Abstract
Drug resistance in bacteria and cancer is a growing problem that decreases drug treatment effectiveness and increases the severity of bacterial infections as well as cancer mortality. Due to their high sensitivity, low cost, and rapid analysis time, electrochemical methods have been increasingly employed to tackle this challenge throughout the last decade. This review covers literature on the electrochemical characterization of antibiotics and chemotherapeutic drugs, as well as advances in analyzing interactions between drug compounds and biological cells. Recent developments towards the quantitative detection of drug resistance in bacteria and cancer by electrochemistry are discussed, and the use of specialized electrochemical instrumentation, such as scanning electrochemical microscopy, is highlighted.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
The rapid spread of drug resistance in bacteria as well as cancer has developed into a significant threat to the global public health.1 According to the World Health Organization (WHO), antibiotic resistance is present in every country,2 and various national and international health organizations, including the United Nations, the Infectious Diseases Society of America, as well as the Public Health Agency of Canada, have called for the urgent development of new treatment and diagnostic strategies.3,4 The Centers for Disease Control and Prevention reports approximately 10 million deaths worldwide each year in connection with antibiotic resistance.5 Similarly, drug resistance in cancer is believed to be responsible for treatment failure in up to 90% of metastatic cancer patients.6 Cellular resistance mechanisms in both bacteria and cancer include cell membrane protein modifications, intracellular drug target alterations, and the over-expression of efflux pumps.7–9 The latter are the result of an over-expression of efflux pump proteins, which enable cells to expel drugs rapidly from the cell interior, before these compounds can take effective action.8 Drug compounds and efflux pump proteins have recently caught the attention by the electrochemical community to develop new methodologies to understand and detect drug resistance in both bacteria and cancer by electrochemistry.
In recent years, the innovation of electrochemical sensors has attracted immense attention, due to their high sensitivity, rapid analysis and ability to analyze complex samples, such as urine and blood. Although no sensor for the point-of-care detection of antibiotic resistance has been proposed so far, electrochemical sensors have become a powerful tool in various fields, such as environmental monitoring,10 biotechnology,11 and industrial process control.12,13 The advantages are clear: Electrochemical sensors are sensitive, inexpensive, and offer the detection of an analyte within seconds. These properties make electrochemistry attractive for its use in medical applications14 and a number of research articles emerged over the last decade that represent useful first approaches towards the analysis of drug resistance by electrochemistry.
In order to detect drug resistance by electrochemistry, suitable target analytes have to be identified and their interaction with biological cells needs to be understood. This review presents a comprehensive overview of the characterization of drug compounds by electrochemistry during the last decade. A literature review is provided on characterization studies on antibiotics as well as anti-cancer drugs. This forms the fundamental basis for ongoing electrochemical studies on drug resistance. Research articles are discussed investigating interactions between drugs and biological cells. These drug interactions studies have been a significant focus of interest not only for understanding the mechanism of action of drugs, but also for the design and development of novel pharmaceutical drug candidates.15 We further present electrochemical approaches, which aim to detect drug resistance in living cells and to further our understanding of drug resistance in bacteria and cancer. A discussion is presented on the most promising electrochemical approaches and techniques, and this review highlights potential target points for future research strategies.
Literature Review
Electrochemical characterization of drug compounds
This section provides an overview of recent publications that are electrochemically characterizing individual drug compounds, which are widely prescribed to treat bacterial infections or cancer. We first discuss electrochemical studies on antibiotics before presenting useful characterization studies on anti-cancer drugs, highlighting successful techniques and electrode materials.
Electrochemical studies on antibiotics
In order to study drug resistance electrochemically, the redox properties of drug compounds must be well understood. Based on their chemical structure, FDA approved antibiotics are commonly categorized into eight different classes, as summarized in Table I.16–19 Since 2009, electrochemical investigations focused mostly on β-lactams,20–28 aminoglycoside,29,30 quinolones10–13,31–43 and macrolides44–50 due to their effectiveness against a large number of bacterial diseases. In the following, we discuss some of the most commonly prescribed antibiotics and their electrochemical properties to evaluate their usefulness for electrochemical live cell studies.
Table I. Classes of antibiotics reported in literature.16–19
| Class Name | Structure | Mechanism of Action |
|---|---|---|
| β-Lactam |

|
Inhibit bacterial cell wall biosynthesis |
| Aminoglycosides |
|
Inhibit bacterial protein synthesis leading to cell death |
| Glycopeptides |
|
Inhibit bacterial cell wall biosynthesis |
| Quinolones |
|
Inhibit bacterial DNA replication |
| Oxazolidinones |
|
Inhibit bacterial protein synthesis |
| Tetracyclines |

|
Inhibit bacterial protein synthesis |
| Macrolides |
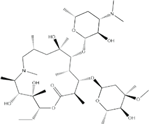
|
Inhibit bacterial protein synthesis |
| Lipopeptides |

|
Disrupt bacterial cell membrane |
Amoxicillin. The antibiotic class of β-lactams is widely used to prevent and treat various bacterial infections in humans and the field of agriculture.23 Amoxicillin is a member of this class20,22 and is the p-hydroxy analogue of ampicillin. It contains variable side chains, which are responsible for its rapid absorption by the human body. The electrochemistry of amoxicillin was investigated by the author Ağin in 201624 using glassy carbon electrodes (GCEs), which were modified by electropolymerization of acridine orange. The electrochemical behavior of amoxicillin was characterized by cyclic voltammetry (CV), revealing an irreversible oxidation peak at 0.92 V vs Ag/AgCl at a scan rate of 100 mV s−1. This study suggests that the pH of the analyte solution has a profound effect on the peak current and potential. An optimal pH of 5.0 was reported for the determination of amoxicillin and a linear relationship between the anodic peak current as a function of the square root of the scan rate indicated a diffusion controlled process. In this study, differential pulse voltammetry (DPV) and square wave voltammetry (SWV) revealed a limit of detection (LOD) of 1.87 × 10−9 M and 1.55 × 10−8 M, respectively. No interferences were reported in this publication.
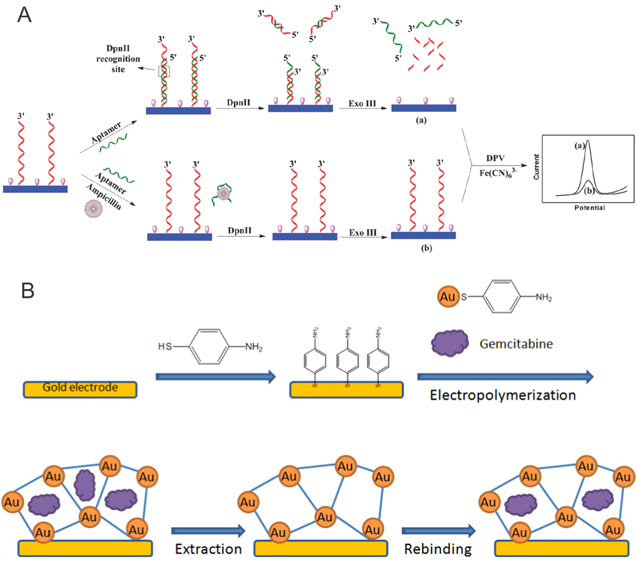
Ampicillin. An interesting strategy to detect ampicillin has been proposed by Wang et al. using aptamer based differential pulse voltammetry.23 In this approach, a GCE was modified with double stranded DNA (dsDNA) containing an ampicillin aptamer sequence (Fig. 1a). Differential pulse voltammograms showed a pronounced reduction peak. This method resulted in an impressive detection limit of 3.2 × 10−11 M and ampicillin was detected in real samples of milk and water.
Figure 1. Representative examples for successful electrode modification for bioelectroanalytical analyses. (a) Electrode modifications with DNA as proposed by Wang et al.23 (b) Molecular imprinted polymer modified electrodes as reprinted by Florea et al.51
Download figure:
Standard image High-resolution imageCiprofloxacin (CIP). Belonging to the second generation of fluoroquinolones, CIP is used worldwide as an antimicrobial agent to treat infections in humans and livestock.39,40 Some antibiotics, such as CIP, can react with metal ions to form stable complexes. Several studies have been conducted to electrochemically characterize CIP.10–13,37–40 Its detection through the complexation of CIP with Cd2+ was reported by Jun et al.40 A well-defined electrochemical signal was recorded at graphene modified GCEs as a result of the complexation of Cd2+ by CIP. The concentration of CIP was found inversely proportional to the electrochemical signal and an irreversible oxidation peak of CIP in various electrolyte solutions, including acetate buffer solution (ABS), phosphate buffer saline (PBS) and sulphuric acid, was observed. GCE modifications using graphene resulted in a significantly enhanced anodic peak in the presence of Cd2+ ions. Overall, this method showed good reproducibility and high selectivity with a detection limit of 5.9 × 10−8 M. The main advantage of this approach is that the electrode did not suffer from electrode fouling which is commonly observed during the direct determination of CIP at bare GCEs. This method successfully detected CIP rapidly and sensitively in pharmaceuticals and urine samples.
Norfloxacin (NFX). This fluoroquinolone is commonly used to treat gastrointestinal, urinary and respiratory tract infections.36 As shown by Ke-Jing et al., NFX was detected at multi-wall carbon nanotube (MWCNT)/nafion film-coated GCEs during voltammetry.36 NFX was determined by linear sweep voltammetry at a potential range of 0.3 to 1.4 V vs Ag/AgCl, which resulted in a well-defined irreversible oxidation peak current at a peak potential of 1.11 V vs Ag/AgCl. The relationship between the peak current and the scan rate at a range of 10–250 mV s−1 demonstrated that mass transfer occurs through an adsorption controlled process and that the pH of the supporting electrolyte solution has an important influence on the electrochemical reaction. The highest oxidation peak was recorded in HAc–NH4Ac buffer solution at a pH value of 4.4. This method has been used in pharmaceuticals and urine samples with an excellent detection limit of 5 × 10−8 M. Only minimal fouling effects were observed during this procedure.
Tobramycin. Tobramycin (TOB) is an aminoglycoside, which is effective against both Gram-positive and Gram-negative bacterial infections.29 A modified GCE was prepared by electropolymerization of pyrrole (Py) in the presence of tobramycin to form a molecularly imprinted polymer film on the GCE surface (PPy/GCE). The general approach of fabricating cavities in a polymer matrix based on a specific template molecule is known as Molecularly Imprinted Polymer (MIP) technique and has found useful application in a variety of research fields including electrochemistry, analytical chemistry and biology.52–54 In the work of Gupta et al., mass transfer occurs through a diffusion controlled process where TOB interacts with pyrrole and results in an oxidation peak at 0.72 V vs Ag/AgCl. Modified TOB imprinted PPy/GCEs were electrochemically characterized by cyclic voltammetry in the presence of potassium ferricyanide in KCl and compared to a bare GCE. The reversible redox peak of potassium ferricyanide, which is observed on the bare GCE, is not shown on the modified GCE due to the presence of the polymer. Square wave voltammetry was performed revealing a favorable detection limit of 1.4 × 10−10 M.29
Neomycin. Another example for the electrochemical characterization of aminoglycosides is neomycin, which has gained considerable attention due to its effectiveness against Gram-negative and Gram-positive bacteria to treat gastrointestinal infections and mastitis in livestock animals.30 Neomycin exhibits a well-defined reduction peak at a potential of −0.2 V vs Ag/AgCl as shown by Hamnca et al. Square wave voltammetry was carried out at the surface of a polyamic acid/graphene oxide (GO)/screen-printed carbon electrode (SPCE) for the electrochemical detection of neomycin in aqueous solutions. The reduction peak current was shown to increase with increasing concentration of neomycin until the catalytic current enhancement effect at the electrode surface reached a saturation level. The limit of detection was found to be 1.07 × 10−6 M. This approach was applied to detect neomycin in urine samples, which presents a reliable and rapid alternative to common chromatography techniques.30
Azithromycin. An interesting molecule of the macrolides class is azithromycin (AZM), which is effective against Gram-negative and Gram-positive bacterial infections.45,49 AZM is highly effective against upper and lower respiratory acute tract infections such as bronchitis, pneumonia, sinusitis, pharyngitis, tonsillitis and otitis, sexually transmitted diseases, skin and soft tissue infections.45,48,49 The electrochemical behavior of AZM has been investigated by cyclic voltammetry on MIP modified carbon paste (CP) electrodes (MIP/CPs) and non-molecularly imprinted polymer (NIP) modified CP electrode (NIP/CPs). Voltammograms show an irreversible oxidation peak at 0.8 V vs Ag/AgCl due to the oxidation of one dimethyl-amino group on the desosamine sugar part of the molecule. This work gives the lowest detection limit of 2.3 × 10−11 M, which compares favorable over any other voltammetry methods.44
Other existing electrochemical studies on antibiotics not discussed in detail, are summarized in Table II.
Table II. Summary of the recent literature on antibiotics, characterized by electrochemistry. Articles denoted with a star symbol are discussed in detail in this review. RGO = reduced graphine oxide; POT (SDS) = poly(o-toluidine) (sodium dodecyl sulphate); CB = carno black; DHP = dihexadecylphosphate; CTAB = cetyltrimethylammonium bromide; BDD = boron doped diamond; GCP = glassy carbon paste; PoAP = poly(o- aminophenol); PEDOT:PSS = poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate); G = graphene; ATP = 4-aminothiophenol; ABA = 4-aminobenzoic acid (4-ABA); IL-G = ionic liquid- graphene; ZSM = mesoporous zeolitic material.
| Classes | Antibiotics | Method of Analysis | LOD [M] | Electrode modification | References |
|---|---|---|---|---|---|
| Β-Lactams | Ampicillin | DPV | 3.2 × 10−11 | dsDNA/AMP aptamer | |
| penicillin | CV | 8.0 × 10−16 | RGO/AuNP | 55 | |
| CV | 1.1 × 10−5 | multisegment nanoparticles | 56 | ||
| Amoxicillin | CV | 6.0 × 10−7 | POT(SDS) | 20 | |
| CV | 5.0 × 10−6 | Ni/CR | 27 | ||
| SWV | 9.0 × 10−6 | AuNP-PdNP-RGO | 28 | ||
| SWV | 1.2 × 10−7 | CB DHP | 25 | ||
| CV | 1.9 × 10−9 | poly acridine orange | |||
| Aminoglycosides | Neomycin | SWV | 1.1 × 10−6 | Polyamic acid/GO | 30 |
| Tobramycin | CV | 1.4 × 10−10 | Polypyrrole | ||
| Quinolones | Ciprofloxacin | CV, ASV | 5.9 × 10−8 | Graphene | |
| CV, DPV | 5.0 × 10−8 | CTAB | 12 | ||
| CV | 1.2 × 10−8 | MgFe2O4-MWCNT | 11 | ||
| CV, LSV | 9.0 × 10−7 | MWCNT | 39 | ||
| CV | GO | 10 | |||
| CV | 3.3 × 10−6 | BDD | 38 | ||
| SWV | 3.3 × 10−8 | GCP | 37 | ||
| CV | 6.0 × 10−6 | MWCNT | 13 | ||
| Levofloxacin | DPV | 1.0 × 10−6 | PoAP/MWCNT | 43 | |
| CV, SWV | 1.4 × 10−8 | AgNPs-CB-PEDOT:PSS | 42 | ||
| CV, DPV | 5.3 × 10−7 | MIP/G-AuNPs | 41 | ||
| CV, SWV | 2.9 × 10−6 | BDD | 34 | ||
| CV | 1.0 × 10−8 | AgNP | 33 | ||
| Norfloxacin | SWV | 3.4 × 10−8 | Polyamic acid/GO | 30 | |
| LSV | 5.0 × 10−8 | MWCNT | 36 | ||
| Enrofloxacin | LSV | 5.0 × 10−7 | MWCNT | 39 | |
| Ofloxacin | CV, SW-AdAsV | 1.8 × 10−10, 2.4 × 10−10 | MWCNT | 32 | |
| CV, DPV | 1.0 × 10−9 | AuNPs/ATP/ABA | 35 | ||
| Oxazolidonones | Linezolid | DPV | 1.5 × 10−7 | bare GCE | 57 |
| Tetracyclines | Tetracycline | CV | 1.5 × 10−5 | multisegment nanoparticles | 56 |
| Macrolides | Azithromycin | CV | bare AuE | 50 | |
| DPV | 7.0 × 10−8 | MgCr2O4/MWCNT | 49 | ||
| CV | MWCNT-DHP | 46 | |||
| CV | bare GCE | 47 | |||
| CV | 1.3 × 10−9 | Ru(bpy)3/ ZSM-5/Nafion | 48 | ||
| CV, DPV | 1.9 × 10−7 | IL-G | 45 | ||
| CV | 2.3 × 10−11 | MIP |
Electrochemical studies on cancer drugs
Anti-cancer drugs that were electrochemically characterized in the last decade are categorized into four groups: alkylating agents, antimetabolites, cytotoxic antibiotics, and inhibitors. While alkylating agents, such as cisplatin lead to cell death in cancer by binding to DNA, antimetabolites, such as 6-mercaptopurine and 5-fluorouracil, mimic metabolites to interfere with processes required for cell division.58,59 Cytotoxic antibiotics, such as doxorubicin are drugs, which are used to treat cancer in addition to their antimicrobial properties.58,59 The presented group of inhibitors consists of chemotherapeutic drugs able to inhibit various enzymes. For example, etoposide can interfere with the enzyme topoisomerase II, which is involved in relieving stress on DNA, induced by supercoiling during cell division.60 The following section discusses recent studies on these four anti-cancer drug categories.
6-mercaptopurine (6-MP). Antimetabolite drugs have been studied extensively using electrochemical methods in the past decade, due to their common use in treating various types of cancers.51,61–77 6-MP is an anti-cancer drug used to treat leukemia.76 There have been several attempts to electrochemically detect 6-MP,68,72,76 such as the recent publication of Kumar et al.,68 who developed a method that reaches a detection limit of 7.2 × 10−10 M in blood plasma. In this work, the authors synthesize nitrogen-doped hollow carbon nanospheres, coated with Palladium (N-HCNS-Pd) and MIPs. These modifications help to improve the effective area and roughness factor of the electrode, which increases its sensitivity. The modified hollow carbon nanospheres are held on an ionic liquid (IL) modified pencil graphite electrode ( PGE) via electrostatic interaction. The authors utilize differential pulse anodic stripping voltammetry (DPASV) to detect 6-MP. A diffusion controlled and irreversible process for the 6-MP oxidation is presented, which involves two protons and two electrons. Notably, these electrode modifications are stable for 3 weeks if stored at room temperature and in dark conditions.68
Other electrode modifications, such as using biomolecules are less stable, but equally valuable due to their effectiveness, as shown by Pattar et al. in 2015. In this manuscript the authors detect 5-fluorouracil (5-FU), an antimetabolite drug used to treat various cancer kinds, such as colon cancer,62 stomach cancer,62 breast cancer,62 and cervical cancer.62 Pattar et al. employ chitosan, a chemically processed form of chitin64,65,67,75 in combination with reduced graphene oxide to modify GCEs. This combination creates a rougher electrode surface and results in an electrocatalytic effect to achieve low detection limits. By applying staircase voltammetry (SCV), an impressive LOD of 1.24 × 10−9 M was achieved.64
Other successful electrode modification strategies involve the use of nanoparticles,61,66 ionic liquids,63 graphene oxide and carbon-nanotubes,71 methylene blue (MTB),67 and the use of copper electrodes.77 Important to point out is the work by Hadi et al. in 2017, utilizing a combination of graphene oxide and carbon nanotubes to modify GCEs and SPCEs. This method shows the widest analytical dynamic linear range from 0.05 to 1200 × 10−6 M, which is shown useful for the determination of 5-FU in human plasma.71
Gemcitabine (GMB) is an anti-cancer drug which is classified as an antimetabolite. Electrochemical detection of GMB can be achieved through the use of bare gold electrodes (AuE), as shown by the authors Naik et al. in 2013.73 Detection of drugs at bare electrodes is commonly favorable, because it saves time and costs, but often times results in lower detection limits. Complex electrode modifications to detect GMB using a microporous metal organic framework (MMOF) (MMOF/AuNP/Au electrode) has been proposed by Florea et al. in 2015.51 These authors construct a molecularly imprinted MMOF by electropolymerizing p-aminothophenol (PATP) equipped with gold nanoparticles (AuNP) on a gold electrode surface in the presence of GMB (Fig. 1b). GMB molecules act directly as templates to create complementary pores in the framework. After the electropolymerization, GMB is extracted, leaving empty binding sites. To analyze samples, the modified electrode is immersed in a solution with GMB, which will bind to complementary sites in the network. This binding will enhance the reduction peak of an electrochemical probe (ferrocyanide/ ferricyanide) and thus, GMB is detected indirectly. This method results in a LOD of 3.4 × 10−15 M. This method provides not only high specificity but also high sensitivity compared to the detection at bare electrodes.51
Cytotoxic antibiotics are antibiotics that are found to have anti-tumor activities. These antibiotics have many mechanisms of action. The subgroup of anthracyclines has been studied in the literature and contains antibiotics, doxorubicin78,79 and daunorubicin,80 extracted from Streptomyces bacteria. Doxorubicin (DOX) was detected using voltammetry81 as well as electrochemical impedance spectroscopy (EIS).78,79 Rezaei et al. developed two different methods to utilize immunosensor and EIS to detect doxorubicin at very low concentrations. While one method uses gold electrodes modified with thiol base sol-gel (TBSol-Gel), gold nanoparticles (AuNP), and monoclonal antibody (MAb) (MAb/AuNP/TBSol-Gel/AuE),79 the other method modifies a stainless steel electrode (SSE) with 3-aminopropyltriethoxysilane (APTES), AuNP, and Mab (MAb/AuNP/APTES/SSE).78 Both strategies follow a similar detection principle. Firstly, an electrode is coated with an insulating layer (TBSol-Gel or APTES) at the electrode surface. Secondly, AuNPs are deposited on the electrode by immersing the electrode in HAuCl4 solution for 180 s at −200 mV (with Ag/AgCl as reference electrode). After that, the newly modified electrode is incubated in a doxorubicin MAb. Finally, the electrode is immersed in a 1% bovine serum albumin (BSA) to block non-specific active site. The modified electrode is immersed in a standard solution of DOX prior to sample measurements. The charge transfer resistance, which is obtained from Nyquist diagrams, is used as a signal. The MAb/AuNP/TBSol-Gel/Au and the MAb/AuNP/APTES/SS electrode result in an LOD of 1.7 × 10−13 M and 3.1 × 10−12 M, respectively.
Other cytotoxic antibiotics that were studied in the past decade include epirubicin,82 daunorubicin,80 and valrubicin.83 A common theme in these three studies is the combination of carbon nanotubes with nanoparticles and other components to detect drugs. Shams et al.82 introduce a GCE modified with silver decorated MWCNT composite (Ag-MWCNT/GCE). By using square wave voltammetry, this work detects epirubicin with a LOD of 1.0 × 10−9 M. Kong et al. 201980 prepared a nanocomposite from nitrogen decorated reduced graphene oxide and single-walled carbon nanotubes. This nanocomposite is loaded with platinum nanoparticles (PtNP) and is then used to modify a GCE (N-rGO-SWCNTs-Pt/GCE). At optimized conditions, this method achieved an LOD of 5.7 × 10−9 M for daunorubicin. In 2015, Hajian et al.83 fabricated an electrochemical sensor based on gold electrode modified with gold nanoparticles/ethylenediamine (EDA)/multi-walled carbon nanotubes (AuNPs/EDA/MWCNTs/AuE). This sensor can detect valrubicin with an LOD of 1.8 × 10−8 M in citrate buffer (pH 4.0) and 0.1 M KCl.
Alkylating agents include temodal,84 ifosfamide,85 and cisplatin.86,87 Temodal was recently detected by the authors Jahandari et al. in 2019, who propose the use of gold nanoparticles (AuNP) and dsDNA to modify a pencil graphite electrode.84 One unique feature of this work is the use of computational chemistry to study the intercalations between temodal and guanine in dsDNA structure, which is used to detect the drug. In particular, this interaction between drug and dsDNA decreases the current of the oxidation peak of the guanine base. This method achieved an LOD of 1.0 × 10−9 M.
Other chemotherapeutic drugs that have been electrochemical studied in the past, but are not reviewed in detail here are etoposide85 and the tyrosine kinase inhibitor erlotinib.74 A summary of anti-cancer drugs investigated by electrochemistry is presented in Table III.
Table III. Recent electrochemical work on anti-cancer drugs detection by electrochemistry. Articles denoted with a star symbol are discussed in detail in this review. ([Co(phen)3]3+ = cobalt (III) trisphenanthroline complex; BMPA = biopolymer from babassu mesocarp modified with phthalic anhydride; PFR = porphyran; PANINT = polyaniline nanotube; CuSAE = Copper solid amalgam electrode; AdSLSV = adsorptive stripping linear sweep voltammetry; Pd@PtNP = mesoporous Palladium and Platinum core–shell nanoparticles; AdSSWV = adsorptive stripping square wave voltammetry; PTP = polythiophene; N-rGO = nitrogen-doped reduced graphene oxide; GST = Glutathione-s-transferase; Au-Pd@rGO = gold, palladium and reduced graphene oxide nanocomposite; PUFIX = polyurethane; PPHF = polypropylene hollow fiber).
| Classes | Drug | Electrode modification | Method of analysis | LOD [M] | References |
|---|---|---|---|---|---|
| Antimetabolite | 6-Mercaptopurine | MWCNT Paste electrode | LSV | 1.0 × 10−7 | 76 |
| [Co(phen)3]3+-GO-dsDNA/GCE | DPV | 1.5 × 10−8 | 72 | ||
| N-HCNS-Pd-MIP/IL-PGE | DPASV | 7.2 × 10−10 | |||
| 5-Fluorouracil | Glucose/CPE | CV, DPV | 5.2 × 10−9 | 62 | |
| BMPA/Flexible AuE | CV, SWV | 3.4 × 10−7 | 65 | ||
| Reduced GO-CS/GCE | CV, SCV, SWV | 1.2 × 10−9 | |||
| AuNP-MWCNT-CS/GCE | CV, DPV | 2.0 × 10−8 | 75 | ||
| AuNP-PFR/CPE | CV, DPV | 6.7 × 10−7 | 66 | ||
| PANINT-AgNP/PGE | DPV | 6.0 × 10−8 | 61 | ||
| IL/CPE | CV, DPV | 1.3 × 10−8 | 63 | ||
| GO-MWCNT/GCE and SPCE | CV, SWV | 1.6 × 10−8 | |||
| CuSAE | CV, AdSLSV | 1.2 × 10−9 | 77 | ||
| MTB/CPE | CV, DPV | 2.0 × 10−9 | 67 | ||
| Gemcitabine | AuE | DPV | 6.0 × 10−8 | 73 | |
| MMOF-AuNP/AuE | LSV | 3.0 × 10−15 | |||
| Cytotoxic antibiotic | Doxorubicin | MAb-AuNP-TBSol-Gel/AuE | EIS | 1.7 × 10−13 | |
| Mab-AuNP-APTES/SSE | EIS | 3.1 × 10−12 | |||
| Pd@PtNP-MWCNT/ GCE | AdSSWV | 8.6 × 10−10 | 81 | ||
| Mitoxantrone | dsDNA-MWCNT-AgNP-PTP/GCE | DPV | 1.3 × 10−8 | 88 | |
| Epirubicin | Ag-MWCNT/GCE | SWV, CV | 1.0 × 10−9 | ||
| Daunorubicin | N-rGO-SWCNT-Pt/GCE | DPV | 5.7 × 10−9 | ||
| Valrubicin | AuNP-EDA-MWCNT/AuE | CV | 1.8 × 10−8 | ||
| Alkylating agents | Cisplatin | GST/CPE | CV, SWV | 8.8 × 10−6 | 87 |
| MWCNT/SPCE | CV, DPV | 4.6 × 10−6 | 86 | ||
| Temodal | dsDNA-AuNP/PGE | DPV | 1.0 × 10−9 | ||
| Inhibitors | Etoposide | Au-Pd@rGO-L-Cysteine/PGE | DPV | 7.2 × 10−10 | |
| Erlotinib | MWCNT-PUFIX-PPHF/PGE | DPV | 2.0 × 10−8 | 74 | |
| Irinotecan | GCE | CV | 1.1 × 10−10 | 89 | |
| Roscovitine | PGE or SPCE | SWV | PGE: 2.0 × 10−7 | 90 | |
| SPCE: 1.5 × 10−7 |
Drug interactions studies
Drug interactions studies have been of significant interest to understand the mechanism of drug-cell interactions, which is crucial for the design of novel and effective pharmaceutical drugs.15 Most of the proposed drug interactions studies in literature focus on the interaction of a drug with DNA molecules.15,91–94 Triggered by an effective drug, DNA often times is susceptible to various intracellular redox reactions, which lead to DNA damage and cause pathological changes in cells.15 Drug molecules bind with DNA either covalently or non-covalently.92 Non-covalent interactions with DNA occurs primarily in three modes which include groove binding interactions, electrostatic interaction from the exterior sugar phosphate backbone and intercalations between the stacked base pairs of the ds DNA.15,91,92 Non-electrochemical techniques that have been used to study drug-DNA interactions, include fluorescence spectroscopy,95 UV-spectrophotometry,96 circular dichroism,96 and mass spectrometry97 The electrochemical investigation of interactions between DNA and drug compounds represents a rapid, and reliable alternative, involving mostly DNA-modified electrodes. Thereby, monitoring the oxidation of DNA base pairs enables the direct detection of drug-DNA interactions on the electrode surface.98
Due to its well-defined electrochemical properties,40 CIP is an ideal candidate for the investigation of CIP-cell interactions. CIP is known to interact with enzyme bound DNA complexes and triggers conformational changes, which block the DNA replication process resulting in rapid bacterial cell death.99 Garbellini et al. studied the interaction of CIP with dsDNA in solution using boron-doped diamond (CPT BDD) electrodes and square-wave voltammetry.40 Employing calf thymus DNA, which consist of 41.9% guanine-cytosine and 58.1% adenine-thymine base pairs, a decrease in the CIP oxidation peak current and an anodic shift of peak potential is observed in the presence of dsDNA, which indicates the interaction of CIP with dsDNA molecules. In the presence of dsDNA, the decrease in peak current reveals the reduced amount of free drug concentrations due to the formation of CIP-DNA complexes. Monitoring the interaction time, the authors conclude a possible CIP interaction with DNA occurs by intercalation.92
Gemcitabine (GMB) is an antimetabolite, and structurally analog to the cytosine nucleoside, and is commonly used to treat a variety of tumors, such as pancreatic cancer, lung cancer, breast cancer, bladder cancer, and brain cancer.98 During its cell metabolic pathway, GMB is converted to inactivated 2',2'-difluorodeoxyuridine (dfdU) by the action of plasma and liver cytidine deaminase and ultimately causes apoptosis by incorporating into DNA. In the work of Tig et al., a poly(2,6-pyridinedicarboxylicacid) modified GCE (GCE/P(PDCA)) is used to elucidate the interaction between GMB and DNA molecules by using differential pulse voltammetry whereby the guanine oxidation current signal is used as an indicator. A decrease in the oxidation peak current for the guanine base pair with increasing concentrations of GMB was observed. The authors propose that this decrease in the guanine oxidation peak current could be explained as a binding of the GMB with dsDNA at the GCE/P(PDCA) surface, which leads to damage in the oxidizable groups of the DNA electroactive nitrogenous bases. By observing the shifting of peak potentials, conclusions can be drawn about the mode of interaction. A shift towards anodic and cathodic potentials of the oxidation peak indicates intercalation or electrostatic interactions, respectively. However, no measurable shift in the peak potential of guanine oxidation was observed, which suggests that binding of GMB with dsDNA is neither intercalative nor due to electrostatic interactions.98
Curcumin is a plant derived phytochemical compound which exhibits anti-cancer, antioxidant, anti-inflammatory, anti-proliferation effects. Because of its strong antioxidant effects, it is considered as a promising anti-cancer compound.100 Suhito et al.100 proposed electrochemical strategies to measure the effects of curcumin on glioblastoma cells U87MG, an aggressive tumor (Fig. 2). The authors used Indium Tin Oxide (ITO) as working electrode which was modified by gold nanoparticles and silver nanoparticles (AgNP) to enhance the electrocatalytic surface, which is further treated with cysteine-containing branched arginyl-glycyl-aspartic acid (RGD-MAP-C) peptides to increase cell adhesion property and growth. Differential pulse voltammetry and cell viability assays were performed after 2 days of curcumin treatment at various concentrations. The result showed a clear decrease in cell viability with increasing curcumin concentration. Voltammograms of DPV experiments revealed a decrease of the oxidation peak indicating an anti-cancer effect of curcumin in U87MG cells. The authors concluded that the proposed electrochemical method is more sensitive, accurate, and free from optical interferences compared to traditional colorimetric methods.
Figure 2. Schematic illustration of electrochemical detection of anti-cancer effects of curcumin with human glioblastoma cells as proposed by Suhito et al.100
Download figure:
Standard image High-resolution imageGanciclovir (GCV) is used as an antiviral drug against herpes viruses, varicella-zoster virus, cytomegalovirus and Epstein-Barr virus.15 In the literature it is reported that GCV is intracellularly converted to its triphosphate form, which inhibits DNA polymerase to elongate the viral DNA during replication by inhibiting the incorporation of deoxyguanosine triphosphate into growing viral DNA.15 Once the pyrophosphate is released, GCV monophosphate causes a slower replication process of viral DNA by incorporating into the end of a growing chain. Paimard et al. investigated the drug-DNA interaction of GCV on the surface of GCEs, modified with Fe3O4/cMWCNTs/GCE using cyclic voltammetry. Voltammograms show an oxidation peak of GCV at 0.93 V vs Ag/AgCl at the Fe3O4/cMWCNTs/GCE. The authors observed a decrease in the GCV anodic peak current and a positive shift in the peak potential at the surface of the modified electrode in the presence of DNA. This decrease in the oxidation peak current can be attributed to a drug-DNA interaction, which is thought to cause a decrease of equilibrium concentration of the free GCV due to GCV-DNA interaction. The positive shift in the GCV oxidation peak potential was explained as an intercalative binding of GCV with DNA molecules.15
Investigation of drug resistance by electrochemistry
Over the last decade, electrochemical research in the area of drug resistance has gone far beyond the characterization of drug compounds, which is nonetheless a crucial first step in recognizing drug resistance electrochemically. The following section discusses approaches based on redox mediators as drug resistance indicators, employed during standard electrochemical methods, such as voltammetry, and specialized instrumentation, such as Scanning Electrochemical Microscopy (SECM).
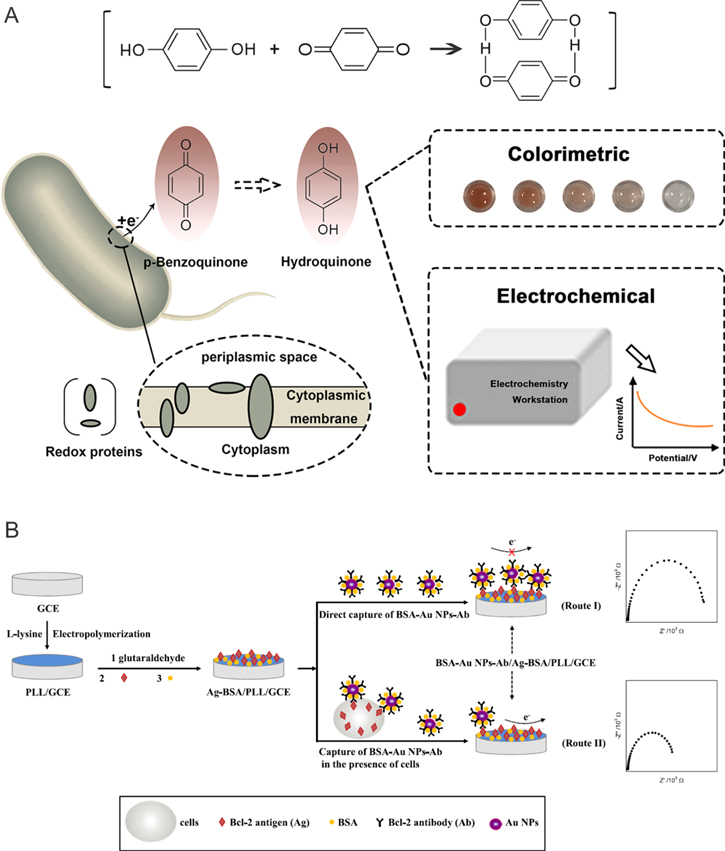
One of the most recent strategies to detect drug resistance in bacteria was reported by Sun et al. in 2019, who employed p-Benzoquinone (BQ) as a redox mediator and indicator for drug resistance in Escherichia coli (Fig. 4).5 BQ acts as an electron mediator during bacterial respiration events. Thereby, BQ is enzymatically reduced to hydroquinone (HQ), which diffuses from the cell towards an electrode, where BQ is regenerated at the electrode surface. Monitored by cyclic voltammetry, this mechanism was revealed as a reversible electron transfer reaction. For the quantitative detection of antibiotic resistance, the authors employed bacteria, resistant to trimethoprim, a folic acid synthesis inhibitor, which is known to inhibit the reduction of dihydrofolic acid to tetrahydrofolic acid through binding with dihydrofolate reductase, which leads to the blockage bacterial DNA biosynthesis.5 During cyclic voltammetry, an increase in peak current was observed with increased resistance phenotype in E. coli. The authors concluded that this method is potentially capable of differentiating between wild type E. coli and antibiotic resistant E. coli with the same concentration, but more studies are needed for the detection in real and complex samples.5
The detection of drug resistance in cancer cells has been approached by studying proteins, which are responsible for an increased number of drug efflux pumps when over expressed. Approaches in recent years include impedance spectroscopy and voltammetry, mostly through antigen/antibody modifications of electrodes101,102 and specialized instrumentation, such as SECM to monitor drug resistance activity in living cells.103–107 The most recent quantitative electrochemical approaches to measure drug resistance in living cells are discussed in the following section.
In 2015, Chen et al. assessed the expression of Bcl-2 proteins using an electrochemical immunoassay as outlined in Fig. 3.101 In this approach, GCEs were modified with L-Lysine through electropolymerization (PLL/GCE). Functionalized electrodes were then immersed in a glutaraldehyde aqueous solution for 1 h. Finally, these electrodes were further modified with a Bcl-2 antigen (Bcl-2Ag) and BSA to block nonspecific binding sites. To detect the expression of Bcl-2 proteins, this modified electrode (Bcl-2Ag/BSA/PLL/GCE) is exposed to a solution containing gold nanoparticles (AuNP) coated with BSA and Bcl-2 antibody (Bcl-2Ab) in the absence of cells (blank), or in the present of drug resistance or drug sensitive cells for 3 h. The use of an antibody ensures the specificity of the proposed method. Electrochemical impedance spectroscopy (EIS) revealed changes in charge-transfer resistance between samples. In the absence of cells, the Bcl-2Ab/BSA/AuNP is able to bind to the Bcl-2Ag/BSA/PLL/GCE as it is modified with the antibody. Hence, the charge transfer resistance is lower than in samples containing cells, because of a competition between the electrode surface and the cells as both have Bcl-2Ag. The more the Bcl-2 proteins are expressed, the more Bcl-2Ab/BSA/AuNP bind to cells and hence, the fewer Bcl-2Ab/BSA/AuNP bind to the electrode. Impedance measurements revealed a decrease of charge transfer resistance as the expression of Bcl-2 proteins increases. Since drug resistant cells express more Bcl-2 proteins than drug sensitive cells, the charge transfer resistance measured in the former is smaller than that of the latter.
Figure 3. Schematic representation of electrochemical approaches towards the detection of drug resistance in (a) bacteria (taken from Sun et al.5) and (b) tumor cells, as presented by Chen et al.101
Download figure:
Standard image High-resolution imageTo investigate drug resistance activity rather than protein expression, cellular drug uptake was monitored in leukemia cells K562 and its multidrug resistant variant K562/A02.107 This approach by Zhang et al. in 2011 used carbon nanotube-modified GCEs (CNTs/GCE) to detect daunorubicin resistance caused by an over-expression of P-glycoprotein (MDR). This modified electrode is immersed in daunorubicin solutions containing cells. The authors hypothesize that sensitive cells have a higher drug uptake than resistant cells, which leads to a lower concentration of daunorubicin in suspensions of drug sensitive cells compared to daunorubicin suspensions of drug resistant cells. The results show a decrease in peak current with increasing concentrations of sensitive cells.
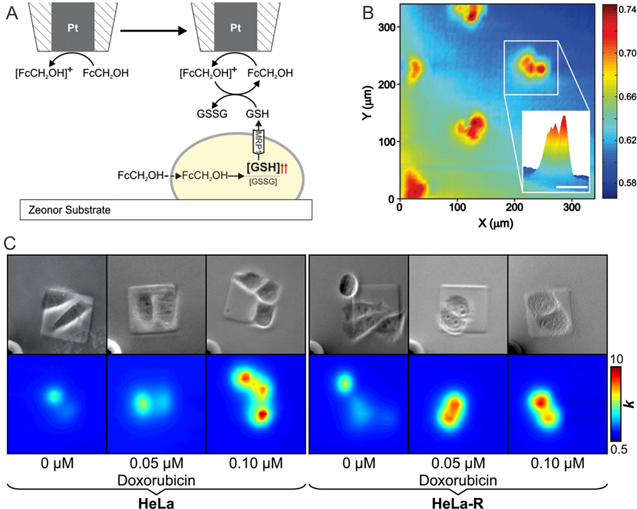
To study cells under physiological conditions and as close to their natural environment as possible, specialized electrochemical instrumentation, such as SECM, has been advanced and customized specifically for the analysis of living cells.108–111 In 2011, Kuss et al. reported a differential electrochemical response during SECM measurements between wild type cells and cells overexpressing the multidrug resistance associated protein 1 (MRP1) in the presence of the redox mediator ferrocenemethanol (FcCH2OH) (Figs. 4a–4b). MRP1 efflux pumps have the ability to expel drugs, and drugs in conjugation with the reduced form of glutathione (GSH), from the cell interior. In the literature MRP1 is known as an independent prognostic indicator and its expression is strongly predictive of the survival rate of cancer patients, which makes it an interesting target for drug resistance detection.112 Using flow cytometry, the authors showed that FcCH2OH increases the intracellular concentration of GSH and propose a mechanism (Fig. 4a) of action in which GSH is expelled from the cells at a higher concentration and reacts with ferrocenium methanol, the oxidized form of FcCH2OH ([FcCH2OH]+), regenerating FcCH2OH in solution. As a result, during SECM studies, an increase in electrochemical current was recorded when the microelectrode passes cells due to the enhanced oxidation of FcCH2OH at the microelectrode (Fig. 4b). Adenocarcinoma cervical cancer (HeLa) cells over expressing MRP1 showed a lower electrochemical current signal. The importance of glutathione as an antioxidant87 suggests that it is possible to monitor the redox state of cells through this FcCH2OH-glutathione relationship.
Figure 4. Electrochemical quantification of drug resistance using SECM imaging. (a) The relationship of FcCH2OH and GSH was used as drug resistant indicator as reported by Kuss et al.105 to electrochemically image HeLa cells by SECM (b) (scale bar: 50 μm.). C) Detection of multidrug resistance protein expression by Polcari et al.113
Download figure:
Standard image High-resolution imageBuilding on their previous work, Kuss et al. successfully extracted an apparent heterogeneous rate constant for drug resistant and nonresistant HeLa cells from experimental SECM data using cell permeable and impermeable redox mediators.105 This work determined the samples' apparent heterogeneous rate constant, independent from their topography, which until this point remained a challenge to the SECM community, and presents the first step towards a quantification of multidrug resistance on the single cell level in human cancer cells by SECM. Further publications by the authors in 2015104,106 demonstrated the application of SECM to cancer cells exposed to green tea catechins (GTC), which are known for their antioxidant properties, and the sensitive monitoring of the cells' electrochemical response over time. In this work, the authors report a simulation model based on the electrode scan velocity during SECM studies for high scan rates and slow scan rates, which enables the determination of the apparent heterogeneous rate constant for cells within minutes. This presents a significant advantage over previous works as results will be more reliable, because cells can be imaged faster and before environmental changes potentially affect the cells metabolism during the experiment. Employing the established relationship between FcCH2OH and GSH, the authors reported an increase in current when HeLa cells were exposed to GTC. The authors suggested this behavior is due to an enhanced efflux of GTC and GSH as a potential defense mechanism to prevent cell death. The electrochemical current signal re-adjusted to a control value after the removal of GTC from the solution. Although no drug resistant cells were monitored during this study, this work represents an interesting approach to potentially monitor the initiation of drug resistance in cancer in the future by SECM.
The correlation between over-expression of proteins related to efflux pumps, such as MRP1, and functional activity of cells was investigated in 2017 by Polcari et al. By exposing wild type and drug resistant HeLa cells to a doxorubicin drug challenge (Fig. 4c), the authors monitored the apparent heterogeneous rate constant by SECM and obtained information about the expression of MRP1 by flow cytometry.113 Comparison of data from both instrumental approaches showed that functional activity and expression do not directly correlate.
Discussion, Conclusions and Future Prospects
The development of antibiotics and chemotherapeutics is one of the greatest scientific achievements of the 20th century, enabling the treatment of various infectious diseases and saving many lives of cancer patients.114 Unfortunately, the emerge of drug resistance due to an overuse of antibiotics or ineffective treatment strategies has evolved to one of the greatest medical obstacles.114–116 To date no electrochemical sensor for the accurate determination of antibiotics in the environment, or the food industry has reached the commercial market and no chemical sensor for the routine detection of antibiotic resistance in patients has been proposed.
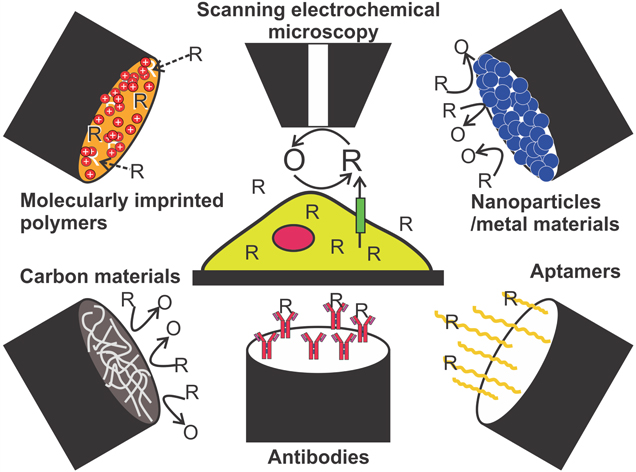
As outlined in this review, many common antibiotics, such as amoxicillin, ciprofloxacin, azithromycin, ampicillin have been successfully characterized using electrochemical techniques, such as cyclic voltammetry11–13,20–22,24,27,31,45,47,48 in various buffer solutions. Figure 6 presents an illustrative summary of in detail discussed electrode modifications and instrumental approaches towards the electrochemical detection of analytes, such as cell metabolites and drug compounds. These techniques reach incredibly low detection limits, down to the sub-nanomolar range.23,29,32,55 However, analyzes in buffer solutions do not accurately represent conditions in environmental samples or biological fluids. Inorganic and organic species present in pharmaceutical and biological samples can interfere with the detection of target drug compounds. A few studies12,31,36 considered such interferences with antibiotics, such as CIP.37 Interferences by various species such as Sr2+, Cd2+, Ba2+, Cr, Ni, Ca, Cu, acetate, lysine, L-glutamic acid, L-serine, L-histidine were studied by Lida et al. and results show influences on the electrochemical signal of CIP of about 5%, which demonstrates the importance of interference studies in quantitative analytical electrochemistry. The analysis of real samples, such as urine and blood is important to exclude interferences by common biomolecules present in samples of such highly complex composition. Interference studies become unavoidable especially when detecting multiple drugs simultaneously. Furthermore, although excellent detection limits have been achieved by electrochemistry during in vitro studies, no relationship to expected concentrations in vivo has been made in the literature. Potential application of electrochemical techniques for in vivo studies of drug detection should address the applicability of the proposed methods to therapeutic concentrations, which usually lie at the order of 1–7 mg kg−1 for individual dosages.117
Combined administration of several chemotherapeutics is considered to increase the effectiveness of the treatment, to minimize side effects, and prevent drug resistance to a specific drug. However, this strategy can lead to multidrug resistance in tumor cells with different drug-unspecific resistance mechanisms, such as MRP1 efflux pumps.81,85 Hence, it is helpful to develop methods for the simultaneous detection of common drug combinations. Out of the literature reviewed in this work, only three studies have focused on the simultaneous detection of drug compounds. Hatamluyi et al. in 201885 detected ifosfamide, an alkylating agent, and etoposide, a topoisomerase II inhibitor, simultaneously. The achieved detection limit of ifosfamide and etoposide are 9.210 × 10−9 M and 0.718 × 10−9 M, respectively. Figure 5 demonstrates the simultaneous detection of these two drugs. Furthermore, Es'haghi et al. presented a method to monitor concentrations of capecitabine, an antimetabolite, and erlotinib, a tyrosine kinase inhibitor, in 2019. Kalambate et al. detected doxorubicin, an anthracycline antibiotics. and dasatinib, a tyrosine kinase inhibitor, simultaneously.81 These studies are great examples for the analysis of mixtures of drugs by relatively simple electrode modifications and many similar studies on new investigational drug compounds, such as antibiotic hybrids, are expected to follow.
Figure 5. Simultaneous electrochemical detection of both anti-cancer drugs IFO and ETO as developed by B. Hatamluyi et al.85
Download figure:
Standard image High-resolution imageFigure 6. Schematic summary of commonly used electrode modifications and techniques to detect analytes, such as cell metabolites or drug compounds. R = reduced species; O = oxidized species.
Download figure:
Standard image High-resolution imageThe increase of drug resistance in Gram-negative bacteria in particular is a major cause for concern,118,119 as many Gram-negatives cause serious infections, such as pneumonia, and few antibiotics effective against Gram-negatives have been developed due to their innate defense mechanisms including low outer membrane permeability and high number of efflux pumps. Thus, with the rise of drug resistance, many infections caused by Gram-negative bacteria have become untreatable.120 The development of antibiotic hybrids has gained significant attention, because reports show that some antibiotic hybrids have the ability to increase the efficacy of other antibiotics, which are unable to cross the cell membrane on their own in Gram-negative bacteria.121 These hybrid molecules represent interesting future targets for the quantitative detection of drug compounds by electrochemistry.
Although the presented review focuses on the direct detection of drug compounds at electrodes, it should be noted that other strategies have emerged to test the efficiency and antimicrobial properties of alternative treatment strategies by electrochemistry. The use of nanomaterials, such as silver nanoparticles, is one example. Arrassi et al. in 2017122 proposed a holistic approach to release a desired amount of silver ions into human cells. Thereby, linear sweep voltammetry was performed to determine the precise amount of silver ions on conductive surfaces. Unfortunately, the low selectivity of silver was found to affect the growth of human cells surrounding the implants and ineffective antibacterial effects leads to the formation of a bacterial biofilm, which was reported immune to antibacterial drugs.
Drug resistance is a complex phenomenon associated with different cellular mechanisms, such as intracellular drug inactivation, drug target alteration, drug efflux pumps, cell death inhibition, and DNA damage repair.9 Targeting single mechanism to combat this issue is therefore often unsuccessful. Efflux pumps mediated resistance is one of the most electrochemically studied mechanisms, because electrochemistry can easily quantify the flux of electrochemically active species.9 However, literature shows that efflux pump protein expression is not directly correlated to a profound drug resistance phenotype.123 Hence, more studies are expected to focus on other drug resistance mechanism rather than drug efflux. The detection of metabolic molecules released from cells by electrochemistry therefore presents an excellent approach to tackle the phenomenon of drug resistance. Combinatorial approaches that incorporate electrochemical methods into the biochemistry toolbox are needed to address both multi-mechanism drug resistance and the analysis of complex samples.
In summary, electrochemistry with its high sensitivity, rapid data output and cost-efficient procedures represents an immense potential to aid in both the detection of resistance and to further the understanding of underlying cellular mechanisms that ultimately lead to a drug resistant phenotype in cells. The development of devices able to detect drug compounds and drug resistance rapidly, sensitively and inexpensively would be of major benefit for clinical diagnoses, disease control, environmental monitoring and food safety. Although no immediate device for the detection of drug resistance has been developed yet, the last decade has provided fundamental steps towards the understanding of electroactive drug compounds and the recognition of drug resistance activity by electrochemistry.
Acknowledgments
This work was supported by the New Frontiers in Research Fund Program as well as Research Manitoba under a New Investigator Operating Grant.