Abstract
Silicon anodes offer a very promising approach to boost the energy density of lithium-ion batteries. While silicon anodes show a high capacity and, depending on the system, a good cycle stability in half-cells vs lithium, their integration in industrially applicable lithium-ion full-cells is still challenging. Balancing described as the capacity ratio of negative and positive electrode (n/p ratio) is a crucial necessity for the successful design of lithium-ion batteries. In this work, three different silicon based anode systems, namely carbon coated silicon nanowires, columnar silicon thin films and silicon-carbon void structures are compared in LIB full cells containing NMC111 cathodes. By varying the areal capacity of the NMC111 cathode, the influence of the balancing was investigated over a broad n/p range of 0.8−3.2. The aim was to find an ideal compromise between lithium plating suppression, high cycling stability and maximized energy density. To underline the high volumetric energy density, the columnar silicon thin films are additionally analyzed in multilayered pouch cells with NMC622 and NMC811 cathodes resulting in 605 Wh L−1 and 135 Wh kg−1 and even 806 Wh L−1 and 183 Wh kg−1 as demonstrated on stack level.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
In terms of increasing the energy density of storage devices, the state-of-the-art lithium-ion battery using a graphite anode is driven to its limits.1,2 To take the next step towards a new generation of lithium-ion batteries, silicon is an attractive anode material.2 The abundant and non-toxic silicon has the highest lithiation capacity (3579 mAh g−1Si, 8303 mAh cm−3, Li15Si4) among all materials forming lithium alloys and a low delithiation voltage around 0.4 V vs Li/Li+.3–5 Moreover, techniques to generate silicon materials are established. Unfortunately, silicon undergoes a large undesirable volume expansion during lithiation and delithiation leading to pulverization and loss of contact between silicon and the current collector. This negatively affects the capacity retention and the Coulombic efficiency (CE).4,6–9 Moreover, the solid electrolyte interphase (SEI) formed on the silicon surface by electrolyte decomposition is cracked by the mechanical stress of the volume change. It is formed repeatedly consuming lithium ions and electrolyte leading to cell dry-out.10–13 Several strategies based on nanostructured silicon like silicon nanowires,14–17 silicon particles,18–23 columnar silicon thin films24–27 have been reported so far. To stabilize the SEI and improve the electrical contact, silicon-carbon composites with a free volume between the silicon core and the carbon shell have been introduced.28–35 Overall, silicon based anodes with application-relevant areal capacities and high cycle stabilities provide a high capacity retention in half-cells vs a lithium anode where irreversible capacity loss is masked by the lithium excess though.14,25,36,37 Logically, the next step is the integration of these silicon based anodes in lithium-ion full-cells.1,38 Graphene silicon composites with 15%–60% silicon are the most widely investigated Si containing anodes in full-cells during the last years.39–52 Furthermore, composites of carbon black and silicon particles (SiNPs),53–58 carbon covered silicon particles59,60 and silicon coated carbon fibers16,61 have been already tested in full-cells. Pure silicon anode structures without carbon and binders like silicon thin films are rarely used.25,62–66 Besides NCA,25,39,51,52,55,61,65,67 LCO,16,43,51,56,60,65,68 LFP40,45,49,53,62,65 and LNMO,54,63,64 NCM41,42,44,46–48,50,57–59,66,69 is the most frequently used cathode for the full-cells comprising silicon based anodes. Nevertheless, only a few scientific articles focus on the balancing of lithium-ion full-cells with Si based anodes.59,66 More importantly, the research findings being gained so far are hardly comparable with each other due to different cell set-ups (different coins cells or Swagelok types) and un-realistic thick glass-fiber separators soaked with electrolyte excess reducing the energy density. In order to transfer results from coin to prototype cell level, application-relevant separators should be used. The balancing described by the n/p ratio is defined as the capacity ratio of negative to positive electrode (n/p ratio) and is crucial for the performance of the lithium-ion full-cells.70 Importantly, lithium plating should be prevented due to the irreversible loss of active lithium ions and possible internal short-circuits leading to safety issues.71 On the other hand, a higher oversized anode is detrimental for the energy density of the resulting cells.72 Kierzeck et al.'s59 investigation of Si–C composite coupled with NMC cathode shows that a slightly oversized anode improves the stability compared to n/p ∼1, and that the anode achieves a higher initial capacity than a larger oversized Si–C anode at the same time.59 Herein, three different representative silicon nanostructures (silicon nanowires, columnar silicon thin films, and silicon-carbon void structures) with an application-relevant separator and areal capacity above 2 mAh cm−2 are matched with a NMC111 cathode in coin cells under comparative conditions. By varying the mass loading of the NMC111 cathode, a wide range of n/p ratios between 0.8–3.2 is realized to investigate the influence of the balancing on the cycle stability and to define an optimal n/p ratio preventing lithium plating and irreversible lithium loss. The results reveal an outstanding potential of the columnar silicon thin film anode multilayered pouch cells. Therefore, these anodes were also evaluated vs NMC622 and NMC811 cathodes to demonstrate the high volumetric energy density.
Experimental
Silicon-carbon composite anode
20.31 g SiNPs (Alfa Aesar, 98%, APS ≥ 50 nm) and 59.60 g PVB (B60HH, Mowital) were mixed with a mortar mill (PULVERISETTE 2, Retsch) for 10 min and heated for 30 min at 190 °C. 70.23 g Si@PVB composite was ground with the mortar mill for 10 min and suspended in 310 ml deionized water and 34 ml ethanol. 101.07 g sucrose (Sigma Aldrich) and 6.8 g 2.5 M sodium hydroxide (Carl Roth) solution were added under stirring. The mixture was heated for 3 h at 100 °C and 6 h at 160 °C. The ground compound was heated under argon flow with 10 K min−1 to 850 °C and kept for 2 h. A water based slurry containing 80 wt% Si–C, 10 wt% multiwalled carbon nanotubes (MWCNT, Nanocyl7000, 90%) and 10 wt% styrene-butadiene rubber (SBR, Targray, 15%) was prepared with a mixer mill (MM400, Retsch) at 25 Hz for 15 min. It was coated on a copper foil (9 μm) with an automatic film applicator (BYK) and the coatings were dried at 80 °C for 2 h. The resulting Si–C anodes have a density of 0.6 g cm−3 and a loading of 4.3−4.8 mg cm−2.
Columnar silicon thin film anode
Columnar silicon thin films with a silicon loading of 0.85 mg cm-2 were prepared by magnetron sputtering (Multi Sputter Lab 600, VTD Vakuumtechnik Dresden GmbH) of silicon (99.99%) onto a 10 μm roughened copper foil with copper dendrites (SE-Cu58 Schlenk Metallfolien GmbH & Co. KG) at 10−6 mbar. The a-Si anodes have a thickness of 10 μm and a density of 0.85 g cm−3.
Carbon coated silicon nanowires
Au nanoparticles were used as catalyst for the Si-NW and were deposited onto pyrolytic graphite sheets (Panasonic EYG-S121803 and EYG-S121807) via thermal evaporation of Au (5N purity) in a customized evaporation system (Bestec GmbH, base pressure 5 · 10−7 mbar). In a customized low power chemical vapor deposition (CVD) furnace (ATV-Tech GmbH, base pressure 5 · 10−2 mbar) Si-NW were grown with a precursor gas mixture of SiH4:H2 (1:5, 5N:5N purity) and a pressure of 150 mbar. Subsequently, a pyrolytic carbon layer was grown onto the Si-NW in the same furnace at 820 °C with a gas mixture of C2H4:N2 (3:10, 5N:5N purity) at a pressure of 100 mbar. The steps Au deposition, Si-NW growth, carbon layer growth were executed repeatedly.
Lithium nickel manganese cobalt oxide (NMC111) cathode
A NMP based slurry comprising 91.4% NMC111 (TODA), 4.4% carbon black (Timcal) and 4.1% PVDF (Polymers France) was coated on a 30 μm thick aluminum foil. The coating thickness was varied to obtain loadings of 8.5, 9.0, 14.5, 16.0, 17.0, 17.5, 19.5, 20.0, 23.0, 25.5 and 28.0 mg cm−2, which ensure a broad areal capacity range between 1.2–3.6 mAh cm−2. The NMC cathodes were calandered to a porosity of 38% and a density of ∼2.4 g cm−3.
Structural characterization
The structure of Si-NW was imaged by the scanning electron microscope (SEM) ZEISS GEMINI LEO 1560 with a Bruker in-lens detector and 5 kV acceleration voltage. The SEM JSM-6610LV from JEOL with a secondary electron detector and 5−10 kV acceleration voltage was used to analyze the columnar silicon thin films. The nanoscale structure and morphology of the Si–C was examined with the transmission electron microscope (TEM) JEM-2100 from JEOL (200 kV acceleration voltage). To quantify the silicon content of the Si–C, it was heated under argon with 10 K min−1 to 500 °C and held for 30 min and heated again with 5 K min−1 to 1000 °C and held for 30 min using a Netzsch STA 409 PC/PG simultaneous thermal analyzer.
Half-cell testing
The Si and the NMC electrodes (diameter 12 mm) were dried at 80 °C under vacuum for 12 h and were tested vs a lithium anode (99.9%, diameter 16.5 mm, 250 μm thick, MTI Corporation). In an argon filled glovebox (MBraun) with less than 0.1 ppm O2 and H2O. CR2016 coin cells (MTI Corp.) were assembled with the Al2O3 impregnated polyethylene terephthalate separator FS3002 by Freudenberg (diameter 19 mm, 22 μm thick), a stainless steel spacer (1 mm thick) and 30 μl LP30 + 10% fluoroethylene carbonate (FEC). LP30 (99.9%, Solvionic SA) contains 1 M lithium hexafluorophosphate (LiPF6) in 1:1 (v/v) ethylene carbonate and dimethyl carbonate. Through the galvanostatic cycling, which was performed with a BaSyTec CTS cell test system, the Si/Li half-cells were discharged (lithiated) and charged (delithiated) with a C-rate of C/20 during the first cycle and C/10 during the following cycles between 10 mV−1 V. The C-rate was based on the theoretical capacity of the silicon (3579 mAh g−1Si). During the formation, the NMC/Li half-cells were cycled two times with a C-rate of C/10 between 3−4.3 V while a CV step until C/100 (1C = 140 mA g−1 based on the mass of NMC) was applied during charge and discharge. Two cycles with a rate of C/5 and CV step until C/50 followed. The subsequent cycling was performed with C/5 between 3.0−4.3 V, and a CV step was just applied at the end of the charge.
Full-cell testing
The balancing is determined based on the ratio of the areal capacity of the anode to the cathode which are deduced from the theoretical capacity of the silicon (3579 mAh g−1Si) and from the practical capacity of NMC111 (140 mA g−1NMC). This results in a wide range of the n/p ratios between 0.8−4.2. CR2016 coin cells containing a dried Si anode (diameter 16 mm) and NMC111 cathode (diameter 15 mm) were assembled in an argon filled glovebox (MBraun) with less than 0.1 ppm O2 and H2O. An Al2O3 impregnated polyethylene terephthalate separator FS3002 from Freudenberg (diameter 19 mm, 23 μm thick), a stainless steel spacer (1 mm thick) and 40 μl LP30 + 10% FEC (99.9%, Solvionic SA) are used. Before full-cell testing, the Si–C anodes were assembled in a half-cell and lithiated/delithiated between 50 mV–1 V with a current density of 0.1 mA cm−2 in the 1st cycle and 0.5 mA cm−1 in the following cycle to previously form a SEI. The galvanostatic cycling was performed with a BaSyTec CTS cell test system. The formation of the NMC111/Si-NW full-cells contains two cycles with C/10 and two cycles with C/5 between 3−4.3 V and a CV step until 10% of the cathode capacity (140 mAh g−1NMC) during charge and discharge. The subsequent cycling in the same voltage range was performed with C/5. To increase the capacity, the NMC111/a-Si and the NMC111/Si_C full-cells were charged until 2.4 V and a constant voltage step until 10% of the theoretical capacity was introduced. After the full-cell testing, the cells were disassembled and rebuilt in half-cells with a fresh lithium chip and electrolyte. The same test procedures as for the half-cell characterization but without formation cycles were used.
Prototype cells
Multilayered pouch cells (71 × 46 mm2) were assembled in a glovebox using a double-sided silicon thin film anode (0.91 mg cm-2, 3.3 mAh cm−2, 0.91 g cm−3). As cathode was used either NMC811 (12.6 mg cm-2, 2 mAh cm−2, 2.8 g cm−3) purchased from Custom Cells or NMC622 (14.2 mg cm-2, 2.3 mAh cm−2, 2.5 g cm−3) which was processed similar to the NMC111 cathode. The NMC622/a-Si pouch cells contain the same separator as the coin cells and 1.65 ml of the electrolyte LP30 + 10% FEC. A thinner polyethylene separator (12 μm) and 1 ml LP30 +10% FEC are used in the NMC811/a-Si pouch cells. For the pouch cell, the test procedure was similar to the coin cell testing.
Results and Discussion
Production and characterization of representative silicon nanostructures
Three different silicon structures shown in Fig. 1 are compared, namely (1) silicon nanowires (Si-NW), (2) amorphous columnar silicon thin films (a-Si), and (3) nanostructured silicon-carbon composite void structures (Si–C). Si-NW are deposited onto pyrolytic graphite sheets and coated with a pyrolytic carbon layer. These synthesis steps are repeated to achieve a branched tree-like structure (supplementary Fig. S1, available online at stacks.iop.org/JES/167/020516/mmedia) and a silicon loading of 0.8−1.4 mg cm−2. The a-Si with a silicon loading of 0.9 mg cm−2 are achieved by sputtering Si onto copper foil with a rough layer of copper dendrites.24 The Si–C containing 28% Si is synthesized via a simple, potentially scalable route without hydrofluoric acid treatment similar to a process as described elsewhere.73 Polyvinylbutyral (PVB) is herein used as intermediate template which could be removed completely during pyrolysis to create tailored voids between SiNPs and carbon shells. In contrast to the Si-NW and the a-Si, the Si–C could be easily incorporated in the slurry coating process which is actually used for LIB.
Figure 1. SEM or TEM image and schematic illustration of the lithiation of silicon nanowires (Si-NW, a), columnar silicon thin films (a-Si, b) and silicon-carbon void structures (Si–C, c) and the resulting SEI morphology (green).
Download figure:
Standard image High-resolution imageAll three Si nanostructures were galvanostatically cycled in half-cells vs a Li anode (Fig. 2). The a-Si thin film has the highest specific capacity. In the second cycle, 3313 mAh g−1Si are achieved, which is close to the theoretical capacity (3579 mAh g−1). The Si-NW and the Si–C electrode have a specific capacity above 2100 mAh g−1Si in the second cycle. The capacities based on the mass of the electrode without current collector and the areal capacities are descripted in the ESI (Fig. S2). All anodes have a practically applicable areal capacity above 2 mAh cm−2. During the 1st cycle, there is the initial formation of the SEI which results in an initial Coulombic efficiency (ICE) lower than 100% and a high irreversible capacity. Due to the relatively low surface to volume ratio compared to the other two structures, the a-Si thin films have the highest ICE of 94.2% followed by the Si-NW with 90.3%. The Si–C was synthesized by sucrose as precursor which is known to form porous irregular carbon coatings, increasing the number of reactive sites and lowering the ICE to 45.3%.73–75 As shown in Fig. 2b, the initial irreversible capacity of the Si–C is much higher than the ones of the a-Si and Si-NW. Therefore, we decided to (pre)cycle the Si–C anode in a half-cell vs lithium before full-cell testing to form the SEI. In conclusion, all three silicon structures show relatively high capacity retention and application-relevant areal capacity.
Figure 2. Specific discharge capacities, Coulombic efficiencies (CE) (a) and accumulated irreversible capacities (b) of the Si-NW, the a-Si and the Si–C electrode vs a lithium anode in a half-cell set-up.
Download figure:
Standard image High-resolution imageElectrochemical performance of the NMC111/Si full-cells
The three representative Si anodes are matched with a NMC111 cathode, which shows a stable capacity of 140 mAh g−1NMC in half-cells (Fig. S3). Areal capacities between 1.2−3.6 mAh cm−2 were realized by adapting the mass loading (8.5−28 mg cm−2). The n/p ratio is determined based on the ratio of the areal capacity of the anode to the cathode which are deduced from the theoretical capacity of the silicon (3579 mAh g−1Si) for a better comparability of the three silicon systems and from the practical capacity of NMC111 (140 mA g−1NMC). Therefore, a wide range of the n/p ratios between 0.8−4.2 is covered. For better clarity and to compensate the error in the determination of the silicon loading, the individual n/p ratios are summarized to n/p ranges with the same characteristics. In the following sections, the influence of the balancing on the cycle stability is discussed to define an optimal n/p ratio preventing lithium plating and increase the energy density.
NMC111/Si-NW full-cells
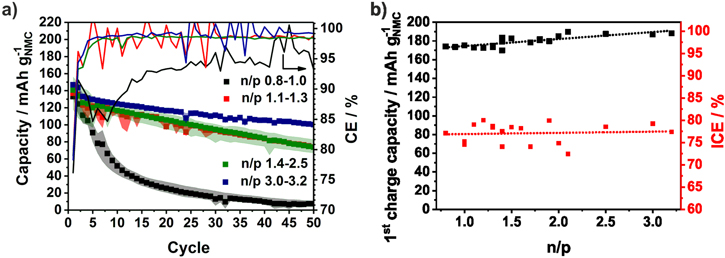
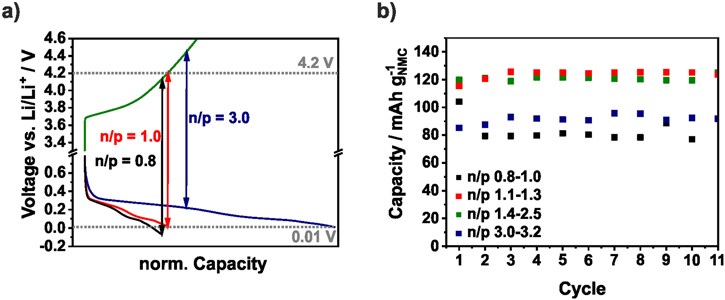
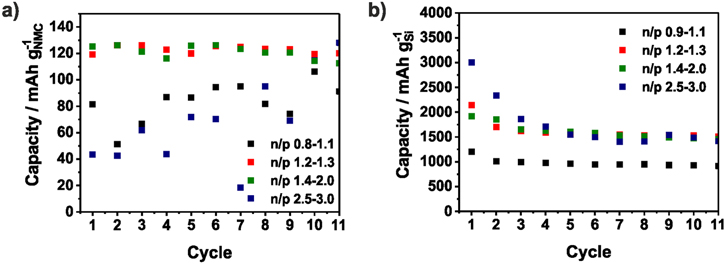
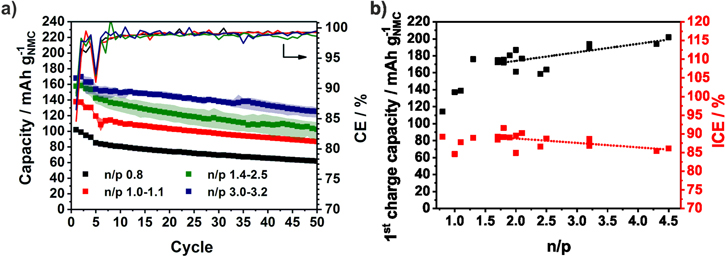
In Fig. 3, the specific capacities of the NMC111/Si-NW full-cells based on the mass of NMC are averaged over several coin cells of the same n/p range. The colored area contains the minimum and the maximum values of the capacity. For better clarity, the averaged CE is shown without error bars. In cells with an n/p ratio below 1.1 the capacity drops directly. Due to the higher capacity of the positive electrode, the negative electrode is charged below 0 V vs Li/Li+ and lithium plating takes place. This loss of active lithium ions causes the capacity fading and the low CE.72 There is no obvious difference between the n/p ranges 1.1−1.3 and 1.4−2.5. Only during the 1st cycle, the capacity slightly rises with increasing n/p ratio. However, with n/p ≥ 3.0 the capacity retention is significantly improved and 100 mAh g−1NMC could be reached after 50 cycles. Since the Si-NW anode is highly oversized, it is only partially lithiated and only around 1200 mAh g−1Si are utilized. In several works, a similar limitation of the capacity of silicon electrodes is applied and the electrode stability in half-cells could be significantly improved.76 Nevertheless, the higher anode mass has to be taken into account which decreases the energy density. In addition, as it can be seen from the voltage profiles of the NMC cathode and Si anode derived from the half-cell tests in Fig. 4a the upper voltage of the cathode is increased by the higher voltage of the Si anode if it is not completely utilized (n/p = 3). In case of an oversized NMC cathode the potential of the Si anodes falls below 0 V and lithium is plated. Ideally, both electrodes are balanced and the cut-off voltage of the NMC111 cathode is around 4.2 V. A similar correlation was described by Kasnatscheew et al.77 for LIB full-cells with graphite anodes. The influence of the Si potential vs Li/Li+ could be seen in Fig. 3b where the 1st charge capacity increased with the n/p ratio. The ICE seemed to be independent from n/p at around 77%.
Figure 3. Specific discharge capacities and Coulombic efficiencies of NMC111/Si-NW full-cells (a). The data of duplicate cells with the same n/p range are averaged and the colored area contains the minimum and the maximum values. First charge capacities and initial Coulombic efficiencies (ICE) depending on the n/p ratio (b).
Download figure:
Standard image High-resolution imageFigure 4. Schematic illustration of the voltage profiles of the NMC111 cathode and a Si anode in half cells (a) and averaged discharge capacities (b) of NMC cathodes in rebuilt half-cells after full-cell testing (NMC111/Si-NW) with different n/p ratios.
Download figure:
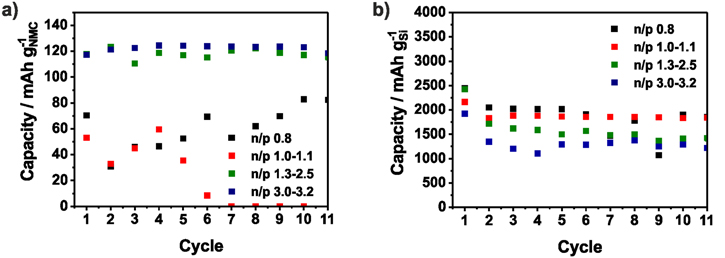
Standard image High-resolution imageTo investigate the electrode degradation during the full-cell test, after 50 cycles both electrodes were reassembled in half-cells. Indeed, an n/p ≤ 1.0 leads to cathode degradation and a capacity of less than 100 mAh g−1NMC could be provided (Fig. 4b). With an n/p ratio between 1.1−1.7, a stable capacity of 125 mAh g−1NMC could be achieved which matches with the results from the previous half-cell testing (Fig. S3). A lower capacity is reached with an n/p ratio ≥1.8. As discussed before, the charge cut-off voltage at the cathode increased with the n/p ratio and above 4.6 V, both the NMC111 cathode and the organic electrolyte begin to degrade (Fig. S3). Nevertheless, these cells are more stable indicating that improved Si anode stability and the lower lithium losses have a large influence on the performance. It should be mentioned that the Si-NW are partially detached from the carbon current collector which is pictured in Fig. S4. Therefore, the utilized capacity is lower than 1000 mAh g−1Si in the reassembled cells (Fig. S5).
NMC111/a-Si full-cells
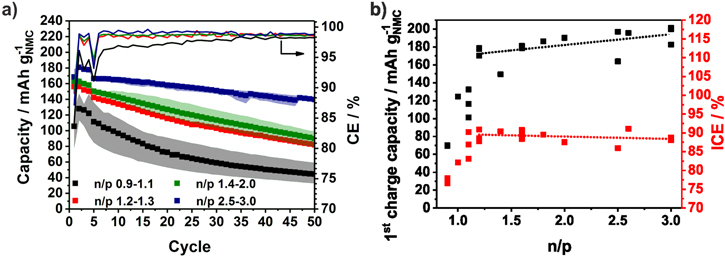
The specific capacities and the CE of the NMC111/a-Si full-cells are shown in Fig. 5a. Compared to the NMC111/Si-NW full-cells, the lower cut-off voltage is changed from 3 V to 2.4 V and a constant voltage step is introduced during charging in order to increase the capacity and also the energy density of the full-cell. While the NMC111/Si-NW full-cells show a stable capacity above an n/p ratio of 1.0, in case of the NMC/a-Si full-cells, the n/p ratio needs to be higher than 1.1 to prevent lithium plating (Fig. 6a). There are no significant differences between the n/p ranges of 1.2−1.3 and 1.4−2.0 in the first cycle when ∼160 mAh g−1NMC are achieved (Fig. 5a). However, if the silicon anode is only partially lithiated (n/p ≥ 2.5), a higher capacity and significant improved capacity retention of more than 80% after 50 cycles (168 mAh g−1NMC) is reached (Fig. 6c). The initial CE from the NMC111/a-Si cell with n/p ≥ 1.2 is independent of the n/p ratio around 90% and therefore higher than that of the NMC111/Si-NW cell (∼77%) indicating a lower irreversible capacity (Fig. 5b). Nevertheless, the specific charge capacity follows the same trend as in the NMC111/Si-NW full-cell and increases with the n/p ratio. Due to the sloping portion of anode voltage profile and the only partially utilized anode, the upper voltage of the cathode is increased. This is why the capacity of the full-cells with n/p 1.4–2.0 is slightly higher than that of the full-cells with n/p 1.2−1.3. Both electrodes after 50 cycles are depicted in Fig. S6 and results of the rebuilt half-cells are shown in Fig. 7. While the NMC cathodes originated from full-cells with n/p between 1.2−2.0 show a stable capacity of 125 mAh g−1NMC in the half-cells, the capacity of the NMC cathodes for the full-cell with lower or higher n/p ratios are lower than 100 mAh g−1NMC. If n/p ≥ 2.5, the NMC is driven to a critical upper cut-off voltage (Fig. 4a). In case of the full-cell with n/p ≤ 1.1, the reason for the cathode degradation is not clear and should be analyzed in further investigations. The a-Si anodes used in the full-cells with an n/p ratio between 1.2−3.0 show a lithiation capacity (discharge capacity) below 2000 mAh g−1Si (Fig. 7b), implying ∼1000 mAh g−1Si reduced capacity than in the preceding half-cell test (Fig. 2). A reason for this finding is the mechanical degradation of the a-Si film during the cycling. Due to the lithium plating the Si electrodes from the full-cells with an n/p ≤ 1.1 degrades and achieved only 1000 mAh g−1Si in rebuilt half-cells.
Figure 5. Specific discharge capacities and Coulombic efficiencies of NMC111/a-Si full-cells (a). The data of duplicate cells with the same n/p range are averaged and the colored area contains the minimum and the maximum values. First charge capacities and initial Coulombic efficiencies (ICE) depending on the n/p ratio (b).
Download figure:
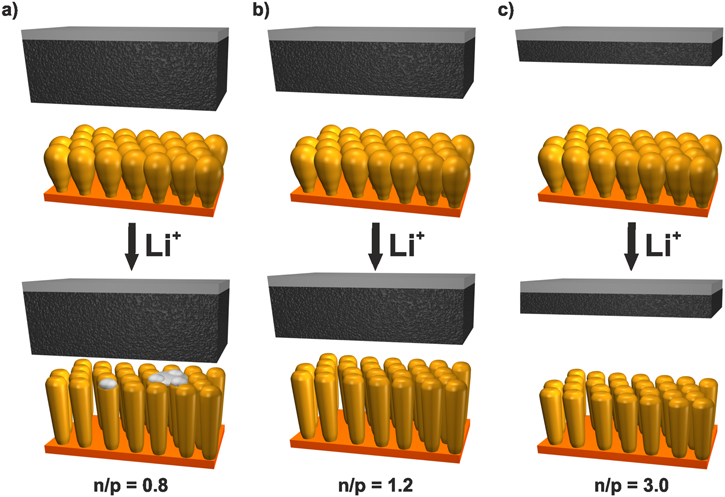
Standard image High-resolution imageFigure 6. Schematic illustration of the NMC111/a-Si full cell with oversized positive electrode where lithium is plated (a), slightly oversized negative electrode (b) and largely oversized negative electrode which is thereby just partially utilized (c).
Download figure:
Standard image High-resolution imageFigure 7. Averaged discharge capacities of the NMC cathodes (a) and the a-Si anodes (b) in rebuilt half-cells after full-cell testing (NMC111/a-Si) with varying n/p ratios.
Download figure:
Standard image High-resolution imageNMC111/Si–C full-cells
As already mentioned, the Si–C anodes were (pre)cycled in a half-cell before full-cell testing to form the SEI, otherwise a large amount of the lithium stored in the NMC cathode would be consumed by the initial SEI formation (Fig. S7). Figure 8 shows the averaged discharge and charge capacity and the resulting CE as a function of the n/p ratio. The NMC111/Si–C full-cells with n/p = 0.8 have a low discharge capacity of approx. 100 mAhg−1NMC due to the lithium plating which is usually accompanied by irreversible lithium loss. With an n/p of 1.0−1.1, a reasonable capacity utilization of 137 mAh g−1NMC in the 1st cycle is reached, indicating that the lithium plating could be prevented, as expected, by the increased anode areal capacity. Using a higher oversized negative electrode (n/p = 1.7−2.5) the capacity of the NMC111/Si–C full-cells with n/p 1.7−2.5 is increased to 158 mAh g−1NMC in the 1st cycle and degrades to 103 mAh g−1NMC after 50 cycles. According to the prior results, the highest capacity (168 mAh g−1NMC in the 1st cycle and 137 mAh g−1NMC in the 50th cycle) could be achieved with an n/p ratio above 3.0. Due to the precycling and the resulting initial SEI formation on the Si–C anode in a preceding half-cell operation, an ICE of 85%–92% similar to ICE of the NMC111/a-Si full-cells and higher than the ICE the NMC111/Si-NW full-cells is achieved. As shown before, the 1stcharge capacity rises with the n/p ratio due to the voltage profile, the partially utilized negative anode and causes the higher capacity. Figure 9 contains the results of the rebuilt half-cells of the Si anodes and NMC cathodes after cycling. Just with high n/p ratios ≥1.7, the NMC cathode provides a capacity of 125 mAh g−1NMC comparable with the previous half-cell test. Below an n/p ratio of 1.7, the capacity of the NMC111 cathodes is lower than 90 mAh g−1NMC in the rebuilt half-cells. In contrast, all Si–C anodes show a stable capacity around 1800 mAh g−1NMC during the first cycles of the half-cell test which underlines the structural stability of the Si–C anode (Fig. S8).
Figure 8. Specific discharge capacities and Coulombic efficiencies of NMC111/Si–C full-cells (a). The data of several duplicate cells with the same n/p range are averaged and the colored area contains the minimum and the maximum values. First charge capacities and initial Coulombic efficiencies (ICE) depending on the n/p ratio (b).
Download figure:
Standard image High-resolution imageFigure 9. Averaged discharge capacities of the NMC cathodes (a) and the Si–C anodes (b) in rebuilt half-cells after full-cell testing (NMC111/Si–C) with varying n/p ratios.
Download figure:
Standard image High-resolution imageComparison and estimation of the energy density based on the coin cell testing
The energy density is a crucial parameter that needs to be considered when discussing novel anode concepts. Usually, the practically usable energy densities could not be deduced directly from the theoretical energy densities of the active materials.78 Here, we compare the energy density on stack level of the three full-cell concepts and the influence of the n/p ratio.
Figure 10 shows the cell design and Fig. 11 the resulting weight and volume distribution and the volumetric and specific energy density of the three full-cell concepts with a minimal n/p ratio based on the calculation descripted in the supporting information. It has to be considered, that the increase in thickness during lithiation is not taken into consideration, because it could not be undoubtedly deduced from coin cell investigations. We are aware that this is a crucial parameter and therefore, investigations with multilayered pouch cells are described in the following chapter.
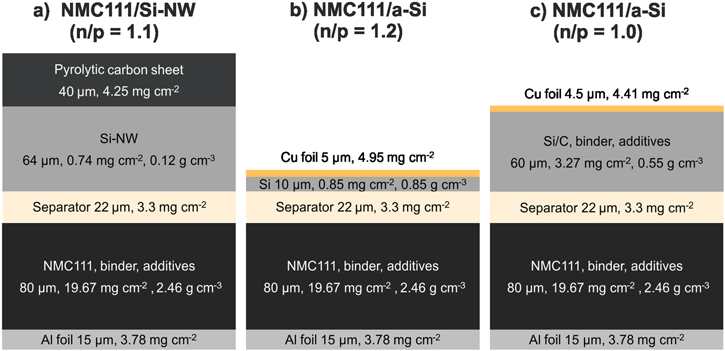
Figure 10. Characteristics of the NMC111/Si full-cells components with a Si-NW, (a), a-Si (d) and Si–C (c) anode and a minimal n/p ratio without lithium plating.
Download figure:
Standard image High-resolution imageFigure 11. Theoretical calculation of the volumetric/specific energy density with the corresponding volume (a)–(c) and weight distribution (d)–(f) of the cell components for NMC111/Si full-cells with a Si-NW (a), (d), a-Si (b), (e) and Si–C (c), (f) anode and a minimal n/p ratio without lithium plating.
Download figure:
Standard image High-resolution imageThe Si-NW have a very low density which result in a high dead volume filled with electrolyte, and a large electrode thickness. Moreover, the used pyrolytic carbon sheet is much thicker than the conventionally used copper foil. This is why the NMC111/Si-NW full-cells have the lowest volumetric energy density with 382 Wh L−1. Due to the dense columnar structure of the a-Si, a comparatively high volumetric energy density of 713 Wh L−1 is achieved. The overall porosity (76%) of the Si–C is lower than the porosity (95%) of the Si-NW approach. Despite the relatively low silicon content of 23%, a volumetric energy density of 466 Wh L−1 could be achieved due to the thin copper current collector. The NMC111/a-Si full-cell has the highest specific energy density of 256 Wh kg−1 followed by the NMC111/Si-NW full-cell with 232 Wh kg−1 and NMC111/Si–C full-cell with 217 Wh kg−1. However, it has to be considered that the volumetric and especially the specific energy density of the three full-cell concepts is strongly influenced by the NMC111 cathode which has a volumetric fraction of 36%–60% and a mass fraction of 50%–53%. Therefore, NCA and nickel-rich NMC materials like NMC622 or NMC811 with much higher capacities (180−210 mAh g−1) and higher areal loading and mass density (3 g cm−3) are an effective option to further increase the volumetric energy density, especially in combination with disruptive silicon anode concepts.78–80 If an optimal NCA cathode according to the energy calculation of lithium-ion batteries by Betz et al.78 with a density of 3.05 g cm−3 and a loading of 13 mg cm−2 is assumed, the energy density of the full-cells improves enormously (Fig. S9). While the NCA/Si-NW and the NCA/Si–C full-cells could reach 519 W L−1 and 336 Wh kg−1 or 681 W L−1 and 304 Wh kg−1, respectively, and with the a-Si anode even 1119 Wh L−1 and 326 Wh kg−1 are achievable. However, the concepts in this work are highly limited by the cathode and energy densities of state-of-art batteries from Panasonic with and 683 Wh L−1 260 Wh kg−1 and where a NCA cathode and a carbon/silicon anode is probably used cannot be exceeded.81
In Fig. 12, the cycle stability for varying n/p ratios in the three investigated systems based on the coin cell testing is shown. As expected and already discussed, an oversized silicon anode is beneficial for the cycle stability, but decreases the overall energy density of the cell. In case of the NMC111/Si-NW and the NMC111/Si–C full-cells, the increased capacity could not compensate the higher anode volume or weight (Fig. 12). However, the cycle stability of NMC111/a-Si full-cell could be significantly improved through the strongly oversized a-Si anode. Consequently, the volumetric energy density and the specific density of the full-cell with n/p = 2.6:1 is maintained after approx. 25−30 cycles, whereas full-cells with the minimal n/p ratio of 1.2:1 principally show an accelerated degradation.
Figure 12. Estimated volumetric energy densities (a) and specific energy densities (b) of NMC111/Si full-cells depending on the Si anode structure and the n/p ratio.
Download figure:
Standard image High-resolution imagePrototype cells
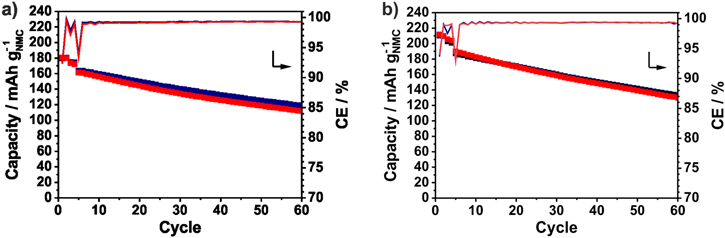
The evaluation of new battery materials in multilayered pouch cells is essential for the targeted application, especially due to new effects caused by double-sided electrode coatings and the different wetting and pressure situation, especially for electrodes that undergo volume changes. Summarizing, the electrochemical performance of the a-Si anode stands out against the other two investigated Si systems by the high volumetric energy density and are further investigated in prototype cells with NMC622 and NMC811 cathodes. As shown in Fig. 13, the NMC622/a-Si pouch cell and the NMC811/a-Si pouch cells reaches a higher capacity of 180 mAh g−1NMC or 210 mAh g−1NMC, respectively, due to the higher Ni content of the NMC cathode. In both pouch cells, higher capacity retention than in the coin cells is reached indicating a lower irreversible capacity by side reactions or electrode degradation. Additionally, the pressure distribution differs drastically in pouch compared to coin cells. After 50 cycles, indeed 70% (125 mAh g−1NMC) of the initial capacity could be achieved in the NMC622/a-Si pouch cells and the NMC811/a-Si delivers still 67% (142 mAh g−1NMC). Consequently, a high initial CE of 92%–94% and of 99.2%–99.4% in the following cycles is achieved. Figures S10 and S11 display the pouch cells and their components after cycling. A deformation of the a-Si anodes similar to the coin cells and previous studies is observed.24 Due to the volume change of the silicon, a large mechanic stress is applied on the copper foil which leads to the microscopic structural changes. Consequently, a few amount of silicon is delaminated and attached to the separator. The energy density of the prototype cells is calculated on stack level considering inactive components like the separator and the current collectors. To allow a better comparability with previous results the pouch foil and the taps are not considered. By using the NMC622 and the NMC811 cathode material the capacity could be increased while the cathode weight and thickness is reduced at the same time. As a consequence, the multilayered NMC622/a-Si pouch cell achieves 605 Wh L−1 and 135 Wh kg−1. In case of the NMC811/a-Si pouch cell, a thinner separator (12 μm) is used resulting in a relatively high volumetric energy density of 806 Wh L−1 and a reasonable specific energy density of 183 Wh kg−1 on stack level.
Figure 13. Specific discharge capacities and Coulombic efficiencies of the NMC622/a-Si prototype cells (a) with a n/p of 1.4 containing two double-sided a-Si anode, a double-sided and two single-sided NMC622 cathodes and of the NMC811/a-Si prototype cells (b) with a n/p of 1.9 containing a double-sided a-Si anode and two single-sided NMC811 cathodes.
Download figure:
Standard image High-resolution imageConclusions
Three representative nanostructured silicon anode systems (Carbon coated silicon nanowires (Si-NW), columnar silicon thin films (a-Si) and silicon-carbon void structures (Si–C)) were successfully coupled with NMC111 cathodes with various mass loadings to investigate the influence of the balancing on the full-cell performance.
The minimal n/p ratio which is needed to prevent lithium plating for each Si anode system was determined. Independent of the Si structure, the cycle stability of the full-cell could be increased significantly by a three times oversized Si anode. The reason for this is the limited lithiation of the Si anode improving its stability during cycling, which is already known for half-cells. The three full-cell set-ups were compared in terms of the theoretical energy density of practical pouch cell stacks based on the material characteristics before cycling. The highest volumetric energy density of 713 Wh L−1 are estimated for the NMC111/a-Si-full cells. Based on these results, the a-Si anodes were successfully cycled in multilayered pouch cells with NMC622 and NMC811 cathodes, respectively. A high capacity of 210 mAh g−1NMC, an improved CE (99.2%–99.4%), and cycle stability compared to the coin cell testing was achieved resulting in a high volumetric energy density as high as 806 Wh L−1.
Summarizing, this study reveals decisive specifications preventing lithium plating in viable full-cells with maximized specific energy and high cycle stability as a function of silicon anode architecture and its interaction with all cell components.
Acknowledgments
This research has received funding from the Federal Ministry of Education and Research through the project BamoSa (support code 03X4637) and KaSiLi (support code 03XP0254). We would like to thank A. Urbanski (Leibniz Institute of Polymer Research (IPF) Dresden e.V.) and A. Omar (Leibniz Institute for Solid State and Material Research (IFW) Dresden e.V.) for useful discussions on polymers and electrolytes and J. Strangalies (Fraunhofer Institute for Material and Beam Technology IWS) for performing SEM measurements and P. Fleischer (Fraunhofer Institute for Material and Beam Technology IWS) manufacturing the pouch cells.