Abstract
In this research study, novel hydrogel composite films were constructed using different ratios of poly (vinyl alcohol) (PVA)/kappa-carrageenan (KC) (PVA90/KC10%, PVA80/KC20%, PVA70/KC30%, PVA60/KC40%) crosslinked with glutaraldehyde (0.025%) and investigated their physicochemical characteristics such as mechanical, thermal, morphological, swelling behaviour, and cell viability. SEM and FTIR revealed that surface morphology changed to heterogeneous and the presence of molecular interaction among the polymers. PVA90KC10 and PVA60KC40 exhibited smaller and larger pores on surface respectively. The change in the proportion of PVA and KC also triggered the tensile strength (Ts) of the film and the highest Ts observed were 21.60 MPa for PVA60KC40. Moreover, the thermal analysis showed three-phase degradation, and an increase in KC40 concentration results inversely proportional to a decrease in the rate of thermal degradation. Further, swelling and in-vitro biodegradation study confirmed the enhanced perseverance of water uptake for PVA60KC40 (286%) due to pores structure of the hydrogel film and PVA and KC alone degraded faster as compare to other films results suggested higher concentration of PVA90KC10 showed lower degradation rate and highest for PVA60KC40 about 6% and 22% respectively. Further, the cell viability was studied with MTT assay method by using NIH3T3 and HEK-293 cells for biocompatibility study revealed NIH3T3 cells were more biocompatible than HEK-293 and cell viability percent for PVA60KC40 showed the highest cell attachment about 99%. Overall corroborating data obtained from the study attested to the average swelling, appreciable mechanical characters, good interaction between molecules, and cell viability of the constructed PVA/KC hydrogel film, these all characters pave to be used as a potential template for biomedical applications such as tissue engineering and drug delivery.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
In recent years, biopolymer-based biomaterials have seen much progress as many researchers proceed to construct and alter the materials to reach the global requirement for biomedical discipline. Perhaps, progress in the field of polymer, hydrogel material triggered the extensive research interest for drug delivery and scaffold as a supporting material, roles that require controllable and precise polymer material for functioning in tissue engineering applications [1]. In this sense, wide attempts have been developed to construct hydrogel using natural gum and synthesized biodegradable polymers. Such polymers are degradable analogous of materials that have received much attention by minimizing the second surgery in tissue regeneration. Looking at hydrogel they are hydrophilic in nature which able to absorb water or physiological liquid for a long period without dissolution of the matrix. Many research results revealed the breakdown of the synthetic polymer material is associated with the healing and functioning of tissue by the controlled release of biomolecules when compared to non-degradable based hydrogel [2]. Further degradation of hydrogel tailored the physical properties which is an integral part of biopolymer fabricated as properties such as the content of fluid, mechanical properties, and permeability has been increased the stability of the hydrogel for drug release as well as cellular function and growth for tissue regeneration, such hydrogels-based matrix analyzed extensively for biomedical applications as an implants such as a drug delivery matrix and tissue regeneration scaffolding template [3–8]. Hybrid-based hydrogels have gained much attention because of their biocompatibility, flexibility. In this background, expanding literature and library of biopolymer-based synthetic and natural polymers block copolymer of poly (vinyl alcohol) (PVA) and kappa-carrageenan (KC) has appeared as a valuable biodegradable polymer material because of their highly manageable physical and chemical properties.
PVA is a biocompatible and water-soluble polymer employed widely in the food industry area, biomedicine, and pharmaceuticals [9]. Its intense property attracts biomedical applications [10] because of hydrogen bond association which easily forms gels. In PVA hydroxyl groups play a vital role for hydrogen bonding to form significant properties viz, its crystallinity range, maximum water solubility, and wide crystal modulus [11]. Ezequiel et al and Gholam et al worked on PVA/Chitosan cross-linked film for biomedical application [2, 12]. However, its bioactivity is being poor, hence together PVA and natural gums opened a new path of advanced study. The blending of KC has explored a new way in the field of biomedical because of its advantageous properties like non-toxicity and gel-forming nature.
Kappa-carrageenan is a linear polysaccharide sulfated galactan extracted from algae (red seaweeds) and classified into wide varieties based on the number and position of the sulphate groups [13]. KC comprises units of (1, 3)-D-galactopyranose and (1, 4)-3, 6- anhidro-α-D-galactopyranose and with sulfate groups [14]. They tend to acts as good gelling agents and easily film-forming substance [15–17]. The reactive hydroxyl and sulfate groups of KC tend to make it hydrophilic and able to modify structures to form a hydrogel network by combining with other polymers to tune the stability of gels. This natural polymer has many applications in pharma, food, drug delivery [18], biomedical application [19], wound dressing [20] tissue engineering [21] bacteria inactivation [22] as a super absorbent [23]. In this scenario, the hybrid-based polymer material can lead to an alternative way to enhance the physical properties for desired applications. Based on crosslinking method, researchers have reported the work on KC with PVA composite film [24], KC with nano clay for drug release [25], and KC/PVA gamma-irradiated hydrogel film [26].
The research work aims to develop and measure the chemical, mechanical and physical properties of novel hydrogel film based on PVA and KC crosslinked with glutaraldehyde. In addition, different blend ratios were prepared to optimize the morphological, spectroscopic, and mechanical aspects, swelling performance, biodegradation rate, and biocompatibility behaviour of hydrogels. Further, a cell compatibility study was performed using MTT assay in order to calculate the ability of matrix to the attachment of cells and proliferation for tissue engineering applications.
Materials and methods
Materials
Poly (vinyl alcohol) (molecular weight of 1,70,000) was gained from Spectrum India, and KC was purchased from TCI Chemicals Pvt Ltd., Japan. Glutaraldehyde was purchased from Spectrochem, Mumbai, India, and Lysozyme enzyme (ex. white egg) and phosphate buffer saline (PBS) from SRL Pvt Ltd. India.
Preparation of poly (vinyl alcohol) and k-carrageenan film
Preparation of PVA solution was obtained by dissolving the PVA in the weight percent ratio of (100, 90, 80, 70, and 60% w/w) in DI water and stirred on a magnetic stirrer at 75 ± 2 °C and attempted to cool in room temperature. Similarly, the solution of KC prepared by dissolving increasing weight percent (10, 20, 30, 40, and 100% w/w) was prepared in distilled water and stirred for 4 h until obtaining a homogenous viscous solution. After PVA solution and KC were combined and mixed well then added crosslink agent glutaraldehyde (0.025%) and kept overnight stirring at room temperature and the total weight of the mixture was maintained to 2 gm. After a complete homogeneous solution of PVA/KC, then subjected to sonication to remove the bubbles, later solution was poured on the petri plates and permitted to evaporate the hydrogel and formed gel films were dried under an oven at 40 °C for 12 h to complete the removal of solvent from petri plates. Films were removed from the petri plates washed with ethanol and PBS (Phosphate Buffer Saline) later kept in a polythene bag and stored in a desiccator. Samples were named PVA90/KC10, PVA80/KC20, PVA70/KC30, PVA60/KC40, and also pure PVA and KC films were prepared for comparative study. Plausible schematic interaction between PVA and KC crosslinked with glutaraldehyde is presented in figure 1.
Figure 1. Schematic representation of reaction between PVA and KC Cross-linked with glutaraldehyde.
Download figure:
Standard image High-resolution imageCharacterization
Mechanical behaviour
The mechanical properties of the film samples were determined by using a universal testing machine (UTM, LLOYD Instrument). Tensile strength (TS), elongation at break (Eb), and Youngs Modulus (YM) were determined according to ASTM D882-91 (ASTM, 2009). The hydrogel films were stretched to a rate of crosshead speed of 5 mm min−1 at room temperature.
Scanning electron microscopy (SEM)
The surface morphology of the hydrogel films was examined by field emission scanning electron microscopy (FESEM) JSM- 6360, JEOL, Germany. Images of hydrogel were collected by using a voltage of 10 kV. Samples were sputter-coated with a gold layer and by using double-sided sticky carbon tapes samples were mounted on metal stubs.
Fourier transform infrared spectroscopy (FTIR)
The molecular interaction between components was successfully analyzed with an attenuated total reflection (ATR) method of IR spectrometer FT-IR spectroscopy-attenuated total reflection, Prestige 21, Shimadzu, Japan.
Atomic force microscopy
Atomic force microscopy (AFM) images were investigated by software Nanosurf Easyscan 2, Switzerland, and instruments were used to obtain the morphological structure of the hydrogel film. Topographic figures were viewed by contact angle mode.
Thermogravimetric analysis (TGA)
A thermal investigation of the material was performed under equipment SDT Q600 V20.9 TA instruments. The samples weight about 5–10 mg and warmed up to 700 °C at a heating rate of 10 °C min−1 under N2 atmosphere (flow rate 100 ml min−1).
Water contact angle (WCA)
WCA measurements were examined to collect information on the nature of hydrophilicity of the polymeric material (samples). The instrument used for water contact is named SEO Phoenix instrument.
Swelling studies
The swelling behaviour of the samples was analyzed in phosphate buffer saline (PBS) media at different intervals of time. The weight of wet (Ws, swollen weight) and dry weight (Wd, dried weight) film samples were measured. Then, calculate the index of swelling by using the following equation.
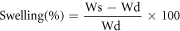
In vitro enzyme-biodegradability
A study of enzyme degradation was performed in the PBS/lysozyme enzyme media. A known quantity (Wi) of prepared hydrogel films were immersed in phosphate buffer solution at 37 °C ± 2 (pH 7.4) containing enzyme lysozyme (Muramidase, white hen egg) enzyme (g/ml) in a water bath. Films were removed after a week, later dried, and noted the weight of dried films (Wf). The loss of weight percent calculated using the following formula.
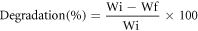
Cell viability assay (MTT)
Cell viability study was performed using a protocol of MTT (MTT Cell Proliferation Assay Instruction Guide –ATCC, VA, USA) 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) as a substrate. Briefly, human embryonic kidney-293 cells (HEK-293) were cultured in the flask T-25, and then trypsinized and aspirated in a centrifuge tube (5 ml). The cell pellets were obtained by centrifugation at 300 × g, after a number of cells were counted and regulated with Dulbecco's Modified Eagle Medium (DMEM) high glucose (HG) medium, such that using a suspension of 200 μl, which contained 1 × 104 cells approximately. The hydrogel samples were cut and placed into the wells. Each well (96 well microtitre plate) added 200 μl of cell suspension and later incubated at temperature 37 °C and atmosphere 5% CO2 for 24 h. Then spent medium was aspirated and were added 200 μl of a medium that contained MTT reagent (10%) to each well to produce a final concentration of 0.5 mg ml−1 and incubated at the temperature 37 °C and atmosphere 5% CO2 for 3 h. After the cultured medium was removed without altered the crystals formed. Then 100 μl of solubilization solution dimethyl sulfoxide (DMSO) was added and gently plate was shaken in a gyratory shaker to solubilize the formazan, then polymeric material (hydrogel film) were removed from the microtitre plate wells and recorded absorbance by using a microplate reader at 570 nm wavelength, finally calculated the percent of cell viability.
Results and discussion
Mechanical properties
The Ts, Eb%, and YM of the hydrogel films were investigated and stress-strain curves are depicted in figure 2, and the results are reported in table 1. The highest tensile strength showed in the film PVA60/KC40 and lowest Ts for PVA80/KC20 up to 21.71 MPa and 9.99 MPa respectively, as compared to pure PVA hydrogel film yielded 6 MPa, but the Eb% for the pure PVA showed highest 124.58% among all tested films. For instance, the modulus of the hydrogel film sample increased as an increase in the content of kappa-carrageenan from 26.18 MPa (pure PVA100) to 551 MPa (PVA60/KC40). In addition, stress-strain curves indicated that an increase in the content of KC increased Ts up to 21 MPa for PVA60/KC40, at the same time Eb% showed descending order (11.93%), this might be attributed to intermolecular interactions between the PVA and KC. The sample PVA80KC20 exhibited lower Ts among PVA/KC blend films may due to variation in the temperature during the evaporation and drying process.
Figure 2. Stress-strain curves of hydrogel film of pure PVA, KC and hydrogel film (a) PVA90/KC10 (b) PVA80/KC20 (c) PVA70/KC30 (d) PVA600/KC40.
Download figure:
Standard image High-resolution imageTable 1. Mechanical study of the hydrogel film PVA/KC.
| Sample code | Tensile strength (MPa) | Elongation break (%) | Youngs modulus (MPa) |
|---|---|---|---|
| PVA100/KC00 | 6.1 | 124.58 | 26.18 |
| PVA90/KC10 | 11.16 | 97.03 | 91.32 |
| PVA80/KC20 | 9.99 | 28.74 | 173.84 |
| PVA70/KC60 | 18.29 | 17.97 | 341.87 |
| PVA60/KC40 | 21.6 | 11.97 | 551.66 |
| PVA00/KC100 | 21.71 | 1.90 | — |
Pure PVA is soft and flexible and whereas PVA/KC hydrogel film becomes harder with decreased elasticity due to higher crystallinity associated with the KC. The phenomenon revealed that the addition of k-carrageenan made the sample more hard and rigid in the dry state because KC interrelates with PVA to enable stress transfer and hydrogen bonding of KC is the key role associated with self-aggregation that supports the emergence of the rigid network structure. The Eb%, significantly decreased as the KC increased, it indicated the film is less elastic and showed good miscibility, the results of the study have close conformity with FT-IR and SEM. Relatively resemble results were occurred by another group for polysaccharide/PVA hydrogel film in the drug release study [23].
Scanning electron microscopy (SEM) analysis
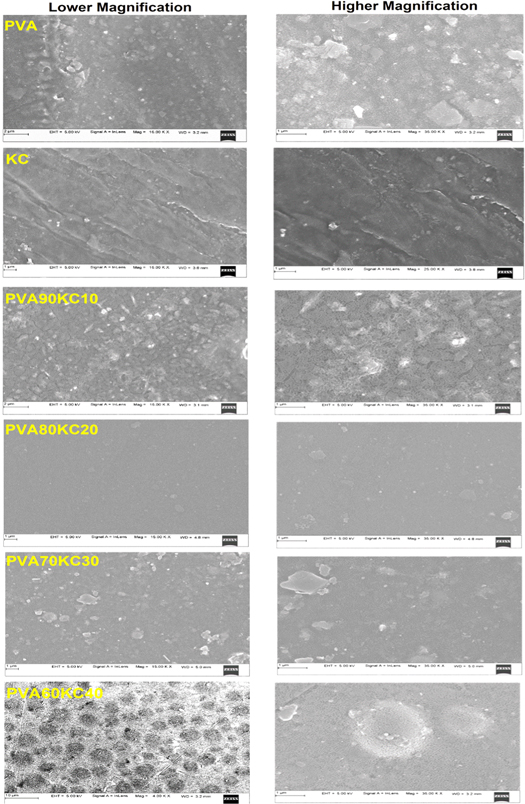
The surface morphological structure of pure and hybrid hydrogel films was depicted in figure 3. The surface morphologies of pure PVA, KC, and PVA/KC blend hydrogel films are significantly different from each other. The pure PVA, KC hydrogel film presented a more or less smooth continuous with uniform and some particles were aggregated on the surface structure of the PVA film, which may be attributed to the phase separation and crystallization [27]. However, PVA/KC hydrogel film showed a rough surface with minute pores present on the surface of film, this might be due to the place of water permeation and the connection points of external stimuli with the hydrophilic group's relation with the KC (SO3 − ). SEM micrographs revealed that the surface structure of the hydrogel film became heterogeneous.
Figure 3. SEM images of lower and higher magnification of pure PVA and KC, and hydrogel films PVA90KC10, PVA80KC20, PVA70KC30, and PVA60KC40.
Download figure:
Standard image High-resolution imageMicrometre sized agglomerations were found most probably due to the dispersion of KC particles in the PVA/KC film. Hydrogel film surface exhibited heterogeneous morphology with a rough surface after mixing with PVA and KC, which indicates the appreciable interaction between PVA and KC. The results have good conformity with other reports showed in the literature for KC mixed with agar [28]. At higher magnification, some scattered bulges and ridges with highly network form were confirmed which are likely to bring about by a few phase separations that may have obtained due to different crosslinking kinetics of KC compared to PVA (Figure PVA90/KC10 and PVA60/KC40). Few researchers noted similar results in the literature [29]. Overall SEM results suggested that the PVA/KC samples were successfully crosslinked by glutaraldehyde which showed the absence of surface holes but observed ridge formation in PVA90/KC10. This elaborated the formation of rough surface and phase segregation with network formation as compared to other proportions of PVA/KC (80:20 and 70:30). We predicted that significant changes were recorded in PVA60/KC40. Results show the formation of some droplet structures due to miscibility and high compatibility between crosslinking agent glutaraldehyde and PVA/KC film. In summary KC in lower concentration was mostly physically entangled but formed a network with glutaraldehyde in a higher concentration of KC [30].
Fourier transform infrared spectroscopy (FT-IR) analysis
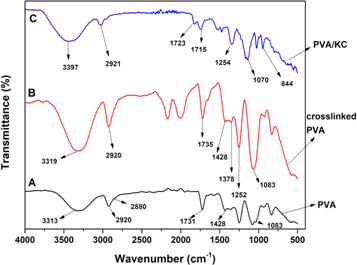
Figure 4 summarizes the FT-IR spectra of hydrogel film PVA, PVA/KC, and crosslinked PVA/KC films. Certainly, the spectrum of the PVA hydrogel film displayed typical hydroxyl and acetate group peaks [31]. Briefly, the peak observed between 3,450 to 3,200 cm−1 is related to the stretching O–H group and stretching band of C–H noticed at 2920 cm−1 and 2880 cm−1. The peak occurred at 1083 cm−1 associated with O–H bending. The peak occurred at 1428 cm−1 and 1378 cm−1 corresponds to bending and wagging of CH2 vibrations respectively [32, 33] and the peaks 1731 for pure PVA and crosslinked PVA 1735 to 1720 cm−1 corresponded to C=O and C–O by acetate group of PVA [34, 35]. The incorporation of KC induced many changes to the vibrational peaks of the PVA film. The hydroxyl groups present in KC chains may cause a shift in the peak value and possible crosslink (GA) has also influenced PVA/KC film. The FTIR spectrum of the film PVA/KC crosslinked GA is shown in figure 4. It is observed that tested film samples of PVA/KC exhibited an intense absorption peak at 1254 cm−1, related to S–O of sulphate esters from KC. Similarly, the occurrence of peaks at 844 cm−1 is corresponding to galactose-4-sulphate. However, the presence of a band at 1070 cm−1 belongs to the presence of glycoside linkage (C–O–C (3, 6anhydro-D-Galactose) [36, 37]. The sharp peak observed at 2921 cm−1 is associated with C–H alkyl stretching and −OH stretching appears at between 3319–3397 cm−1. However, −OH stretching band (3600–3674 cm−1) disappeared in the spectra of PVA/KC film. We believed that non-bonded −OH from PVA might have involved in the crosslinking reaction. Further, it is also noted that there is no increase in the C=O band in the vicinity of 1715 cm−1, it is assumed that the aldehyde groups of GA are may consumed in crosslinking reaction with −OH groups from PVA and KC.
Figure 4. Spectroscopy of pure PVA, crosslinked PVA, and PVA/KC.
Download figure:
Standard image High-resolution imageAtomic force microscopy (AFM)
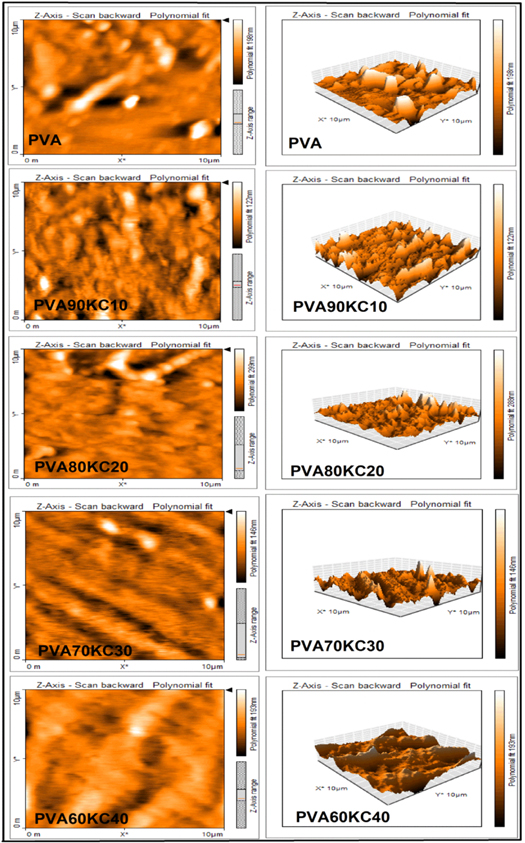
The AFM hydrogel film images of different ratios were shown in figure 5. The PVA/KC films with composition 60/40 showed a decrease in roughness than pure PVA. The presence of a higher concentration of PVA showed an increase in the surface roughness (PVA90/KC10). It suggests that KC is miscible well at a molecular range when present in the high concentration with lower PVA concentration (PVA60/KC40) exhibited small micrometric ridges with a uniform surface. At a higher concentration of PVA (PVA90/KC10), dispersion and aggregation of PVA were observed in the film matrix which related to the rough surface and phase separation, the obtained results are consistent with the SEM.
Figure 5. Atomic Force Microscopy (AFM) images of pure PVA and different concentrations of hydrogel films PVA/KC.
Download figure:
Standard image High-resolution imageThermal properties
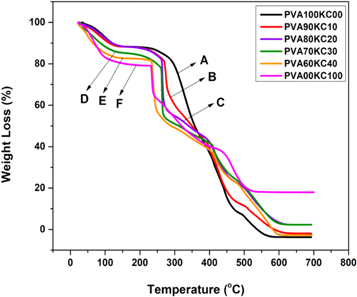
TGA curves of PVA, KC pure, and PVA/KC blend are depicted in figure 6. It is clear from the thermal analysis that the weight of hydrogel films was decreased as the temperature increased. The significant differences were recorded in the thermal degradation of pure polyvinyl alcohol (A) and kappa-carrageenan (F) when compared to PVA/KC (B, C, D, and E). The thermogram of PVA exhibited a three-step degradation process. The peak occurred around 270 °C in PVA this might be associated with the breakage of cross-links during the first phase of decomposition. Similar results were also reported by other researchers [38]. The major degradation process in the second phase occurred from 250 °C to 450 °C, but phase degradation gradually decreased in blend films compared to PVA. In all blend films the second degradation process phase occurred at 240 °C–430 °C, 230 °C−410 °C, 250 °C–400 °C and 220 °C–240 °C for PVA/KC (B, C, D, and E respectively). In PVA a third degradation step occurred at 470 °C−550 °C, which is attributed to the by-products generated by poly (vinyl alcohol) during the thermal degradation process [39]. In contrast, third thermal degradation occurred at 530 °C, 510 °C, 505 °C and 500 °C for PVA/KC (B, C, D, and E respectively) due to a residue of the film and also ash content in all blend film. It is slightly higher when compared to PVA and this could be possibly due to KC which consists of SO3 − group which was left as ash. The overall result suggests that increased concentration of KC with respect to a decreased ratio of PVA in blend leads to decreased stability of the hydrogel film (PVA/KC). We believed that the PVA concentration has a significant influence on the stability of matrix.
Figure 6. TGA curves of (A) Pure PVA, (B) blend PVA90KC10, (C) PVA80KC20, (D) PVA70KC30, (E) PVA60KC40, and (F) KC.
Download figure:
Standard image High-resolution imageWater contact angle studies
The water contact angle of the PVA pure and hydrogel film (PVA/KC) are illustrated in figure 7. The hydrogel film (PVA90/KC10) showed the highest contact angle of 83° which indicated that affinity towards water increased to a small extent with the increased concentration of KC (PVA60/KC40, 71°). This suggests that hydrophilicity increased with an increase in the concentration of KC and decrease in the concentration PVA as compare to pure PVA (showed 85°). Similar results were reported by Fanrong et al [24]. The lowest contact angle was observed for the PVA60/KC40 film which implies that the KC significantly improved the wettability of the sample surface.
Figure 7. Water contact angle of (A) pure PVA, (B) PVA90KC10, and (C) PVA60KC40 and the size of the drop 1 mm.
Download figure:
Standard image High-resolution imageSwelling behaviour
Fluid absorption behaviour studies are of significance for the preliminary analysis of biodegradable materials. For fluid uptake quantification, all the specimens of the PVA/KC hydrogel films with different weight ratios (PVA100/KC00, PVA90KC10, PVA80KC20, PVA70/KC30, PVA60/KC40, and PVA00/KC100) were prepared as described in the previous section. Swelling behaviour experiment conducted with pure PVA, KC, and cross-linked PVA/KC with GA. A typical swelling behaviour is shown in figures 8(a) and (b). The swelling phenomenon happened to each cured film after dipping in PBS at different intervals of time (1, 2, 4, & 8 h) at room temperature. Briefly, the observed results indicated that initial rapid mass uptake was observed approximately at 1 h, followed by mass stabilization over a 3 h period. Visual examination of the samples also showed an appreciable increase in volume. The pure PVA and KC exhibited higher water uptake with 307 and 750% respectively, as illustrated in figure 8(a) (a) and (f), and in the cross-linked hydrogel film the mixture of PVA and KC reduced the swelling rate. Perhaps, the highest swelling rate exhibited for the film PVA60KC40 (286%) and lowest for PVA80KC20 (196%). Overall results indicated that hydrogel film (B, C, D, and E) maintained their water uptake behaviour up to 2 h as compared to PVA and KC pure are initially increased the water uptake up to 1 h. Later on, decreased due to higher mobility of interconnected molecules and finally, the swelling ratio percent of the polymeric material was measured using the following formula [40].
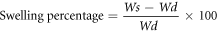
Where Wd and Ws represent the weight of hydrogel film i.e. dried and water uptake before and after immersed in PBS media respectively.
Figure 8. (a) Swelling percent images of pure PVA100 (A), blend films PVA90/KC10 (B), PVA80/KC20 (C), PVA70/KC30 (D), and PVA60/KC40 (E), and pure KC (F). (b) Images of before and after degradation of pure and blend film PVA/KC. Scale range of the hydrogel films 1 cm × 1 cm.
Download figure:
Standard image High-resolution imageBiodegradation study
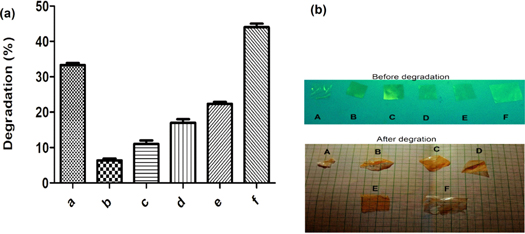
The bio-degradation study was conducted in PBS media with lysozyme enzyme. The dried hydrogel films were cut and the initial weight was recorded. Then films were placed in lysozyme/PBS (g/ml) and incubated at 37 ± 1 °C in a water bath. The wet films were dried and weighed after two weeks (14 days). The resulted data obtained from the degradation study were reported in figure 9. The lowest degradation rate of the PVA/KC film was 6% for PVA90KC20 and the highest 22% for PVA60KC40 after 14 days, this degradation was less than pure components PVA and KC. The overall result of the study indicated that KC concentration in the film was more affected on the mobility of molecules leads to easy degradation of film. The degradation percentage was measured using the following equation [40].
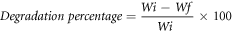
Where Wi and Wf represent the initial and final weight of film, respectively.
Figure 9. (a) Enzyme degradation percent images of pure PVA100 (a and A), blend films PVA90/KC10 (b and B) PVA80/KC20 (c and C) PVA70/KC30 (d and D), and PVA60/KC40 (e and E) and pure KC (f and F) after 14 d. (b) Images of before and after degradation of pure PVA and KC with blend film PVA/KC. Scale range of the hydrogel films 1 cm × 1 cm.
Download figure:
Standard image High-resolution imageMTT assay
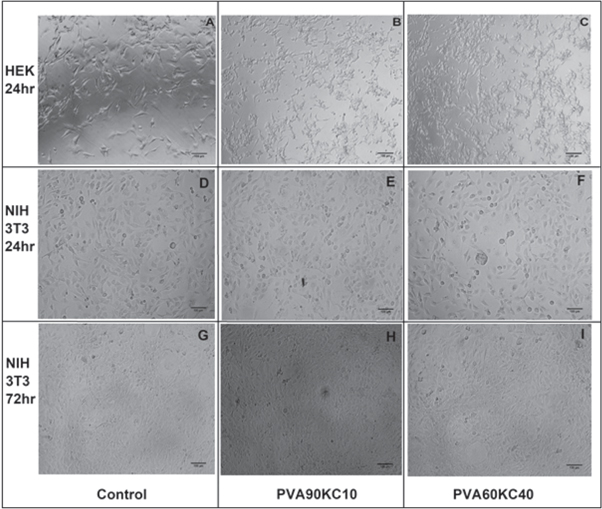
Biocompatibility or viability study was analyzed with MTT after 24 h cells seed by incorporating HEK-293 and NIH3T3 fibroblast cell line. Figures 10 and 11 show the cell viability (%) and cell attachment of optical images, these samples showed comparable acceptable biocompatibility. Finally, cells seeded on a culture plate contain without sample as a control. Results from this study showed that cell viability percent varying from PVA90KC10 (90.1%) to PVA60KC40 (70%) (Compared to control as 100%) for HEK-293 and NIH3T3 about 86% (PVA90KC10) to 98% (PVA60KC40), in this comparison NIH3T3 showed more compatible than HEK-293. For further study extended to the proliferation of cells on hydrogel film by using NIH3T3 from 24 to 72 h, the study revealed that cell growth was increased about 92 and 99% for PVA90KC10 and PVA60KC40 respectively, this indicated that cells are having viable tendency over the matrices. It is believed that a large number of cell attachments over the matrices due to micrometric ridges and rough surface structure of the film and also because of more number of chemical cues to the cell for attachment and growth over the hydrogel film. Results were related to the growth of cells on a polymeric material that starts with the attachment of cells on a template, layout, proliferates, and differentiate [41]. An overall study result suggested NIH3T3 fibroblast cells are more biocompatible than HEK-293 cells.
Figure 10. (a) Cell viability percent of PVA/KC hydrogel film (a) combined result of HEK-293 and NIH3T3 fibroblast for 24 h of the sample (A) control, (B, D) PVA90CMC10, (C, E) PVA60KC40, (b) NIH3T3 fibroblast for 24 and 72 h of the sample (a) Control, (b, c) PVA90KC10, and (d, e) PVA60KC40.
Download figure:
Standard image High-resolution imageFigure 11. Optical microscopic images of PVA/KC A, D and G-Control, B, E and H- PVA90KC10, and C, F and I- PVA60KC40. Scale bar 100 μm.
Download figure:
Standard image High-resolution imageConclusion
The present study focussed on the tuning of hydrogel films with various amounts of PVA and KC. The tested films were obtained using glutaraldehyde as a cross-linker and interactions among the PVA/KC components and physical properties were measured by FT-IR, UTM, SEM, AFM, and TGA. Further biocompatibility was analyzed with NIH3T3 fibroblast and HEK-293 cell line. The hydrogel films prepared with a higher content of KC and PVA (PVA90KC10 and PVA60KC40) showed significantly increased properties. The study proved that the film PVA60KC40 was capable to stimulate and directional the growth of NIH3T3 cells as compare to HEK-293 cells due to its mechanical and biocompatible properties. The results obtained from the research study confirmed the potential of the PVA/KC film and this might be advantageous to act as a suitable scaffold for biomedical applications such as wound healing and skin tissue engineering.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).