Abstract
Lithium powder is safely prepared by the droplet emulsion technique and compacted to provide a lithium powder anode. Using the compacted Li powder anode, high specific energy density cells are assembled with a nonlithiated cathode such as  . The capacity and cycle capability of a lithium powder/
. The capacity and cycle capability of a lithium powder/ cell are tested. The lithium powder cell is found to maintain its initial capacity for more than 100 cycles, whereas the lithium foil cell exhibited capacity fading after 37 cycles. The lithium powder with a double-sided cathode cell, whose cathode/anode ratio is about 1/10 in capacity, shows a capacity of
cell are tested. The lithium powder cell is found to maintain its initial capacity for more than 100 cycles, whereas the lithium foil cell exhibited capacity fading after 37 cycles. The lithium powder with a double-sided cathode cell, whose cathode/anode ratio is about 1/10 in capacity, shows a capacity of  at a rate of C/2 for over 40 cycles. The morphological changes of a Li foil and a Li powder anode after 50 cycles are observed by scanning electron microscopy. Impedance behavior of the powder anode cell is also observed and compared to that of the foil cell.
at a rate of C/2 for over 40 cycles. The morphological changes of a Li foil and a Li powder anode after 50 cycles are observed by scanning electron microscopy. Impedance behavior of the powder anode cell is also observed and compared to that of the foil cell.
Export citation and abstract BibTeX RIS
Rechargeable batteries using lithium metal as the negative electrode theoretically offer the greatest promise. Nevertheless, their widespread use has been obstructed due to their intrinsic problems, such as the reactivity of lithium, electrolyte depletion, and dendritic growth during cycling.1–3 For these reasons, although lithium-ion batteries are currently commercialized, their capacity is restricted to that of graphite because the lithium ions are intercalated in the graphitized carbon anode. Therefore, it is expected that they will soon become inadequate to meet the expectations of the future market.4 Consequently, the concept of high surface area lithium may hold the key to the future success of lithium rechargeable batteries. Thus, due to its high surface area, the lithium powder electrode is one of the candidates for advanced lithium rechargeable batteries.
In a previous work, it was revealed that the reactive surface area of a compacted lithium powder electrode was roughly 6 times that of a comparable foil electrode.5 The typical microstructure of a compacted lithium powder electrode is shown in Fig. 1, where the porous characteristics of the lithium powders can be clearly observed. This larger reactive surface area lowers the effective current density in the electrode surface, which suppresses the dendrite formation and reduces the electrolyte depletion of the lithium powder cell during cycling. As a result, the cycling efficiency of the lithium powder cell was improved by  , while its cycle life was more than double that of the lithium foil cell.6–8 However, the results of previous studies using a lithium powder electrode were obtained only in a symmetric cell or half-cell system. The lithium powder electrode has not been applied to a full cell, and the capacity and cycle behavior of the lithium powder cell have not been reported quantitatively. To conduct experiments using a full cell, the selection of the cathode material is very important. That is, the selected cathode material should not include lithium, and the cell should be capable of handling the large electric charge of lithium. For this reason,
, while its cycle life was more than double that of the lithium foil cell.6–8 However, the results of previous studies using a lithium powder electrode were obtained only in a symmetric cell or half-cell system. The lithium powder electrode has not been applied to a full cell, and the capacity and cycle behavior of the lithium powder cell have not been reported quantitatively. To conduct experiments using a full cell, the selection of the cathode material is very important. That is, the selected cathode material should not include lithium, and the cell should be capable of handling the large electric charge of lithium. For this reason,  was chosen in this study as the cathode material to meet the former requirement, and a double-sided cathode cell was fabricated to meet the latter. The double-sided cathode cell is schematically shown in Fig. 2. Therefore, the electrochemical performance of a lithium powder electrode was investigated in a
was chosen in this study as the cathode material to meet the former requirement, and a double-sided cathode cell was fabricated to meet the latter. The double-sided cathode cell is schematically shown in Fig. 2. Therefore, the electrochemical performance of a lithium powder electrode was investigated in a  rechargeable battery. A lithium powder cell was prepared to determine its cycle capability and to measure its achievable capacity quantitatively. The test results were also compared to those of a lithium foil cell.
rechargeable battery. A lithium powder cell was prepared to determine its cycle capability and to measure its achievable capacity quantitatively. The test results were also compared to those of a lithium foil cell.
Figure 1. Morphology of the compacted lithium powder electrode.
Figure 2. Schematic illustration of the double-sided cathode cell.
Experimental
Lithium powders were made safely by the droplet emulsion technique (DET). The DET process has been presented in detail elsewhere.9–11 The lithium powders have a diameter of  to
to  . To form them into the shape of an electrode, the lithium powders were compacted in a
. To form them into the shape of an electrode, the lithium powders were compacted in a  square by pressing them onto an stainless steel (SUS) mesh with a pressure of
square by pressing them onto an stainless steel (SUS) mesh with a pressure of  (Fig. 1). The surface area of the lithium powder electrode, determined by the linear sweep voltammetry method,5 was roughly 6 times that of a foil electrode and had a porosity of 11.8% as measured using image analysis with Image-Pro (Media Cybernetics, MD) based on a quantitative microscopic theory.8
(Fig. 1). The surface area of the lithium powder electrode, determined by the linear sweep voltammetry method,5 was roughly 6 times that of a foil electrode and had a porosity of 11.8% as measured using image analysis with Image-Pro (Media Cybernetics, MD) based on a quantitative microscopic theory.8
Two kinds of lithium electrodes were used as the anode. One was the as-received lithium foil electrode obtained from Cyprus Co. (CO, 99.9%). The other was a compacted lithium powder electrode manufactured in the laboratory. As a cathode, a mixture of  comprising 85 wt %
comprising 85 wt %  , 10 wt % Ketjen black, and 5 wt % poly(vinylidene fluoride) (PVDF) was used. A premixed mixture of
, 10 wt % Ketjen black, and 5 wt % poly(vinylidene fluoride) (PVDF) was used. A premixed mixture of  and Ketjen black was mixed with PVDF in
and Ketjen black was mixed with PVDF in  -methyl-2-pyrrolidone using a homogenizer to provide the
-methyl-2-pyrrolidone using a homogenizer to provide the  paste, which in turn was cast on aluminum foil. The cathode was dried in a fan-circulated oven, roll-pressed, and dried again in a vacuum oven. The electrolyte consisted of propylene carbonate (PC) solutions containing 1 M
paste, which in turn was cast on aluminum foil. The cathode was dried in a fan-circulated oven, roll-pressed, and dried again in a vacuum oven. The electrolyte consisted of propylene carbonate (PC) solutions containing 1 M  , and polypropylene was used as a separator. The various parts were assembled to form a pouch-type cell.
, and polypropylene was used as a separator. The various parts were assembled to form a pouch-type cell.
Cycle tests were performed for both the lithium foil and the lithium powder cells. The cells were discharged with a cutoff voltage of 2.7 V, and then charged with an upper potential limit of 4.0 V. The cells were cycled at a constant current density (C/2 rate) of  during cycling. After cycling, both of the lithium electrodes were washed with pure PC to remove the residual electrolyte and then dried in an argon-filled glove box at room temperature. To observe the morphological changes, the morphology of the lithium deposited on the lithium electrodes was characterized after the 50th cycle using a field emission scanning electron microscope (SEM) (Horiber 7200-H).
during cycling. After cycling, both of the lithium electrodes were washed with pure PC to remove the residual electrolyte and then dried in an argon-filled glove box at room temperature. To observe the morphological changes, the morphology of the lithium deposited on the lithium electrodes was characterized after the 50th cycle using a field emission scanning electron microscope (SEM) (Horiber 7200-H).
The electrochemical behaviors of the lithium electrodes were also analyzed by impedance measurements (Solartron 1255 frequency response analyzer), which were performed at open-circuit voltage. The amplitude of the applied alternating potential was ±5 mV, and its frequency was varied from 100 kHz to 0.01 Hz at room temperature.
A double-sided cathode cell was also fabricated to maximize the capacity of the lithium powder cell and the cathode/anode (C/A) capacity ratio. The C/A ratio of the regular cell is about 1/40, while that of the double-sided cathode cell is about 1/10 in capacity. Because of the morphology of the cell and an anode pressing method, the C/A ratio in the double-sided cathode cell increased 5 times. The  paste was cast on aluminum foil with a thickness of
paste was cast on aluminum foil with a thickness of  . An anode was prepared through the compaction of the lithium powder on an SUS mesh. The lithium powder weighs about 2 mg per unit area
. An anode was prepared through the compaction of the lithium powder on an SUS mesh. The lithium powder weighs about 2 mg per unit area  . The double-sided cathode cell is schematically shown in Fig. 2. The same experiments were carried out at a constant current density of
. The double-sided cathode cell is schematically shown in Fig. 2. The same experiments were carried out at a constant current density of  in the potential range from 2.7 to 4.0 V. For the double-sided cell, the C-rate was expressed differently by each mass of electrode. In other words, this current density corresponds to a C/8 rate on the anode side and a C/2 rate on the cathode side.
in the potential range from 2.7 to 4.0 V. For the double-sided cell, the C-rate was expressed differently by each mass of electrode. In other words, this current density corresponds to a C/8 rate on the anode side and a C/2 rate on the cathode side.
Results and Discussion
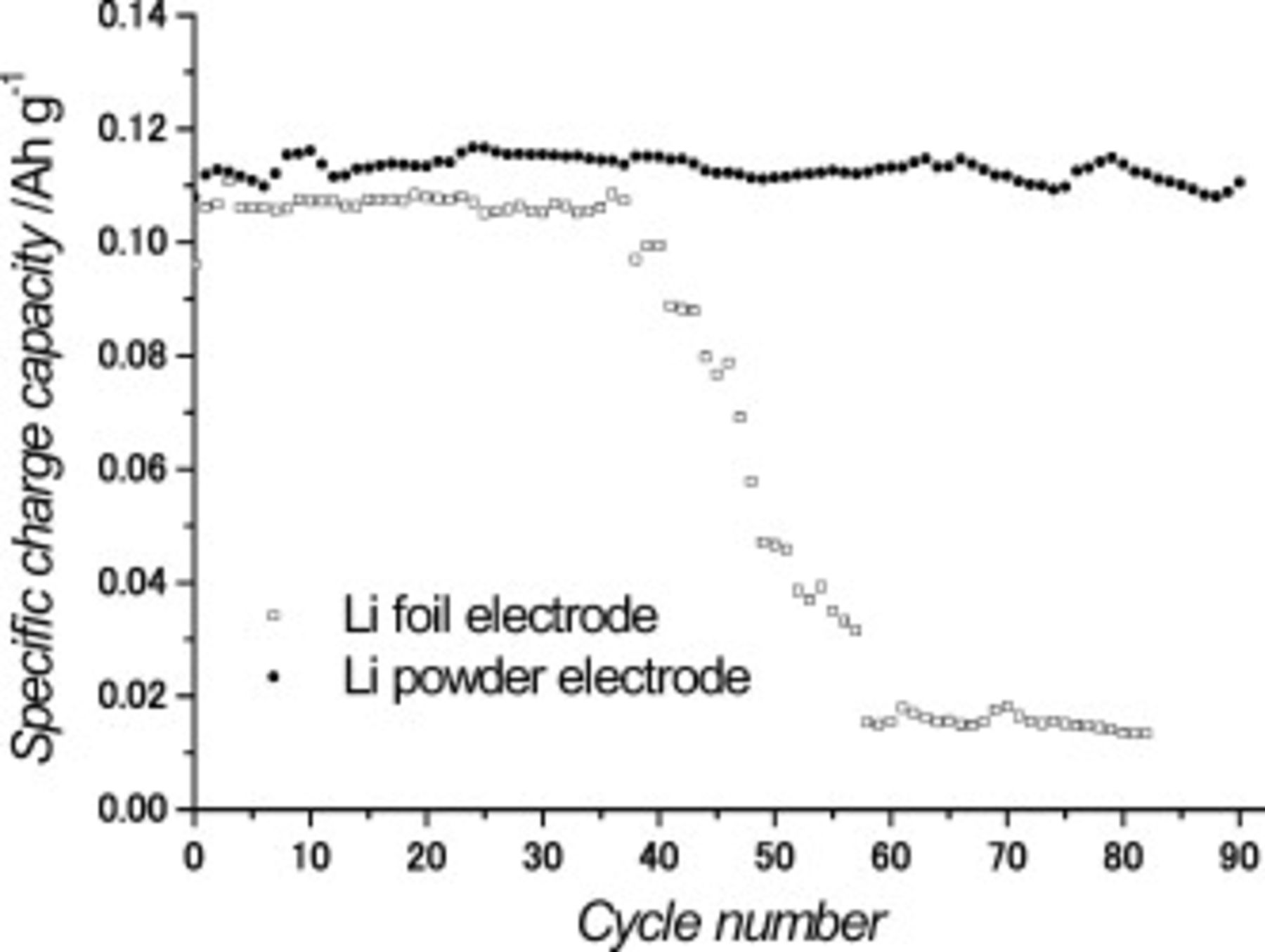
Two kinds of lithium cells (foil and powder) were cycled at a C/2 rate corresponding to a current density of  . The cells were discharged with a cutoff voltage of 2.7 V, and then charged with an upper potential limit of 4.0 V. Figure 3 shows the specific charge capacity per cathode mass in the lithium foil and powder cells during cycling. At the first cycle, the capacity was lower than that of the other cycles because the manufactured cathode (a
. The cells were discharged with a cutoff voltage of 2.7 V, and then charged with an upper potential limit of 4.0 V. Figure 3 shows the specific charge capacity per cathode mass in the lithium foil and powder cells during cycling. At the first cycle, the capacity was lower than that of the other cycles because the manufactured cathode (a  mixture) did not have a layered structure yet. After the first cycle, however, the cells appeared to have a considerably uniform and stable capacity, which is close to the theoretical value of the cathode material. The charge capacity of the lithium powder cell was slightly higher than that of the lithium foil cell. Because the reactive surface area of the lithium powder electrode was larger than that of the lithium foil electrode, the lithium foil and powder electrodes exhibited different effective current densities when the cells were cycled at constant current density. Also, although both of the cells had the same configuration and were examined under the same experimental conditions, the lithium powder cell exhibited a higher charge.
mixture) did not have a layered structure yet. After the first cycle, however, the cells appeared to have a considerably uniform and stable capacity, which is close to the theoretical value of the cathode material. The charge capacity of the lithium powder cell was slightly higher than that of the lithium foil cell. Because the reactive surface area of the lithium powder electrode was larger than that of the lithium foil electrode, the lithium foil and powder electrodes exhibited different effective current densities when the cells were cycled at constant current density. Also, although both of the cells had the same configuration and were examined under the same experimental conditions, the lithium powder cell exhibited a higher charge.
Figure 3. Specific charge capacity in the lithium foil and lithium powder cells at C/2 rate  .
.
As shown in Fig. 3, the lithium powder cell maintained its initial capacity for more than 100 cycles, whereas the capacity of the lithium foil cell decreased dramatically after 37 cycles. This sudden capacity drop of the lithium foil cell may indicate the occurrence of dendritic growth on the lithium electrode surface. Generally, when lithium foil cells are discharged, the lithium dendrite sometimes becomes cut and isolated from the lithium surface.12–14 This means that the active anode material is consumed, and the cell resistance is increased during cycling. As a result, its cyclability becomes poor.15–17 Meanwhile, the lithium powder cell exhibited good capacity retention even under relatively high current conditions (C/2 rate), which agrees with the previous research which indicated that the cyclability of the lithium powder cell was better than that of the lithium foil cell in a half-cell system.6
To examine the cause of the improved cyclability in the lithium powder cell, SEM observation and impedance analysis were performed. After the 50th cycle, the morphology of the lithium metal electrodes was observed by SEM. Figure 4 shows the SEM images on the surface of the lithium foil and powder electrode after the 50th cycle at a C/2 rate. For the lithium foil electrode, significant lithium dendritic growth was observed and scattered pits were also localized in a limited area on the lithium surface (Fig. 4a). At high magnification, the morphology of these deposits resembled that of a mass, in which a bunch of dendrites were twisted and aggregated.18–21 Compared with the lithium foil electrode, there remained almost no dendrites on the surface of the lithium powder electrode, and the individual powder particles retained a lump shape, if not their original spherical shape. Also, the connections between most of the particles were retained (Fig. 4b). From these results, it was concluded that the dendritic growth was considerably suppressed until the 100th cycle in the lithium powder electrode.
Figure 4. SEM images of the (a) lithium foil surface and (b) lithium powder surface after the 50th cycle at C/2 rate  .
.
Figure 5 shows the Cole–Cole plots of the lithium foil and powder electrodes. The resistance of the surface film on the lithium electrode was determined from the diameter of the first semicircle in the Cole–Cole plot in accordance with a previous paper.22, 23 The interfacial resistance of the lithium powder electrode was slightly higher than that of the lithium foil electrode before cycling (Fig. 5a). A very large amount of surface film [solid electrolyte interface (SEI)] was formed on the lithium powder electrode at the beginning due to its large reactive surface area. Therefore, the initial interfacial resistance of the lithium powder electrode was larger than that of the lithium foil electrode.6 On the contrary, the interfacial resistance of the lithium powder electrode was  times lower than that of the lithium foil electrode after the 50th cycle (Fig. 5b). It means that the lithium powder electrode was restrained from further reaction with electrolyte because a tight and protective SEI was preferentially formed on the lithium powder electrode at the beginning. From the SEM observation and impedance measurements, the improvement of the cyclability in the lithium powder cell was possibly caused by a combination of the suppression of dendritic growth and the decrease of the interfacial resistance.
times lower than that of the lithium foil electrode after the 50th cycle (Fig. 5b). It means that the lithium powder electrode was restrained from further reaction with electrolyte because a tight and protective SEI was preferentially formed on the lithium powder electrode at the beginning. From the SEM observation and impedance measurements, the improvement of the cyclability in the lithium powder cell was possibly caused by a combination of the suppression of dendritic growth and the decrease of the interfacial resistance.
Figure 5. Cole–Cole plot for the lithium powder and lithium foil electrodes (a) before cycling and (b) after the 50th cycle.
The achievable capacity record of the Li powder cell was also studied because the need for high capacity was the major reason for choosing lithium metal as the anode material. Because the cathode (a  mixture) has a very low capacity
mixture) has a very low capacity  compared with the lithium anode
compared with the lithium anode  , the mass of the lithium powder is too high for cell designs, such as a general pouch cell. Therefore, the cell design must be that of a double-sided cell (Fig. 2) which is able to increase the cathode mass and to decrease the anode mass. After the fabrication of the double-sided cathode cell, cycle tests were carried out at a constant current density of
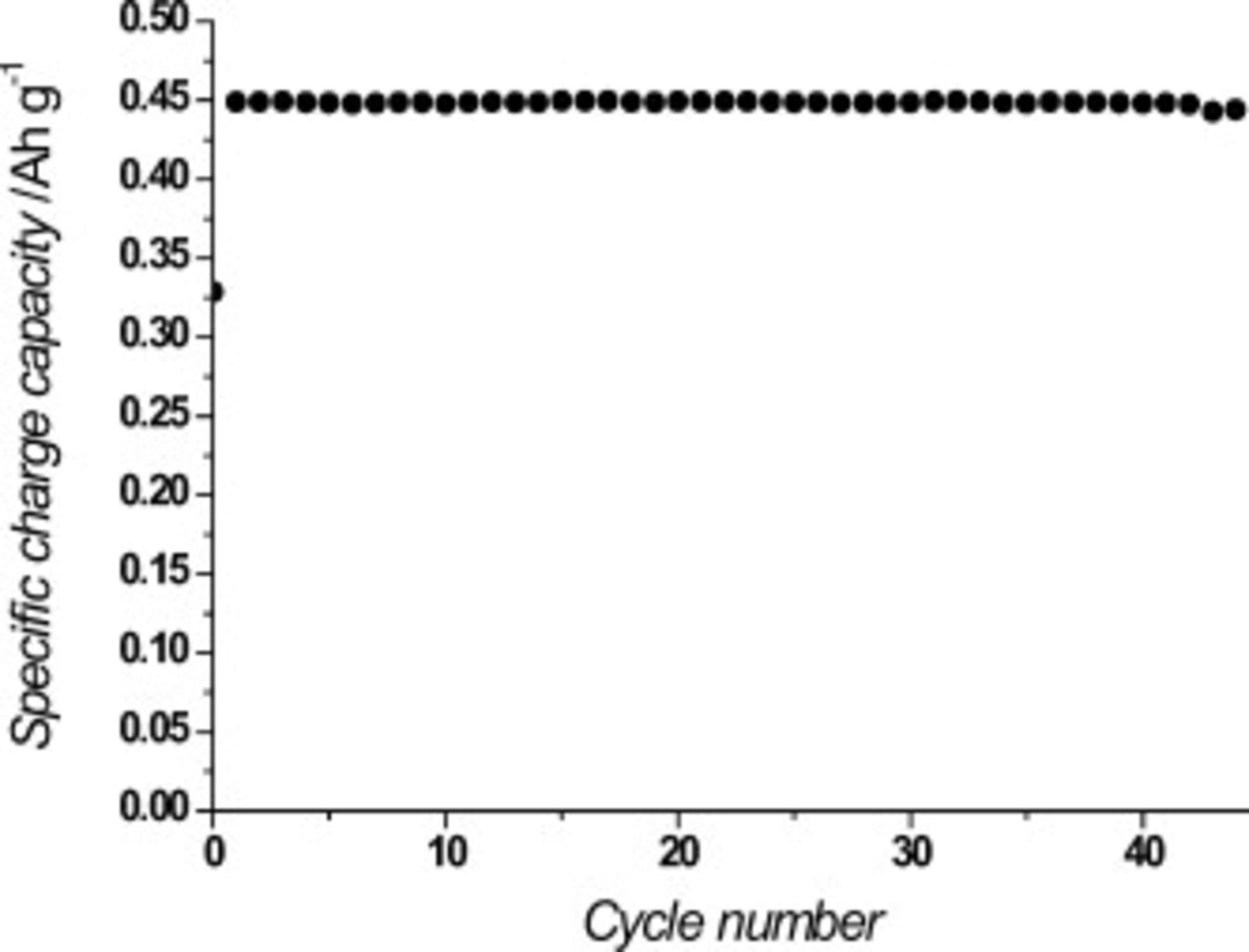
, the mass of the lithium powder is too high for cell designs, such as a general pouch cell. Therefore, the cell design must be that of a double-sided cell (Fig. 2) which is able to increase the cathode mass and to decrease the anode mass. After the fabrication of the double-sided cathode cell, cycle tests were carried out at a constant current density of  corresponding to a C/8 rate on the anode side and a C/2 rate on the cathode side. Figure 6 shows the specific charge capacity in the double-sided cathode cell using the lithium powder electrode during cycling. Its average capacity was about
corresponding to a C/8 rate on the anode side and a C/2 rate on the cathode side. Figure 6 shows the specific charge capacity in the double-sided cathode cell using the lithium powder electrode during cycling. Its average capacity was about  based on the weight of lithium anode, and good capacity retention was demonstrated for more than 40 cycles. Although it is still limited by the restricted capacity of the
based on the weight of lithium anode, and good capacity retention was demonstrated for more than 40 cycles. Although it is still limited by the restricted capacity of the  cathode, the cell shows a reasonably high capacity and sustains the capacity for pretty long cycles. This promising result suggests that if the cathode can be thickened without any loss in its ion conductivity or its intrinsic capacity can be raised, a cell having a capacity greater than that of the current commercial cells could be obtained using the lithium powder anode.
cathode, the cell shows a reasonably high capacity and sustains the capacity for pretty long cycles. This promising result suggests that if the cathode can be thickened without any loss in its ion conductivity or its intrinsic capacity can be raised, a cell having a capacity greater than that of the current commercial cells could be obtained using the lithium powder anode.
Figure 6. Specific charge capacity per anode mass in double-sided cell at C/8 rate.
Conclusions
Lithium powder was safely prepared by the DET and compacted to provide a compacted lithium powder anode. The compacted lithium powder was superior to the lithium foil as the anode material of a lithium secondary battery. The lithium powder cell maintained its initial capacity for more than 100 cycles. To maximize the capacity of the lithium powder, a cathode double-sided cell was fabricated, and its cell capacity of  was maintained for more than 40 cycles. In conclusion, a lithium metal rechargeable battery which has a high specific energy density was developed using a lithium powder anode by selecting a cathode material which has a large electric charge and developing a suitable cell design. That is, the careful selection of the cathode and proper cell design can further increase the capacity of the lithium powder cell.
was maintained for more than 40 cycles. In conclusion, a lithium metal rechargeable battery which has a high specific energy density was developed using a lithium powder anode by selecting a cathode material which has a large electric charge and developing a suitable cell design. That is, the careful selection of the cathode and proper cell design can further increase the capacity of the lithium powder cell.
Acknowledgments
This work was supported by the Korea Research Foundation grant funded by the Korean government (KRF-2008-313-D01288) and by the Korea University grant.
Korea University assisted in meeting the publication costs of this article.