Abstract
The present study employs a tungsten tracer incorporated into the aluminum substrate to investigate the development of porosity in anodic alumina formed in phosphoric acid electrolyte. An unusual inversion of the tracer distribution is revealed as the tracer layer traverses the barrier region. Although initially incorporated into the barrier layer at locations beneath pore bases, associated with the scalloped metal/oxide interface, the tracer at these locations subsequently lags behind that found at the cell walls. The behavior is contrary to expectations of a field-assisted dissolution model of pore development, with usual migration behaviors of film species in the barrier layer. However, the findings are consistent with pore formation due mainly to flow of alumina from the barrier layer toward the cell walls, driven by film growth stresses. Flow of film material can also account for the presence of phosphorus species in the film and the increased thickness of the film relative to that of the oxidized metal.
Export citation and abstract BibTeX RIS
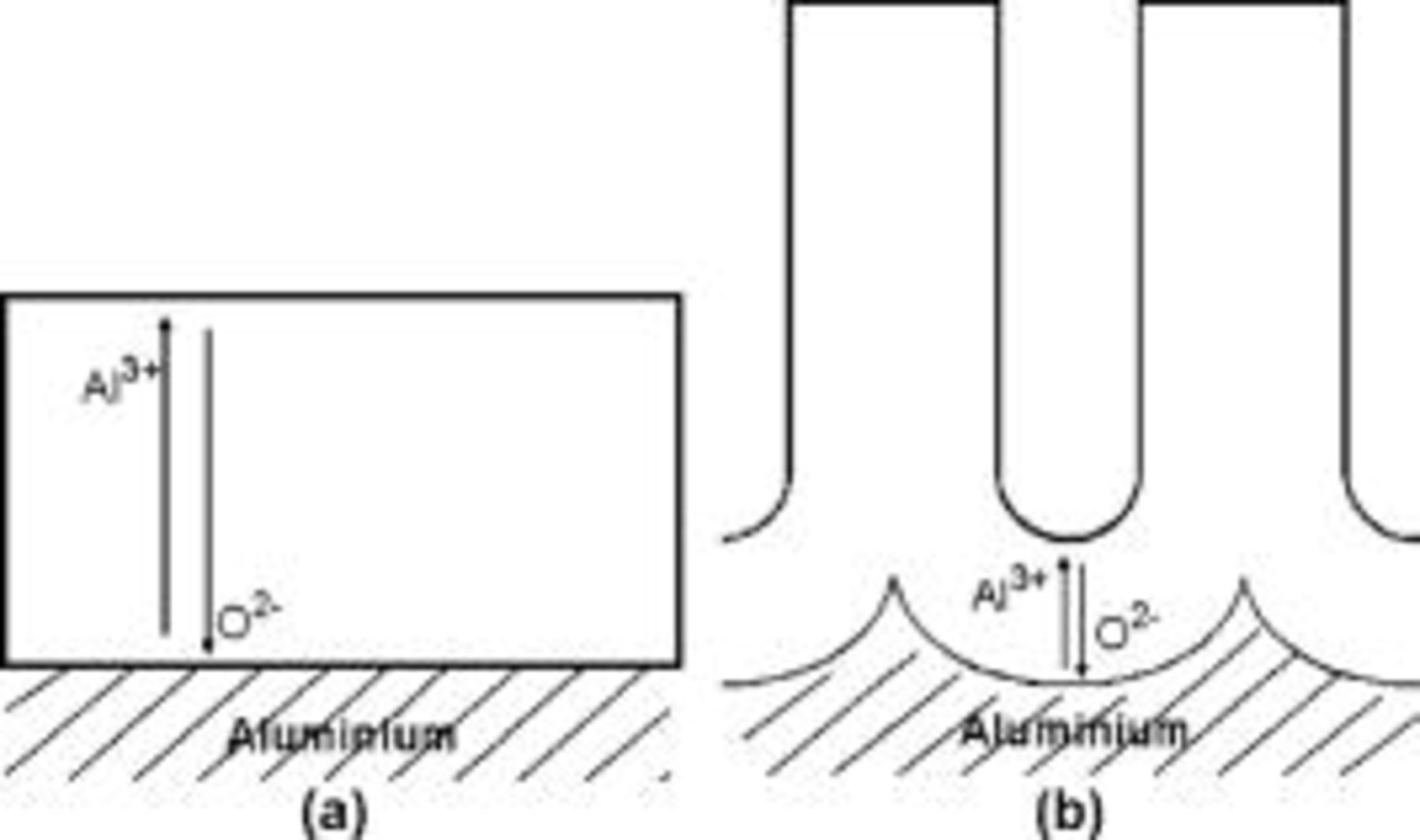
Anodic alumina films are used extensively in protection and functionalization of aluminum alloys, in electronics through aerospace to architecture. The films are usually formed in aqueous electrolytes, with two morphological types recognized that depend upon composition of the electrolyte, pH, current density, voltage, temperature, etc. (Fig. 1).1–4 Barrier films consist of compact, amorphous alumina of uniform thickness, up to a few hundred nanometers. Porous films comprise a thin barrier layer next to the metal and an outer layer of porous alumina, up to tens of  thick.2, 3 The pores are of approximately cylindrical section and extend from the film surface to the barrier layer. The thickness of the barrier layer and the diameter of the pores are related to the forming voltage, with ratios of
thick.2, 3 The pores are of approximately cylindrical section and extend from the film surface to the barrier layer. The thickness of the barrier layer and the diameter of the pores are related to the forming voltage, with ratios of  , while the thickness of the porous layer depends primarily upon the anodizing charge for a particular current density. The porosity has often been explained by massively increased dissolution of the alumina at the pore base under the high electric field of the barrier layer.5 An early suggestion was made of a role of oxide flow in the dissolution process, with flow occurring due to electrostriction stresses, estimated to be of the order
, while the thickness of the porous layer depends primarily upon the anodizing charge for a particular current density. The porosity has often been explained by massively increased dissolution of the alumina at the pore base under the high electric field of the barrier layer.5 An early suggestion was made of a role of oxide flow in the dissolution process, with flow occurring due to electrostriction stresses, estimated to be of the order  and sufficient to deform oxides.6 Notably, the nanoscale pore dimensions and self-regulating pore arrangements are attractive to the optoelectronic and photonics fields, with much activity being directed toward the development of nanopore arrays.7–10
and sufficient to deform oxides.6 Notably, the nanoscale pore dimensions and self-regulating pore arrangements are attractive to the optoelectronic and photonics fields, with much activity being directed toward the development of nanopore arrays.7–10
Figure 1. Schematic diagrams illustrating the morphology of, and ionic migration in (a) barrier and (b) porous anodic films on aluminum.
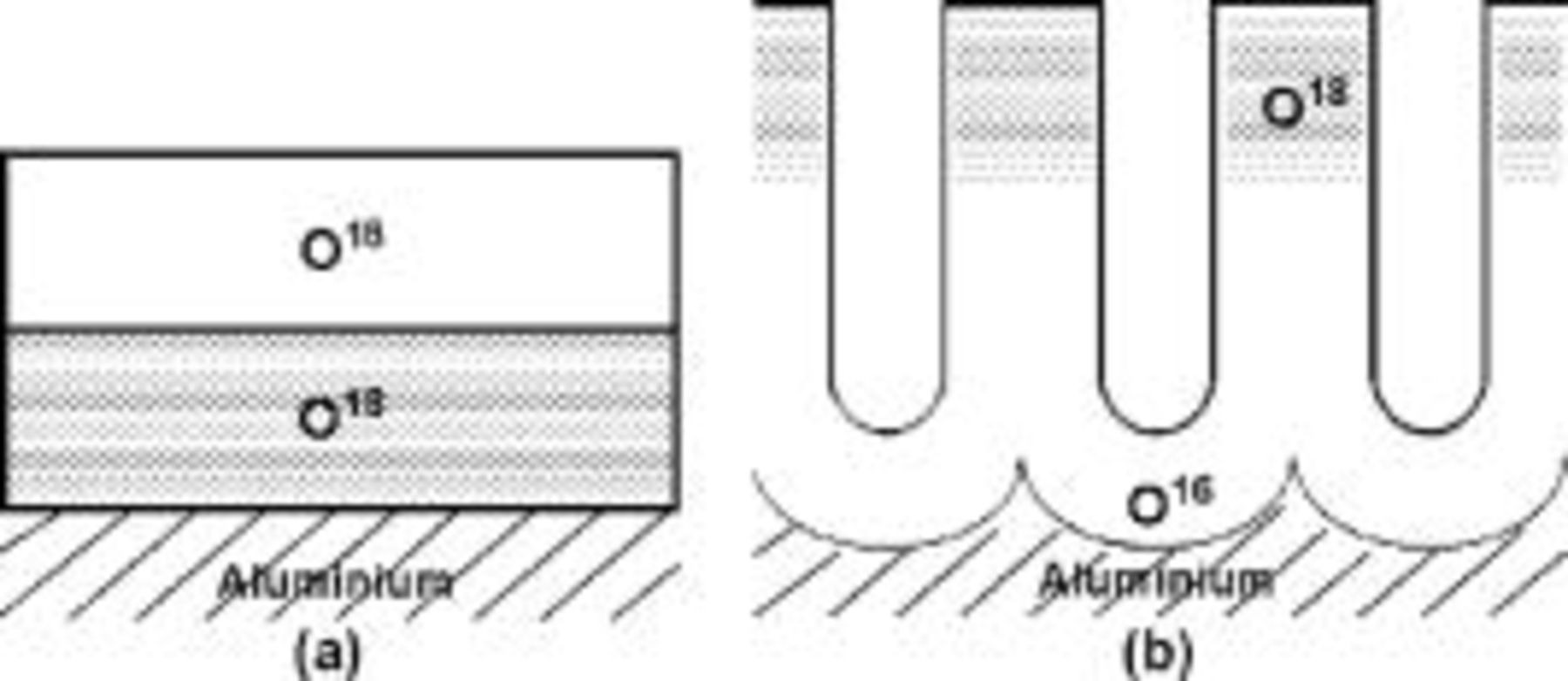
Some previous key studies are of particular relevance to porous alumina growth. During formation of anodic alumina, the ionic current in the film is exponentially dependent upon the electric field.11 Further, movements of oxygen ions during barrier film growth conserve ionic order.12 Thus, when a film is grown in  -enriched electrolyte followed by growth in a nonenriched electrolyte, the
-enriched electrolyte followed by growth in a nonenriched electrolyte, the  is located next to the metal (Fig. 2a). Both
is located next to the metal (Fig. 2a). Both  and
and  ions migrate in anodic alumina, with 40% of the ionic current carried by the cations and the remainder by the anions (Fig. 3a and 3b).13 The ionic transport involves movements of practically all of the ions of the film, resulting in a highly labile film material.14 Other
ions migrate in anodic alumina, with 40% of the ionic current carried by the cations and the remainder by the anions (Fig. 3a and 3b).13 The ionic transport involves movements of practically all of the ions of the film, resulting in a highly labile film material.14 Other  tracer studies revealed contributions of cations and anions to ionic transport in porous films similar to that in barrier films.15, 16 Remarkably, no loss of tracer occurred from porous films, which was attributed to oxide decomposition at the pore base and reincorporation of released oxygen into new oxide.16 Further, the first incorporated oxygen tracer was found at the film surface, which was attributed to short-circuit transport (Fig. 2b). Tracer studies have also indicated that the number of oxygen ions in porous anodic alumina accounts for about 60% of the ionic current for typical conditions of anodizing in sulfuric acid electrolyte.16 The remainder is associated with loss of
tracer studies revealed contributions of cations and anions to ionic transport in porous films similar to that in barrier films.15, 16 Remarkably, no loss of tracer occurred from porous films, which was attributed to oxide decomposition at the pore base and reincorporation of released oxygen into new oxide.16 Further, the first incorporated oxygen tracer was found at the film surface, which was attributed to short-circuit transport (Fig. 2b). Tracer studies have also indicated that the number of oxygen ions in porous anodic alumina accounts for about 60% of the ionic current for typical conditions of anodizing in sulfuric acid electrolyte.16 The remainder is associated with loss of  ions to the electrolyte by field-assisted ejection and dissolution processes. A similar efficiency is recognized from practical experience of aluminum alloys.17
ions to the electrolyte by field-assisted ejection and dissolution processes. A similar efficiency is recognized from practical experience of aluminum alloys.17
Figure 2. Schematic diagrams illustrating findings of oxygen tracer experiments for the growth of (a) barrier and (b) porous anodic films on aluminum. In each case, the anodizing is carried out first in electrolyte enriched in  and second in electrolyte enriched in
and second in electrolyte enriched in  .
.
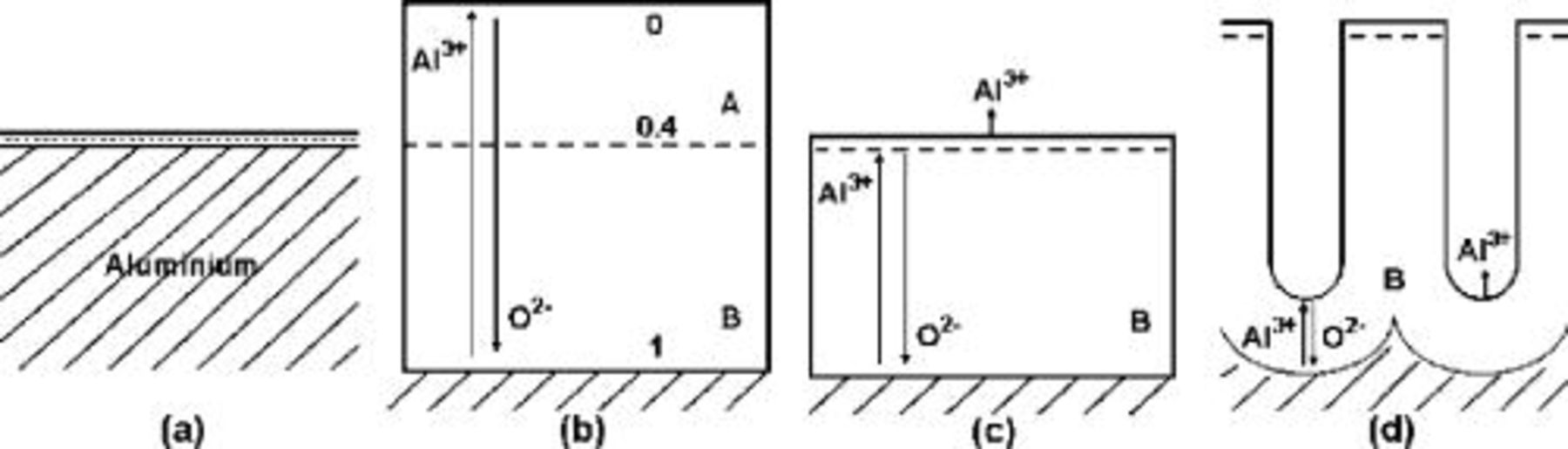
Figure 3. Schematic diagrams illustrating dimensional changes of an aluminum specimen following anodizing. (a) Initial aluminum, with a thin air-formed oxide film. An immobile marker layer, shown as a dashed line, is incorporated into the oxide film. (b) Anodized at 100% efficiency, with formation of a barrier anodic film. The marker layer is located at a depth of 40% of the film thickness, in a plane corresponding to that of the original metal surface. (c) Anodized at just above 60% efficiency, with formation of a barrier anodic film. The plane of the marker layer is the same as in (a, b).  ions are ejected to the electrolyte at the film surface. (d) Anodized at 60% efficiency, with formation of a porous anodic film. The marker plane is located above that of the original metal surface.
ions are ejected to the electrolyte at the film surface. (d) Anodized at 60% efficiency, with formation of a porous anodic film. The marker plane is located above that of the original metal surface.
Marker studies have revealed that the critical condition for pore initiation in anodic alumina occurs when no film material forms at the film surface, i.e., all  ions that migrate to the film surface are ejected to the electrolyte.4 This occurs at an efficiency of about 60%, when the volume of formed oxide is approximately equal to the volume of metal consumed (Fig. 3c). Pore initiation was attributed to the instability of the film surface when subjected to field-assisted dissolution of alumina at local regions where the field was enhanced.
ions that migrate to the film surface are ejected to the electrolyte.4 This occurs at an efficiency of about 60%, when the volume of formed oxide is approximately equal to the volume of metal consumed (Fig. 3c). Pore initiation was attributed to the instability of the film surface when subjected to field-assisted dissolution of alumina at local regions where the field was enhanced.
Of relevance to present interests, an observed increased thickness of porous alumina relative to the metal consumed, by a factor of about 1.35, has been associated with displacement of film material above the original metal surface (Fig. 3d).18 Further, growing anodic films can undergo extensive plastic deformation, as evident from the penetration of fine fingers of anodic tungsten oxide through anodic alumina19 and expansion of gas bubbles within anodic alumina,20 the latter occurring at stress levels of a similar order to those of electrostriction.6 Anodic tantala has also disclosed extensive ductility during its formation.21
The present study investigates further porous film growth on aluminum, using a tracer method. The approach involves incorporation of a fine band of tungsten into the aluminum substrate, followed by observation of the behavior of the tracer when it enters the anodic film. Tungsten is an ideal tracer, because the tungsten species are slow moving within the anodic film compared with  ions22 and are readily apparent using transmission electron microscopy (TEM) due to the strong atomic number contrast.
ions22 and are readily apparent using transmission electron microscopy (TEM) due to the strong atomic number contrast.
Experimental
Aluminum layers were deposited by magnetron sputtering upon specimens of electropolished aluminum, of dimensions  , that had been anodized to
, that had been anodized to  at
at  in
in  ammonium pentaborate solution at
ammonium pentaborate solution at  . These anodizing conditions result in a
. These anodizing conditions result in a  thick, amorphous barrier film, which provides a flat surface for deposition of the aluminum. The sputtering was carried out in an Atom Tech system, with targets of 99.99% aluminum and 99.9% tungsten. The system was first evacuated to
thick, amorphous barrier film, which provides a flat surface for deposition of the aluminum. The sputtering was carried out in an Atom Tech system, with targets of 99.99% aluminum and 99.9% tungsten. The system was first evacuated to  , with aluminum then deposited in 99.999% argon at
, with aluminum then deposited in 99.999% argon at  . During sputtering of the aluminum, the tungsten target was activated briefly to form the tracer layer, of
. During sputtering of the aluminum, the tungsten target was activated briefly to form the tracer layer, of  thickness, in the middle of the aluminum layer. The average composition of the tracer band corresponds to about
thickness, in the middle of the aluminum layer. The average composition of the tracer band corresponds to about  for the selected conditions of sputtering. Specimens were subsequently anodized at
for the selected conditions of sputtering. Specimens were subsequently anodized at  in
in  phosphoric acid solution at
phosphoric acid solution at  ,which develops a steady voltage of about
,which develops a steady voltage of about  . Anodizing employed a two-electrode cell, with an aluminum cathode. For comparison purposes, selected specimens were anodized at
. Anodizing employed a two-electrode cell, with an aluminum cathode. For comparison purposes, selected specimens were anodized at  in
in  ammonium pentaborate solution at
ammonium pentaborate solution at  , which results in barrier film formation at close to 100% efficiency. Ultramicrotomed sections, nominally
, which results in barrier film formation at close to 100% efficiency. Ultramicrotomed sections, nominally  thick, of the aluminum and anodic films were later examined by TEM using a JEOL 2000 FX II instrument operated at
thick, of the aluminum and anodic films were later examined by TEM using a JEOL 2000 FX II instrument operated at  . The movement of the tracer through the anodic oxide was then followed.
. The movement of the tracer through the anodic oxide was then followed.
Results and Discussion
Behavior of the tungsten tracer
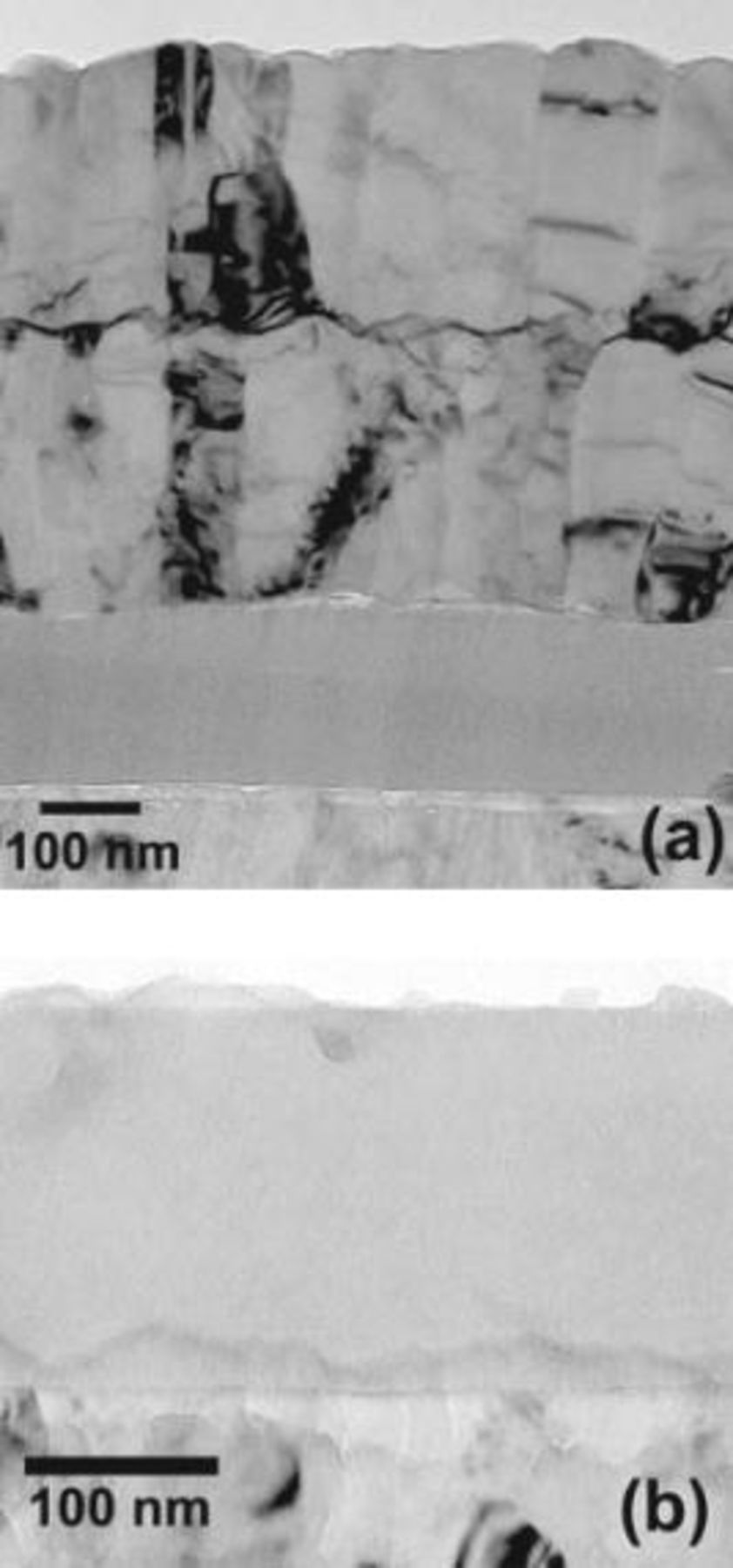
Transmission electron micrographs reveal the tungsten tracer, by atomic number contrast, as a fine, dark band, about  thick, in the columnar-grained sputtering-deposited aluminum (Fig. 4a). The band is wavy due to the fine-scale roughness of the aluminum surface during deposition of the aluminum, which is typical for the conditions of sputtering. The tracer layer remains relatively flat, apart mainly from the influences of the roughness, and of uniform contrast following incorporation into the barrier film formed in ammonium pentaborate electrolyte (Fig. 4b), where it resides as units of
thick, in the columnar-grained sputtering-deposited aluminum (Fig. 4a). The band is wavy due to the fine-scale roughness of the aluminum surface during deposition of the aluminum, which is typical for the conditions of sputtering. The tracer layer remains relatively flat, apart mainly from the influences of the roughness, and of uniform contrast following incorporation into the barrier film formed in ammonium pentaborate electrolyte (Fig. 4b), where it resides as units of  in the amorphous alumina.22
in the amorphous alumina.22
Figure 4. Transmission electron micrographs of ultramicrotomed sections of aluminum with a tungsten tracer layer. (a) Before anodizing. (b) Anodized to  at
at  in
in  ammonium pentaborate solution at
ammonium pentaborate solution at  .
.
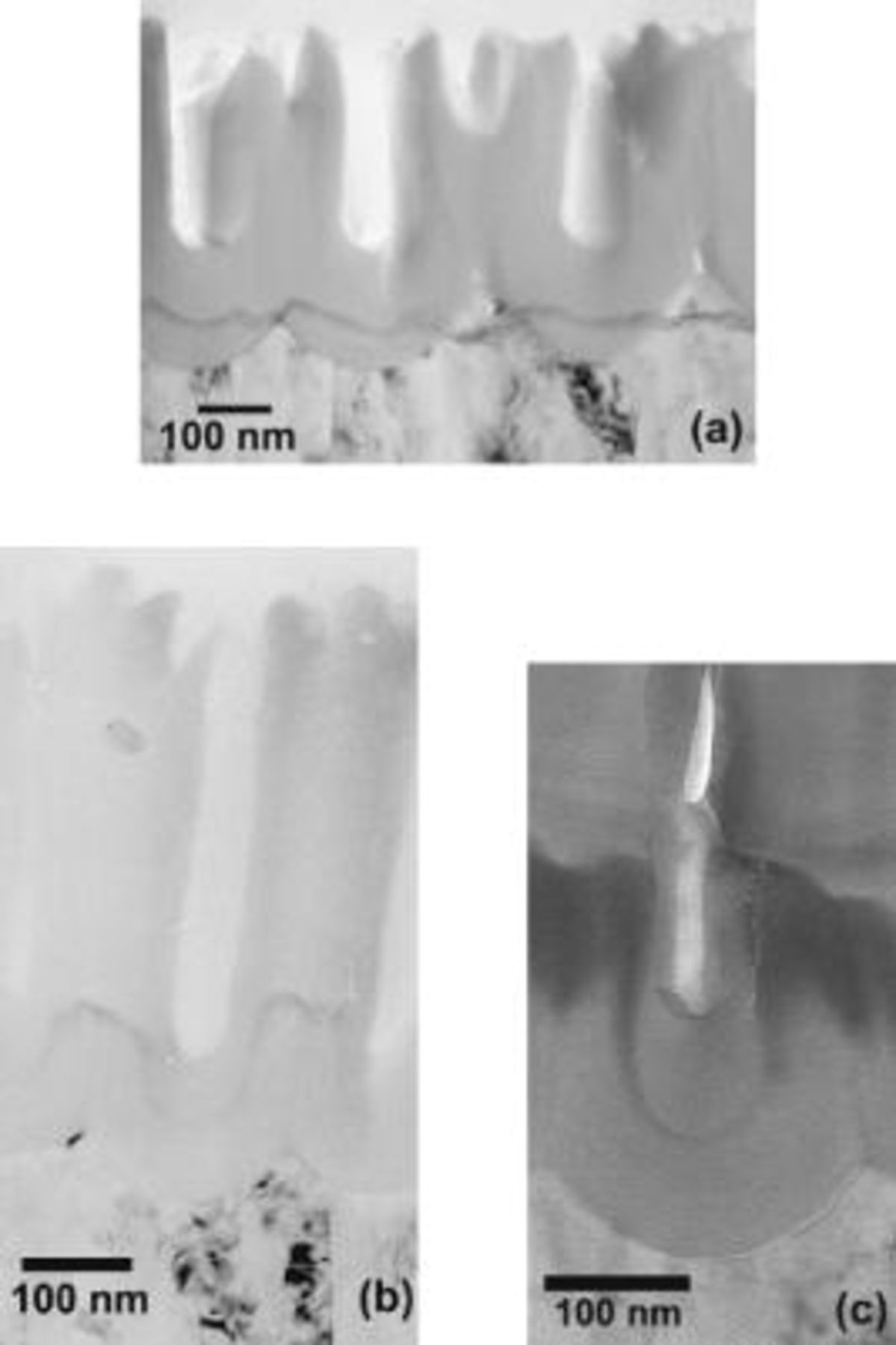
A specimen anodized for  in the phosphoric acid electrolyte captures the tracer at the early stages of incorporation into the porous anodic film (Fig. 5a). In some locations, the tracer is present within the barrier layer, with a separation from the metal/oxide interface of up to about 30% of the thickness of the barrier layer. At other locations, mainly at the peaks in the scalloped metal/oxide interface, the tracer lies within the metal. The thickness of the tracer in the oxide exceeds that in the metal due to the volume increase on conversion of metal into oxide. There may be some additional spreading of the tracer due to local current channeling, associated with the lower ionic resistivity of the tungsten-rich oxide relative to that of the alumina.19 The distribution of the tracer is consistent with its first incorporation into the film at the scalloped regions beneath pores bases, which first intersect the tracer band. The film thickness is
in the phosphoric acid electrolyte captures the tracer at the early stages of incorporation into the porous anodic film (Fig. 5a). In some locations, the tracer is present within the barrier layer, with a separation from the metal/oxide interface of up to about 30% of the thickness of the barrier layer. At other locations, mainly at the peaks in the scalloped metal/oxide interface, the tracer lies within the metal. The thickness of the tracer in the oxide exceeds that in the metal due to the volume increase on conversion of metal into oxide. There may be some additional spreading of the tracer due to local current channeling, associated with the lower ionic resistivity of the tungsten-rich oxide relative to that of the alumina.19 The distribution of the tracer is consistent with its first incorporation into the film at the scalloped regions beneath pores bases, which first intersect the tracer band. The film thickness is  , while the thickness of metal that has been oxidized, determined from the charge passed in anodizing the aluminum for
, while the thickness of metal that has been oxidized, determined from the charge passed in anodizing the aluminum for  , i.e.,
, i.e.,  , is
, is  . Thus, the thickness of the film exceeds the thickness of consumed aluminum by a factor of about 1.38. Behavior of particular interest occurred following further anodizing, to
. Thus, the thickness of the film exceeds the thickness of consumed aluminum by a factor of about 1.38. Behavior of particular interest occurred following further anodizing, to  , when the tracer was located fully within the barrier region of the porous film.
, when the tracer was located fully within the barrier region of the porous film.
Figure 5. Transmission electron micrographs of ultramicrotomed sections of aluminum with a tungsten tracer layer. (a) Anodized for  at
at  in
in  phosphoric acid solution at
phosphoric acid solution at  . (b) As (a), but anodized for
. (b) As (a), but anodized for  . (c) As (b), but with a tracer layer of increased thickness.
. (c) As (b), but with a tracer layer of increased thickness.
Importantly, and contrasting with the relatively flat tracer band of the aluminum substrate, the band of oxidized tracer species was severely distorted within the porous alumina (Fig. 5b), with the tracer at the cell wall regions being located ahead of the tracer beneath the pores. Further, there was a slight downward bending of the tracer band near the cell boundaries. Notably, a diminished contrast indicates a reduced concentration of tungsten in the tracer region beneath the pores. The findings indicate an inversion of the distribution of tungsten within the barrier region, with the first incorporated tungsten, originally at the base of the troughs in the metal/oxide interface, eventually lagging the tungsten incorporated into the cell wall regions. The commencement of the inversion is suggested in Fig. 5a, with the tungsten immediately beneath pores tending to lie slightly below adjacent tungsten, despite tungsten species migrating outward in anodic alumina. Further to the dark appearance of the tracer layer, other contrast variations in the film are evident, such as the slight darkening of the outer region of film material along the cell walls adjacent to the pore of Fig. 5b. However, such variations were not replicated in all cells, and may reflect nonuniformity of the section due to cutting of the porous film material rather than alteration of film composition.
The distribution of tungsten was confirmed for a second specimen with an original tracer layer of increased thickness, about  , to enhance the visibility of the tracer in the film sections (Fig. 5c). The lagging and reduced contrast of the tracer band beneath the pore bases with respect to that in the cell walls were again evident. Further, the tungsten tracer band is continuous within the film, which indicates that there is no transformation of the tungsten species to an anionic type. Such transformation would lead to separation of the cationic and anionic tungsten distributions, due to the opposite directions of tracer migration.
, to enhance the visibility of the tracer in the film sections (Fig. 5c). The lagging and reduced contrast of the tracer band beneath the pore bases with respect to that in the cell walls were again evident. Further, the tungsten tracer band is continuous within the film, which indicates that there is no transformation of the tungsten species to an anionic type. Such transformation would lead to separation of the cationic and anionic tungsten distributions, due to the opposite directions of tracer migration.
According to the conventional model of film growth, the tracer is incorporated initially into the film at regions immediately beneath pores, where the scalloped metal/film interface first intersects the tracer layer. Final incorporation occurs at the cell boundary regions. Within the anodic alumina, the tungsten migrates relatively slowly outward. Thus, the first incorporated tungsten should lie ahead of that finally incorporated into the film. However, the tracer layer is inverted with respect to such expectation, remaining relatively far from the pore base, when elsewhere the tracer has reached the cell walls.
Behavior of incorporated phosphorus species
During anodizing in phosphoric acid electrolyte under the present conditions, phosphorus species are incorporated into the film and are located in an outer region of the barrier layer and the cell walls.23 The inner film region, found next to the metal and at the cell boundaries, consists of phosphorus-free anodic alumina. The thickness of the inner region is about 50% of that of the outer region. A thicker, phosphorus-free layer has been reported for the barrier layer beneath the pores than at the cell boundaries.24 Further, differences in phosphorus concentration within the outer, phosphorus-containing region have been indicated from variations in dielectric constants derived from reflectivity measurements.24 Additionally, an increased water content has been suggested near the pore surface from crystallization studies in the TEM.25 The distributions of species derived from electrolyte anions within anodic alumina are related mainly to their migration behaviors.26 Phosphorus species migrate inward in barrier films more slowly than  ions,27 which results in their location up to a depth of about 40–80% of the film thickness, depending upon the solution pH.27 While such barrier films thicken with time, the barrier layer of a porous film changes negligibly in thickness during anodizing in the steady voltage region. According to the dissolution model, the barrier layer growth is determined by the migration rate of the
ions,27 which results in their location up to a depth of about 40–80% of the film thickness, depending upon the solution pH.27 While such barrier films thicken with time, the barrier layer of a porous film changes negligibly in thickness during anodizing in the steady voltage region. According to the dissolution model, the barrier layer growth is determined by the migration rate of the  ions, while thinning by field-assisted dissolution maintains a constant thickness. However, due to more rapid inward migration of
ions, while thinning by field-assisted dissolution maintains a constant thickness. However, due to more rapid inward migration of  ions than that of phosphorus species, the inner, relatively pure alumina should thicken, while the phosphorus-containing region should diminish due to its dissolution at the pore base. Thus, phosphorus species would be eliminated from the barrier layer.
ions than that of phosphorus species, the inner, relatively pure alumina should thicken, while the phosphorus-containing region should diminish due to its dissolution at the pore base. Thus, phosphorus species would be eliminated from the barrier layer.
Revised growth model
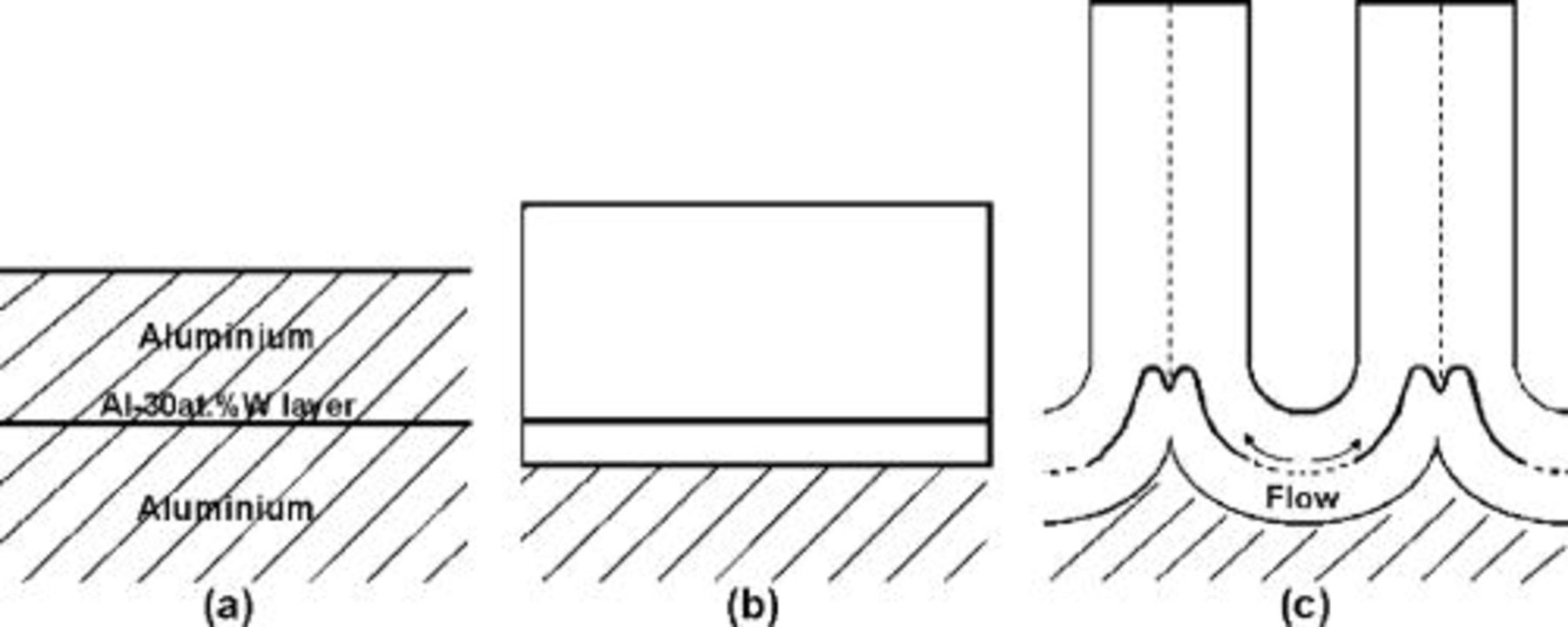
An alternative process to field-assisted dissolution is required to maintain a constant thickness of the barrier layer during the growth of the porous film, while allowing for incorporation and migration of phosphorus species and expansion of the film relative to the thickness of the metal consumed. The simplest possibility is a field-assisted flow of material from the barrier region to the pore walls (Fig. 6). Such flow is compatible with observations of film plasticity14, 19–21 and with the magnitude of electrostriction stress.6 The flow is restricted to the region of ionic transport in the barrier region and is linked to the migration process. The movement of the tracer layer through the barrier region toward the pore base will depend upon the outward migration of  ions and the flow of material above, below, and within the tracer layer. Because the tungsten migrates relatively slowly, most of the tungsten should be displaced to the cell wall region before it can reach the pore base. The displaced material contributes to the increased film thickness with respect to the thickness of oxidized aluminum, by a factor of about 1.35 for the present anodizing conditions.18
ions and the flow of material above, below, and within the tracer layer. Because the tungsten migrates relatively slowly, most of the tungsten should be displaced to the cell wall region before it can reach the pore base. The displaced material contributes to the increased film thickness with respect to the thickness of oxidized aluminum, by a factor of about 1.35 for the present anodizing conditions.18
Figure 6. Schematic diagram illustrating the findings of tracer experiments. (a) Initial aluminum, with a tungsten tracer layer. (b) The tracer layer following incorporation into a barrier anodic film. The layer remains flat. (c) The distorted tracer layer following incorporation into the barrier layer region of a porous anodic film. The distortion arises from flow of anodic oxide from the barrier layer region at the base of the pores toward the cell walls. In both types of anodic film, the tungsten is incorporated into the alumina as units of  . The
. The  ions are much slower moving outward in the film than
ions are much slower moving outward in the film than  ions.
ions.
The flow mechanism of pore generation allows for incorporation of phosphorus species into the film, with phosphorus species also being transported toward the cell walls, in addition to their inward migration in the barrier layer. The flow of film material is also compatible with inversion of isotopic order in oxygen tracer studies of porous films and the absence of tracer loss,16 with initially incorporated tracer being displaced to the cell walls. The presence of phosphorus and oxygen tracer species can be accommodated because no film material undergoes field-assisted dissolution, the only losses being field-ejected  ions. Self-organization of pores is also envisaged through the redistribution of stress that follows attainment of a symmetrical pattern of pores.28
ions. Self-organization of pores is also envisaged through the redistribution of stress that follows attainment of a symmetrical pattern of pores.28
Flow can also account for observations of the initiation of pores in phosphoric acid electrolyte, which is accompanied by local thickening of the initial barrier layer.29 At the critical condition for pore generation in a barrier film, the anodic alumina grows at the metal/oxide interface. At sites of locally reduced film thickness, associated with the topography of the initial aluminum, the local electric field is enhanced, thereby increasing the electrostriction stresses and displacing alumina toward adjacent lower stress regions, where the alumina thickens. The displacement reduces the field at the thickened regions and increases the field, and also current density, at the thinner regions, thus stabilizing the pore.
The revised model refocuses attention on the role of electrolyte anions in establishment of the film morphology, which has generally been related to chemical influences on field-assisted dissolution of the alumina. The diameter of pores increases in order of sulfuric acid, oxalic acid, and phosphoric acid anodizing, with the films containing incorporated anion species.23 These electrolytes are also often preferred to obtain self-ordering. Further, the resultant films undergo significant expansion relative to the oxidized metal, with a dependence upon current density.30 Such observations suggest pore morphologies may reflect the influences of anion species on the electric field, film plasticity, and the consequent deformation behavior. Notably, porous films formed in borax and chromic acid electrolytes that are free of incorporated electrolyte species have a less regular morphology.31, 32 Further work is now being undertaken in order to understand the transport of species within the barrier layer and interactions between flow, migration, and compositions of the phosphorus-free and phosphorus-containing film layers, including modifications of transport due to the simultaneous presence of tungsten species.
Conclusions
An inversion of the distribution of a tungsten tracer layer occurs during its transit of the barrier layer region of porous anodic alumina films formed in phosphoric acid solution. A flow model is suggested to account for the tracer distribution. According to the model, pores are generated by flow of material in the barrier region, from beneath the pores toward the pore walls, under the influence of growth stresses. The flow results in a significantly increased thickness of the anodic film relative to that of the oxidized metal. The model is also compatible with incorporation of phosphorus species into the film, earlier observations of retention of oxygen tracers during film growth, and the local thickening of barrier film during pore initiation. An important role of anion species incorporated into the film from the electrolyte is suggested.
Acknowledgment
The authors are grateful to the Engineering and Physical Sciences Research Council (U.K.) for support of this work .