium ion conductor and lithium metal. (a) Non-reactive and thermodynamically stable interface; (b) reactive and mixed conducting interphase (MCI); (c) reactive and metastable solid-electrolyte interphase (SEI). Reprinted with permission from Ref. 100, Copyright 2015, Elsevier B.V.
Download figure:
Standard image High-resolution imageLithium anode/sulfide SSEs interface
The properties of the interface between solid electrolytes and electrode materials are of vital importance for the performance of ASSLBs. The reactions between lithium metal electrodes and the solid electrolytes can lead to the formation of compounds that either facilitate or block the ion transfer kinetics. Sulfide-based solid electrolytes are relatively soft and deformable materials, and their ionic conductivities are comparatively high. Nevertheless, most highly conductive sulfide-type solid electrolytes (such as Li7P3S11, Li10GeP2S12) have an unstable interface against lithium metal, forming a new interface layer which is commonly composed of Li2S, Li3P, etc.34,101 Another key challenge is the formation of Li dendrite along the voids and grain boundaries in these solid electrolytes although these materials have much stronger mechanical strength than Li metal.102 The interfacial reactions of sulfide-based electrolyte toward lithium anode affect electrochemical performance more severely than that toward cathode. Therefore, finding an effective strategy to solve the Li/sulfide solid electrolytes interface problem remains a challenge to realize better electrochemical performance of ASSLBs.
Experimental studies on lithium anode/sulfide SSEs interfaces
The Li7P3S11 (composed of Li3PS4 and Li4P2S7) is a Li2S-P2S5 system compound with triclinic crystal structure of slightly distorted PS43− tetrahedra and P2S74− di-tetrahedra. Among the different LPS materials, glass ceramics with the composition 70Li2S–30P2S5 (Li7P3S11) show the highest conductivity of up to 1.7 × 10−2 S cm−1.33 The tetragonal structure Li10GeP2S12 (composed of Li3PS4 and Li4GeS4), which is Li2S-GeS2-P2S5 system, consists of negatively charged PS43− and GeS44− tetrahedral, which are surrounded by Li ions for charge compensation.103 It exhibits the highest lithium ionic conductivity of 1.2 × 10−2 S cm−1 at 27 °C. Argyrodites of the type Li6PS5X (X = Cl, Br, I), which exhibit high lithium ion conductivities in the range of 10−2 to 10−3 S cm−1 at room temperature, are considered as suitable candidates for the fabrication of ASSLBs facilitating Li metal anodes. The thermodynamic stability of the different argyrodites in contact with lithium metal is of practical interest for long-term stability of ASSLBs. When contact with Li-metal electrode, the sulfide solid electrolytes decomposed forming new chemical species on the interface that affect the interface resistance.
To clarify whether sulfidic ceramics are stable in contact with lithium metal and which products are formed at the interface, S. Wenzel et al. investigated the formation of an interphase between Li7P3S11 and lithium metal by a combined analytical approach, comprising in situ photoelectron spectroscopy (in situ XPS) and time-dependent electrochemical impedance spectroscopy.104 Mostly, the stability of solid electrolytes in contact with lithium metal is investigated by electrochemical impedance spectroscopy (EIS) or cyclic voltammetry (CV), both of which allow to monitor interphase formation in situ but do not provide chemical information. Combining impedance spectroscopy with in situ XPS allows the chemical identification of reaction products and gives information about the type of interphase. The measurements clearly revealed the growth of an interfacial reaction zone consisting of the decomposition products Li2S and Li3P at the Li/Li7P3S11 interface.105
S. Wenzel et al. also obtained the data on the stability of Li6PS5X (X = Cl, Br, I) in contact with Li metal from an in situ X-ray photoemission technique in combination with time-resolved impedance spectroscopy.106 They reported that Li6PS5X decomposes into multiple phases composed of Li2S, Li3P, possibly LiP, and LiX. In situ XPS in combination with time-resolved electrochemical measurements offers detailed information on the chemical reactions at the Li/LGPS interface. Contacting with Li-metal electrode, the Li10GeP2S12 decomposed to form Li2S, Li3P, and Li−Ge alloys at the Li/Li10GeP2S12 interface.101
The interphase formation reactions between sulfide SSEs and Li-metal were suggested as the followings93,101,104,106:
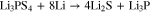
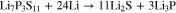

or

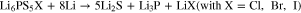
The formation of Li2S as major product, which exhibits a relatively low ionic conductivity (∼10−13 S cm−1 for bulk Li2S), the reduction interphase layer (Li2S, Li3P, LiX or Li-Ge alloy ) has much lower ionic conductivity, increasing the Li/SSEs interfacial resistance. The stability of lithium binary compounds vs lithium anode is calculated from experimental thermodynamic data. The stability window (V vs Li metal) is in the sequence: Li3P < Li2S < LiI < Li2O < LiBr < LiCl < LiF.107 The formation of Li2S and the instability of Li3P at the interface degrade the interfacial properties between solid electrolyte and metal anode. Thus the interface modification will be required to form a proper low-impedance interface to apply sulfide SSEs in ASSLBs.
A. Kato et al. revealed that the Au thin film between Li metal and Li3PS4 electrolytes prevented void generation after the initial Li dissolution and increased the sites for Li deposition. The more uniform morphology changes of Li metal contributed to the better reversibility of Li utilization.93 D. Xie et al. also prepared Sb2O5-doped β-Li3PS4 solid electrolytes with good stability against Li metal. The glass-ceramic Li3P0.98Sb0.02S3.95O0.05 exhibited an excellent stability, could be due to existence of the oxygen ions in the structure, suppressing the side reactions between sulfide electrolyte and lithium.108
To suppress the Li dendrite growth, R. Xu et al. used LiF (or LiI) layer at the interface between Li and sulfide electrolyte and penetrated methoxyperfluorobutane (HFE) (or I solution) inside of sulfide electrolyte. The effect of LiF and LiI interphase layer on the interface resistance and Li dendrite suppression were evaluated in a symmetrical Li/electrolyte/Li cells using LiF (or LiI) coated Li, and HFE (or I) coated/infiltrated Li7P3S11 electrolyte. The interfacial resistance was evaluated by electrochemical impedance spectroscopy (EIS). Their results indicated that the LiF and LiI interphase between Li and electrolyte effectively suppressed the side reactions and dramatically reduces the interfacial resistance. In comparison, LiF was easier to form dense/uniform layer on lithium metal and it had advantages over LiI in maintaining interface stability and inhibiting the growth of lithium dendrite.11
L. Cong et al. used perfluoropolyethers (PFPEs) interfacial stabilizers coating between Li anode and Li10GeP2S12 composite solid-state electrolyte, dramatically improving the interfacial compatibility with Li anode. The SEM and XPS analyses revealed that the PFPEs coating in situ form a LiF-rich solid electrolyte interphase layer, not only facilitating solid-solid interfacial contacts, but also promoting the fast Li+ mobility in electrode/electrolyte interfaces.109
J. Zhang et al. fabricated Li6PS5Cl/poly(ethylene oxide) composite solid electrolytes by liquid-phase process. The Li6PS5Cl/PEO composite SSEs could effectively inhibit the interfacial reactions and lithium dendrite formation when the PEO content reached the optimal value. With Li6PS5Cl/5 wt% PEO SSE the as-assembled sandwich-type LiNi0·8Co0·1Mn0·1O2/composite SSE/Li battery exhibited enhanced cycling performance with a capacity retention rate of 91% over 200 cycles at 0.05 °C and 30 °C.110
Theoretical simulation of lithium anode/sulfide SSEs interfaces
Recently, research efforts have focused on the study and design of materials with high ionic conductivity and the understanding of ion transport mechanisms by computational methods. The quantum mechanics-based methods, density functional theory (DFT), and experimental tools have enabled the study of Li-metal/SSE interfaces.90,111,112 Camacho-Forero and coworkers used density functional theory (DFT) and ab initio molecular dynamics (AIMD) simulations to learn more about the Li-metal/sulfide-type SSEs (LGPS, Li2P2S6, β-Li3PS4, and Li7P3S11) interfacial chemistry.113 They found that, in general, the SSE/Li interface was very unstable and induced the formation of multiple solid phases such as Li2S, Li3P, Li17Ge4, etc. The anions that were close to the interface quickly decomposed during the DFT optimization, S–P bond breaking and Li3P and Li2S forming when Li2P2S6 and Li7P3S11 reacted with Li-metal. In case of LGPS, S–P and S–Ge bonds cleavaging, Li17Ge4, Li3P, and Li2S formed from LGPS reduction reaction with the Li-metal. These results are in good agreement with in situ XPS observations of sulfide-type SSEs instability in contact with Li metal.101,105 The compounds that are formed in the interface are likely to increase the ionic transport resistance. They also found that artificial Li2S thin-film on Li-metal had a strong passivation effect at the interface. However, ionic transport kinetics studies of this and other thin film layers are required in order to gain a more complete understanding of their effect in the overall performance of the batteries.
Li anode/oxide SSEs interface
In the case of the oxide-based SSEs, the primary advantages are their stability in air and better stability with respect to Li metal and the high voltage cathodes. The drawbacks are their lower ionic conductivity, poorer processability, and higher density. For example, the garnet-type cubic Li7La3Zr2O12 (LLZO) is stable against Li metal. However, compared to the sulfide-based SSEs, LLZO tends to be brittle, which makes it harder to maintain a tight solid-solid interfacial contact.
Li anode/LLZO SSEs interface
The interface modification at the Li anode/solid electrolyte interface was shown to be a useful technique for improving the cycle stability and the rate of a charge-discharge reaction of ASSLBs. Strategies for improving the solid-solid interfacial issues include forming an intermediate Li-metal alloy that changes the wettability of the garnet surface to develop a good contact between the Li metal anode and garnet electrolyte with Au,114 or Ge115 coatings on LLZO substrate, and decreasing the interfacial impedance using aluminum oxide (Al2O3).116 Moreover, it has been shown the effect that mixing LLZO with a polymer to improve its flexibility,117 introducing Li3PO4 as an additive in garnet-type Li6.5La3Zr1.5Ta0.5O12 could improve the interfacial compatibility and suppress Li-dendrite formation.118
C.-L. Tsai et al. synthesized Ta-substituted LLZO (Li6.6La3Zr1.6Ta0.4O12) via solid-state reaction. Gold (Au) buffer layers (∼20 nm) were sputtered onto both sides of the LLZO pellets for Li dendrite prevention. The interface resistance was dramatically reduced by using thin layers of Au buffer to improve the contact between LLZO and Li electrodes, which prevented the Li dendrite growth due to a more homogeneous current distribution.114 Depositing a thin germanium (Ge) (20 nm) layer on the garnet LLZO (Li6.85La2.9Ca0.1Zr1.75Nb0.25O12) could reduce the garnet/Li-metal interfacial resistance. By applying this approach, the garnet/Li-metal interfacial resistance decreased from ∼900 Ω cm2 to ∼115 Ω cm2 due to an alloying reaction between the Li metal and the Ge.115
X. Han et al. effectively addressed the large interfacial impedance between a lithium metal anode and the garnet Li7La2.75Ca0.25Zr1.75Nb0.25O12 electrolyte using ultra thin aluminium oxide (Al2O3) by atomic layer deposition (ALD) technique. As in Fig. 2a, a schematic of the wetting behavior between garnet and Li metal illustrated that without surface modification, garnet had insufficient physical contact with Li metal. With the ALD coating, the ultra thin Al2O3 layer helped the molten Li metal to coat the garnet surface with no interfacial void space. The SEM images in Fig. 2b clearly demonstrated the enhancement of interfacial contact by applying the ALD-Al2O3 ultra thin layer on garnet surface. A significant decrease of interfacial impedance, from 1710 Ω cm2 down to 1 Ω cm2, was observed at room temperature, effectively negating the lithium metal/garnet interfacial impedance. The reduction revealed that the oxide coating enabled wetting of metallic lithium in contact with the garnet electrolyte surface and the lithiated-alumina interface allowed effective lithium ion transport between the lithium metal anode and garnet electrolyte.116
Figure 2. Characterizations of garnet solid-state electrolyte/Li metal interface. (a) Schematic of the wetting behavior of garnet surface with molten Li. (b) SEM images of the garnet solid-state electrolyte/Li metal interface. Without ALD-Al2O3 coating, garnet has a poor interfacial contact with Li metal even on heating. With the help of ALD-Al2O3 coating on garnet, Li metal can uniformly bond with garnet at the interface on heating. Reprinted with permission from Ref. 116, Copyright 2017, Macmillan Publishers Limited.
Download figure:
Standard image High-resolution imageJ. B. Goodenough and co-workers prepared a solid polymer electrolyte membrane composed of lithium poly(acrylamide-2-methyl-1-propane-sulfonate) (PAS) and polyethylene oxide (PEO). The PEO-PAS was coated on garnet electrolyte of Li6.5La3Zr1.5Ta0.5O12 (LLZTO) to make a polymer-ceramic single-ion conducting solid electrolyte. The interfacial impedance of a Li/PEO-PAS/LLZTO/PEO-PAS/Li cell demonstrated a dramatically lower overall resistance, from around 5000 Ω for Li/LLZTO/Li to less than 400 Ω. The impedance curves of Li/PEO-PAS/LLZTO/PEO-PAS/Li symmetric cells during the lithium plating-stripping cycles at a constant current didn't exhibit any obvious change. After cycling, the lithium-metal anode was carefully peeled off and observed with SEM. The lithium-metal surface showed a uniform layer of deposited lithium without obvious dendrite formation, which indicates that the PEO-PAS coating layer not only effectively lowered the interfacial impedance, but also successfully suppressed dendrite formation owing to a more uniform interface and Li+ homogeneous flux across the interface.117
B. Xu et al. had introduced Li3PO4 as a second phase additive for Li6.5La3Zr1.5Ta0.5O12 garnet electrolyte and resulted in decreased interfacial resistance and Li/garnet/Li cell successfully cycled at a current density of 0.1 mA cm−2 at 60 °C for 60 h.118 The outstanding interface stability can be attributed to the in situ reaction of the Li flux with Li3PO4 to form a self-limiting and ion-conducting interphase Li3P near the grain boundaries.
The interfacial layers, like alumina (Al2O3) and gold (Au), which can increase the wettability of lithium metal anode with LLZO and provide a good contact between the garnet-type electrolyte and lithium metal anode, were prepared by ALD or sputter which are too expensive and complex to be adopted for large scale applications. Y. Tian et al. prepared a composite electrolyte composed of Li6.75La3Zr1.75Ta0.25O12 (LLZTO) particles embedded in the amorphous Li3OCl (2 wt%) by grounding LLZTO and Li3OCl powders, pressing the solid-state mixture into the pellet, reheating at 350 °C for 12 h and then quenching in a glove box to room temperature. As shown in Fig. 3, during the charge-discharge process of Li/LLZTO-2 wt% Li3OCl/Li cell, the Li3OCl in composite in situ reacts with the lithium metal to form a stable and dense interfacial layer, which not only greatly decreases the interfacial resistance between the electrolyte and lithium metal electrode, but also effectively suppresses the growth of lithium dendrite on the surface of lithium metal anode during lithium plating-striping.119 R. Sudo et al. investigated the effect of the Al2O3 content in LLZO on the interface behavior with lithium metal. Garnet-type lithium conducting solid electrolytes with the nominal Li7La3Zr2O12 composition and with various Al2O3 contents were prepared by solid state reaction at 1180 °C. The interface resistance between lithium metal and Al2O3-doped LLZO was significantly dependent on the Al2O3 content. The lowest grain boundary resistance and interface resistance were obtained for 0.5 wt% Al2O3-doped LLZO, with the highest relative density of 93.7%.120
Figure 3. Schematics of Li deposition behavior using (a) LLZTO particles solid state electrolyte and (b) LLZTO-2wt% Li3OCl composite solid state electrolyte, formation of interfacial layer between LLZTO-2wt% Li3OCl and Li metal by in situ reaction of Li3OCl and Li metal. Reprinted with permission from Ref. 119, Copyright 2018, Elsevier B.V.
Download figure:
Standard image High-resolution imageThe environment effect on interface resistance between lithium metal and LLZO are important for ASSLBs assemble and their usage. Recently, the Li/LLZO interface stability was observed under different environmental conditions, like temperature and current density,121 air exposure,122 moisture (relative humidity) and carbon dioxide.123 It was demonstrated that by heating to 175 °C, the Li/LLZO interface resistance decreased dramatically (from 5822 Ω cm2 (room temperature) down to 514 Ω cm2 (175 °C)) and the maximum sustainable current density of the Li/LLZO interface was increased evidently from 30 °C to 175 °C.121 It was demonstrated that LLZO reacted with humid air,the most favorable pathway involving protonation of LLZO and formation of an LiOH intermediate, subsequent exposure to CO2 converting the LiOH to Li2CO3. Moreover, the Li/LLZO interfacial resistance was correlated to LLZO surface chemistry. It was shown that the formation of contamination layers at the LLZO surface significantly increases in the Li/LLZO interfacial impedance. These results suggest that the LLZO can be synthesized, and densified in ambient air, measures must be taken to prevent subtle surface contamination to enable relatively low Li/LLZO interfacial resistance.
Lithium anode/LiPON interface
A. Schwöbel and coworkers studied interface layer formation between LiPON and metallic lithium using an in situ X-ray photoemission spectroscopy (in situ XPS) surface science approach. Because of the buried nature of the interface and the XPS surface sensitivity, they had used a surface science approach to analyze the interface with in situ XPS by exposing LiPON film stepwise to lithium in ultrahigh vacuum. Therefore at each exposure XPS measurements were conducted. They concluded that the chemical reactions lead to the decomposition into smaller units like Li3PO4, Li3P, Li3N and Li2O, and that the interface reactions were not continuous, but quickly vanished due to the formation of a suitable passivation layer, which meant that the chemical reactions were finished after a certain period of time and not all the LiPON was destroyed. And that the disrupted interface region must be thin enough to allow Li-ion transfer. Meanwhile, they believed that in situ experiments combined with the highly surface sensitive XPS yield a valuable amount of information about the structure and the chemical composition directly at the interface.103,107
Li anode/SPEs interface
Solid polymer electrolytes (SPEs) are light-weight, flexible, and processable. The SPE acts as both an ion conduction medium and a separator. The interface layers between the SPE and the Li electrode should allow lithium ions to pass through and passivate the electrode surface to reduce undesirable side reactions. Many factors influence the stability of the interface formed by PEO-based SPEs in contact with lithium metal electrodes.
G. B. Appetecchi et al. investigated how the electrolyte preparation procedure, the filler addition, and the cell assembly process influenced the interfacial properties of PEO-LiCF3SO3/Li cells. Firstly, using their preparation procedure under controlled environment could obtain completely dry high-purity PEO-based polymer electrolyte characterized by excellent interfacial compatibility with the Li anode. Secondly, the addition of fillers (γ-LiAlO2 or C) in the composite polymer electrolytes only slightly improved the interfacial compatibility with the Li electrode if the preparation procedure and overall working environment were optimized and controlled. Finally, the battery assembly procedure was a critical factor for the establishment of a highly stable lithium anode/SPE interface.124 In addition, the interface resistance depended on the properties of added fillers and lithium salts (LiX). The PEO with LiClO4 electrolyte contacted with lithium metal showed the high interfacial resistance of 1000 Ω cm2 at 70 °C for 25 d. In contrast, the interface resistance between lithium metal and PEO with Li (CF3SO2)2N was as low as 67 Ω cm2 after contacting at 80 °C for 30 d. The interface stability and the lithium ion conductivity were improved by addition of a small amount of ferroelectric BaTiO3 as the filler into the PEO + LiX electrolyte. The interfacial resistance of Li/PEO + Li (CF3SO2)2N +BaTiO3/Li had maintained less than 50 Ω cm2 and stored at 80 °C for a long period.125
The inorganic/organic composite solid electrolyte, combination of polymer and inorganic electrolyte, is reported that the film-forming performance and the interface contact of such composite electrolyte membrane with Li electrode can be improved. C. Wang et al. used a Li1.5Al0.5Ge1.5(PO4)3-poly(ethylene oxide) (LAGP-PEO) composite solid electrolyte and a PEO-(lithium bis(trifluoromethane)sulfonimide) (PEO-LiTFSI) modified lithium metal anode in ASSLBs to suppress the formation of lithium dendrites. The PEO-LiTFSI film modified on the anode possessed good mechanical properties and interfacial contact between the lithium anode and solid electrolyte. In addition, the formation of lithium dendrites could be effectively inhibited as the LAGP-PEO composite solid electrolyte was combined with the PEO film on the Li anode. The assembled Li-PEO-LiTFSI∣LAGP-PEO∣LiMn0.8Fe0.2PO4 all-solid-state cell delivered an initial discharge capacity of 160.8 mAh g–1 and exhibited good cycling stability and rate performance at 50 °C.126 C. Wang and coworkers used Li phosphorous oxynitride (LiPON) modified Li anode and LAGP-PEO(LiTFSI) composite solid electrolyte in ASSLBs as well. The LiPON film with a thickness of 500 nm exhibited satisfactory interface property between Li metal anode and the LAGP-PEO(LiTFSI) solid electrolyte. The LiPON film provided a uniform Li+ flux across the interface and effectively inhibited the formation of Li dendrites in ASSLBs. The assembled ASSB Li(LiPON)∣LAGP-PEO(LiTFSI)∣LiFePO4 delivered an initial discharge capacity of 152.4 mAh·g−1 and exhibited good cycling stability and rate performance at 50 °C.127
C. Yang et al. utilized the PEO-garnet Li7La3Zr1.75Nb0.25O12 hybrid ion-conducting membrane coated on Li metal as a protective layer to stabilize the Li/electrolyte interface and mitigate the growth of Li dendrites. The dendrite-inhibiting effect of the ion-conducting protective layer was visually evidenced by in situ microscopy using planar batteries. The protective Li metal anode exhibited excellent cycling stability for a cycle life as long as 1000 h, and also shown a high Coulombic efficiency (∼99.5%) in a full cell with a LiFePO4 cathode.128
X. Xu et al. introduced a hybrid solid electrolyte with Li7P3S11 being wrapped by polyethylene oxide-LiClO4 (PEO-LiClO4), which served as conductive bridge between the Li7P3S11 particles. The polymer layer of PEO-LiClO4 could isolate lithium metal and Li7P3S11 solid electrolyte, suppressing the reaction between lithium anode and Li7P3S11 electrolyte. Therefore, the hybrid solid electrolyte Li7P3S11-PEO-LiClO4 showed excellent interfacial compatibility with lithium foil. The Li-S battery with this hybrid electrolyte exhibited much improved electrochemical performance with better cycling stability and higher Coulombic efficiency.129
Except for PEO-based SPEs, polycarbonate-based polyurethane polymer electrolyte's interfacial stability against Li-metal anode was evaluated recently. J. Bao et al. synthesized polycarbonate-based polyurethanes (PCPU) and studied the thermal stability, mechanical and electrochemical properties of PCPU-based electrolytes. Interfacial stability against Li of PCPU-20% LiTFSI was compared with that of PEO-20% LiTFSI. The results showed that the PCPU-20% LiTFSI SPE exhibited better stability during the 14 d storage than the PEO-based one. Thus, the PCPU-based SPE possessed a good compatibility with Li metal electrode at 60 °C.77 The PPC (poly propylene carbonate) polycarbonate-based polymer electrolyte, CPPC (PPC-LiTFSI-cellulose) SPE, was synthesized by C. Wang and coworkers. The interfacial evolution of Li metal/CPPC-SPE was performed to monitor the impedance of Li/CPPC-SPE/Li symmetrical cells every 2 h at the activation process of 80 °C. The tremendous resistance at the initial stage decreased to 20 Ω cm2 and reached a steady value after 16 h, indicated that there might exist some reactions between CPPC-SPE and Li-metal electrode through the activation process, which led to the significant reduction of both the bulk resistance and the interfacial resistance. Furthermore, the results demonstrated that cellulose could limit the degradation to a beneficial extent at the interface.130 Thus the polycarbonate-based SPE can improve the contact between electrolyte and lithium metal and reduce the interfacial resistance, leading to the promotion of the stability, integrity and security of ASSLBs system.
Cathode/Solid Electrolyte Interface
Despite of the promising prospect of all-solid state batteries, the main hurdle for developing successful ASSLBs is in minimizing the interfacial impedances between the SSEs and the electrodes, especially for the cathode/SSEs interfaces. The interfacial impedances primarily dominate the rate capability and cycling stability of the ASSLBs. Most SSEs react with cathode active materials and need to be protected by coating protecting layer on the cathode materials. It is necessary to form a good ionic conduction path through the solid interface between cathode and electrolyte, and to reduce the strain/stress at the electrode/electrolyte interface due to the volume change during lithiation/delithiation. Lower interfacial electrode/electrolyte resistances have been obtained by means of the deposition of solid electrolyte thin layer on the cathode active materials. For example, Li2S–P2S5 and Li6PS5Cl solid electrolytes layers have been deposited on LiCoO2 particles by means of the pulsed laser deposition (PLD) and solution procedures, respectively.10,131
Cathode/sulfide-based solid electrolyte interface
The high resistance at the cathode/sulfide-based electrolyte interface had been the main obstacles of the commercial applicability of ASSLBs, owing to the poor compatibility of electrode/electrolyte interface. The interfacial modifications on either electrodes or SSE are necessary to reduce the interfacial impedance and extending the electrochemical window beyond 4.2 V (vs Li/Li+). Lower interfacial resistances have been obtained by means of interface design, the composite cathodes, and bi-layer electrolyte construction.
Coated cathode
C. Vinado and coworkers studied the interfacial behavior of Li10SnP2S12 (LSPS) SSE with Li3NbO4-coated LiCoO2 cathode, which the Li3NbO4 layer was coated by atomic layer deposition (ALD).132 Their study showed that the ALD coating largely improved the electrochemical performance of ASSLBs with the LSPS SSE. It is obvious that the ultrathin Li3NbO4 coating layer largely impedes the atomic inter-diffusion, resulting in much thinner interphase layer and hence lower interfacial resistance compared with uncoated particles (as shown in Fig. 4). It is demonstrated that surface protection of the cathode materials is necessary, at least for the layered cathodes (i.e. LCO, NCA (LiNi0.8Co0.15Al0.05O2), NMC(LiNi1−x−yMnxCoyO2)) and that the ALD coating with ultrathin oxide-based Li-ion conductors is effective in not only protecting the active-material/SE interface, but also reducing interfacial resistance.133 Using LiNbO3 coated LiCoO2 as cathode, methoxyperfluorobutane (HFE) coated/infiltrated Li7P3S11 glass-ceramic as electrolyte, and LiF coated Li metal as anode, the electrochemical performances of the LiNbO3@LiCoO2∣Li7P3S11∣Li ASSLB showed a high reversible discharge capacity of 118.9 mAh g−1 at 0.1 mA cm−2 and retained 96.8 mAh g−1 after 100 cycles.11 Other coated cathodes, like Li2O–SiO2 glasses coated LiCoO2,134 Li4Ti5O12 coated LiNi0.8Co0.15Al0.05O2,135 LiAlO2 coated Li(Ni1/3Mn1/3Co1/3)O2,136 Li3PO4 coated LiNi0.5Mn1.5O4 electrode,137 Li2MoO4 coated Li[Ni0.8Co0.15Al0.05]O2,138 LiTaO3 coated on the LiNi1/3Co1/3Mn1/3O2 (NMC) cathode,139 were shown that the coatings were effective in suppressing the side reaction between the cathode and the sulfide electrolyte and improving interfacial stability.
Figure 4. (1). TEM images and corresponding EDX line scans of (a) LCO/LSPS and (b) coated LCO/LSPS interfaces. (2) (a) Rate capability of LCO/LSPS/Li-In and coated LCO/LSPS/Li-In. (b) Voltage profiles at different rates of LCO/LSPS/Li-In (dashed lines) and c-LCO/LSPS/Li-In (solid lines). Reprinted with permission from Ref. 132, Copyright 2018, Elsevier B.V.
Download figure:
Standard image High-resolution imageComposite cathode
The composite cathodes for ASSLBs based on sulfide solid electrolytes have been proven to be effective to improve the battery performance.140 The composite cathodes are typically prepared by mixing the active material, the sulfide-based solid electrolytes and conductive additive. This is because sulfide materials could provide of good intimate contact with composite electrodes through a simple cold pressing due to its low Young's modulus. Composite cathode with high content of an active material could be prepared by a liquid phase process assisted by a dispersant agent to produce a better electrode and electrolyte interface. A composite cathode was prepared by the dispersion of LiNi1/3Mn1/3Co1/3O2 particles and carbon additive in the Li6PS5Cl-solution containing dispersant agent and subsequent drying at 180 °C. Bulk-type ASSB fabricated with the composite cathode containing 89 wt% of the active material showed an initial discharge capacity of 110 mAh g−1 at 25 °C and maintained 95% discharge capacity after 15 cycles.141 Other composite electrodes using sulfide-based SSE coated on active materials by solution processes, for example, cathode materials of Li6PS5Cl-coated LiCoO2,142 Li6PS5Br-coated LiCo1/3Ni1/3Mn1/3O2,42 Li6PS5Cl-coated Li2S,143 Li6PS5Cl-coated MoS2,144 and Li6PS5Cl-coated LiNi0.8Co0.1Mn0.1O2,145 showed good electrode/electrolyte interface and improved electrochemical performance. All-solid state cells with these composite electrodes have been proved to achieve better electrochemical performance compared to non-coated active materials.
Bi-layer electrolyte construction
The key for realization of ASSLBs is to find an ideal SSE with high conductivity, wide electrochemical window and excellent compatibility with both cathode and anode. To solve interfacial problems of sulfide solid electrolytes like LGPS, the bi-layer construction of sulfide electrolytes was reported recently. J. E. Trevey et al. firstly revealed that ASSLBs constructed with the bi-layer sulfide electrolyte (Li∣77.5Li2S-22.5P2S5/Li3.3Ge0.3P0.7S4∣LiCoO2) exhibited a better electrochemical performance than those with the single layer electrolyte. They revealed that the different electrolyte configurations (Li∣(Li2S-P2S5)∣LiCoO2, Li∣(Li2S-P2S5/Li2S-GeS2-P2S5)∣LiCoO2) resulted in different electrochemical performance for ASSLBs.146 The Li10GeP2S12 (LGPS) and glass-ceramic Li3PS4 (LPS) bi-layer construction was reported by B. R. Shin et al. The combined use of the TiS2-LGPS cathode and the LGPS-LPS SSE bi-layer where LPS forms an interface with the Li-In anode ((TiS2-LGPS)∣(LGPS-LPS)∣Li-In cell) resulted in the best overall performance at 30 °C, exhibiting a capacity of ∼60 mAh g−1 at 20 °C.147
Based on the bi-layer electrolyte several ASSLBs with different electrode materials were reported.148–150 The Li∣75% Li2S-24% P2S5-1% P2O5/Li10GeP2S12∣Fe3S4 @Li7P3S11 ASSLB exhibited higher discharge capacity and better rate capability, the discharge capacity remained at a high value of 1001 mAh g−1 at a current density of 0.1 A g−1 after 200 cycles.148 The Li∣70%Li2S-29%P2S5−1%P2O5/Li10GeP2S12∣FeS ASSB showed reversible discharge capacity of 550 mAh g−1 after 50 cycles at a current density of 0.1 A g−1 and exhibited superior rate performances.149 D. Xie et al. prepared Sb2O5 doped β-Li3PS4 glass-ceramic electrolytes, and the sample of Li3P0.98Sb0.02S3.95O0.05 exhibited the highest ionic conductivity of 1.08 × 10−3 S cm−1 at room temperature. The constructed LiCoO2∣LGPS/Li3P0.98Sb0.02S3.95O0.05∣Li ASSB showed high discharge capacity of 133 mAh g−1 at 0.1 C (1 C = 120 mA g−1) in the range of 3.0–4.3 V vs Li/Li+) at 25 °C. Moreover, the ASSLBs with the doped glass-ceramic bi-layer electrolyte were still workable at −10 °C.108
Cathode/oxide-based solid electrolyte interface
Among the various kinds of solid electrolytes, oxide-based solid electrolytes are safe and chemically stable because of their lower reactivity with gaseous components in the air. Among them the garnet-type cubic Li7La3Zr2O12 (LLZO) ceramics and their doped variants like Li6.25La3Zr2Al0.25O12 etc possess chemical stability and ionic conductivity of ∼10−4 S cm−1. The most critical issue towards ASSLBs is high interfacial resistance of the LLZO-related solid electrolytes with electrodes, especially at room temperature, due to their rigid ceramic nature and poor contact between SSEs and electrodes. Therefore, the cathode interface has become the main challenge to develop garnet-based ASSLBs. Recently, several strategies have been performed in terms of composite electrolyte,151 intermediate coating,152–155 thermal annealing,156,157 and composite cathode150,158 etc, for decreasing the interfacial resistance.
Composite electrolyte
S. Hao et al. developed a new LLZO-based composite electrolyte by LiBr incorporation into Li6.25La3Zr2Al0.25O12 (LLZO). The LiBr, which has much lower melting point than LLZO, would serve as a binder for interaction and densification among LLZO grains owing to the flow characteristic of liquid LiBr during high-temperature sintering. Due to good wetting properties among LLZO grains, large contact area, and effective bonding effect, the incorporation of LiBr into LLZO matrix could thereby give rise to fast Li-ion migration in the LLZO-based electrolytes. Moreover, the interfacial resistance between the LLZO-based electrolyte and electrode would be reduced by improving wetting properties and effective contact at the microscopic level.151 The LiCoO2∣LLZO-based composite electrolyte∣Li full cells delivered a specific discharge capacity of 121.5 mAh g−1 in the first cycle, and achieved capacity retention of 91.8% at the 70th cycles. X. Yan et al. prepared Li7La3Zr2O12 (LLZO) composite solid electrolyte by a novel nanoparticle slurry coating method. The thin film solid electrolyte, composed of LLZO, LiN(CF3SO2)2 (LiTFSI) Li-salt and some additives, was obtained by a wet coating method. The Li∣LLZO composite solid electrolyte∣LCO cell showed a discharge capacity of 119.3 mAh g−1 at a current density of 0.21 mA cm−2 after 45 cycles at room temperature.152
Intermediate coating
When garnet Li7La3Zr2O12 (LLZO) is combined with a common positive electrode LiCoO2, mutual diffusion of Co, La, and Zr between LLZO and LiCoO2 occurs at the interface during a thermal treatment after depositing thin LiCoO2 films on LLZO pellets. It is demonstrated that reaction phases due to mutual diffusion are easily formed between LLZO and LiCoO2 at the interface.153 In this case, electrolyte/electrode interface modification by intermediate coating has been suggested as an effective way to reduce interfacial resistivity.
T. Kato et al. deposited a thin Nb metal layer on Li7La3Zr2O12 (LLZO) pellet by radio frequency magnetron sputtering in an Ar atmosphere at room temperature. A thermal treatment following deposition of a Nb metal layer onto LLZO produced a low-resistivity amorphous layer. The interfacial resistivity between LLZO and LiCoO2 decreased from 2600 Ω·cm2 to 150 Ω·cm2 depending on the thickness of the Nb metal layer. The interface modification approach using a Nb interlayer dramatically improved the discharge capacity and rate capability of a ASSLB.154 S. Ohta and coworkers constructed a ASSB by screen-printing process using Nb doped LLZO (LLZONb) as a solid electrolyte and Li3BO3 as a intermediate layer between the LiCoO2 (LCO) active cathode material and LLZONb. The Li3BO3, a lithium ion conductor, has a low melting point (ca. 700 °C) and is expected to act as a bonding material at the interface by sintering. With Li3BO3 layer sufficient interface contact between the LCO cathode layer and the LLZONb solid electrolyte can be easily achieved by an annealing process.155
C. Wang et al. demonstrated an effective cathode/garnet interface with mixed ionic-electronic conductor through a unique slow pre-lithiation process, which dramatically improved the conductivity of a cathode for both electrons and ions as well as the cathode/garnet interface. A composite cathode of titanium sulfide (TiS2) mixed with carbon nanotubes was slowly pre-lithiated, resulting in more than 20 times lower interfacial resistance. The all solid state batteries of Li metal anode∣garnet (Li6.75La2.75Ca0.25Zr1.75Nb0.25O12 ) SSE∣TiS2 composite cathode can work at high temperatures from 100 °C to 150 °C for 400 cycles at current densities up to 1 mA cm−2.156
Thermal annealing
To address the high interfacial resistance between the solid electrolyte and cathode, B. Liu et al. developed a rapid thermal annealing method to melt the cathode and form a continuous contact. The ASSLB using thermally stable Li7La2.75Ca0.25Zr1.75Nb0.25O12 garnet SSE, lithium metal anode, and V2O5 cathode was demonstrated to operate at 100 °C high-temperature with 97% Coulombic efficiency and reliable safety as well as stable cycling performance. The resulting interfacial resistance of the solid electrolyte and V2O5 cathode was significantly decreased from 2.5 × 104 to 71 Ω·cm2 at room temperature and from 170 to 31 Ω·cm2 at 100 °C.157 E. A. Ilina et al. deposited 30Li2O·47.5V2O5·22.5B2O3 glassy cathode on Li7La3Zr2O12 substrate applied to lithium middle-temperature ASSLBs. The interface features between cathode and SSE at various crystallizing temperatures (650 °C–850 °C) and holding times (0.5–5 min) of cathode on LLZO substrate was investigated. The cathode deposited on LLZO annealed at a proper temperature and holding time can create the optimal interface and good contact between electrode and electrolyte.159
Composite cathodes
The composite cathode, which contains solid electrolyte to the cathode active material to improve ionic and electronic conduction, is another way to obtain a low electrode/electrolyte interfacial resistance. M. He et al. fabricated LiFePO4 based flexible composite cathodes by introducing conductive frameworks consisting of succinonitrile and lithium salt LiTFSI, significantly improving the contact performance and interface stability between garnet solid electrolyte and LiFePO4 cathode. The introduction of such flexible frameworks not only enables close contact between the cathode and the stiff garnet-structured Li6.375La3Zr1.375Nb0.625O12 SSE, but also bridges every electrode and electrolyte particles together forming interconnected three dimensional ionic conductive paths. The ASSB of Li∣SSE∣LiFePO4 with the flexible composite cathodes demonstrated an initial discharge capacity of 149.8 mAh g−1 and the Coulombic efficiency of 99% after 100 cycles at 0.05 C at room temperature.150 W. Zha et al. applied wet coating and hot pressing technique to form a continuous and homogeneous electrolyte layer on a cathode layer. The LLZTO-based electrolyte contained 90 wt% Li6.4La3Zr1.4Ta0.6O12 (LLZTO) powder and 10 wt% PEO-LiTFSI. The composite cathode contained 55 wt% LiFePO4, 20 wt% LLZTO powder, 10 wt% Super-P, and 15 wt% PEO-LiTFSI. The Li∣90LLZTO-10PEO∣LiFePO4 batteries delivered a discharge specific capacity of 148.3 mAh·g−1 after 50 cycles at 60 °C and a discharge specific capacity of 96.0 mAh·g−1 after 50 cycles at 25 °C.158
Summary and Perspectives of All Solid-state Lithium Batteries
Advanced batteries play a basic role in promoting the development of consumer electronics, electrical vehicles, stationary storage and the smart grid, and other emerging applications. Increasing energy density and power density, improving safety and cycle life of the batteries are highly desirable but difficult to achieve. All solid-state batteries have recently attracted great interest as potentially safe and stable high-energy storage systems, but still remain a number of key technological challenges. Interfacial resistance between electrode layer and solid electrolyte layer is very large due to poor physical contact. Physical contact between SSE phase and active particles in the electrode layer becomes worse upon cycling. Many efforts have been focused on the development of high Li-ions conductive solid electrolytes, including sulfide-based and oxide-based inorganic solid electrolytes, as well as polymer and composite solid electrolytes. Nevertheless many fundamental scientific problems for ASSLBs are still not very clear, such as transport mechanism of ions in solid electrolytes, across interfaces, and in electrode composites at atomic level. The solid electrolytes and the electrolyte/electrode interfaces are needed extensive basic investigations to understand their intrinsic conducting mechanism and interfacial properties by various complementary techniques, like solid state NMR technique,56,93 in situ X-ray photoelectron spectroscopy (in situ XPS),95 the time-of-flight secondary ion mass spectrometry (ToF-SIMS),160 in situ transmission electron microscopy (in situ TEM)161,162 to atomic-scale observation of structural evolution of cathode material, electrolyte and their interfaces during electrochemical cycling, and in situ probing station installed in the SEM to investigate the lithium plating/striping behavior at the interface between contacted solid electrolyte and Li electrode.163
A few strategies such as interlayer addition and interface modification have also already been explored to suppress the Li dendrite formation in Li anode/sulfide-SSE interfaces. Such as LiF and LiI interphase layer were effective on the reduction of interface resistance and Li dendrite suppression. The composite solid electrolytes of Li6PS5Cl/PEO could effectively inhibit the interfacial reactions and lithium dendrite formation. The interface modification at the Li anode/oxide-SSE interface was shown to be a useful technique for improving the cycle stability and the rate of a charge-discharge reaction of ASSLBs. Strategies for improving the Li-metal/oxide-SSE interfacial issues, including Au or Ge, aluminum oxide (Al2O3) coatings on LLZO substrate, introducing Li3PO4 as an additive in LLZTO etc, have been shown good contact between the Li metal anode and garnet electrolyte that improves the interfacial compatibility and suppresses Li-dendrite formation.
The cathode/solid electrolyte interface modifications on either electrodes or SSEs are necessary to reduce the interfacial impedance. Lower interfacial resistances have been obtained by means of interface coating, the composite cathodes fabrication, and bi-layer electrolyte construction for the cathode/sulfide SSEs interface. Several strategies have been performed in terms of composite electrolyte, intermediate coating, thermal annealing, and composite cathode, for decreasing the cathode/LLZO interfacial resistance.
Recent years, there has been great progress on the efforts of improving energy density, safety and cycling life of many types of ASSLBs. On one hand, we believe that the interfacial modification engineering will become more and more important in ASSLBs to improve their battery performances. On the other hand, new understandings on fundamental scientific problems for ASSLBs and interfacial engineering processes will bring ASSLBs much closer to commercial reality. Using the latest technologies including advanced analyse techniques, nano-structural modification of the interfaces will solve the interface issues and make breakthrough on innovative new generation batteries, not only all solid-state Li metal-intercalation cathode batteries, but also "5V-class" ASSLBs, Li–S and Li–O2/air batteries, even new system like employing LiBH4-based hydride solid-state electrolyte in solid-state Li metal battery.164–169
Acknowledgments
The authors acknowledge the financial supports from the Hebei Provincial Natural Science Foundation of China (B2018210126 and B2019210358).





