Abstract
Globally, farmers are seeking advanced precision technology to help transform their practices into a more sustainable and productive agri-tech process. Accurate and real-time soil data has become one of the most valuable resources among farmers. Real-time soil sensor data can be exploited in manners that increase farm production and profit, maintain and increase product quality, promote food security, and ensure environmental protection. Researchers have already attempted to develop real-time in situ soil nutrient sensors based on optical and electrochemical techniques. Of these sensor systems, only a few of them are commercially available for monitoring. In this review, we present both available sensors and sensors under research in agriculture. Then briefly discuss both advantages and challenges to overcome in order to produce systems that deliver real-time quality soil information. Optical and electrochemical sensors are becoming less expensive to manufacture and can provide results that are comparable to laboratory soil analysis. Based on the literature presented here, there still exists a need to understand the effects of soil heterogeneity on the analytical performance of both electrochemical and optical systems when used in situ. By doing so, these sensors can be fully adopted as suitable commercial platforms. Overall, these sensors harness the potential to revolutionize decision management systems in agriculture as internet of things (IoT) soil nutrient sensors.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Over the past six to seven decades, during the green revolution, farmers have met the growing global demand for food using technologies, methods, and regulations that are inefficient, unsustainable, and non-ecofriendly.1 Currently, farmers are using these methods to produce more than three billion metric tons of produce per year,2 feeding a global population of approximately 7.8 billion. In the next thirty years, Earth's population will reach approximately 9 billion, and will require an increase in food production by more than 70 percent.3 To meet this challenge of successfully increasing yield production, the exploitation of agrochemicals and nutrient fertilizers, as much as 190 million metric tons per year,4 has remained the number one method of choice of farmers. However, given the harmful environmental impact due to the excessive and poor management of fertilizer application, agriculture requires the use of advanced technology in order to continue meeting food demands (present and future), while reducing the environmental impact.1 The agri-tech revolution is emerging and aims to use advanced precision technology, such as real-time soil nutrient sensors and IoT, to meet the future demands for food, fiber, and fuel,4 in a more sustainable, efficient, and eco-friendly manner.
Precision agriculture provides remotely sensed data which allows farmers to minimize resource input and maximize yield. Precision agriculture is a data demanding system that allows the farmer to assess the heterogeneity of soil physical and chemical content, geospatial variations, and crop data, for optimizing resource utilization based on acquired field data.5,6 Precision information and advancing technology have given rise to innovative smart decision management systems, such as sensor dictated irrigation. Real-time soil moisture profiles have allowed farmers to derive optimal fertilizer rate application and harvest time. However, the efficiency of decision management systems relies on the rapid accuracy of technology and cost (per sensor/sample).7 There has been a significant rise in research and development of precision agriculture technologies to monitor pH, salinity, moisture content, organic matter, and texture, however, in situ monitoring of soil macronutrients, Nitrogen (N), Phosphorus (P), and Potassium (K) remain a challenge.8
N, P, and K are known to be responsible for assimilating proteins, synthesizing metabolic energy, and producing ATP. They are considered the most essential macronutrients. Although they naturally exist in many forms throughout the soil environment, these nutrients are often exploited by humans in the form of fertilizers. Variations of soil nutrient and chemical composition are found spatially throughout agricultural farms and natural environments. These nutrient variations range from depleted soils to excessively fertile soils. As nutrients become limited, plants respond by reducing growth and altering their aspects for nutrient acquisition, utilization and morphology to maximize and acquire the limited resources.9 When excess nutrients are available, those macro-nutrients which are not consumed by plants are leached into ground and surface waters during rainfall and irrigation events. Surface water uses mainly impacted by nutrient pollution include municipal and private water supplies, recreational waters for swimming and boating, cold and warm water fisheries, agricultural water supplies, and navigational waters. It has been noted that the accumulation of these nutrients in waters has harmful effects on humans10 and biodiversity,11 examples include cancer-causing drinking waters and decreased marine species population due to eutrophication. In addition, substantial economic loss and costs have been associated with excessive anthropogenic nutrient loading and harmful algal blooms (HAB's). Declining fishing and boating activities as a result of nutrient pollution and HAB's, has caused over $1 billion in losses each year to tourism. The EPA has also expressed their encouragement for states to immediately prioritize management actions for nutrient pollution in watersheds and water bodies.12 Thus, due to the impact of excess soil nutrients, there is a need for an affordable system that can continuously and accurately monitor soil conditions and movement of their macronutrients in real-time.
Traditional soil nutrient measurement practices employ the random grid sample technique to obtain soil cores. The cores are then packaged and transported to a laboratory. Next, the process will require expensive lab instrumentation and additional extractant solutions for further chemical analysis. These practices can include Kjeldahl wet digestion, dumas combustion, and gas chromatography (GC) mass spectrometry. In addition, ion-selective electrodes and ion-selective field-effect transistors are also used in the laboratory but their potential for in situ analysis in field settings will be further discussed in a later section. Although the lab methods are highly accurate, they lack the ability to deliver continuous real-time analysis of farm soil. They are also challenged with time per sample and cost per sample, two major challenges encountered by large scale farmers seeking to map their terrain. With additional emphasis, farm terrain is variable, meaning what is present on one farmland may not be the same for another within the terrain. Therefore, accurate, high-density, in situ monitoring of soil conditions, such as the ones listed in the subsequent sections, hold a great advantage over traditional lab methods.
Soil nutrient sensors
Advanced technology and communications are allowing faster and stable transmission of sensor data worldwide, and are becoming more affordable due to miniaturization and lower material costs. As shown in Table I, the literature reveals various types of sensors, both commercially available and under development, to measure soil chemical properties.8,11,13 These can be employed in either map-based14 or real-time systems. Among all the available techniques, the majority of in situ soil sensing involves optical and, or, electrochemical methods (Fig. 1). In this review, optical and electrochemical sensors for detecting soil nutrients are highlighted. Optical sensors use spectroscopy based on reflectance spectroscopy to identify the magnitude of reflected and absorbed energy by soil nutrient ions. Electrochemical sensors for soil nutrient determination function by using ion-selective electrodes to initiate a current or voltage output that reflects the concentration of target ions.
Figure 1. Flow chart for real-time soil nutrient sensors used in precision agriculture.
Download figure:
Standard image High-resolution imageTable I. Types of soil nutrient sensors.
| Sensor concept | Status of development | Current results | Key references |
|---|---|---|---|
| Vis-NIR | Laboratory/field | Soil pH and nutrients | (26–28) |
| Vis-MIR | Laboratory | Soil mineral nitrogen | (24–25) |
| ATR Spec | Laboratory/field | Soil Nutrients | (28) |
| Raman | Laboratory/field | Soil Nutrients | (29) |
| ISE | Laboratory/field | Soil pH and nutrients | (39–44) |
| ISFET | Laboratory/field | Soil pH and nutrients | (6–7), (18) |
Optical sensor: Vis-IR, ATR, and Raman spectroscopy
Researchers are investigating the use of optic sensors for field measurements of soil nutrients in a rapid and nondestructive manner. Infrared spectroscopy (IRS) is a technique used to determine the structure of compounds, both inorganic and organic. This has been accomplished by using an ultraviolet, visible, and infrared polychromatic radiation source to quantify light energy absorbed and, or diffusely reflected by soil nutrients. To further understand the phenomenon of infrared spectroscopy, we must understand that a) IR spectroscopy is a technique that analyzes molecular vibrations, and b) the principle of IR spectroscopy is based on the theory of simple harmonic oscillation. An example of a simple harmonic oscillator system would be the shared bonds between two atoms. These spring-like bonds display resonant characteristics dependent upon the "spring" constant which describes the force between them, and the atomic weight at the end of each bond. Thus, the oscillation of a particular chemical bond coincides with a unique frequency and intensities of energy. Further, these unique frequencies of oscillation between bonds are complementary to particular wavenumbers cm−1 (Table II), sharing a positive correlation. As seen in Fig. 2, incident radiation is projected upon a sample at similar vibrational frequencies shared between the bonds. This radiation becomes adsorbed while others are intently reflected. A spectrophotometer records the reflectance, forming a reflectance spectra that reveals the magnitude of energy captured as a function of wavelength. Therefore, a molecule can be identified by comparing its absorption peak to a database of spectrums. Some of these sensors have been designed to be highly portable and can offer a full spectrum in less than 1 second.15 These sensors have also been integrated into microfluidic devices such as the one presented in.16 The researchers produced a lab on chip integrated with a light emitting diode and photoresistor to measure the changes in absorption and detect levels of ammonia and amino acids. The microfluidic device was said to respond to a wide range of concentrations and posses a limit of detection as low as 2 parts per million.
Figure 2. Illustration of IR spectroscopy. As incident light is directed to molecular bonds between two atomic masses, the energy is either absorbed, transmitted, or reflected.
Download figure:
Standard image High-resolution imageTable II. Compound bond and wavenumber.
| Compound | Bond | Wavenumber |
|---|---|---|
| Alkenes | C = C | 1610-1680 |
| Aldehydes, ketones, esters | C = O | 1680-1750 |
| Acids (hydrogen bonds) | O–H | 2500-3300 |
| Alkanes, alkenes, arenes | C–H | 2840-3095 |
| Alcohols, phenols | O–H | 3230-3670 |
| Primary amines | N–H | 3350-3500 |
Literature lists17,18 and explores19–23 many soil properties such as moisture, organic matter, nutrients, and pH, that can be identified rapidly and cost efficiently using diffuse reflectance spectroscopy. Investigations by24,25 explored the use and range of NIR for quickly measuring nutrients, then later employed the use of MIR to increase the accuracy of this system. Researchers in26 highlighted the use of both Vis-NIR and Vis-MIR for soil nitrogen analysis, however, these measurements were made in a laboratory on prepared soil samples. In,27,28 researchers explored the use of NIRS for on the go nutrient-sensing using soil penetrating probes. These probes transmit and receive light using fiber optic cables and optic aperture panes adjoined with soil. The sensor devices were interfaced with farm vehicles to map the variability of soil parameters while traversing the farm terrain.
Attenuated total reflectance spectroscopy
Attenuated total reflectance (ATR) spectroscopy operates using the same principles of infrared energy absorption by particles and molecular bonds. However, instead of targeting samples with infrared spectrums and acquiring the diffuse reflectance, a crystal directly adjoining a target receives the incident IR energy (Fig. 3). The crystal is usually made of diamond, zinc selenide, geranium, and, or thallium iodide, however certain criteria should be considered before selecting the proper ATR crystal, such as the refractive index, spectral range, and physical and chemical properties of the sample. When incident radiation is directed upon the crystal, an evanescent field is created between the crystal and sample due to the incident reflection. The energy then departs the ATR crystal and travels toward a spectrometer. Here, a spectrum will be produced for the sample. Linker et al. used ATR in the 2,500–50,000 nm spectrum to identify nitrate from various types of soil pastes.29 The use of ATRS for the detection of soil nutrients requires little sample preparation, however, there still is a great challenge minimizing the interferences caused by soil moisture and existing minerals. In addition, ATRS instrumentation is expensive and delicate, therefore it is not practical for use in field settings.
Figure 3. A simple illustration of ATR spectroscopy. As an IR beam is directed into the ATR device it is reflected from a series reflectors that direct the incident light toward the ATR crystal. The crystal, in contact with a soil sample, reflects the light, creating an evanescence. The reflected energy exits the ATR crystal it is directed toward a spectrometer to produce a reflectance spectrum for the sample.
Download figure:
Standard image High-resolution imageRaman spectroscopy
Raman spectroscopy is a technique that involves measuring the changes in wavelength and intensity of scattered light upon interaction with a sample. Incident light energy is absorbed and reemitted from the sample at different frequencies known as the Raman scatter or Raman effect. The Raman spectrum from the observed sample is a unique fingerprint of a sample that offers information about the chemical structure and identity of a sample, the polymorphism, intrinsic strain, and sample contamination. In30 researchers have explored and developed a portable Raman spectroscopy device and method which provided good results for the determination of phosphorus and other nutrients in soils and vegetation. The device is also capable of detecting nutrients in both wet and dry soils and contains compartments for drying, sieving, and grinding soil samples. This eliminates further laboratory needs and provides in situ determination. The device also features an on-board microprocessor which allows the data acquired by the spectrometer to calculate the concentration of phosphorus within the sample.
The advantages of optical sensors are that they are a highly flexible form of analysis, capable of measuring irregular surfaces non-destructively and requires little to no sample preparation. Such an example is illustrated in Fig. 4. This method of measurement eliminates the need for additional sample solutions and preparations such as extractants, sample grinding, and drying. It has also been noticed that these reproducible measurements can be produced at an extremely low cost.13 Since measurements are taken in a non-destructive manner5 and they can be deployed subsoil for rapid in situ real-time analysis, this makes them perfect for in situ soil measurements. Collecting proper soil samples is a critical aspect of soil testing which must consider the spatial distribution, depth, and time of day. Therefore large scale deployment of these devices has the potential to offer a higher resolution of nutrient-sensing within the terrain.
Figure 4. Illustrated above is a TN prototype from An, X. et al. for detecting total N; (b) comparison between predicted and measured values of soil TN content.
Download figure:
Standard image High-resolution imageAlthough these sensors are becoming increasingly popular and less expensive to assemble, they are still affected by many soil physical and chemical properties to different degrees.31 While exploring MIR for soil nutrient sensing,25 discovered that measurements in desiccated soils were extremely difficult. Later,29 and23 recommended soils be prepared moist and paste-like to facilitate adequate soil-crystal contact for improving results. A recent study by Viscarra revealed the effects of moisture content and the environment when deriving proximal sensed Vis-IR data. Researchers also state that in order to successfully implement in situ NIRS, calibration is required for each site, which can potentially increase sample preparation time and cost.
Electrochemical sensors: ISFET's and ISE's
Electrochemical sensors are a common approach to understanding the chemical content of a solution and are becoming increasingly popular due to their miniature size and ease of integration into autonomous systems. The electrochemical device functions by coupling a chemically selective layer, known as a recognition element, to an electrochemical transducer. When the recognition layer is in contact with the ion of interest, the transducer converts the chemical energy of the selective membrane interaction into an electrical signal. The electrical techniques used for the transduction of the target analytes allows electrochemical sensors to be further organized into subcategories. These categories include potentiometric, which measures the changes in membrane potential; conductometric for measuring changes in conductance; impedimetric sensors which measure changes in impedance, and amperometric sensors for measuring changes of current at the sensing membrane. The two most employed electrochemical sensors for determining soil nutrients are the ion-selective field-effect transistors and ion-selective electrodes.
Ion selective electrode
Just as the name implies, ion-selective electrodes are conductive pieces of matter with a specialized membrane (glass/polymer) whose potential reflects the concentration of the selective target being measured. These ion concentrations are determined by calculating the voltage drop across the membrane using the Nernst equation. The Nernst equation is by far the most fundamental equations to the understanding of electrochemical cells. Formulated by German scientist Walther Nernst, this equation considers standard potential, temperature, and concentrations in an electrochemical reaction to relate with the potential of the working electrode.
The Nernst equation is depicted as:
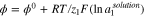
where ϕ is the electrode potential, ϕ0 is the standard value of the potential, a1 is concentration and z1 is the target ion charge number, R is the gas constant, T is the absolute temperature, and F is the Faraday constant. Usually, in a galvanic cell, the difference between two electrodes submerged in a solution is measured as the electromotive force (EMF). EMF is described as that source of energy which enables electrons to move around an electric circuit32 and is determined by the difference of oxidizer and reducer potentials33 in a reduction-oxidation reaction. Under certain conditions, when the reference potential remains constant in a galvanic system and the active electrode follows the Nernst equation, the EMF equation can be described as:
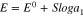
where E is the measured EMF, E0 is the standard EMF value at aI = 1, and S is the response slope. The slope is usually S25 = 59.18/z1 mV at 25 C. However, according to literature the slope can differ with freedom of about 0.2–2 mV.34 The slope is derived by obtaining a calibration curve using at least 3 standards. Once this is done the ISE can be used to measure concentrations of target analytes in solutions. The sensitivity can be calculated by dividing the linear part of the calibration slope by the surface area of the working electrode.
ISE's are proven to have a wide dynamic range, appearing to distinguish between variations in concentrations of residual nitrogen (0.1–10,000 ppm N) and nutrients. ISE's are traditionally employed in the laboratory facilities to obtain nutrient measurements. A common approach for using ISE's are integrating them into microfluidic structures. Microfluidics allows the controlling of liquid samples at micro and pico levels while the integrated ISE's allows rapid analysis of nutrients. In,35 researchers presented a flow loop microfluidic device integrated with an ISE sensor for in situ analysis of phosphate.
Electrochemical sensors can be employed in a variety of environments. Literature reveals that majority of electrochemical sensors are used to detect nutrients, toxins, and pollutants in waters and aqueous solutions.36–40 However, ISE's have also been known to gather rapid measurements in slurries, unfiltered soil extract and naturally moist soils.7,41,42. The portable multi ion measurement system developed in41 allowed site specific nutrient analysis and characterization of the spatial variability of surface soil nutrients. Researchers in7 produced a system for on-the-go mapping of nutrients using direct soil measurement methods that allowed nutrient electrodes to distinguish between very high and very low concentrations. In addition to aqueous solutions, moist soils and slurries, electrochemical sensors have also been used to determine ionic concentrations in gaseous forms.
There has been numerous reports on various membrane materials and recognition elements used to electrochemically quantify soil nutrient concentrations. Though many recognition elements for nutrient detection exists, the most popular membranes are based on PVC-ligand membranes and molecular imprinted polymers. Glass membrane based ISE's are popularly used for determining pH, but not commonly reported for detecting nutrients. Some of the most popular membranes for detecting nutrients include the use of doped polymer membranes such as Nitrogen doped polypyrrole (N-doped PPy) for the detection of soil nitrogen, tetradodecylammonium o-nitrophenyl octyl ether (TDDA-NPOE) based electrodes for detecting nitrate, valinomycin-bis(2- ethylhexyl) sebacate (DOS) based electrodes for detecting potassium, and cobalt rod-based electrodes for measuring phosphate.43 MIP based membranes for detecting soil nitrogen has been explored in.44 In44 the electrochemical doping was used to fabricate a nitrate sensor. The researchers further increased the sensitivity of their sensor by doping polypyrrole nanowires. Optimal membrane compositions and preparation conditions have been determined for polypyrrole (PPy) for determining soil nitrate concentrations.45 The researchers reported that te lifetime of the membranes can also be extended by adding appreciable amounts of plastisizers to the membranes. Overall, due to the ease of preparation, literature finds that MIPs and PVC are suitable for fabrication selective ISE's.
Ion selective field effect transistor
Ion-selective field-effect transistors (ISFET's) are simply ISE's joined with a field-effect transistor (FET) and can also be referred to as chemically modified field-effect transistors (CHEMFET's). The function and structure of a FET are quite interesting, as it uses an electric field within a region of the device to control the flow of current and is comprised of three terminals; the source, drain, and gate. There are two types of FET devices, p-type and n-type. The device type dictates which carrier conducts the flow of current. There are two carrier types, electrons, and holes. In an n-type FET, a gate receiving a positive voltage causes a carrier depletion region due to the repelling of holes in the region beneath the gate. As the holes are repelled, electrons are pulled from the substrate into the channel region, assembling a bridge between the source and drain. Now, as a voltage is applied between the drain and source, electrons can flow freely through this channel. Quite the opposite effect occurs within a p-type device, as a positive voltage will deplete the carriers and reduce the conductance. By applying voltage to the gate, an electric field can be generated to control the amount of charge in the channel and therefore influence the conductivity of the channel. This applied voltage is referred to as the threshold voltage, as it represents the number of volts required to sufficiently accumulate a quantity of electrons for a conductive channel. In the case of a CHEMFET or ISFET, the ISE membrane is appended to the insulating layer of the FET, thereby chemically modulating the threshold voltage as a function of the concentration within the solution.
The use of ISFET's with flow injection analysis (FIA) has been discussed in.6 Researchers have investigated technology to successfully measure soil nutrients from prepared extracts. The system based on the multi-ISFET technology featured a rapid extraction ISFET system for real-time in-field measurements of soil nitrogen in less than 5 seconds. Literature has also discusses ISFET devices that have demonstrated the ability to analyze various soil nutrients,7 however, like many previous devices the sensor relies on a extracton system. It is reported that the flow injection analysis system helps to mitigate ISFET sensor drift and increase the efficiency and performance of the sensor system. Compared to ISE's, ISFET's are relatively smaller in dimensions, have a higher signal to noise ratio, and rapid response time.
Disadvantages/advantages
Disadvantages of the ion-selective electrodes are that it requires frequent calibration,46 may require additional extraction solutions, and relies on soil moisture for accurate nutrient readings. Another disadvantage is that nutrient sensor response is affected by various properties of soil such as the textural class, particle size, volumetric water, organic matter, and interfering ions listed in Hofmeister's series.47 Consequently, the limited success of these sensors are due to the measurement inconsistencies caused by the above disadvantages. Also, the effective use of electrochemical sensors for determining soil nutrients requires some type of procedure or device for nutrient extraction and rinsing agent for the electrochemical sensor which creates a further lengthy process for analysis. In addition to the sensor requiring periodic rinsing, it is recommended that the user have an understanding of soil texture and physical parameters for advanced calibration. Further, the development of highly selective membranes is needed, as this can also improve the accuracy of in situ soil nitrate ISE's for nutrient determination.
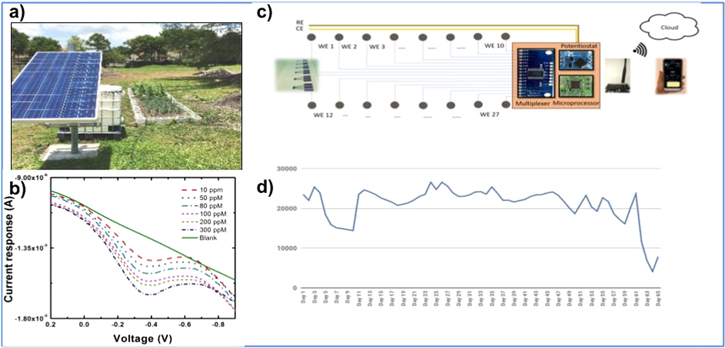
Literature pertaining to this sensor system reveals that the ion-selective electrodes can make an ideal platform for in situ soil nutrient measurements. These soil sensor measurements can be acquired in a rapid manner with lab-grade accuracy.48 The measurements can also be performed in slurry solutions, unfiltered extractants, and naturally moist soils. Additionally, electrochemical sensors can be fabricated using low cost materials, such as the ammonia sensor based on paper substrates reported in.49 Further, printed sensor platforms can be mass-produced using common industrial printing techniques,50 then interfaced to a single microcontroller for real-time soil nutrient analysis,51 as shown in Fig. 5.
Figure 5. (a) Solar powered IoT sensor garden from Y. Mekonnen et. al. The IoT farm system also features sensor dictated irrigation (44); (b) nitrate calibration response using fabricated in situ nitrate ISE (43); (c) Layout of the IoT enabled soil sensor sheet system from L. Burton et al.; (d) Real-time soil moisture content data used to correlate with in situ soil nitrate sensor.
Download figure:
Standard image High-resolution imageIoT soil nutrient sensors
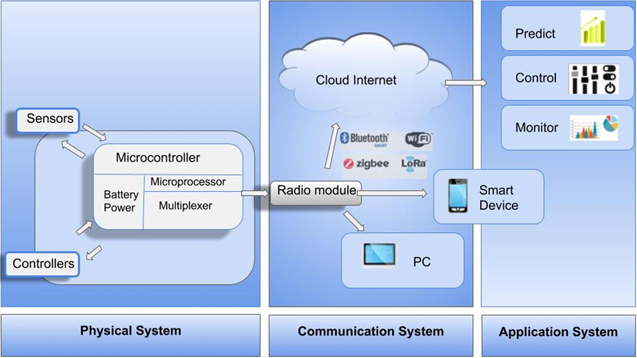
A system of devices or objects assigned unique identifier tags and exercises the ability to transmit and, or receive signal over a network can be referred to as the internet of things (IoT). an interesting concept behind this system is that its devices can function perfectly without human-to-human, or, human-computer interaction. The unique identifier which bridges devices to the cloud, giving the in situ soil nutrient sensor systems the advantage of real-time data visualization and sharing. The support of communication technologies such as SMS for text messaging, WiFi internet, ZigBee, and LoRa low powered long-range radio modules have allowed innovative systems for control, prediction, and monitoring of many soil parameters. This is done in a manner where interaction between the sensor systems and soil can be handled via a web application. As seen in Fig. 6, a typical IoT sensor system consists of a sensor interfaced with a microcontroller; the microcontroller relies on some battery source to power its function of commands from the microprocessor and is also interfaced with a radio module.52 The radio module, which holds the unique numeric identification or IP address, transmits and receives data over a network to a cloud, smart device or personal computer. The personal computer and cloud web application can feature deep learning and algorithms for predicting yield and crop growth parameters. The microcontroler and wireless radio module configuration was also used in,53 where researchers deployed a wireless sensor micro heater network for smart agriculture using. In addition, real-time soil nutrient monitoring techniques have been used to improve crop cultivation and production efficiency in precision agriculture with the use of additional actuator systems for control, such as sensor dictated fertigation. Real-time, in-field, automated soil monitoring systems have been further explored in.51,52,54,55
Figure 6. Schematic of a typical IoT sensor system for real-time monitoring.
Download figure:
Standard image High-resolution imageResearchers in54 developed a soil nutrient mapping system capable of rapidly assessing large-scale nitrate variations with lab-grade accuracy. The system is usually attached to heavy farm vehicles for mapping soil conditions as it traverses farm terrain. Later, they implemented validation tests of their nitrate extraction and measurement system. The authors found the level of agreement between the system's nitrate extraction subunit and standard laboratory measurements to be excellent.
In,51 smart gardening IoT soil sheets were developed and implemented in limestone derived Udorthent soils in south florid. The inkjet sensor printed paper sheets allowed real-time analysis and measurement data were transmitted via Waspmote, ZigBee, cloud server and mobile smart device. The author's latest explorations and further research aim to better identify influences of soil heterogeneity such as texture, organic matter, and moisture on the performance of the in situ polymer sensor systems.
Researchers in55 developed a micro total analysis system and mobile microchip capillary electrophoresis device for field detection of nutrient extracts. The lab-on-a-chip (LOC) systems allowed fluid handling, activation, and observation of chemical reactions in the soil. Although the system is criticized for its lengthier sample and measurement time, compared to other on the go proximal sensor systems, the device produces measurement readings that are comparable to laboratory analysis at a much lower cost.
Although not specifically intended for soil nutrient analysis, researchers in56 employed an inexpensive sensing platform for in situ monitoring of water and nutrients that also provided decision management for a smart irrigation system. Perhaps future work would allow researchers to adopt methods for precision agricultural practices. In addition to their use in precision agriculture, a real-time in situ system allows site-specific monitoring of pollutants for environmentalists. Such site-specific data has the potential to improve and better enforce environmental legislature.13
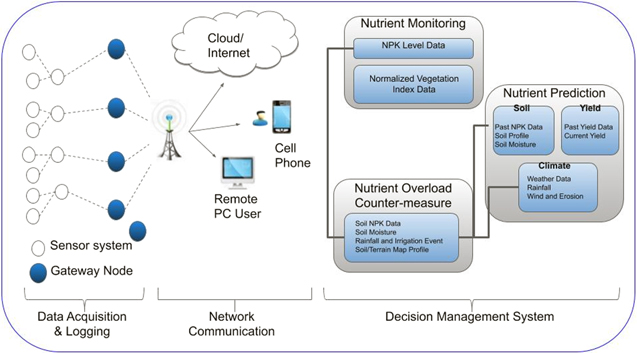
The interest of real-time in situ sensors and IoT systems has increased in agriculture, especially as farmers demand greater decision management systems for improving yield production and nutrient fertilizer application. A decision support systems incorporate various data elements such as climate, irrigation, crop genetics, energy, land terrain, human activity, and economic resources to provide an analysis of how their interrelations influence productivity. As shown in Fig. 7, a typical decision management system for nutrient application would include deployable sensor systems for acquiring real-time soil nutrient conditions. The sensor systems then forward the data to a gateway node, where the data can be logged or transmitted to a cloud, cellular or PC network. After this, the data can be further analyzed using machine learning, and neural networks to help predict soil nutrient requirements and present countermeasures for the overloading of nutrient fertilizers. The current state of the art decision management system, SMART Fertilizer Management, is a cloud-based software that offers nutrient requirement data for over 250 different crops. According to SMART, the platform helps farmers maximize yields an average of 15 percent, save costs and increase profits.
Figure 7. Schematic of a decision management system for nutrient fertilizers.
Download figure:
Standard image High-resolution imageConclusions and Future Perspectives
Given all the sensors mentioned in the review, there exists an urgent need for incorporating them with IoT and Artificial Intelligence. This would allow real-time monitoring and control of farm systems, thus making it easier for farmers to optimize production and minimize resource utilization. The literature identifies that due to the excessive utilization of fertilizers and pollution effects on the environment, a growing concern of pollution has warranted the need for technologies that can better monitor soil nutrients and their fate. The conventional laboratory methods may offer highly accurate analysis of soil chemistry, in situ based soil nutrient sensors that offer real-time feedback are needed in order to truly increase the efficiency of farming and managing the environment. As compared to conventional lab instruments for soil nutrient analysis, we find that in situ based sensors are more advantageous due to their low-cost, and high-density measurement capability for large-area soil nutrient mapping. Although these sensors are becoming less expensive to manufacture and can provide comparable results to laboratory soil analysis, there still exists a need to understand the effects of soil heterogeneity on the response of both optical and electrochemical sensors. More durable and accurate sensor systems that consider the effects of soils heterogeneity and offer soil specific calibrations are needed to move toward commercializing these platforms. When interfaced with long-range low powered radio modules, these sensor platforms are able to implement real-time prediction, control, and decision management for large scale precision agricultural practices. As the saying goes, the revolution will be live! In the case of agriculture, the revolution will be real-time.
Acknowledgments
The author would like to acknowledge support from the FEF McKnight, FIU CREST CACHe, and the NSF Bridge to the Doctorate fellowship.