Abstract
In the carbon nanotubes film/graphene heterostructure decorated with catalytic Pt nanoparticles using atomic layer deposition (Pt-NPs/CNTs/Gr) H2 sensors, the CNT film determines the effective sensing area and the signal transport to Gr channel. The former requires a large CNT aspect ratio for a higher sensing area while the latter demands high electric conductivity for efficient charge transport. Considering the CNT's aspect ratio decreases, while its conductivity increases (i.e., bandgap decreases), with the CNT diameter, it is important to understand how quantitatively these effects impact the performance of the Pt-NPs/CNTs/Gr nanohybrids sensors. Motivated by this, this work presents a systematic study of the Pt-NPs/CNTs/Gr H2 sensor performance with the CNT films made from different constituent CNTs of diameters ranging from 1 nm for single-wall CNTs, to 2 nm for double-wall CNTs, and to 10–30 nm for multi-wall CNTs (MWCNTs). By measuring the morphology and electric conductivity of SWCNT, DWCNT and MWCNT films, this work aims to reveal the quantitative correlation between the sensor performance and relevant CNT properties. Interestingly, the best performance is obtained on Pt-NPs/MWCNTs/Gr H2 sensors, which can be attributed to the compromise of the effective sensing area and electric conductivity on MWCNT films and illustrates the importance of optimizing sensor design.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The utilization of carbon nanotubes (CNTs) as the sensing materials for detection of various gas molecules has been explored for over a decade [1, 2]. Liquid sensors based on CNTs such as alcoholic sensor have also been reported [3, 4]. CNT films or networks can provide a large effective surface area for adsorption/desorption of gas molecules, and the follow-up electron transfer driven by the interactions between gas molecules and π electrons of CNT [5]. This leads to a charge doping effect on CNTs and hence a change in the resistance of the CNT film, which is measured as the sensor's response to gas molecules. However the weak physisorption of most gas molecules on CNTs and the associated poor sensing performance for gas sensors based on pristine CNTs [6, 7] have prompted introduction of nanostructured catalytic metals such as gold (Au), silver (Ag), platinum (Pt), and palladium (Pd) on CNTs. The decoration with catalytic Pt metal nanostructures was especially suitable for enhancing H2 sensing by providing much stronger chemisorption of H2 molecules and the follow-up dissociation of H-H bond [8], lowering the work function of Pt and consequently resulting in charge transfer from hydrogen to CNT [9]. While improved performance was reported on CNT-based hydrogen sensors with Pt catalyst [9–11] further enhancement of sensitivity through optimization of the sensor design is important.
In a recent work [12], we have developed a 3D electrode architecture consisting of single-wall CNTs on graphene (SWCNT/Gr). The implementation of Gr of high charge mobility at room temperature [13, 14] has been found to effectively improve charge (signal) transfer by directing the charges from SWCNTs to electrodes through Gr, instead of through multiple CNT-CNT junctions to electrodes, which present hurdles to charge transfer in CNT only sensors. In addition, Gr is a semimetal and forms an Ohmic contact with common electrodes such as Au or Pd. [15–17]. Therefore, H2 sensors consisting of a CNT film/graphene heterostructure 3D electrode decorated with catalytic Pt nanoparticles using atomic layer deposition (Pt-NPs/CNTs/Gr) combine the advantages of large effective sensing surface area of CNT films, the catalytic benefit of the conformally coated Pt-NPs, and high mobility signal transport through CNT/Gr 3D electrode. On the Pt-NP/SWCNT/Gr H2 sensors, enhanced H2 sensitivity by more than 50% of magnitude has been observed as compared to their counterparts without Gr [12]. This result has revealed the critical importance of engineering the signal transfer on the 3D CNT/Gr electrodes while raises a question on whether SWCNT films are optimal for charge transfer. In fact, SWCNTs were chosen primarily because SWCNTs have the largest possible effective sensing area due to the highest aspect ratio (length versus diameter) among all CNTs with comparable lengths. However, SWCNTs tend to form bundles of an average diameter of ∼10 nm, which considerably reduces the effective surface area of the SWCNT films [12]. In addition, most as-made SWCNTs using chemical vapor deposition are unpurified with about 2/3 semiconductive and 1/3 metallic SWCNTs. The inter-SWCNT junctions between the two kinds of SWCNTs are Schottky junctions and can hinder the charge transport [18] in SWCNT-based H2 sensors. This means that SWCNTs may suffer limitations in reaching the optimal sensing surface area expected and poor charge transfer in the ideal case, both are critical parameters for high-performance H2 sensors. Considering the bandgap of CNTs decreases inversely with the diameter of the CNTs [19, 20], higher conductivity is anticipated in CNTs with larger diameters including double-wall CNTs (DWCNTs) and multi-wall CNTs (MWCNTs). In addition, the issue of bundling would become less serious with decreasing aspect ratios as the CNT diameter increases. Considering both high effective sensing surface area and high conductivity are required for high-performance of the Pt-NPs/CNT/Gr nanohybrid H2 sensors, a quantitative assessment of the effects of the CNT diameter and electrical conductivity on the sensor performance is important to obtaining optimal sensor design. Motivated by this, the objective of this work is to carry out a systematic investigation of the Pt-NPs/CNT/Gr nanohybrid H2 sensor performance in correlation with the CNT morphology and electric conductance when the constituent CNT diameter in CNT films is increased from ∼1 nm for SWCNTs, to ∼2 nm for DWCNTs, and to ∼10 nm–30 nm in MWCNTs. Interestingly, H2 sensitivity has been found to be sensitively affected by the CNT selection and the highest H2 sensitivity has been achieved on Pt-NPs/MWCNT/Gr nanohybrid H2 sensors in which the MWCNTs have ∼10 nm in diameter.
2. Materials and methods
The Pt-NPs/CNT/Gr device fabrication involves four major steps of metal electrode deposition, graphene transfer, CNT film transfer and Pt-NP coating. In the first step, evaporation of Au electrodes was made on SiO2/Si substrates. The dimensions of the Au electrodes are 4 mm (length) × 2 mm (width) with a distance of 0.3 mm (regarded as device channel length) between neighboring Au bars. In the second step, a single-layer graphene strip was transferred onto the SiO2/Si substrates with pre-fabricated Au electrodes (figure 1(a)). The graphene was synthesized using chemical vapor deposition (CVD) on commercial copper foils (Sigma-Aldrich) at ∼1000 °C. The details of graphene growth and transfer were reported in previous works [21, 22]. In the third step, the CNT film was transferred on top of graphene to form the 3D CNT/Gr nanohybrid (figure 1(b)). The SWCNT, DWCNT and two kinds of MWCNT films studied in this work were prepared using a vacuum filtration method from dispersed CNT suspension solutions [23, 24]. This thicknesses of the CNT films were controlled by the filtration time or the total amount of CNTs based on the pre-calibrated rates for filtration from the corresponding CNT suspension solutions as we reported previously [12, 25]. Commercial (CheapTubes, Inc) SWCNTs (1–2 nm in diameter), DWCNTs (2–3 nm in diameter), smaller MWCNTs (10 nm) with CNT diameter of 10 nm, and larger MWCNTs (10–30 nm) due to the broader range of the CNT diameter were used to make CNT films of ∼500 nm in thickness. The CNT lengths are in the range of 5–10 μm. In the fourth step, the Pt-NPs, shown as grey spheres, were decorated conformally on the CNT/Gr electrodes using atomic layer deposition (ALD) by using alternating exposures to MeCpPtMe3 (Sigma-Aldrich) and oxygen pulses at 310 °C [12] (figure 1(c)). It should be noted that the Pt-NPs was found to nucleate on CNTs at low ALD pulse numbers and merge into a continuous Pt film with much reduced catalytic effect [12]. In this work, 20 ALD cycles (20 c) was selected in ALD Pt for Pt-NPs with an optimal catalytic effect in H2 sensing.
Figure 1. (a)–(c) Schematic illustration of the Pt-NPs/CNTs/graphene H2 gas sensor fabrication process: (a) Gr transferred on Si substrate with two pre-fabricated Au electrodes. (b) CNT films transferred on top of Gr. The CNT diameters are 1–2 nm for SWCNTs, 2–3 nm for DWCNTs, 10 or 30 nm for MWCNTs. (c) ALD coated Pt-NPs (grey spheres) on CNT/Gr. Raman spectra of (d) Gr, (e) SWCNTs, (f) DWCNTs, and (g) MWCNTs films.
Download figure:
Standard image High-resolution imageScanning electron microscopy (SEM) images and Energy-dispersive x-ray spectroscopy (EDS) spectra of the CNT/Gr nanohybrid samples with different cycle numbers of ALD-Pt coating were taken on Hitachi SU8230 Ultra-high Resolution Scanning Electron Microscope to extract the information of sample morphology and Pt element distribution. A WiTec Alpha300 confocal micro-Raman system equipped with a piezoelectric sample stage was used to collect Raman spectra and maps of graphene and CNTs on the CNT/Gr devices. Typically, a 488 nm laser was used as excitation light source in Raman spectroscopy and imaging. A Digital Instruments Multimode AFM system with a Nanoscope IIIa controller using standard silicon nitride cantilevers (NanoAndMore USA, k = 0.06, 0.27 N m−1) was used in contact mode with scan rates from 1–3 Hz to collect atomic force microscopy (AFM) images on the sample. For consistency, AFM images were collected from at least 3 different locations on each sample and the CNT dimensions were measured by averaging cross-sectional measurements of at least 20 nanotubes. The ultraviolet (UV) light (360 nm–400 nm) was used to activate the surface of CNT/graphene. The photoresponse of UV-irradiated sensor was measured to investigate the effect of UV on the performance of our sensor. The use of UV was to desorb air molecules from the CNT/graphene surface which allow more room for H2 gas adsorption.
The H2 response and sensitivity were characterized at room temperature in a vacuum chamber (volume ∼500 cm3) under a mixed H2/N2 gas flow. The ALD-Pt-NPs/CNT/Gr sensors were mounted on a sample stage inside the chamber with a multi-pin electric feedthrough for the electric connection of the sample to external electronics. Before the measurement, the chamber was purged with a N2 flow for about an hour to remove residual gas molecules. The concentration of H2 was controlled by controlling the flow ratio of H2 and N2 gases using an MKS four-channel flowrate controller (MKS 946). In this work, the concentration of H2 was varied in the range of 1% to 20% via changing the volume ratio of H2 to N2 buffer gases for the device sensitivity characterization. Current-time (I-t) curves were recorded on the Pt-NPs/CNT/Gr sensors at a constant bias voltage (V) using a CH Instruments CHI660D electrochemical workstation in response to the H2 flow on and off in the vacuum chamber. The I-t curves were later converted to resistance-time (R-t) curves using Ohm's law R = V/I where V was set 0.1 V in this work for the responsivity calculation. Resistance as function of temperature (R-T) curves were taken CNT films using a Keithley 224 current source (providing the bias currents) and a Keithley 2182 dc voltmeter (to record the voltage generated across the sample). Each device was exposed to H2 gas for 1320 s (H2 ON), followed with being in N2 atmosphere for recover. The response of the device can return to its original state after a short exposure to air at room temperature (∼22 °C).
3. Results and discussions
The architecture and fabrication procedure (details in Experimental) of Pt-NPs/CNT/Gr nanohybrid are schematically shown in figure 1. In this work three different types of CNTs - SWCNT, DWCNT and MWCNT - were compared. Gr is a semimetal and forms an Ohmic contact with Au electrodes as demonstrated in the linear I-V characteristic (figure S1 (available online at stacks.iop.org/NANOX/3/035004/mmedia)). The Raman spectra of Gr and the three different types of CNTs are shown in figure 1(d)–(g) respectively. The Raman spectrum of Gr exhibits the typical G peak at ∼1604.9 cm−1 and 2D peak at ∼2692.5 cm−1. The G and 2D peaks are related to the doubly degenerate zone center E2 g mode at the Brillouin zone center and the second order of zone-boundary phonons, respectively [26]. The absence of the D peak at ∼1352.5 cm−1 confirms that the Gr is the high quality with negligible defects. In addition, the high intensity ratio of the 2D peak to G peak of 2.3 indicates that the Gr is single-layer. In the Raman spectrum for SWCNT (figure 1(e)), the G peak splits into two peaks (G- and G+ peaks) at 1496 cm−1 and 1521 cm−1 respectively in SWCNTs due to the curvature of the graphene sheet in the SWCNTs [27, 28]. Another distinctive peak is located at ∼2833 cm−1 corresponding to the 2D-band for SWCNTs [8]. The D peak at 1215 cm−1 associated with the presence of in-plane defects on SWCNTs, possibly the growth defects that occurred during CVD synthesis of SWCNTs, is visible with low intensity, suggesting the defects are minor [29]. A single peak at 192.4 cm−1 (the inset of figure 1(e)) is attributed to the radial breathing mode (RBM) of the SWCNTs [30, 31]. Due to a large diameter of the MWCNTs and DWCNTs, the characteristic RBM peak associated to smaller CNTs becomes too weak to be seen [32]. Therefore, the Raman spectra for DWCNT (figure 1(f)) and MWCNT (figure 1(g)) films look similar. In both cases, two distinct peaks at 1521 cm−1 (G peak) and 2838 cm−1 (2D peak) are clearly visible as anticipated from the high crystallinity of the DWCNTs and MWCNTs in addition to the D peak at 1215 cm−1. Again, the low intensity of the D peak suggests the defects in DWCNTs and MWCNTs are insignificant.
The optical microscope images of the SWCNT, DWCNT and MWCNT (10 nm) films are displayed in figures 2(a)–(c), showing homogeneity over relatively large area of the film. The optical images and the Raman maps of a representative MWCNT (10 nm)/Gr device before and after the CNT was transferred on Gr are shown in figure S2 from which a uniform Gr channel and the CNT film on top can be seen clearly. The AFM images shown in figures 2(d)–(f) reveal porous structures of the CNT films at a microscopic scale, which is desired for gas sensors to provide a large sensing surface area, for the SWCNT, DWCNT, MWCNT (10 nm in diameter). Specifically, the diameter of the tube-like features, determined from the AFM cross-sectional heights, are 10 ± 7 nm, 15 ± 6 nm and 16 ± 7 nm respectively for the SWCNT, DWCNT and MWCNT shown in figures 2(d)–(f), respectively. In the two former cases, the diameters measured for the tube-like features in these images are larger considerably than the anticipated for the constituent SWCNT and DWCNT, indicating the CNT bundling in these two cases. In the MWCNT, the issue of bundling is much less of concern since the AFM measured diameter is close to the anticipated one for the MWCNTs of 10 nm in diameter. Figures 2(g)–(l) display the SEM images of the SWCNT/Gr, DWCNT/Gr, MWCNT (10 nm)/Gr nanohybrids decorated with ALD Pt-NPs. Figures 2(g)–(i) are the zoom-out SEM images of the same samples, illustrating the network structure of CNT films. As seen in figure 2(g), SWCNTs tend to assemble into bundles with the diameter considerably larger than 1–2 nm. However, the amount of CNT bundling is expected to be less in the samples of DWCNT and MWCNT films because of their smaller aspect ratios and hence better dispersibility in water than their SWCNT counterpart's. Less bundling represents more effective sensor surface area, which is important to the gas sensor performance. In all three samples, Pt-NPs are uniformly and sparsely distributed surrounding the walls of CNTs. The diameter of Pt-NPs is a few nanometers and the mass fraction (wt%) is in the range of 1%-4% from EDS (figure S3), which is consistent with our previous result [12].
Figure 2. Optical, AFM, and SEM images of: (a), (d), (g), (j)) Pt-NPs/SWCNT/Gr, (b), (e), (h), (k) DWCNT/Gr, and (c), (f), (i), (l) MWCNT (10 nm)/Gr samples. Ruler bars for figures 2(g)–(i) and (j)–(l) are 250 nm and 200 nm, respectively.
Download figure:
Standard image High-resolution imageThe responsivity (or sensitivity) of the Pt-NPs/CNTs/Gr devices to H2 gas is defined as
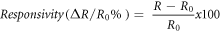
Where R0 and R are the resistances of the sensor before and after the exposure to H2, respectively [33]. Figure 3(a) compares the dynamic response to 10% of H2 gas of the Pt-NPs/SWCNTs/Gr (black), Pt-NPs/DWCNTs/Gr (red), Pt-NPs/MWCNTs (10 nm)/Gr (green), and Pt-NPs/MWCNTs (10–30 nm)/Gr (blue) devices. The Pt-NPs/MWCNTs (10 nm)/graphene device exhibits the best sensitivity of 26% among the four samples. The Pt-NPs/SWCNTs/Gr device shows a slightly lower sensitivity of 24%. This could be attributed to the bundling of SWCNTs to form a larger diameter similar to that of MWCNTs (10 nm), resulting in a comparable sensor surface area to that of the MWCNTs (10 nm). The Pt-NPs/DWCNTs/Gr device shows a considerably lower H2 responsivity of 16% while the lowest responsivity <10% was measured on the Pt-NPs/MWCNTs (10–30 nm)/Gr device.
Figure 3. (a) Responsivity of H2 gas sensor with 10% of H2 of Pt-NPs/CNT/graphene sensors using four different kinds of CNTs: SWCNTs (black), DWCNTs (red), MWCNTs (10 nm, green), and MWCNTs (10–30 nm, blue). (b) R-T curves of the SWCNTs, DWCNTs, and MWCNTs (10 nm) and MWCNTs (10 nm–30 nm) films. (c) Schematic illustration of the sensing mechanism.
Download figure:
Standard image High-resolution imageIn order to understand the difference in the H2 sensitivity, responsivity measured on the four kinds of Pt-NPs/CNTs/Gr devices, figure 3(b) compares the normalized R-T curves of different types of CNT films. Although all four CNT films exhibit semiconductive behavior with resistance increasing with decreasing temperatures, a quantitative difference in the temperature dependence exists. The two MWCNT samples have the lowest temperature dependence due to the metallic behavior of MWCNTs. However, the DWCNT sample has a larger temperature dependence than its SWCNT counterpart does though its Eg is expected to be considerably smaller than the latter's. This may be explained by the reduced surface oxygen doping effect in DWCNTs as compared to SWCNTs, which is well known to dope the CNT and therefore increases the conductivity [18]. Since surface oxygen adsorption and doping is maximized on SWCNTs because the only CNT shell is completely exposed to air, it's not surprising that SWCNT behaves more metallic than DWCNT. Therefore, the difference in H2 responsivity measured on the four kinds of Pt-NPs/CNTs/Gr devices in figure 3(a) could be attributed to the compromise of the effective sensor surface area and CNT network conductance. For H2 sensing, both high surface area and CNT network conductance are desired. With consideration of CNT bundling and the CNT conductance with increasing diameter of the constituent CNTs or CNT bundles in practical CNT films, the best H2 responsivity on the Pt-NPs/MWCNTs (10 nm)/Gr device can be ascribed to the combination of highest effective sensing surface area and conductance in MWCNTs (10 nm) films. Specifically, the SWCNT bundle diameter is comparable to the diameter of the MWCNTs (10 nm). The considerably lower conductance in the latter would lead to better signal transport and hence H2 sensitivity in the Pt-NPs/MWCNTs (10 nm)/Gr device than its counterpart of SWCNTs. This result also suggests that further improvement of sensor performance is possible by removing the bundling effect in purified metallic SWCNTs films in Pt-NPs/SWCNTs/Gr nanohybrids. Figure 3(c) illustrates the working principle of the Pt-NPs/CNTs/Gr devices. The catalytic Pt-NPs assist dissociation of the H2 molecules and the follow up transfer of electrons from the Pt-NPs to the CNT and eventually to Gr, which consequently changes the resistance of the Gr channel as H2 response. The catalytic Pt-NPs assist dissociation of the H2 molecules, and the resulted H atoms dissolve readily into a Pt layer, lowering its work function. This results in electrons transfer from the Pt layer to the CNT and finally to graphene, thus lowers the resistance of CNT and graphene (which are p-type in ambient)[9, 34].
Figure 4(a) compares the H2 responsivity of the Pt-NPs/MWCNTs (10 nm)/Gr sensors as a function of the H2 concentration. The responsivity decreases linearly when the decreasing H2 concentration is anticipated due to the reduction of the number of the H2 molecules absorbed on the Pt-NPs/MWCNTs/Gr sensors [35]. The approximately linear relationship between the H2 responsivity and H2 concentrations can be observed in figure 4(b). Quantitatively, the responsivity decreases from 26% to 7% when the H2 concentration decreases from 10% to 1%. It should be noted that the concentration of 1% of H2 gas is lower than the threshold of inflammable H2 concentration of 4% [36]. In order to probe the reproducibility, the Pt-NPs/MWCNT/Gr nanohybrid H2 sensor was exposed to repeated cycles of 4% H2 gas pulses and figure 4(c) illustrates the dynamic responses measured. Overall, a good repeatability of the Pt-NPs/MWCNTs/Gr H2 gas sensor has been demonstrated with respect to multiple H2 pulse exposures.
Figure 4. (a) Dynamic response of the Pt-NPs/MWCNTs (∼10 nm)/Gr nanohybrids to H2 of different concentrations in the range from 1% to 10%. (b) H2 sensitivity as function of H2 concentration. (c) Dynamic response of Pt-NPs/MWCNTs (∼10 nm)/Gr H2 gas sensor at H2 concentration of 4%.
Download figure:
Standard image High-resolution imageIt should be realized that the H2 responsivity of the Pt-NPs/CNTs/Gr nanohybrids can be further enhanced by activating the CNT surface as shown previously on SWCNTs [25]. Figure 5(a) shows the responsivity of the Pt-NPs/MWCNTs (10 nm)/Gr H2 gas sensor to H2 gas at concentrations of 10% (red) and 2% (blue) respectively. The solid curves correspond to the results taken on the as-prepared device while the dashed curves, on the same device after the device was exposed to nondestructive UV irradiation for 5 min. The responsivity of the device increases from 26% (10%) to 36% (20%) for the H2 concentrations of 10% (2%). This result demonstrates that the UV light (360 nm–400 nm) can effectively induce desorption of air molecules from the surface of MWCNTs and graphene [37–41], which improves the H2 sensing performance of the ALD Pt-NPs/MWCNTs (10 nm)/Gr nanohybrid sensors. A similar trend was also observed for the Pt-NPs/SWCNTs/Gr H2 gas sensor to H2 gas at the H2 concentrations of 10% (red, solid and dashed lines) and 2% (blue, solid and dashed lines), as displayed in figure 5(b). Longer time of exposure (> 5 min) to UV light caused a degradation to Gr and CNTs [25].
Figure 5. Sensitivity of (a) Pt-NPs/MWCNTs (∼10 nm)/Gr H2 gas sensor before (solid line) and after UV irradiation for 5 min (dashed line) at H2 concentrations of 10% (red) and 2% (blue), respectively. (b) Pt-NPs/SWCNTs/Gr H2 gas sensor before (solid line) and after UV irradiation for 5 min (dashed line) at H2 concentrations of 10% (red) and 2% (blue).
Download figure:
Standard image High-resolution imageWhile most H2 sensor characterization was carried out in N2 atmosphere, the practical applications of the H2 sensors may be in ambient. For ambient operation, a few techniques such as UV illumination, increasing the humidity, and the operating the temperature have shown to improve the recovery time of H2 gas sensor [42]. In addition, gate-assistant recovery approach has been used for rapid recovery H2 gas sensors for practical application in ambient [43]. A Al-Diabata et al fabricated CNTs-based H2 gas sensor with a short recovery time and attributed the fast recovery to the large surface to-volume ratio CNTs and the exposure of the device to air for fast recovery [44]. When H2 detection is performed using air as the carrier gas, H2 molecules may interact with oxygen in air to produce water molecules [45, 46] and reduces the H2 molecules adsorbed on the surface of the sensor.
Figure 6 compares the responsivity of the Pt-NPs/MWCNTs (10 nm)/Gr H2 gas sensor to 10% of H2 in air (red) and in N2 (blue) atmosphere. The device indeed exhibits reduced H2 responsivity in air by about 40%. Interestingly, a considerably faster recovery of the H2 gas sensor represents the benefit of the in-air detection of H2 because O2 molecules in air can facilitate desorption of H2 molecules from the surface of the Pt-NPs/CNTs/Gr nanohybrids sensors. This result shows that the Pt-NPs/CNTs/Gr nanohybrids sensors are promising for practical applications of H2 sensing.
Figure 6. Responsivity of Pt-NPs-MWCNT/graphene H2 gas sensor to 10% concentration of H2 in N2 (blue) and in air (red), respectively.
Download figure:
Standard image High-resolution imageTable 1 compares the performance of the Pt-NPs/MWCNT/Gr H2 gas sensor developed in this work with some representative works of CNT-based gas sensor decorated with Pt. Prior works typically adopted either evaporation or solution based chemical reaction for Pt fabrication, which have disadvantages considering these approaches consume significantly more Pt source during the fabrication while not producing a conformal Pt nanostructure coating on CNTs. In contrast, ALD could efficiently provide a conformal coating of the catalytic Pt-NPs on CNT films as shown in this work. Compared to the SWCNT counterpart, the MWCNT film has the advantage of large surface area and high electrical conductance, both are critical to achieving high sensor responsivity. In addition, UV irradiation helps remove CNT surface to promote H2 sensing. All these together leads to the much higher H2 sensitivity up to 36% in our Pt-NPs/MWCNT/Gr H2 gas sensor.
Table 1. Comparison of H2 sensing performance with early reports on Pt decorated CNT-based H2 sensor. Specific responsivity is calculated by dividing H2 responsivity by the H2 concentration.
| Sensor platform | Pt fabrication method | H2 concentration | H2 responsivity | Specific responsivity | References |
|---|---|---|---|---|---|
| superaligned CNT film pulled from forest | evaporation | 10% | ∼7% | 0.7 | [10] |
| MWCNT | reaction in solution | 4% | 6.5% | 1.625 | [47] |
| MWCNT | reaction in solution | 4% | 8% | 2 | [9] |
| vertically-aligned CNT | sputtering | 1% | 1.1% | 1.1 | [48] |
| SWCNT/graphene nanohybrid | ALD | 10% | 7.5% | 0.75 | [34] |
| MWCNT/graphene nanohybrid after UV irradiation | ALD | 10% | 36% | 3.6 | This Work |
4. Conclusions
In summary, this work has made a comparative study of the H2 sensors performance on Pt-NPs/CNTs/Gr nanohybrids with different films of SWCNTs, DWCNTs, MWCNTs (10 nm) and MWCNTs (10–30 nm) of comparable film thicknesses of ∼500 nm. In these Pt-NPs/CNTs/Gr sensors, the CNT film determines the effective sensing area and the signal transport to Gr channel. With increasing diameter of the CNTs from ∼1 nm for SWCNTs, to ∼2 nm for DWCNTs, and 10 nm and up to 30 nm respectively for two kinds of MWCNTs, the constituent CNT aspect ratio (since the CNT lengths are fixed in the range of 5–10 μm) and the effective sensing area can be systematically varied. On the other hand, CNT conductivity can be also varied systematically considering the CNT bandgap is proportional inversely to the CNT diameter, leading to more metallic CNTs at larger CNT diameters. This study has revealed that the performance of the Pt-NPs/CNTs/Gr H2 sensors is the compromise of the effective sensing area and electric conductivity. Among different CNT assessed, the best performance was observed on Pt-NPs/CNTs/Gr H2 sensors with a MWCNT film of CNT diameter of ∼10 nm. The highest response is about 26% when Pt-NPs/MWCNTs/Gr to H2 concentration of 10% before UV radiation. In comparison with its counterparts using SWCNTs and DWCNTs that surfer CNT bundling, this MWCNTs film has a comparable sensing area but considerably better electrical conductance, revealing the critical importance of the high electric conductivity in the MWCNTs for an efficient charge transfer.
Acknowledgments
This research was supported by Plant Directed Research and Development funds from the Department of Energy's Kansas City National Security Campus, operated and managed by Honeywell Federal Manufacturing and Technologies, LLC. under contract. No. DE-NA-0002839 with the U.S. Department of Energy/National Nuclear Security Administration. The authors also acknowledge support in part by NSF contracts Nos. NSF-DMR-1909292, and NSF-ECCS-1809293. M A acknowledges the support from Umm Al-Qura University.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Supplementary material
See Supplementary Material for optical images and Raman maps of graphene and MWCNT/graphene. Also, EDS spectra of 50 C ALD-Pt @ SWCNT film, DWCNT film, and MWCNT films are included in the Supplementary Material.







