Abstract
Nanoparticles of copper oxide were synthesized by the simple precipitation method. The influence of calcination temperature on the structural characteristics and antibacterial activity of the nanoparticles were evaluated. The nanoparticles were analyzed by Bruner-Emmett-Teller (BET) surface area and pore size analyzer, x-ray diffraction, transmission electron microscope and scanning electron microscope. The findings demonstrated the formation of copper oxide nanoparticles showing a monoclinic phase. Before calcination, the nanoparticles showed a high BET surface area with rod shape morphology and size range between 18–70 nm and after calcination, irregular spherical-like morphology with size range of 20–200 nm was observed. However, it was evident that the BET surface area decreased gradually with increasing calcination temperature, while the nanoparticle size increased forming an irregular spherical shape. Subsequently, the copper oxide nanoparticles demonstrated that they are highly effective for bacteria inactivation. The inactivation activity was found to be more effective with uncalcined nanoparticles than with calcined nanoparticles. This was due to the large nanoparticle sizes and the decrease in surface area obtained after calcination. Thus, it was noted that calcination of the as-prepared nanoparticles significantly affects the structural and antibacterial properties. Hence, for antibacterial application, calcination was not necessary as the nanoparticles showed excellent antibacterial results.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Metal oxide nanoparticles exhibit diverse and fascinating characteristics that offer the possibility of developing new products and have since paved way for broader applications. Among the metal oxides that are being explored in their nanoscale is copper oxide (CuO) nanoparticles [1–6]. Copper oxide nanoparticles are inexpensive materials with high potential in various applications including gas sensors, catalysis, coating, antimicrobial, etc [7–12]. As antimicrobial agents, CuO nanoparticles have more advantages compared to their bulk counterparts in terms of performance, stability and sensitivity [13, 14]. The nanoparticles are also considered to be highly effective on resistant strains of pathogenic bacteria and are of low toxicity which of course depends on several factors. The high antibacterial activity they possess is because of their large surface area and small particle sizes that influence the material to strictly interact with the bacteria cell membranes, hence, causing cell death [14–22]. These properties therefore open new application routes for CuO nanoparticles to be used in other crucial applications such as food packaging, healthcare and water treatment. Copper oxide nanoparticles are prepared by various routes which include sonochemical [23–25], thermal decomposition [26], precipitation [27, 28], electrochemical [29, 30], chemical vapour-phase decomposition [31], hydrothermal [32], microwave [33] and reverse micro emulsions methods [34]. These methods have the potential to synthesize the required CuO nanoparticles, however, some of the disadvantages posed by the methods include high energy consumption, the use of hazardous and expensive stabilizing and reducing agents [34]. In addition, exploiting all these methods for the development and advancement of nanoparticles of various shapes and sizes for specific applications, calcination at high temperatures has drawn significant attention worldwide. With calcination, parameters such as size and distribution of the nanoparticles, morphology and the performance of the nanoparticles could be altered which could subsequently hinder or improve the end application. Calcination is believed to be an effective route to solve the prevailing shortcomings of the synthesis methods. In general, it has been reported that high temperatures used in calcination provide high crystallinity, although it reduces the surface area because of particle growth [35].
Research studies have conducted investigations in the synthesis of CuO nanoparticles and their properties using various methods, but there has been little work devoted to study the properties of CuO nanoparticles after calcination at high temperatures. The morphology, surface area and the size of the nanoparticles are important characteristics as they regulate the physicochemical properties of the material. Therefore, in the current study, a modified precipitation technique was used in the preparation of CuO nanoparticles. The precipitation method exhibits advantages including cost-effective, environmentally friendly, large-scale production and reduced need of equipment or capping agents. The purpose of this study is to investigate the impact calcination temperature has on the structural characteristics of CuO nanoparticles and to test their effectiveness against gram positive bacteria like Staphylococcus aureus and gram negative Pseudomonas aeruginosa and Escherichia coli. The prepared CuO nanoparticles were characterized by Dynamic Light Scattering particle size analyzer, x-ray diffraction, BET surface and pore size analyzer, transmission electron microscope and scanning electron microscope.
2. Materials and methods
2.1. Materials
Copper chloride anhydrous (CuCl2) and sodium hydroxide (NaOH) in analytical grades were supplied by Minema, South Africa. Distilled water was employed as a solvent for all the solutions.
2.2. Synthesis of CuO nanoparticles
For the synthesis, 360 ml of 0.1 M CuCl2 aqueous solution in a round bottom flask was heated to boil. The blue CuCl2 solution was heated at 100 °C and when it started boiling, a solution of 1.0 M NaOH with pH of 12 was gradually added to the boiling solution to form precipitates (the colour of the mixture immediately turned black) and then removed from the heating source to cool it down to room temperature while stirring. The precipitates were centrifuged and washed repeatedly to remove any impurities until the pH reached 7. After drying in an oven at 80 °C overnight, a black powder was produced. Part of the dried sample was calcined in air at 200, 500 and 700 °C (for each calcination temperature, the sample was heated to 100 °C and left for an hour at this temperature and then increased to the desired temperature for 2 h).
2.3. Characterization of CuO nanoparticles
The morphology and particle sizes of CuO nanoparticles were evaluated with transmission electron microscope (TEM, JEOL 2100 F) operating at an accelerating voltage of 200 kV. Ethanol was used to disperse the CuO nanoparticles which were sonicated in a bath sonicator for 5 min. Subsequently, a drop of the dispersion was deposited on a carbon-coated copper grid and left to air dry. Scanning electron microscope (SEM, Zeiss Ariga) was used to evaluate the microstructure of the nanoparticles. To prevent charging, the samples were mounted on aluminum stubs using double-sided carbon tape and sputter coated with carbon. To determine the quantitative elemental composition of the samples, an energy dispersive spectroscope (EDS) coupled to SEM was used. x-ray diffraction (XRD) analyses were carried out by PAnalytical XPERT-PRO diffractometer operating with nickel filtered CuKα as radiation (λ = 1.5406 Å) and an adjustable slit at 45 kV/40 mA to determine the structural phases. The diffraction measurements were collected at a scan range of 2θ = 0−90°. The specific surface area and porosity measurements of the samples were analyzed by Micromeritics TRISTAR II 3000 (USA) surface area analyser applying low temperature (77 K) liquid nitrogen adsorption- desorption isotherms. Firstly, to remove the moisture and adsorbed impurities on the surfaces and pores of the samples, they were degassed overnight under constant flow of N2 gas at 80 °C. Dynamic Light Scattering (DLS) technique was used to evaluate the size distribution of the nanoparticles by dispersing the nanoparticles in Millipore water and sonicating for at least 5 min to deagglomerate the nanoparticles. This was followed by analyzing the suspension by DLS analyzer. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) tests were conducted to measure the Cu2+ ions concentration in the samples by dispersing 200 mg of the sample into 20 ml of distilled water at room temperature and shaking vigorously in a shaking water bath. At different time intervals, leaches were collected, filtered and analyzed.
2.4. Evaluation of bacterial activity using disk diffusion method
To assess the antibacterial effectiveness of CuO nanoparticles, gram negative Escherichia coli (E. coli, ATCC 25922) and Pseudomonas aeruginosa (P. aeruginosa, ATCC 27853) and gram positive Staphylococcus aureus (S. aureus, ATCC 25923) were employed. Selective media agar was first used to verify the bacterial species. In a 100 ml of nutrient broth, a loop of a bacterial sample was injected, mixed and placed in a shaking incubator at 150 rpm and 37 °C for 24 h. The bacterial growth was determined using the pour plate method and nutrient agar. Subsequently, 100 ml of nutrient broth was spiked with 3–5 colonies and incubated at 37 °C for 24 h. For each bacteria culture, 100 μl aliquots were inoculated on nutrient agar and left to set at room temperature for about 15 min. Copper oxide nanoparticles were made into 9 mm pellets by pressing them in a 9 mm ring without adding anything (the pressing was to give shape to the pellets and not to make them rigid) and put at the centre of agar plates for incubation at 37 °C for 24 h. Control plates without the CuO nanoparticles were also included. Antibacterial activity was evaluated by measuring the inhibition zone (mm) surrounding the pellets. All tests were conducted in triplicates and averaged.
3. Results and discussions
3.1. Morphology, size distribution and elemental analyses of CuO nanoparticles
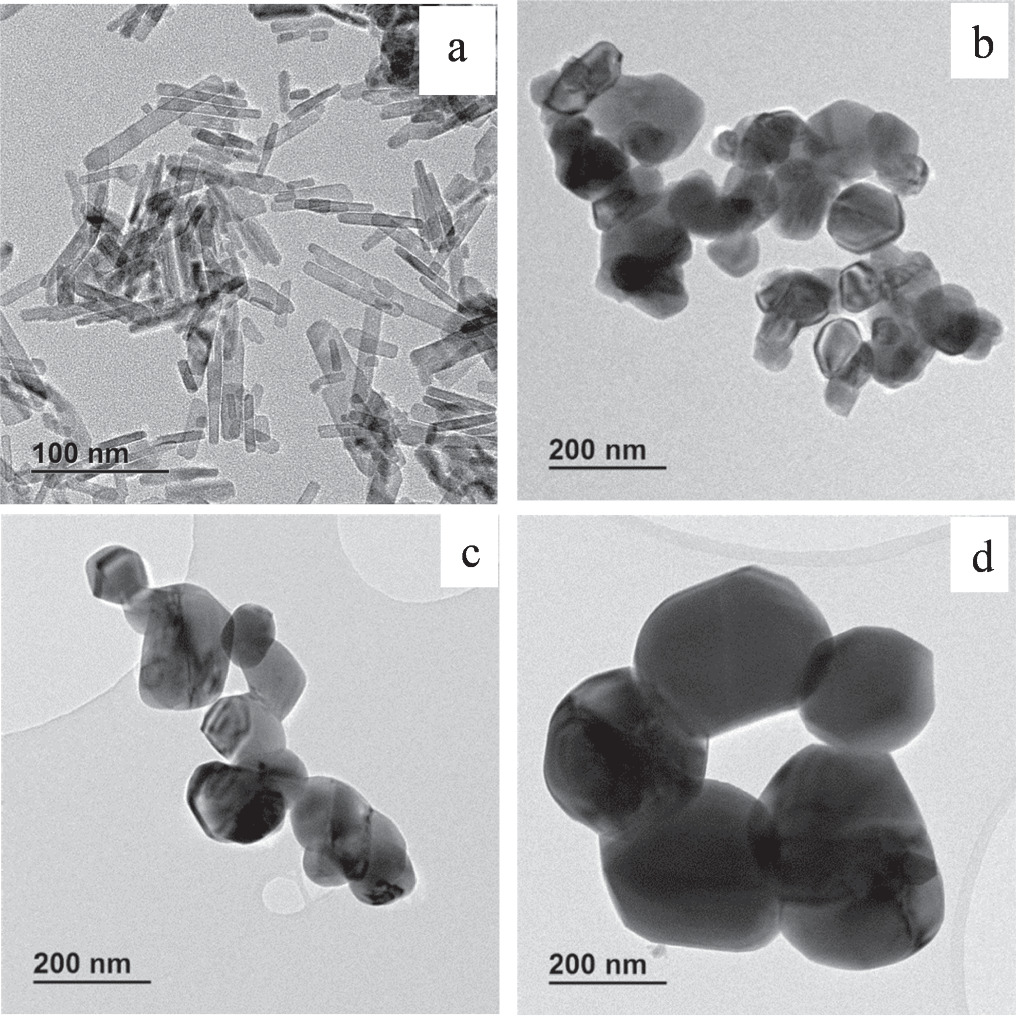
The morphology of CuO nanoparticles was evaluated by TEM and SEM. The TEM images of the nanoparticles are depicted in figure 1, with CuO nanoparticles obtained before calcination given in figure 1(a) (uncalcined CuO nanoparticles denoted as CuO-NPs) and after calcination (CuO-NPs 200, CuO-NPs 500 and CuO-NPs 700 corresponds to samples calcined at 200, 500 and 700 °C, respectively) in figures 1(b)–(d). The sample before calcination shows that the nanoparticles appear as rod shapes with uniform size distribution ranging from 18–70 nm, as shown from figure 2(a). From figures 1(b)–(d), it is evident that the calcined nanoparticles have an irregular and mixed sphere-like shapes along with size distribution from 20–200 nm. Figures 2(b)–(d) illustrates the wide particle size distribution of the calcined nanoparticles. The average size of the nanoparticles was found to be 40.43, 55.09, 72.86 and 103.61 nm for CuO-NPs, CuO-NPs 200, CuO-NPs 500 and CuO-NPs 700, respectively, whereas the standard deviation was 19.35, 30,37, 55,31 and 60 nm for CuO-NPs, CuO-NPs 200, CuO-NPs 500 and CuO-NPs 700, respectively. Direct comparison of all the samples shows that the calcined nanoparticles are prone to agglomeration, whereas the uncalcined nanoparticles are well separated. It is also evident that the high calcination temperature not only led to bigger nanoparticles, but also resulted in the change of the morphology of the CuO nanoparticles from the nanorods to the irregular spherical nanoparticles. Furthermore, SEM micrograph in figure 3(a) confirms the presence of CuO nanoparticles with a rod shape, which is before calcination. The rods are observed to be protruding in figure 3(a) with their tips mostly observed. However, figures 3(b)–(d) shows that most of the nanoparticles have an irregular spherical-like shape and the nanoparticles are not of the same size, which confirms what was observed from TEM and also from the particle size analyses. In addition, the EDS spectrum (figure 4) which is the same for all the samples confirmed the presence of CuO nanoparticles with no other elemental impurities present. Therefore, these results indicate that an increase in the nanoparticle size and morphology change is due to the primary collection of the nanoparticles which were prone to fuse as the temperature increased. These results are in agreement with other published literature regarding calcination of nanoparticles at high temperatures [20]. For instance, Hamad et al [36], synthesized titania (TiO2) nanoparticles using sol–gel-hydrothermal method. They reported that the nanoparticles resulted in an increased crystallite size and phase change after calcination. They attributed the agglomeration of the nanoparticles after calcination to sintering as the crystals grow, yielding larger particles. The authors concluded by saying that calcination in their study was inevitable as they wanted a different phase of the TiO2 nanoparticles, although high temperatures frequently result in particle growth, agglomeration and phase transformation. Shohel et al [37], reported on the preparation of ZnO nanoparticles using a hybrid electrochemical-thermal technique at different calcination temperatures. They reported that the more calcination temperature increased, the more the crystallinity of ZnO also increased. However, the surface area of the nanoparticles decreased with an increase in temperature which they acknowledged was due to sintering as the temperature increased and also caused a reduction in the photocatalytic activity of the nanoparticles. Another study by Kayani et al [38], reported on the effect of calcination on the structural characteristics of ZnO nanoparticles. The study reported on the increased crystallite size that also caused agglomeration between the nanoparticles with increasing temperature. Therefore, from these studies, it is obvious that although calcination at high temperature gives high crystalline nanoparticles and required structural phases, the nanoparticle growth is inevitable and often times the final application of the nanoparticles could either be improved or compromised.
Figure 1. TEM images of (a) CuO-NPs, b CuO-NPs 200, (c) CuO-NPs 500 and (d) CuO-NPs 700.
Download figure:
Standard image High-resolution imageFigure 2. Particle size measurements of (a) CuO-NPs, (b) CuO-NPs 200, (c) CuO-NPs 500 and (d) CuO-NPs 700.
Download figure:
Standard image High-resolution imageFigure 3. SEM images of (a) CuO-NPs, (b) CuO-NPs 200, (c) CuO-NPs 500 and (d) CuO-NPs 700.
Download figure:
Standard image High-resolution imageFigure 4. EDS spectrum of CuO nanoparticles.
Download figure:
Standard image High-resolution image3.2. X-ray diffraction analyses of CuO nanoparticles
The confirmation of CuO nanoparticles formation was conducted by XRD analyses on the prepared samples. figure 5 illustrates the XRD patterns of the prepared CuO nanoparticles and CuO nanoparticles calcined at different temperatures of 200, 500 and 700 °C. From the patterns shown, the samples did not show any obvious changes in the peak position but indicated slight sharpness in the intensity of the calcined nanoparticles indicating that the structure was preserved even after calcination. Corresponding narrow and strong diffraction peaks indicated the well crystalline structure and the monoclinic phase of the nanoparticles. The analyses showed various diffraction peaks at 2θ values of 32.6, 35.53, 38.9, 48.7, 53.1, 58.26, 61.44, 66.01, 68.0 72.2, and 74.8° that were observed in all the patterns and are indexed as (110), (−111), (111), (−202), (020), (202), (−113), (−311), (220), (311), and (004) planes [6, 20, 27, 39, 40]. These results were supported by reports describing CuO powder of the monoclinic structure [6, 20, 27, 39–43]. Notably, there were no other additional peaks after calcination indicating the purity of the CuO nanoparticles and its single phase.
Figure 5. XRD patterns of CuO-NPs, CuO-NPs 200, CuO-NPs 500 and CuO-NPs 700.
Download figure:
Standard image High-resolution image3.3. Surface area and pore size measurements
Table 1 gives the surface area analyses of CuO nanoparticles and calcined CuO nanoparticles at different temperatures. The effect of calcination temperature on both the surface area and pore size is highly evident here. It is notable that the specific surface area and pore sizes gradually decreases when calcination temperature is increased due to the formation of bigger particle sizes between the nanoparticles (as observed from SEM and TEM images); whereas a high specific surface area of CuO nanoparticles before calcination was observed with corresponding smaller particle sizes. The specific surface area before calcination and at different temperatures of 200, 500 and 700 °C were found to be 51.47, 6.71, 6.41 and 0.88 m2 g−1, respectively. The BET results show that although the monoclinic structure of the CuO nanoparticles was preserved during high temperature calcination, the high temperatures had a significant effect on the specific surface area of the nanoparticles which is one of the influencing factors for antibacterial applications. The antibacterial activity is dependent on the surface area and particle sizes of the nanoparticles, with a large surface area and small particle sizes preferred. This is because a higher surface area provides more active sites, whereas small particle sizes can easily interact with the membrane and the cell contents of the microbes. Figure 6 shows the nitrogen adsorption–desorption isotherms measured at 77 K for all the samples. All the isotherms correspond to Type II isotherm, giving a characteristic of mesoporous materials. Furthermore, the isotherms showed an H3 type hysteresis loop which is often correlated to narrow slit-like pores due to agglomerations of the particles [44]. This confirms what was observed with TEM and SEM images. The results also showed a decrease in the adsorbed volume as the calcination temperature increased which also led to the specific surface area reduction. The desorption branch of CuO nanoparticles calcined at 700 °C is quite evident of the structural modification that occurred in both the pore and surface area characteristics. This also confirmed the reduction observed from table 1.
Table 1. Surface area and pore size distribution by BET measurements.
| Sample | Surface area (m2/g) | Pore size (nm) |
|---|---|---|
| CuO-NPs | 51.47 ± 0.68 | 23.66 ± 0.34 |
| CuO-NPs 200 | 6.71 ± 0.98 | 13.79 ± 0.86 |
| CuO-NPs 500 | 6.41 ± 0.47 | 12.59 ± 1.28 |
| CuO-NPs 700 | 0.88 ± 0.28 | 7.48 ± 0.60 |
Figure 6. Nitrogen adsorption–desorption of, (a) CuO-NPs, (b) CuO-NPs 200, (c) CuO-NPs 500 and (d) CuO-NPs 700.
Download figure:
Standard image High-resolution image3.4. Antibacterial activity of CuO nanoparticles
The bacterial effectiveness of CuO nanoparticles was evaluated using S. aureus, E. coli and P. aeruginosa using the disk diffusion technique. The antibacterial effectiveness was assessed in triplicate and averages are given here. The antibacterial activity of the tested bacteria with CuO nanoparticles at different calcination temperatures is given in figures 7 and 8 gives representative photographs of the antibacterial efficacy. From these results, it is obvious that the antibacterial activity results show the effectiveness of CuO nanoparticles to inactivate the tested bacteria. Although the shape of the nanoparticles has been widely reported to have a significant impact on the antibacterial activity of nanomaterials, the nanorods used in this study were found to be more effective for the application as compared to the irregular spherical nanoparticles. The open literature has not given an exact preference on the shape of the nanoparticles, as different morphological structures of the nanoparticles give totally different results depending on the synthesis route. But it is evident that the antibacterial effectiveness is higher when the nanoparticles have a smaller size. Sonia et al [45] successfully prepared CuO nanorods and evaluated their antibacterial activity with S. aureus and Salmonella Typhimurium (S. typhi). The results demonstrated that the CuO nanorods were highly efficient in inhibiting the growth of the tested bacteria. A similar study to the current one was conducted by Tavakoli et al [46], where they used aloe vera extract as the reducing agent to prepare CuO nanoparticles and evaluated the effect of shape and size of the nanoparticles on the antibacterial activity of gram negative E. coli and gram positive S. aureus. The results demonstrated that rod shape CuO nanoparticles had the highest surface area and gave the highest inhibition zone. Although the nanoparticles demonstrated antibacterial activity towards both bacteria, E. coli was observed to be more susceptible to the nanoparticles as compared to S. aureus which was more resistant due to the thick peptidoglycan membrane. Rod shape nanoparticles were found to have the highest surface energy and available active sites. Moreover, the efficacy was also attributed to the difference in the bacteria structural compositions and cell membrane. Qamar et al [47], synthesized CuO nanorods using Momordica charantia fruit extract and evaluated both in vitro and in vivo methods. Of significance was the antibacterial activity of the nanorods to inactivate both gram negative and gram positive human resistant pathogens. Bacillus cereus was observed with the highest inhibition zone. Anwar et al [20], also reported a similar study where they found that the morphology of the CuO nanoparticles changes from rod shape to spherical shape when calcination temperature is increased. In addition, the average crystallite size of the CuO nanoparticles was observed to increase with an increasing calcination temperature. However, the antibacterial activity was observed to increase with an increase in calcination temperature, and E. coli was more susceptible as compared to Bacillus subtilis (B. subtilis). Raza et al [48], synthesized silver nanoparticles (AgNPs) of different sizes and shapes and tested their antibacterial activity. Their results showed that AgNPs with small particle size between 15–50 nm and having sphere-like shape inhibited E. coli and P. aeruginosa with high inhibition zone. They attributed the high bacterial effectiveness to the smaller spherical AgNPs with high surface contact sites, while larger AgNPs were observed with the least activity. In addition, AgNPs with triangular-like shape exhibited low antibacterial effect in comparison to the smaller sphere-like shape AgNPs in the same work. Katwal et al [30], investigated the antimicrobial activity of CuO nanoparticles against bacterial strains of S. aureus and E. coli and fungal strains of Aspergillus nigres and Candida albicans using the growth curve technique. The results indicated that the nanoparticles were effective in inhibiting the growth of the tested strains which they attributed to the nanoparticles attaching to the outer surfaces of the membranes resulting in the disruption of the active transportation and formation of the nucleic acids, etc. The study did not compare the antimicrobial activity at different calcination temperatures, their size or shape; but only reported on the concentration of 50 μg ml−1. Another study by Selvaraj [49], synthesized CuO nanoparticles with size distribution ranging from 70 nm to 90 nm with a spindle shape for the antibacterial activity of clinical strains of P. aeruginosa, S. aureus, E. coli and B. subtilis. The study showed that P. aeruginosa and B. subtilis exhibited the highest inhibition zone while E. coli exhibited the lowest inhibition zone. The results did not show any favouritism of being more effective on either gram negative or gram positive, however, it was observed that gram positive B. subtilis performed much better than gram negative E. coli. Another study by Swarnkar et al [50], synthesized and conducted the antibacterial activity of Cu/Cu2O nanowires against gram positive bacteria (S. aureus and B. subtilis) and gram negative bacteria (E. coli, P. aeruginosa and S. typhi). The size of the nanowires ranged from 200 to 600 nm. The antibacterial activity was found to be concentration dependent and more favourable with gram negative bacteria than gram positive bacteria. The authors attributed the results to the thin cell wall layer of the gram negative bacteria made of peptidoglycan which was more vulnerable to the nanowires. A study by Chauhan et al [33], prepared CuO nanoparticles using the green synthesis approach and microwave irradiation method and their antimicrobial efficiency evaluated against bacterial strains (E. coli and B. subtilis) and yeast strains (Candida albicans). Their results indicated that, although, the nanoparticles were effective in their inhibition of the strains, they were more pronounced to B. subtilis with the highest inhibition zone. Another investigation by Pal et al [51], compared the antibacterial activity of AgNPs with different shapes ranging from spherical, rods and triangular. In their study, the authors reported that a high bactericidal activity was observed with the triangular shape AgNPs. In contrast, the triangular AgNPs were actually larger than the spherical or the rod shape nanoparticles. The authors then argued that the high antibacterial activity by the triangular AgNPs is attributed to the crystal planes and geometric arrangement, where the triangular AgNPs are characterized with high-index facets which led to the highest antibacterial activity compared to the other two shapes. Although Raza et al [48], showed that small and sphere-like AgNPs were optimum in exerting the antibacterial activity in comparison to larger sphere-like and triangular shape AgNPs, Pal et al [51], demonstrated that larger triangular nanoparticles were the best to exert the antibacterial activity in their study. Therefore, it is obvious that the bacterial effectiveness of nanoparticles depends largely on the size and shape of the particles and also a major role is played by the effective surface area of the various structural geometries which is still yet to be understood. Wang et al [52], also compared the bacterial efficacy of Ag2O based on the morphology of the nanoparticles. In their study, they reported that the cubic Ag2O nanoparticles exhibited stronger antibacterial activity to the tested bacteria as compared to octahedral Ag2O nanoparticles. They also attributed the morphology depended antibacterial activity to the exposed facets with different atomic arrangement of the Ag2O, which influences the release of the Ag+ ions. Thus, in the current study, both nanoparticles were observed to inactivate the tested bacteria and high inactivation activity was exerted by the nanorods, which could be attributed to their smaller sizes and high surface area as compared to the irregular spherical nanoparticles which were also larger. The uncalcined CuO nanoparticles were characterised by a high inhibition zone for all the tested bacteria, and this was observed to decrease moderately as the calcination temperature was increased. As mentioned, the decrease in inactivation could be due to an increased nanoparticle size and low surface area where the nanoparticles were not able to interact and penetrate easily through the bacterial cell wall. It is widely acknowledged that the higher the surface area per unit mass, the more active and effective the material becomes. In addition to the surface characteristics of the material, the release of ions plays a major role [19, 21, 53]. The exact bacterial mechanism of CuO nanoparticles is still not yet fully understood, it emerges that several mechanisms have been suggested which include (1) the immediate interaction of CuO nanoparticles and bacterial surfaces, consequently damaging the cell wall of the bacteria. This occurs due to the generation of reactive oxygen species (ROS), which could either be hydroxyl radicals (HO•), hydrogen peroxide, superoxide anions (O2 −) or organic hydroperoxides which are capable of damaging the cell wall leading to cell lysis [53, 54]. And (2) the generation of the Cu2+ ions which may directly attach to the cell membrane, hence, destroying it. The leaching of Cu2+ ions from the nanoparticles is one of the mechanism to induce cytotoxicity to the bacteria. The released Cu2+ ions attach to the carboxylic groups, peptidoglycans or lipopolysaccharides of either gram negative or gram positive bacteria cell walls, disrupting the stability of the cell membranes and damaging the intracellular contents [53, 54]. The antibacterial mechanism in this study could have followed either one of the mentioned mechanisms or the two mechanisms complimented each other and were responsible for the results found. However, the results from this study revealed that gram positive S. aureus which is characterized by a thick cell wall was more susceptible to the CuO nanoparticles where it demonstrated a higher inhibition zone as compared to P. aeruginosa and E. coli characterized with a thin cell wall. This was observed for all CuO nanoparticles before calcination and after calcination. Compared to gram positive bacteria, gram negative bacteria are more vulnerable to antibacterial agents. This is because thick cell walls that characterize gram positive bacteria protects the contents of the cell by preventing and making it challenging for foreign objects to easily penetrate through. Therefore, these results can be explained by the gram positive S. aureus responding better to the CuO nanoparticles than the two gram negative strains used. Jadhav et al [29], reported on the inactivation of S. aureus and E. coli using CuO nanoparticles. Their findings also indicated that S. aureus had a higher bacterial effect than E. coli. Another study by Alswat et al [55], reported on the bacterial efficacy of CuO nanoparticles loaded zeolite assessed using gram positive B. subtilis (B29) and gram negative Salmonella Choleraesuis (ATCC 10708). The synthesized CuO-zeolite composites demonstrated a higher bacterial effectiveness towards B. subtilis. The authors attributed the activity to good dispersion of CuO nanoparticles and the available surface sites interacting with the bacteria species. They did not relate the effectiveness to either the thin cell wall or the thick cell wall bacteria, even though it was obvious that gram positive bacteria were susceptible even with the smallest amount of CuO nanoparticles. Another study by Khashan et al [40], also compared the bacterial efficacy of CuO nanoparticles against S. aureus and E. coli. The study showed improved efficiency of the nanoparticles towards S. aureus than on E. coli. For E. coli, more CuO nanoparticles concentration was required to inhibit the bacteria. Similar results were reported by Guzman et al [21], where they observed that CuO nanoparticles with particle size range of 4.1 ± 1.9 nm were more effective in inhibiting both S. aureus and E. coli, with the results being more pronounced to S. aureus. From the above studies, it is not conclusive why the CuO nanoparticles are better in inactivating gram positive bacteria with higher activity compared to gram negative bacteria. Additionally, the studies did not make any correlation between the shape and the bacterial effect. Dadi et al [18], prepared CuO and ZnO nanoparticles and evaluated their bacterial efficiency against S. aureus, P. aeruginosa and E. coli. Both nanoparticles were observed to have a mean particle size of 3 nm. However, their results showed that ZnO nanoparticles required higher concentration to effectively inhibit the bacteria, whereas with CuO nanoparticles the inhibition started with less concentration. Interestingly, it was noted that the inhibition zone of E. coli and P. aeruginosa was larger than that of S. aureus when using CuO nanoparticles. The study showed that CuO nanoparticles were highly effective in the inhibition of all the tested bacteria as compared to ZnO nanoparticles which the authors attributed to less agglomeration of the nanoparticles and the particle sizes.
Figure 7. Inhibition zone measurements (mm) assessed using agar diffusion method.
Download figure:
Standard image High-resolution imageFigure 8. Representative petri dishes of the antibacterial activity by CuO nanoparticles: The abbreviations used here stands for: EC (Escherichia coli), PA (Pseudomonas aeruginosa) and SA (Staphylococcus aureus).
Download figure:
Standard image High-resolution image3.5. Leaching tests
To confirm the release of Cu2+ ions and quantify its concentration, ICP-AES was used, and the results are given in figure 9. The results shows that Cu2+ ions were leaching into the water and samples of uncalcined CuO nanoparticles leached more as compared to the calcined nanoparticles. The leaching decreased in the order of CuO-NPs >CuO-NPs 200 >CuO-NPs 500 and CuO-NPs 700, with all samples having Cu2+ ions concentration of less than 1 mg l−1. Furthermore, the results showed that calcination reduced the amount of Cu2+ ions released into the water. This also corroborate the results observed on the antibacterial activity of calcined nanoparticles. However, the Cu2+ ions concentration was below the recommended limit of 2 mg l−1 by the World Health Organization (WHO) for drinking water [56]. The leaching of Cu2+ ions into the water confirms the possibility of the antibacterial activity having taken place due to the dissociation of the ions to disrupt the cell walls.
Figure 9. Leaching results of CuO nanoparticles.
Download figure:
Standard image High-resolution imageThe general observation from this study was that all calcination temperatures resulted in bigger nanoparticle sizes of up to 200 nm, where the morphology of the CuO nanoparticles was transformed from the initial rod shape to the irregular spherical shape. Thus, at high calcination temperatures, CuO nanoparticles agglomerated extremely too much, hence, the particle sizes increased. Calcination affected the morphology, particle size, the surface properties and the antibacterial activity. For antibacterial applications, smaller particle sizes and high available surface area of the nanoparticles are desirable as it offers high possibilities for the nanoparticles to interact closely with the surface of the bacteria and cause cell death. Therefore, these results show that calcination is not always ideal for certain properties of the nanoparticles as it negatively affects the final application. Moreover, the synthesis route plays a pivotal role in the shape, size and antibacterial activity of the nanoparticles, as it was observed from the different literature cited. The study has also demonstrated that the morphology of the nanoparticles has a significant influence on the antibacterial activity due to the different surface interfaces characterizing the various chemical and physical adsorption–desorption competencies towards the bacteria ensuring the various antibacterial activities [45].
4. Conclusion
The precipitation technique worked well in the preparation of CuO nanoparticles without using any capping agent. XRD results confirmed the presence of highly pure monoclinic CuO nanoparticles and the EDS data was a supplement to these findings. Interesting observation was done on morphology when the nanoparticles before calcination were rod shape and after calcination the nanoparticles showed an irregular spherical shape with an increased size. Moreover, the uncalcined nanoparticles showed greater antibacterial activity compared to the calcined nanoparticles as a result of a higher surface area and smaller particle size. Therefore, calcination of nanoparticles at high temperatures was observed to have a significant impact on the structural characteristics of the nanoparticles as well as their antibacterial activity. The uncalcined CuO nanoparticles showed excellent antibacterial activity and therefore, there is no need for calcination and these nanoparticles are also promising antibacterial agents for further studies such as disinfection of water, packaging and other industrial applications.
Acknowledgments
The author would like to acknowledge the Department of Science and Innovation and the Council for Scientific and Industrial Research for financial support. The author also wishes to thank the characterization facility team at the Center for Nanostructured and Advanced Materials.
Data availability statement
Data can be shared upon request. The data that support the findings of this study are available upon reasonable request from the authors.
Conflict of interest
The author declares no conflict of interest.