Abstract
A systematic study of the growth of uniform vertically-aligned ZnO nanowires is presented. It is shown how, by careful control over the growth parameters, high quality nanowires of chosen morphology may be synthesized at low temperatures using electro-deposition. The key experimental parameters were the growth temperature, the ZnCl2 concentration in the electrolyte, the applied voltage and the thickness of the catalytic Au film. As a result of optimizing these parameters, ZnO nanowires with tailored dimensions were realized – an essential prerequisite for the use of such nanowires in functional nanoscale devices.
Export citation and abstract BibTeX RIS
Nanoscience and nanotechnology involve studying and working with matter on a nanoscale. Phenomena at the nanoscale are likely to be a completely new world, where properties may not be predictable from those observed at large scales.1–3 Zinc oxide (ZnO) nanostructures have attracted considerable interest owing to their excellent electronic and optical properties.4–8 ZnO with a band gap of 3.37 eV at room temperature and with a high excition binding energy of 60 meV, has been considered a material of choice for use in short-wavelength light-emitting diodes (LEDs), laser diodes and organic LEDs.9 Consequently, in recent years, there has been extensive interest in synthesizing various ZnO nanostructures, including nanowires, nanoribbons and nanotubes.
ZnO nanostructures have been synthesized by a variety of methods, such as chemical vapor deposition,10, 11 metalorganic vapor phase epitaxy,12 arc discharge,13 thermal evaporation, laser ablation,14 template-based deposition,15 and electro-deposition.16, 17 However, many of these methods are not cost effective in terms of producing high quality, aligned, uniform nanowires. We show that synthesis by electrochemical deposition, which is a low temperature growth technique, holds a great deal of promise for preparing high quality ZnO nanowires, vertically oriented on various substrates, and potentially suitable for preparing tailored morphology nanowires (particularly relating to strict dimensional control with good uniformity), that can be easily integrated into device structures.
Studies of electro-deposition of ZnO nanowires have been reported in the literature.16, 17 These studies show the influence of deposition parameters on the dimensions of single nanowires by oxygen reduction and nitrate reduction for the formation of hydroxyl species involved in ZnO precipitation. These papers also studied the influence of a controlled nucleation on step nanowire dimensions, which the ZnO buffer layer was deposited at a relatively low temperature and charge density. Our work builds on these studies and presents a systematic study of the role of deposition parameters on controlling nanowire morphology (and in particular, the nanowire diameter and length). A unique feature of our study is that we simplified the electrodeposition process avoiding using a specific nucleation process for a ZnO buffer, and not using oxygen injection. The key parameters that we report on, are the growth temperature and the ZnCl2 salt concentration in the electrolyte. We discuss the results of this optimization study in terms of a mechanistic model, and also discuss the influence of the growth parameters on synthesizing high quality, vertically oriented nanowires, which would enable the use of these arrays in the fabrication of optoelectronic devices.
Experimental
The electrochemical deposition of ZnO nanowires was performed in a three electrode electrochemical cell with a n-type Si (001) substrate coated with about 15 nm of Ni as an adhesion layer, and a 115 nm Au film as the cathode; a Pt spiral wire was used as the counter electrode and a similar Pt spiral as the reference electrode. All the potentials reported are quoted with respect to the reference electrode. Prior to Au evaporation, a standard cleaning procedure was used for the Si substrates. This involved the removal of any organic contaminants from the substrate surface by cleaning in acetone, alcohol and DI water (17.6 MΩ cm) at 90°C. The electrolyte used for the electrochemical process was an aqueous solution of ZnCl2 (Sigma-Aldrich, purity >98%), to provide Zn2+, and KCl (ACP, purity 99.0–100.5%), as the supporting electrolyte [Alpha Aesar].
The study focused on the influence of ZnCl2 concentration and growth temperature on nanowire dimensions. Two groups of deposition experiments were carried out: (1) varying the ZnCl2 concentration in solution, with molarity ranging from 0.2 to 10 mM, and keeping the concentration of KCl constant at 100 mM, with all electrodepositions conducted at 80°C, and (2) varying the electrodeposition temperature from 55 to 90°C with concentrations of ZnCl2 constant at 5 mM and KCl constant at 100 mM, respectively. The pH of the electrolyte was maintained at 6.3–6.5 at room temperature. In order to facilitate comparison of the as-grown nanostructures, experiments were performed using a constant potential to the reference electrode of 0.95 V, a growth time of 1 h, and a volume of electrolyte of 230 ml. The bias was applied using a Tektronix PS2521G programmable power supply, with bias maintained by adjusting a variable resistor connected in parallel with the electrochemical deposition system. The area of sample immersed in the electrolyte was about 1 cm2. The grown nanowires were characterized using a Field Emission Scanning Electron Microscope (FE-SEM) (Hitachi S-5200) with Energy Dispersive X-ray (EDX) analysis.
ZnO Nanowire Growth Model
In the electrochemical system, we are concerned with processes that influence charge transport. The possible zinc species in the electrolyte include Zn2+ ions, zinc hydroxide complexes including Zn(OH)+, Zn(OH)2,  , and
, and  , and zinc chloride complexes including ZnCl+, ZnCl2, and
, and zinc chloride complexes including ZnCl+, ZnCl2, and  . The predominant species will depend on the pH and the temperature of the solution. In our specific case, in the growth temperature range from 55 to 90°C and pH from 6.3 to 6.5, the major zinc species are Zn2+, ZnCl+, Zn(OH)+, and ZnCl2.18
. The predominant species will depend on the pH and the temperature of the solution. In our specific case, in the growth temperature range from 55 to 90°C and pH from 6.3 to 6.5, the major zinc species are Zn2+, ZnCl+, Zn(OH)+, and ZnCl2.18
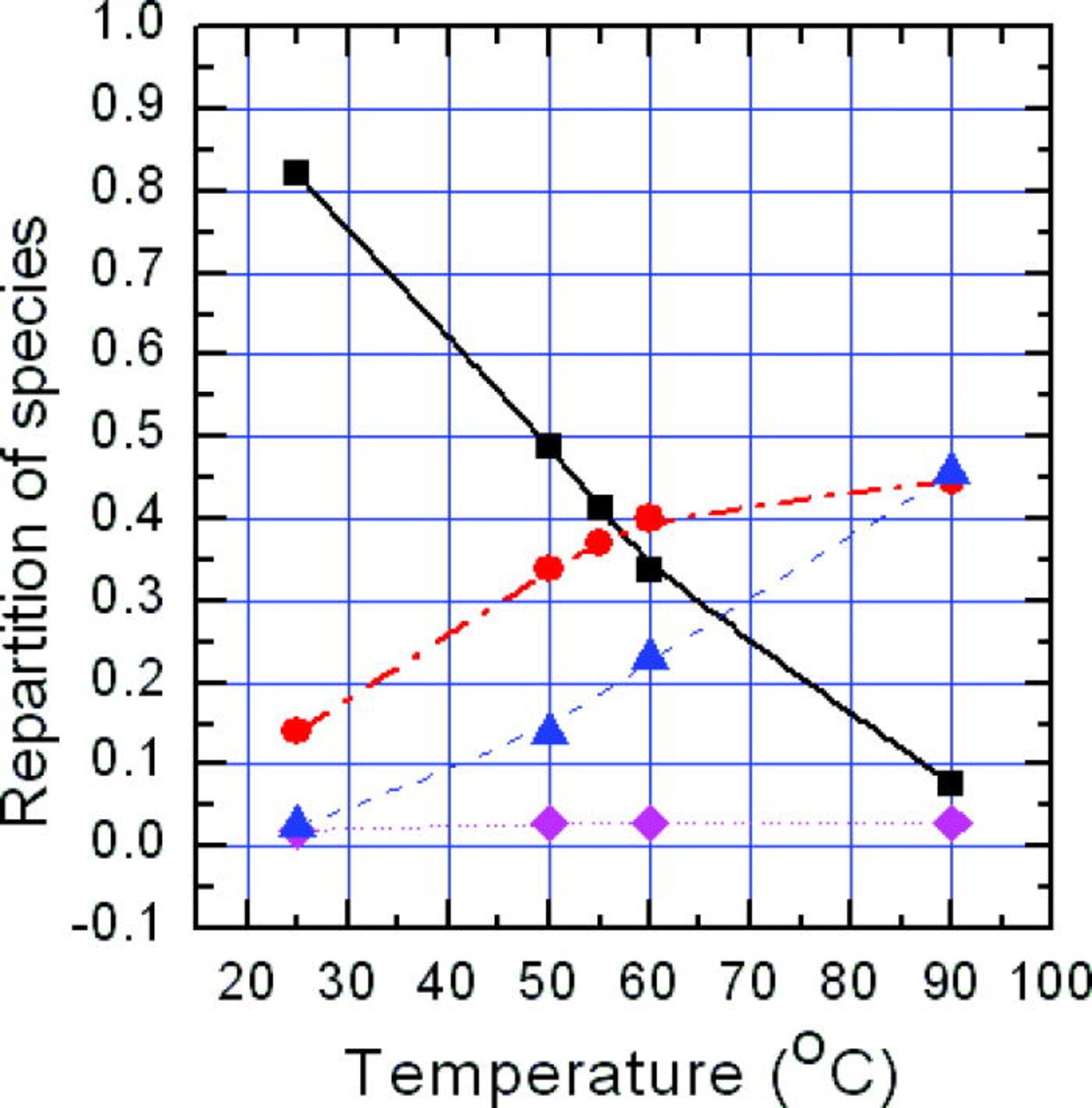
ZnCl2 is a minor constituent in the temperature range from 55 to 90°C. However all of the other major zinc species concentrations vary significantly as the temperature increases, as shown in Fig. 1.19 From the Gibbs free energy, ΔG, for the zinc species present,19 only Zn2+ is less stable than ZnO, the product phase. The polynuclear complexes, ZnCl+, Zn(OH)+, ZnCl2, are all more stable than the product phase and are thus neglected hereafter.
Figure 1. (Color online) The repartition of zinc soluble complexes as a function of solution temperature at pH 6.3. Symbols represent different zinc species: ▪ Zn2+; • ZnCl+; ▴ Zn(OH)+; ♦ ZnCl2.
During electrodeposition, the production of Zn2+ on the anode is described by
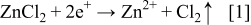
Two possibilities for O2 electro reduction on cathode side have been discussed19, 20 for the case of a Au electrode – these are shown by Eqs. 2, 3 below for the case of acidic and alkali (pH > 6; our case) conditions, respectively
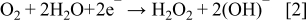
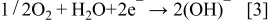
With ZnO formation occurring through the reaction of Zn2+ and OH− ions in solution
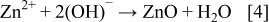
Considering the redox reactions on the counter and working electrodes in the cell, the ZnO synthesis may be described under our conditions by
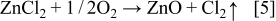
This equation may be expressed in terms of the precursor concentrations as
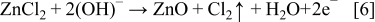
Equation 6 indicates that the precursor ratio, [ZnCl2]/[OH−], should be 1:2 to generate ZnO. In our process, the Zn2+ and OH− concentrations can be estimated from the ZnCl2 concentration, the O2 concentration, the deposition temperature and the current density. Three limiting regimes may be used to describe the electrodeposition process, (1) [Zn2+] ≪ [OH−], (2) [Zn2+] < [OH−], and (3) [Zn2+] ≈ [OH−].
Results and Discussion
Influence of varying the ZnCl2 concentration in the electrolyte
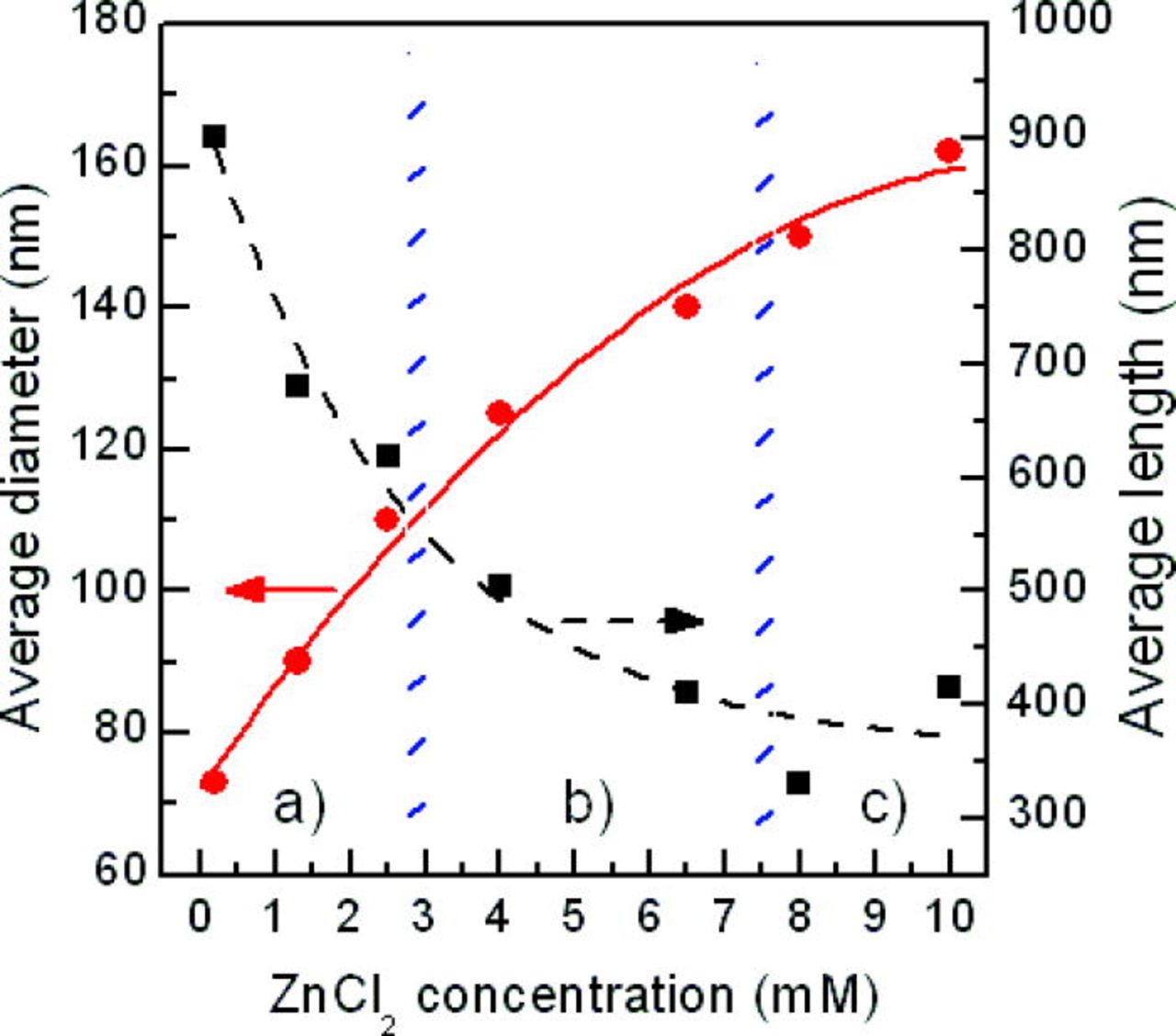
Figure 2 shows the results of varying the ZnCl2 concentration on the dimensions of the nanowires, in terms of their diameter and length. The dimensions of the nanowires were calculated from a statistical analysis of the SEM images. The average nanowire diameter increases, and length decreases, with increasing ZnCl2 concentration, saturating at high ZnCl2 concentrations. This is consistent with the findings of other researchers.21, 22
Figure 2. (Color online) Dependence of nanowire dimensions (diameter and length) on ZnCl2 concentration in electrolyte, at a growth temperature of 80°C and potential to reference electrode of 0.95 V. Also shown are the regimes: (a) [Zn2+] « [OH−], (b) [Zn2+] < [OH−] and (c) [Zn2+] ≈ [OH−]. Symbols represent: • average nanowires diameter (nm) and ▪ average nanowires length (nm).
On application of an electric field, reduction of oxygen occurs, leading to the production of OH− ions on the surface of the working electrode, as well as in the solution. Holes enter the circuit via the counter electrode and oxidize ZnCl2 to form Zn2+ ions and other Zn-related species. In our experiments it was observed that the nanowire electrodeposition process may be divided into two distinct phases: a nucleation stage lasting around 2 min, and a growth phase thereafter.
The nucleation stage on the cathode is a very important step which mostly determines the nanowire diameter and areal density. The nucleation rate depends on the OH− and Zn2+ ion concentrations which depend, in turn, on the current density and electromigration in the electrolyte solution. These depend on the applied potential. If the potential is insufficient, nucleation on the working electrode will not occur. Our experiments show that when an appropriately high potential is applied, even after just 2 min, the diameter of the nanorods achieve a diameter of about half of those grown for 60 min, and the nanorod areal density hardly changes over this period from its initial distribution (established in the first 2 min, for the case of [ZnCl2] at 1 mM and [KCl] at 100 mM, respectively). For a given potential, the higher the Zn2+ ion concentration relative to the OH− concentration, the thicker the nanowires and the lower the areal density. As explained above, only under condition (c) is the desired solution stoichiometry achieved, as described by Eq. 4. In regimes (a) and (b) one is severely and moderately limited, respectively, by the supply of Zn2+ ions relative to the OH− supply. However the volumetric deposition of nanowires is constant at 4.81 ± 2.1)×)10−20 cm3/cm2 of area of substrate with increasing ZnCl2 concentration. This is reflected in the nanowire dimensions and areal density.
A thermochemical analysis of the decomposition of ZnCl2 in 100 mM KCL solution shows that the Zn2+ concentration is quite low – it is about 17% at 80°C and pH 6.3, (see, Fig. 1). In the case of a potential to the reference electrode of 0.95 V, the concentration of electrons, involved in reducing O2 to produce OH−, is calculated from the measured current density. The concentration of electrons is always about three to five orders of magnitude higher than the concentration of Zn2+. Owing to the far lower diffusion coefficient of Zn2+, (6.2–7.4)×)10−6 cm2 s−1 (Refs. 23, 24) in aqueous ZnCl2 solutions as compared with that of molecular oxygen, 3.4)×)10−5 cm2 s−1 (Ref. 25), it is believed that Zn2+ diffusion controls the growth of the ZnO nanowires. In other words, the [ZnCl2] determines the nucleation of the ZnO nanowires once a sufficient bias is applied. This initial critical step determines the initial nanowire diameter and areal density. This areal density remains relatively invariant over the succeeding growth process. As the Zn2+ ions readily react with OH− ions on the tips of the ZnO seeds, ZnO nanowire growth is facilitated. This is predicated by the fact that the wurtzite ZnO crystal structure can be viewed to form from the stacking of two sets tetrahedra – namely, Zn2+ and O2− tetrahedra, and by the sharing of corners, edges or faces of the coordination tetrahedra. Nucleation and growth occurs most favorably along the [0001] direction.22, 26 For every coordination tetrahedron, the terminal vertex of the corner contains an OH− ligand, as shown by IR (Ref. 27) and Raman data.28, 29 An increase in [ZnCl2] in the electrolyte leads to the formation of thicker ZnO nanowires given sufficient OH− on the cathode.
Figure 2 shows three appropriate regimes to describe nanowire formation: (a) [Zn2+] ≪ [OH−], (b) [Zn2+] < [OH−], and (c) [Zn2+] ≈ [OH−]. These conditions correspond to the ZnCl2 concentration being in the following ranges, 0.2–3, 3–7.5, and 7.5–10 mM, respectively. In these three regimes we have the ratio of [Zn2+]/[OH−] as 0.021–0.32, 0.32–0.80, and 0.80–1.06, respectively. In (a), the Zn2+ concentration and transport limit electrochemical reactions and for (b), Zn2+ transport is limited in the reaction process. ZnO nucleation occurs mainly on the tips of the ZnO nanowires in (a), with the diameters being small and the lengths extended. When [ZnCl2] exceeds 7.5 mM in the electrolyte, [Zn2+] is similar to [OH−] surrounding the cathode, with sufficient supply of zinc precursors near the cathode, and the ZnO seeds thicken and elongate.
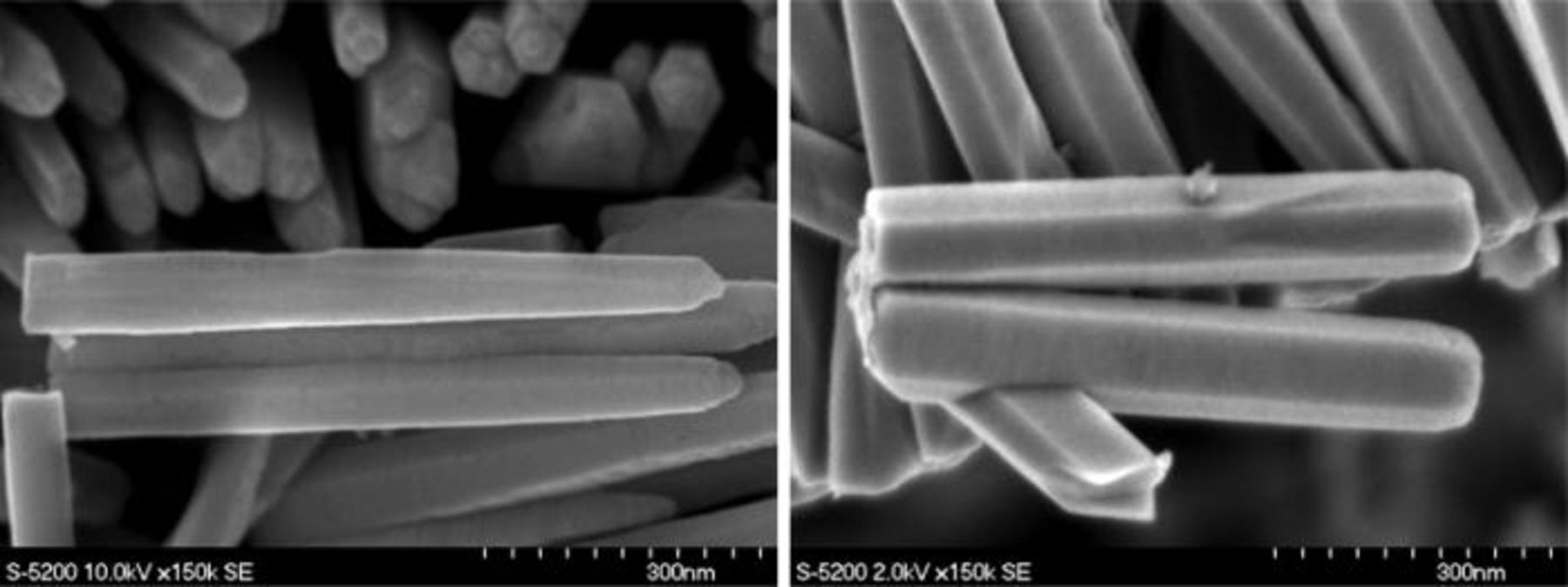
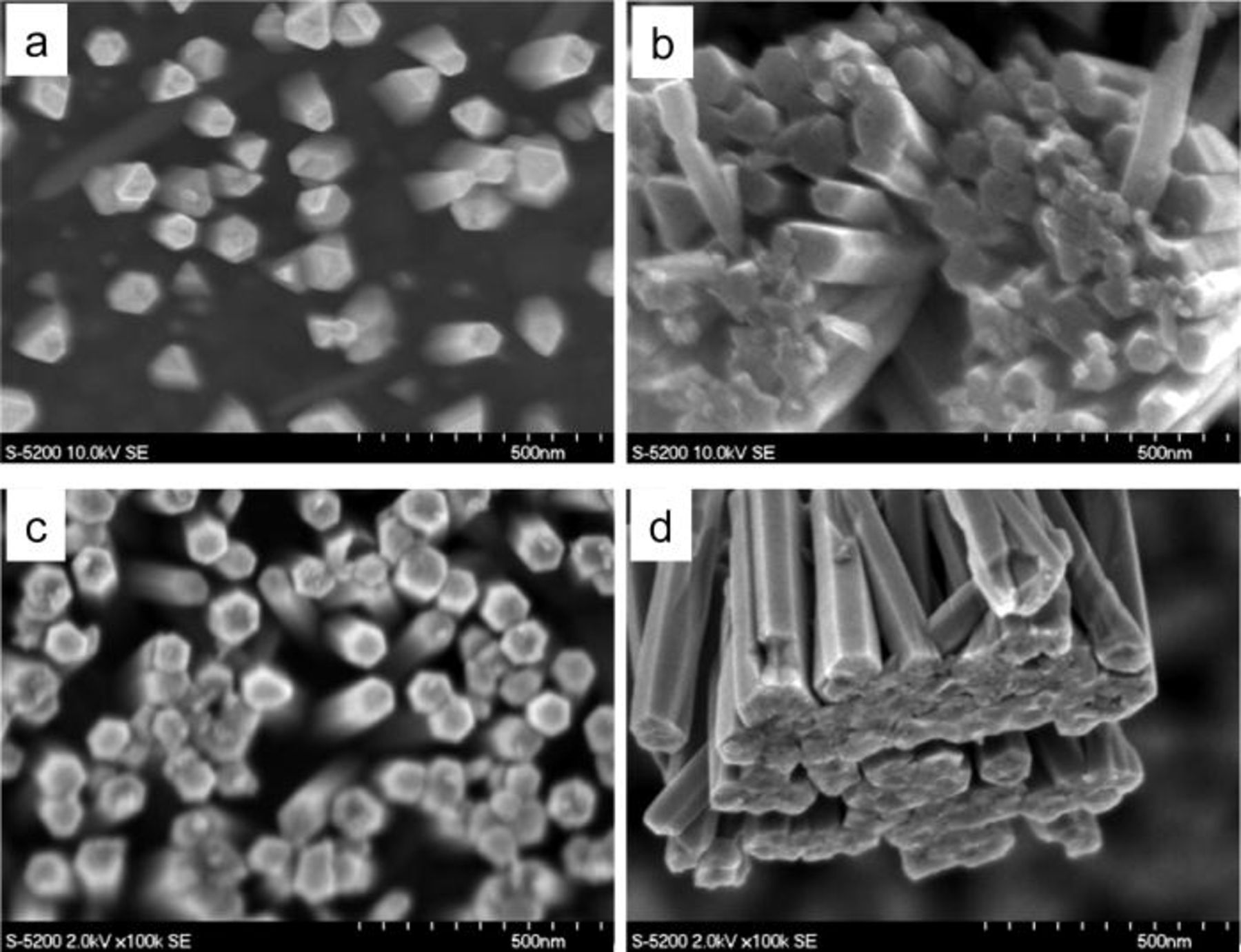
We also observed different nanowire morphologies (i.e., hexagonal columns and hexagonal cones) for different [ZnCl2], as shown in Fig. 3. The discussion of these different morphologies and the role of the associated preparation conditions are beyond the scope of the current paper. Growth conditions including the initial solution pH, concentration of building ions, and exterior potential, all likely contribute to morphological evolution. These parameters will vary the relative growth rate on different facets and hence lead to different morphologies. Figure 4 also shows that nucleation and growth of individual nanowires tends to work in a cooperative fashion, resulting in proximate nanowires coalescing into a pseudo-film, even at low [ZnCl2].
Figure 3. The morphology of nanowires (hexagonal columns and hexagonal cones) for different ZnCl2 concentration in the electrolyte. Left image is for samples grown with ZnCl2 concentrations of 0.5 mM and right for sample grown in 2.8 mM, respectively.
Figure 4. Scanning electron micrographs showing nanowire morphological evolution from relatively independent growth for low ZnCl2 concentrations in electrolyte, to clustered and coalescing growth resulting in pseudo-films for higher Zn concentrations in the electrolyte. a) is top view, [ZnCl2] 0.25 mM, b) bottom view, [ZnCl2] 0.25 mM, c) top view, [ZnCl2] 2.8 mM, and d) bottom view, [ZnCl2] 2.8 mM.
Influence of Synthesis Temperature
To study the influence of synthesis temperature on electrodeposition, electrolyte concentrations of 5 mM for ZnCl2 and 100 mM for KCl were used, with growth temperatures of 55, 60, 65, 70, 80, and 90°C, respectively. Two factors should be considered here – these are (a) the repartition of the species, and (b) the solubility of oxygen in the electrolyte. Since the solubility of zinc species vary depending on the solution pH and temperature, deposition will inevitably depend on these experimental variables. From Fig. 1, it is evident in Zn-Cl-H2O system that the repartitioning of precursors depends sensitively on temperature for a given pH. We can see there is a marked change in the distribution of Zn2+, ZnCl+ and Zn(OH)+ species with temperature. In the temperature range from 55 to 90°C, the Zn2+ fraction dramatically decreases from being the major to the minor species in the solution. The solution temperature also has a significant influence on the decomposition of molecular oxygen. The oxygen solubility, as presented by Hichman,30 decreases markedly, by ∼11% from 55 to 90°C, and so the [OH−] is expected to decrease.
On the basis of repartition of zinc species, as shown in Fig. 1 and the solubility of oxygen at saturation,13 the concentrations of Zn2+ and O2 at 5 mM of [ZnCl2] solution were derived. It was found that the [Zn2+] is more than sufficient to form ZnO from 55 to 70°C, so that the deposition of ZnO is not limited by the Zn2+ concentration. Above 70°C, [Zn2+]/[O2] is less than 2, the theoretical ratio for forming ZnO, and the Zn2+ concentration gradually increases its influence on the process. However for all temperatures from 55 to 90°C, with sufficient ZnCl2 concentration, the Zn2+ concentration is not the key in controlling the precipitation. Indeed, the OH− production rate from O2 is the rate limiting step for the deposition process. At low growth temperatures (e.g., 55°C), [Zn2+] and [O2] are about 2.07 and 0.89 mM, respectively. This corresponds to [Zn2+] ≫ [OH−] close to the cathode, for slow reduction of O2. There is sufficient time for Zn2+ to combine with OH− on the Au film surface to form thicker seeds in the initial nucleation. As the growth temperature increases, the [Zn2+] fraction keeps falling; correspondingly, the OH− production rate rises, such that there is sufficient Zn2+ and OH− to enable crystallization of seeds and deposition of longer nanowires. At even higher growth temperatures than 70°C, the OH− concentration matches that of Zn2+ ions, and thicker seeds grow and lateral and longitudinal deposition increase, providing high growth efficiency.
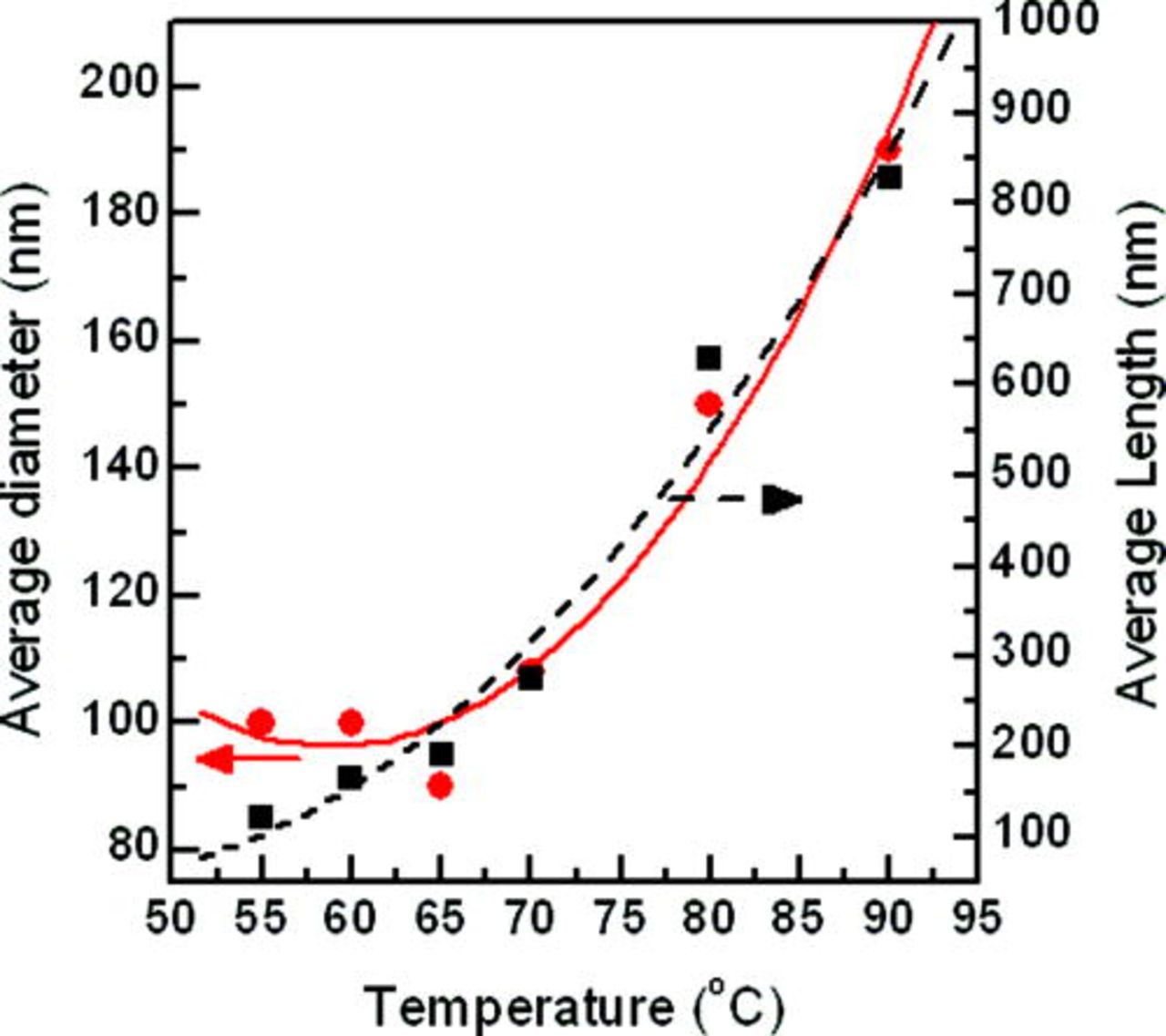
The influence of growth temperature on nanowire dimensions – average nanowire diameter and length versus growth temperature of 55 to 90°C, synthesized in 5 mM of [ZnCl2], are shown in Fig. 5. In the Fig. 5, the two curves are very similar and the dimensions increase with growth temperature.
Figure 5. (Color online) The relationship between ZnO nanowire dimensions, (diameter and length) on growth temperature for six temperatures; 55, 60, 65, 70, 80, and 90°C, using a ZnCl2 concentration in the electrolyte of 5 mM. Symbols represent: • average nanowires diameter (nm) and ▪ average nanowires length (nm).
Conclusion
A systematic study was presented on the role of electro-deposition parameters on preparing tailored dimension vertically-aligned ZnO nanowires. The dimensions, in terms of the diameter and length, and their areal density, were primarily controlled by the growth temperature and ZnCl2 concentration in the electrolyte. Thus, it is possible to tailor the dimensions and have controlled growth of well aligned ZnO nanowires by effective control of the growth parameters.
It was found that the initial 2 min of applied bias are the most important for determining the diameter of ZnO nanowires, as this corresponds to the formation of nuclei which subsequently grow. A study of the electrodeposition mechanism revealed that with sufficient zinc concentration (i.e., 5 mM of [ZnCl2]), the growth process is reaction controlled, reducing O2 to OH− on the cathode, at temperature, below 70°C, and by reaction and transport of Zn2+ to the cathode. In addition, it was observed at 80°C, that electrodeposition is controlled by Zn2+ transport for ZnCl2 concentrations below 3 mM, and controlled by Zn2+ transport and OH− production at high zinc concentrations (3–10 mM).
ACKNOWLEDGMENTS
The authors thank Ilya Gourevich for assisting in scanning electron microscopy. Funding for this work was provided by NSERC, CSA, OCE, and ORF.
University of Toronto assisted in meeting the publication costs of this article.