Abstract
Charge storage in concentrated electrolytes gives redox flow batteries (RFBs) a unique edge in grid-scale energy storage. It also creates a unique challenge in the design of organic molecules as alternatives to expensive vanadium salts. Molecules often come in the form of organic salts, and dissolve by ionic bond breakingand ion solvation, which can hardly be interpreted by "like dissolves like." Here we use anthraquinone sulfonate salts as a model system to investigate factors underlying their aqueous solubility. We synthesize fifteen salts, measure their solubilities with UV–vis spectroscopy, and find that the solubility of the same quinone could change by three orders of magnitude with a change in its counter-cation. The same impact can come from the position and number of the sulfonate groups. We explain the results with the differences in the ion solvation energy and the lattice energy, the latter of which is corroborated by single-crystal X-ray diffraction. We also derive a simple method to semi-quantitatively predict the solubility of a quinone sulfonate salt based on that of a common sulfate salt. The work provides a physical-chemical base for understanding the solubilities of organic salts for the design of high capacity electrolytes for aqueous flow batteries.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Dissolving high concentrations of organic molecules in water has become a ubiquitously important task in the research of redox flow batteries (RFBs). A large variety of organic molecules have been exploited to replace vanadium salts in the most mature RFB systems so that a wide adoption of the technology will be constrained by the high and volatile cost of vanadium salts.1,2 Among them, quinone,3–5 nitroxyl radicals,6 and phenothiazine7 have all demonstrated outstanding performance with low redox potentials, high chemical stabilities, and low permeabilities through ion-selective membranes. Although solubility seems a less important property since RFBs aim at stationary energy storage, a low solubility and a bulky electrolyte will burden a RFB with a high cost of balance-of-site,8 hurting their chance to compete with the ever mature lithium-ion batteries.
However, the approach to design highly soluble molecules has relied largely on the intuition of synthetic chemists in favor of small, highly charged molecules, in a sharp contrast to the methodic designs for other properties like the redox potential. The most comprehensive theoretical study so far is an investigation into the aqueous solubility of quinone by Er et al.9 They applied density functional theory to study the solvation energy of quinone derivatives to elucidate the impact of functional groups. Similar strategies have been employed to predict the solubility of molecules in non-aqueous solvents.10 However, the models, which consider the equilibria between neutral molecules in vacuum and in water, hardly capture the physical process of the dissolution. Most of these molecules have conjugated structures, which take charged functional groups like sulfonate or phosphonate to dissolve well. The dissolution thus involves dissociation after breaking bonds in the solid, rather than the simple solvation of neutral molecules. Research has shown a strong dependence of the solubility of quinone molecules on their counter cations.11,12 Another study has suggested that frustrated formation of ionic crystals could be responsible for a highly soluble bis-quinone derivative,12 stressing the need of considering the solid phase when dealing with solubility.
Here, we use anthraquinone sulfonate salts as model systems to understand factors that determine their aqueous solubility. We synthesize 15 salts and measure with UV–vis spectroscopy their solubilities, falling a wide range of 1 mM to 1.2 M. We further perform single-crystal X-ray diffraction (XRD) to understand the impact from the solid structure of the salts. The results reveal insights into the design of highly soluble molecules for aqueous RFBs.
We focus on anthraquinone sulfonate salts for the following reasons. First, a variety of anthraquinone sulfonates are commercially available. Second, anthraquinone derivatives have demonstrated their potential in aqueous RFBs with rapid charge transfer kinetics, low redox potentials and high solubilities.3,4,13 Third, they represent the typical structure of molecules for RFB applications, a redox-active but hydrophobic core functionalized with hydrophilic, anionic groups. At last, sulfonates have a sufficiently low pKa, so unlike other anionic groups (e.g. hydroxyl), the salts dissociate fully in water, which simplifies the aqueous speciation and eliminates the need to control the pH of the solution.
The three anthraquinone sulfonate molecules studied here are shown in Fig. 1. They are 2,7-anthraquinone-disulfonate (2,7-AQDS2−), 2-anthraquinone-sulfonate (AQS−), and 1,5-anthraquinone-disulfonate (1,5-AQDS2−), all purchased as sodium salts. The cations we choose are Na+, K+, Mg2+, Ba2+ and Ca2+, as they do not hydrolyze in water. With the combination of the above anions and cations, we can investigate how solubility depends on factors such as the number and the position of charged functional groups, the cations, and the structure of the solid salt.
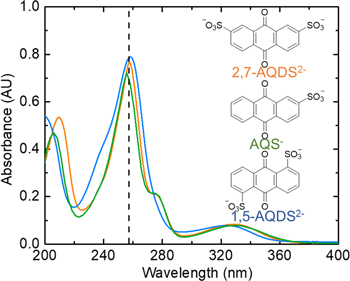
Figure 1. UV–vis spectra of the three quinone molecules (structures shown as insets). The quinone concentrations of the solutions for capturing the spectra are 0.0137, 0.0216, and 0.0142 mM for 2,7-AQDS2−, 1,5-AQDS2−, and AQS−, respectively. The adsorption at ∼260 nm is used for estimating the salt solubility.
Download figure:
Standard image High-resolution imageWe follow two general procedures when preparing the quinone salts. The quinone molecules come as sodium salts, which first need to be purified through recrystallization. Take the preparation of 2,7-AQDS2− salts for examples. We add to a concentrated 2,7-AQDSNa2 solution a small amount of chloride salt with a different cation. If precipitate appears (in the cases of Ca2+ and Ba2+), we will collect it, wash it with deionized water, and dry it for solubility measurement. If the salt dissolves (in the cases of K+ and Mg2+), we will instead use the acid form (2,7-AQDSH2) as the precursor, attained through ion exchanging the sodium salt. For the potassium salt, the acid is reacted with an exact amount of KOH for neutralization. For the magnesium salt, we neutralize the acid an excess amount of 4MgCO3·Mg(OH)2, which does not dissolve in a neutral solution. The supernatants after neutralization thus contain only the metal cation and the quinone, which is confirmed by measuring the pH of the solution. The supernatants are then dried for the salts.
We measure the solubilities with the light absorption of quinone that is independent of its counter cations when diluted to a low concentration. Compared to other common methods of solubility measurements such as gravimetry and elemental analysis, the use of spectroscopy offers a combination of high precision, convenience, and low material consumption, the last of which allows us to examine more than a dozen of compounds within reasonable time. The as-prepared salts are added to water at 50 °C, stirred, until deemed supersaturated. The mixture is then cooled down and filtered. The eluent is diluted for UV–vis spectroscopy. A calibration curve is established with the sodium salts of the quinone molecules. We assume Beer–Lambert's law for dilute solutions of these salts, whose absorbance (A) follows
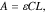
where ε is the molar attenuation coefficient derived from the calibration, C the concentration of the quinone, and l the path length. UV–vis spectra for the three quinone molecules are shown in Fig. 1, where strong adsorption can be found at ∼260 nm, which was chosen to measure the solubility of quinone salts.
Table I summarizes all the results. They fall in a wide range from below 1 mM to above 1 M. The solubility of a salt apparently depends on the following a trend of Mg2+ > Na+ > K+ > Ca2+ > Ba2+, and the quinone anion with a trend of 2,7-AQDS2− ≫ 1,5-AQDS2− > AQS−. It is remarkable that the magnesium salts, instead of the most exploited sodium salts, are the most soluble ones for all three quinone molecules.
Table I. Solubilities of all the quinone salts measured via UV–vis spectroscopy. The error, not shown here, is estimated by repeated measurement to be ∼4.7%, rising from the dilution and the calibration of UV–vis spectroscopy.
| Na+ | K+ | Mg2+ | Ca2+ | Ba2+ | |
|---|---|---|---|---|---|
| 2,7-AQDS2− | 0.740 M | 0.380 M | 1.26 M | 0.0201 M | 1.80 mM |
| AQS− | 0.0197 M | 0.0193 M | 0.0644 M | 2.50 mM | 0.945 mM |
| 1,5-AQDS2− | 0.0720 M | 0.0100 M | 0.0838 M | 1.49 mM | 0.311 mM |
To understand the above trends, we invoke the thermodynamic cycle that separates the dissolution into steps of sublimation and hydration. The Gibbs free energy of dissolution (ΔGdiss) thereby comprises the sum of the hydration energy of ions (ΔGh,i), minus the cavitation energy for water to accommodate the solute (ΔGc) and the lattice energy of the salt being dissolved (ΔGl), as in
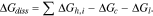
All ΔG's will be discussed in terms of their magnitudes. The contribution of ΔGc is typically small in fully dissociated compounds,14 so we crudely leave it out of the discussion. The hydration energy of cations can be found in literature.15 The hydration energy of the quinone anions and ΔGl's depend on the structures of the anions and the solids, respectively. Our approach here does not account for the inter-dependent nature of these terms of energies, and we will only compare contributions from the same type of ΔG. Nonetheless, we will show below that even with these simplifications, the discussion provides plenty insights into the aqueous solubility of the molecules.
The impact of the metal cations can be understood by considering their hydration energies and the lattice energies. The order of Mg2+ > Na+ > K+ follows the order of  ΔGh,Mg2+ > ΔGh,Na+ > ΔGh,K+,15 where the factor of a half comes from the fact that the dissolution of one mole of quinone anions releases 50% fewer divalent cations. On the other hand, the low solubility of calcium and barium salts, despite the high hydration energies of Ba2+ and Ca2+, are likely the results of the high lattice energies. The lattice is held together by the dispersive forces among the conjugated structures and the Coulombic forces among the ions. The dispersive forces in the salts should be similar to that in anthraquinone, whose lattice energy (114 kJ mol−1)16 is much lower than those of ionic organic salts.17,18 Therefore, the Coulombic forces between the sulfonates and the metal cations should dominate the lattice energies.
ΔGh,Mg2+ > ΔGh,Na+ > ΔGh,K+,15 where the factor of a half comes from the fact that the dissolution of one mole of quinone anions releases 50% fewer divalent cations. On the other hand, the low solubility of calcium and barium salts, despite the high hydration energies of Ba2+ and Ca2+, are likely the results of the high lattice energies. The lattice is held together by the dispersive forces among the conjugated structures and the Coulombic forces among the ions. The dispersive forces in the salts should be similar to that in anthraquinone, whose lattice energy (114 kJ mol−1)16 is much lower than those of ionic organic salts.17,18 Therefore, the Coulombic forces between the sulfonates and the metal cations should dominate the lattice energies.
The above insights allow us to empirically connect the solubilities of a quinone sulfonate salt and a sulfate salt if they share the same metal cation. Underlying this connection is the fact that the dissolutions of the two salts have the same hydration energy of the cation and the assumption that similar Coulombic forces exist in the two solids given the similarity between sulfates and sulfonates. The correlation is demonstrated for all the salts in Fig. 2, where a cation that makes a sulfate soluble also renders a highly soluble quinone sulfonate. This correlation will be a convenient first assessment when we design the composition of a RFB electrolyte, which should by no means be limited to sulfonated quinone.
Figure 2. The solubilities of 2,7-AQDS2−, AQS− and 1,5-AQDS2− salts vs the solubilities of sulfate salts of the same metal cations (labelled in gray). The dashed lines fit to the three groups of data, respectively, based on the least-squares method.
Download figure:
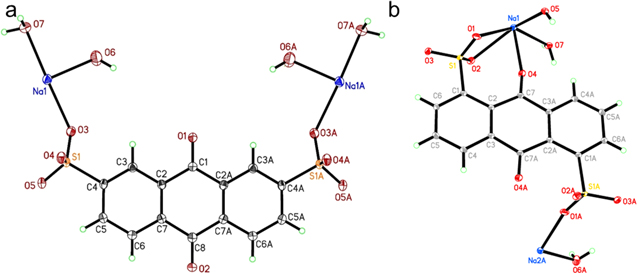
Standard image High-resolution imageThe differences between 2,7-AQDS2− and 1,5-AQDS2− salts root in the lattice energies, as the two anions are unlikely to differ significantly in their hydration energies. To support this interpretation, we grew the single crystals of the two sodium salts from water solutions with an ethanol layer on top. Single-crystal XRD reveals their structures as in Fig. 3. Compared to 2,7-AQDSNa2, 1,5-AQDSNa2 displays more complicated bonds to the sodium cations; the adjacent sulfonate and carbonyl groups chelate one cation, and another sulfonate has a short bond with another cation (Na2A-O1A, 2.2844(14) Å) indicative of a strong Coulombic force. Therefore, slight differences in the positions of functional groups can result in a major difference in the lattice structures, leading to the different solubilities.
Figure 3. The crystal structures of (a) 2,7-AQDSNa2 (CCDC 1967668) and (b) 1,5-AQDSNa2 (CCDC 1967667) at 30% probability levels of the thermal ellipsoids. Selected bond distances (Å) and angles (degree): (a) Na1-O3:2.3759(16); Na1-O6:2.3830(18); Na1-O7: 2.3630(15); and (b) Na1-O1:2.7196(15); Na1-O2:2.5432(14); Na1-O4: 2.4729(12); Na1-O5:2.3111(18); Na1-O7:2.3399(18); Na2A-O1A:2.2844(14); Na2A-O5A:2.4344(19); Na2A-O6A: 2.236(2).
Download figure:
Standard image High-resolution imageThe hydration energies are responsible for the differences between the 2,7-AQDS2− and AQS− salts. Not only is the dissolution of 2,7-AQDS2− favored energetically because of its high charge, it also brings more cations to the solution. The lattice energy does not play an as-important role, since we would expect the divalent 2,7-AQDS2− to result in a more stable crystal. At last, the comparison between the 1,5-AQDS2− and AQS− salts embodies the challenge in predicting their aqueous solubilities, as the benefit from the high hydration energy of the divalent 1,5-AQDS2− is countered by the high lattice energy of the salts, leading to solubilities similar to those of AQS− salts.
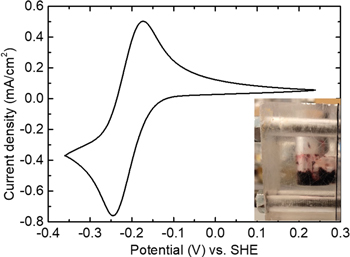
An immediate application of the above results is in the design of a high-capacity neutral quinone electrolyte. 2,7-AQDS2−, the most soluble quinone out of the three, has been applied to the negative sides of acid RFBs.19–21 If applied in a neutral RFB to take advantage of the lower redox potential, the routine choice of a cation would be either Na+ or K+, given the small size, the compatibility with other active materials in the positive side, and the high conductivity in a cation-selective membrane (e.g. Nafion). However, if aimed at a high capacity, our measurement suggests Mg2+. Therefore, we carried out cyclic voltammetry of a dilute solution of 2,7-AQDSMg (Fig. 4), which confirms its electrochemical reversibility.
Figure 4. The cyclic voltammetry of 5 mM 2,7-AQDSMg at 50 mV s−1, supported by MgSO4 on a glassy carbon electrode. The inset shows the red precipitate from the reduction of a 1 M 2,7-AQDSMg solution in an acrylic electrolysis cell.
Download figure:
Standard image High-resolution imageHowever, at a higher concentration, the reduction of 2,7-AQDS2− leads to the precipitation of Mg(OH)2, which precludes its application in a neutral RFB. The reaction goes as
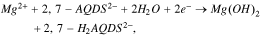
which is spontaneous given the pKa of the reduced quinone and the association constant of Mg(OH)2.22 In another words, the reduced quinone renders the solution weakly basic, where Mg2+ hydrolyzes and precipitates. As a result, when we electrolyze a solution of 1 M 2,7-AQDSMg in a homemade H-cell, we immediately observe precipitation (the inset of Fig. 4), the XRD characterization of which confirmed the formation of solid Mg(OH)2. Although we can buffer the solution to prevent the precipitation, it would introduce a large number of ions to limit the solubility of quinone and defeat the purpose of using 2,7-AQDSMg. The same issue will occur to other quinone molecules, but not to a molecule that does not involve protons in its electrochemical reaction, such as derivatives of methyl viologen.23
We use three sulfonated anthraquinone molecules and fifteen different salts to investigate factors underlying their aqueous solubilities. The results emphasize the need to consider the cations and the lattice structures when designing quinone salts, which likely applies to other redox-active organic salts intended for high-capacity RFB electrolytes. We recommend that a computed descriptor, including the hydration energy and the lattice energy, must be used with precaution when explaining or predicting solubilities. Instead, we suggest using the solubility of common inorganic salts as a convenient tool to estimate the solubility of organic salts and to understand solubility trends.
Experimental
Synthesis
2,7-AQDSNa2 and 1,5-AQDSNa2 were purchased from TCI and AQSNa was purchased from Aladdin, and purified through recrystallization. Their acid forms were prepared by passing saturated solutions of the sodium salts through a column of ion-exchange resin (Amberlyst 15, hydrogen form wet). The barium and calcium salts were precipitated from the addition of their chloride salts to concentrated solutions of the sodium quinone salts. The potassium and magnesium salts were prepared by first neutralizing the quinone acids with KOH and 4MgCO3·Mg(OH)2 and then drying the supernatants. In the neutralization reactions, we made sure that the supernatants only contained the quinone anions and the metal cations by checking the pH. Single crystals of 2,7-AQDSNa2, 1,5-AQDSNa2, and AQSNa were grown from water solutions layered with ethanol.
Characterization
UV–vis spectra were collected in a Lambda 365 ultraviolet−visible spectrophotometer (PerkinElmer). The solubilities were estimated from pre-calibrated molar absorptivities of AQS (50389 @256 nm), 2,7-AQDS (55718 @258 nm), and 1,5-AQDS (36534 @258 nm), respectively. Single-crystal XRD characterization was carried out on a Rigaku-Oxford Diffraction SuperNova diffractometer at 100 K. Cyclic voltammetry was performed in a three-electrode cell with a saturated calomel reference electrode and a Pt-sheet counter electrode by a BioLogic SP-300 potentiostat. Electrolysis was carried out in a home-made, acrylic H-cell. A solution of 2,7-AQDSMg, separated from a MgSO4 solution by an ion-selective membrane (Nafion), was reduced by carbon cloth.
Acknowledgments
The work was supported by the Research Grants Council of the Hong Kong Special Administrative Region, China (T23-601/17-R) and the Science and Technology Innovation Commission, Shenzhen under the grant number JCYJ20170307173900343.