Abstract
For decades, silver has been used as a non-toxic inorganic antimicrobial agent. Silver has a lot of potential in a variety of biological/chemical applications, particularly in the form of nanoparticles (NPs). Eco-friendly synthesis approach for NPs are becoming more common in nanobiotechnology, and the demand for biological synthesis methods is growing, with the goal of eliminating hazardous and polluting agents. Cultures of bacteria, fungi, and algae, plant extracts, and other biomaterials are commonly used for NP synthesis in the 'green synthesis' process. Plant-based green synthesis is a simple, fast, dependable, cost-effective, environmentally sustainable, and one-step method that has a significant advantage over microbial synthesis due to the lengthy process of microbial isolation and pure culture maintenance. In this report, we focussed on phytosynthesis of silver nanoparticles (AgNPs) and their characterization using various techniques such as spectroscopy (UV–vis, FTIR), microscopy (TEM, SEM), X-Ray diffraction (XRD), and other particle analysis. The potential applications of AgNPs in a variety of biological and chemical fields are discussed.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanotechnology is the use of scientific expertise from the physical, chemical, and biological disciplines to manipulate and monitor the matter on a nanoscale. In 1971, Professor Norio Taniguchi from the University of Tokyo introduced the term 'nanotechnology' for the first time [1]. The idea of nanotechnology was first delivered by Richard Feynman in 1959, and explained by Drexler in 1986. In 1981, the cluster science and scanning tunneling microscope (STM) helped the growth of nanotechnology, whereas, fullerenes and carbon nanotubes were discovered concerning the developments of nanotechnology. Nanotechnology emerged from the industrial revolution in the 21st century [1].
The breakthrough in nanotechnology has resulted in advances in material science and electronic devices. Fabrication of nanoparticles (NPs) was one of nanotechnology's early stages of growth. NPs are any materials with at least one dimension in the range of 1 to 100 nanometers. [2, 3]. NPs have different physical and chemical properties than their bulk materials due to reduction at smaller scale. NPs may be formed by metals, minerals, and polymers [4]. Owing to the appearance of quantum effects, NPs vary from their corresponding bulk materials in terms of size and origin of phenomena such as coulomb blockade, superparamagnetism, plasmon resonance, and so on. The high surface-to-volume ratio of NPs is another consequence of their reduced size. NPs exhibit entirely new properties as a result of their precise characteristics, such as small size, large surface area, varying shape, and particle distribution [1].
1.1. Why silver nanoparticles?
One of the first and most obvious questions is that why we are interested in AgNPs and investigating their biological and chemical properties. Metal nanoparticles (MNPs) are being produced from the salts of various metals, including copper (Cu), iron (Fe), gold (Au), silver (Ag), platinum (Pt), palladium (Pd) among others. Amongst various metallic nanoparticles, AgNPs have several advantages. Although many are characterized as 'AgNPs', owing to large ratio of surface to bulk Ag atoms, some contain a high amount of Ag2O. In the last decade, AgNPs have been used in a wide range of electronic and medical devices, surgical instruments, bone cements, surgical masks, and other applications [5–8]. In addition, a particular amount of ionic silver is used to treat wounds. As a result, AgNPs are used to treat wounds and burns [9–11]. Since AgNPs have large scattering cross-sections and surface plasmon resonance, they are used in molecular labeling [12]. Thus, many AgNPs are now being recognized for their wide range of applications [13–15].
2. Methods involved in the synthesis of nanoparticles
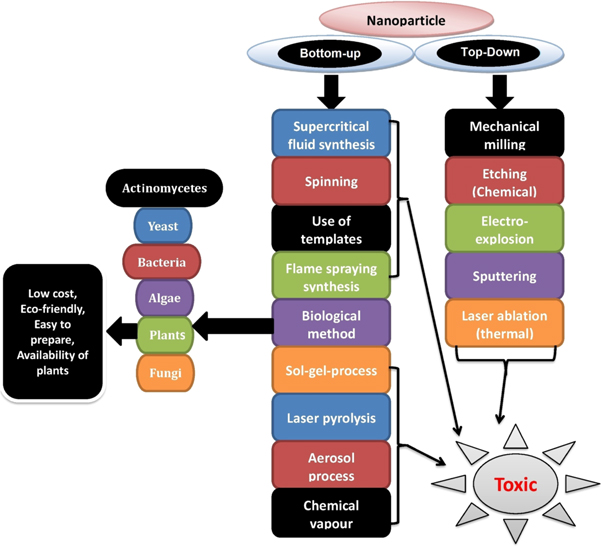
On the basis of precursor used, the nanoparticles are synthesized by mainly two processes, (i) Top-down and (ii) Bottom-up approach. In the Top-down approach, bulk materials are converted to fine nanoparticles mostly by physical methods i.e. milling, grinding, and sputtering techniques. Whereas, in the Bottom-up approach, substances are synthesized from bottom level i.e. atom-by-atom, molecule-by-molecule, and cluster-by-cluster involving physical, chemical and biological processes as shown in figure 1.
Figure 1. Synthesis processes of nanoparticles.
Download figure:
Standard image High-resolution image2.1. Physical methods
The physical approach is one of the common methods, where nanoparticles are synthesized by high energy ball milling (HEBM) [16], laser pyrolysis [17], electrospraying [18], laser ablation [19], and evaporation-condensation [20]. Some other physical techniques are also used for the synthesis of nanoparticles such as electromagnetic radation [21], annealing [22], arc-discharge technique [23], and atomization [24]. The physical approaches mostly based on Top-down phenomena are costly to carry out with more energy requirement i.e. high temperature and pressure. These physical processes are lengthy tasks, and the created NPs have a limited lifespan and poor thermal stability. However, physical modes of synthesis have many benefits over chemical approaches, including the absence of solvent contamination in the prepared thin films and the homogenous dispersion of synthesized nanoparticles [25].
2.2. Chemical methods
The hydrothermal, sol-gel method, microemulsion technique, chemical vapour synthesis, and polyol synthesis techniques are mainly used in chemical approaches. In chemical approaches, some reducing agents are used for reduction, which may be organic or inorganic compounds such as sodium citrate, NaBH4, hydroquinone, elemental hydrogen, and gallic acid. Chemical processes are usually carried out in the solution level, but the result can also be found as a precipitate, so these reactions are referred to as co-precipitation [26].
2.3. Biological methods
Biosynthetic routes are considered ecofriendly because the biological system, including bacteria, actinomycetes, fungi, yeasts, algae, and plants themselves or their active principles, act as a 'bio-laboratory' for the development of pure metal and metal oxide NPs using a biomimetic approach [27]. The main advantage of the biological method is that neither bulk machinery nor external sources of hazardous chemicals are required in the synthesis of nanoparticles, and thus there is no chance of environmental toxicity, in contrast to physico-chemical methods where reducing agents are highly reactive and toxic in nature. In most of the cases, the secondary metabolites of the biological system may act as capping, reducing, and stabilization agents. The deposition of biomolecules on the surface of NPs enables them biocompatible, and thus the bio-based nanostructure opens up a lot of possibilities in biomedicine and other fields. In addition, the biological procedures allow for the development of NPs with unique morphologies and sizes [27]. These bio-based protocols have been termed 'Green synthesis' in nanobiotechnology due to their focus on mild reaction conditions and nontoxic precursors to reduce generated waste and to implement environmental sustainability [28].
2.3.1. Fungi mediated
Various species of fungi like Phaenerocheate chrysosporium [29], Colletotrichum sp. [30], Fusarium oxysporum [31], Aspergillus clavatus [32], and Trichoderma longibrachiatum [33] have been used for the synthesis of nanoparticles due to having high binding and metal-accumulation ability. The synthesis of nanoparticles using fungi is facile and more advantageous than using other microorganisms because mycelial growth can easily be monitored in the laboratory than bacteria and actinomycetes.
2.3.2. Bacteria mediated
Silver, gold and other metallic nanoparticles have been synthesized from different bacterial species. A gram-positive Staphylococcus aureus [34], and gram-negative Acinetobacter calcoaceticus [35] were used to synthesize AgNPs with antimicrobial applications. A rod shaped bacterium Bacillus was preferred by the researchers, and its various species, like B. subtilis [36] B. amyloliqefaciens [37] B. megaterium [38], and B. flexus [39], were used for biosynthesis of the AgNPs. Psychrophilic and mesophilic bacteria were also used for the synthesis of antibacterial AgNPs [40]. The synthesized AgNPs have different shapes i.e., discoidal, spherical, triangular, hexagonal, and cuboidal. Saifuddin and his co-workers synthesized AgNPs from Bacillus subtilis under microwave conditions in the presence of water [41]. Shahverdi group synthesized AgNPs within 5 min by using Escherichia coli, Enterobacter cloacae, and Klebsiella pneumonia [42]. For the desired NPs, bacteria can be manipulated genetically without much difficulty and show high growth rate; however, sophisticated equipments are required to obtain clear filtrate from colloidal broth.
2.3.3. Algae mediated
The algae show fast propagation and capacity to accumulate and reduce inorganic metal ions. Therefore, NPs have also been synthesized by using microalgae, seaweeds and diatoms from the aquatic ecosystem [43]. The antibacterial and cytotoxic O-AgNPs was reported to be synthesized using a blue-green alga, Oscillatoria limnetica [44]. Jena et al synthesized stable AgNPs within 4–16 nm size range by using a microalga, Chlorococcum humicola [45]. Sargassum wightii, a brown marine alga was also reported for extracellular synthesis of AgNPS with antibacterial potential [46].
2.3.4. Plant mediated
The plant mediated green synthetic approach has been emerged a better alternative over abovementioned biological methods due to tedious process of procurement, isolation, purification, and maintenance of microbial culture with costly nutrient media [47]. Further, contamination of culture and less identified biocapping agents are another issues which render microbes and algae, less preferred than plants. Therefore, biosynthesis of AgNPs using plants and their by-products is most desirable among nano-biotechnologists due to easily available precursors, and cost-effective, rapid, eco-friendly, and non-pathogenic protocol. The phytomolecules like amino acids, proteins, terpenoides, polyphenols, favonoides, polysaccharides, vitamins, alkaloids, tannins, saponins, resins, fats and enzymes are playing a significant role as reducing and stabilizing agents in phytosynthesis of NPs [27, 48–50]. Some examples of plant mediated green synthesized AgNPs are shown in table 1.
Table 1. List of some AgNPs with mediated plants and size.
| S.No | NPs | Mediated plants | Size | References |
|---|---|---|---|---|
| 1. | Silver | Adiantum capillus-veneris | 25–37 nm | [51] |
| 2. | Silver | Bryophyllum pinnatum (Lam.) Oken | ~15 nm | [52] |
| 3. | Silver | Pongamia pinnata | 5–15 nm (small) and 22–55 nm (large) | [53] |
| 4. | Silver | Cordia dichotoma | 2–60 nm | [54] |
| 5. | Silver | Rumex hymenosepalus | 2–40nm | [55] |
| 6. | Silver | Eichhornia crassipes | 22 nm | [56] |
| 7. | Silver | Rhizophora mucronata | 60–95nm | [57] |
| 8. | Silver | Azadirachta indica | 15–35nm | [58] |
| 9. | Silver | Chenopodium murale | 30–50nm | [59] |
| 10. | Silver | Lippia citriodora | 15–30 nm | [60] |
| 11. | Silver | Azadirachta indica | 34 nm | [61] |
| 12. | Silver | Murraya koenigii | ~10 nm | [62] |
3. Optimization of plant-mediated synthesis of AgNPs
Phytosynthesis of AgNPs depends upon various factors such as temperature, pH, reaction time, concentration of metal salts, and concentration of plant extracts.
3.1. Effect of reaction time
The reaction time influences the quality, morphology, and properties of silver nanoparticles [63]. For a long incubation period, aggregation and shrinkage can occur [64].
3.2. Effect of plant extracts concentration
The concentration of plant extracts plays a vital role to determine the size and shape of the synthesized silver nanoparticles. Dubey et al synthesized AgNPs and AuNPs by using different concentrations (0.5 ml, 1.0 ml, 2.8 ml and 4.8 ml) of Tanacetum vulgare fruit extract [65]. The absorption peaks of NPs were found to be increased by increasing the concentration of plant extracts [66]. Dwivedi et al synthesized AgNPs and AuNPs from leaf extract of the Chenopodium album, and they observed that particle size was decreased with increasing the concentration of plant extracts [67].
3.3. Effect of silver nitrate (AgNO3) concentration
The concentration of metal salts plays an important role to determine the shape, rate of reduction process, and size of NPs. Dubey et al synthesized AgNPs and AuNPs by using tansy fruit extract with different concentrations of metal ions from 1 to 3 mM [65]. They observed that the large size of NPs was found at a higher concentration of the metal salt. Similarly, when Ag and AuNPs were synthesized by using leaf extract of Chenopodium album, the absorption peaks and particle size were increased with increasing the metal ion concentration. The yields of the synthesized NPs were also increased by increasing the concentration of metal ions [67].
3.4. Effect of pH
The formation of NPs also depends on the pH of the reaction medium [68]. From the literature review, lower pH values favoured the production of larger sized NPs [65, 69]. The larger rod shapes AuNPs were synthesized using Avena sativa at pH 2, whereas smaller sized AuNPs were made at pH 3 and 4 [70]. Spherical shapes of AgNPs were reported by using Cinnamon zeylanicum at high pH 5 and above [71].
3.5. Effect of temperature
The shapes and sizes of the NPs are also affected by the temperature of the reaction mixture. when AuNPs were synthesized using the leaf extract of Cymbopogon flexosus, the resulting shape was nano-triangles at low temperature, whereas spherical shapes were formed at high temperature [72].
4. Applications of green synthesized AgNPs
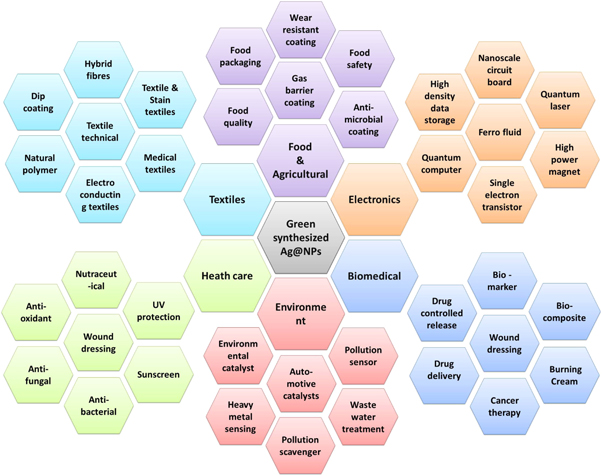
AgNPs have been used in a number of different applications. Figure 2 depicts a few examples of applications in various fields.
Figure 2. Multiple applications of AgNPs.
Download figure:
Standard image High-resolution image4.1. As a photocatalyst for the degradation of synthetic dyes
Degradation of dyes from contaminated water and soil using NPs has recently become a viable option. The photocatalytic activity of AgNPs have been investigated with different types of pollutant dyes such as Methylene blue (MB), Methyl orange (MO), Naphthol orange (NO), Methyl red (MR), and Malachite green (MG), etc in presence of sunlight. Jyoti et al synthesized AgNPs using leaf extracts of Zanthoxylum armatum and investigated their photocatalytic activity against synthetic dyes like MB, MO, MR, and safranine-O [73]. Kolya et al synthesized AgNPs from leaf extract of Amaranthus gangeticus and studied their antimicrobial activity and photocatalytic activity against congo red (CR), an anionic azo dye [74]. Arya et al synthesized AgNPs using leaf extract of Cicer arietinum and evaluated the photocatalytic activity against 4-nitrophenol, CR, and MB [75]. The dyes are degraded by both oxidation and reduction reactions. These dyes have characteristic absorption peaks at certain wavelengths (e.g. MB: 665nm; CR: 497nm; MG: 624nm). After interaction with NPs, gradual decrease in the intensity of absorption occurs due to reduction of dye and formation of intermediate product. The blue color of MB comes from its oxidized state, which becomes colorless when reduced to leuco methylene blue. The AgNPs have been found to serve as mediators in the transfer of electrons, thus degrading the dye [76]. Camellia japonica mediated AgNPs generate ROS, which oxidize Eosin-Y dye and produce intermediate products [77].
4.2. In Hydrogen peroxide (H2O2) sensing
H2O2 is a highly toxic chemical, and even a small amount of H2O2 contamination in food can be harmful to living organisms. AgNPs are said to be able to detect very small amounts of H2O2. According to Mohan et al, when H2O2 was added to AgNPs, free radicals were produced, which oxidized Ag0 to Ag+ and thus reduced the absorbance. Therefore, it is suggested that AgNPs be used as detectors of H2O2 [47]. Raja et al reported phytosynthesis of AgNPs using leaf extract of Calliandra haematocephala and investigated their sensing properties against H2O2 [78]. Aadil et al synthesized AgNPs from Acacia lignin and used as a sensor for the detection of H2O2 [79]. Kumar et al also reported the detection of H2O2 using Euphorbia hirta mediated AgNPs [80].
4.3. In heavy metal sensing
Researchers are concerned about heavy metal pollution in our environment. Various heavy metals like, As, Pb, Hg, Cd, Ni, Al, Va, etc are known to cause carcinogenesis, neurotoxicity, loss of cellular function and cell damage in humans [81]. Phytosynthesized AgNPs are being employed to detect toxic metal cations from contamination soil and industrial effluents. Silver and gold NPs were reported from L-tyrosine, and investigated for their sensing property against heavy toxic metals like Mn2+, Hg2+, and Pb2+ [82]. Firdaus et al synthesized AgNPs from papaya fruit and used them as a sensor against Hg2+ [83]. Tagad et al reported AgNPs using the root extract of Panax Ginseng and investigated their sensing property against heavy metals [84]. AgNPs are used as sensors for the detection of heavy metals in two ways. One is the redox reaction, in which AgNPs are oxidized and heavy metal is reduced. The second way is the formation of a chelation complex between heavy metal and functional groups of phytochemicals adsorbed on NP surface [47].
4.4. In DNA interaction studies
In vitro, AgNPs have been reported to bind with calf thymus DNA (CT-DNA), halting their activity and causing cell damage. Such interactions between nanostructures and DNA have been discussed in a number of papers. The possible interaction between NPs and DNA are electrostatic interaction, intercalation, and groove binding [85]. Ribeiro et al synthesized AgNPs by aqueous extract of black tea, and investigated the CT-DNA binding capacity with AgNPs using the electronic absorption and fluorescence spectroscopies [85]. Rahban et al reported the interaction between AgNPs and CT-DNA using electronic absorption spectroscopy [86]. Roy et al also reported the interaction of biosynthesized AgNPs with CT-DNA [87].
4.5. As cytotoxic agent
AgNPs are found to disrupt normal cell division in somatic cells, and thus used as an antimitotic agent. AgNPs interfere with mitotic events in cells, and as a result, cells die due to decrease in the progress of S-phase, blockage of the G2 phase, and arrest of M phase with reduced expression of cyclins/cdks. NPs cause structural or numerical changes in chromosomes, resulting in their deformation and reconstruction. The viscosity of cytosol is affected by AgNPs, resulting in irregular spindle activity and aberration of chromosomes [88, 89].
Jacob et al reported AgNPs from leaf extract of Piper longum and studied their cytotoxic activity [90]. AgNPs, synthesized by using leaf extract of Rauvolfia tetraphylla, were investigated for their genotoxic effects showing chromosomal aberrations in the root cells of Allium cepa [91]. Researchers also reported cytotoxicity of same NPs against MCF7 and A549 cell lines following MTT assay. Suman et al reported AgNPs using the root of Morinda citrifolia and investigated the cytotoxic activity on the HeLa cells [92].
4.6. As antimicrobial agent
Silver metal ions are well known for their antiseptic properties, and some of the plant extracts have antimicrobial properties as well. AgNPs have significant antibacterial, antifungal, and antiviral effects. Owing to their small size, AgNPs and released Ag + have ability to penetrate the cell wall and plasma membrane of bacterial cell, inactivate respiratory enzymes, and disrupt ATP formation. Likewise, AgNPs interrupt the electron transport chain and produce ROS, bind to DNA and prevent replication, denature ribosomes and inhibit protein synthesis, lyse the plasma membrane, causing release of cytoplasm and organelles, and ultimately, cell death [93]. The antimicrobial effects of ampicillin and other antibiotics were found to be enhanced in combination with AgNPs [94]. Therefore, AgNPs have significantly more antimicrobial activity than their source materials (i.e. Ag salt and plant extract). Phytosynthesized AgNPs using plant extract of Chenopodium murale were investigated for their antibacterial activity against Staphylococcus aureus [59]. Similarly, Eclipta alba mediated AgNPs were evaluated for antibacterial activity against Pseudomonas aeruginosa, S. aureus, and Escherichia coli [95].
4.7. As anti-cancer agent
Cancer is a life-threatening illness that is responsible for the majority of deaths worldwide. As a result, one of the most ardent targets is the discovery of powerful and successful anticancer drugs. Now a day, scientists have paid close attention to AgNPs in cancer therapy because of their special properties. These AgNPs have shown to be effective against a variety of cancer cell lines. Kuppusamy et al synthesized and characterized AgNPs from the plant extract of Commelina nudiflora, and investigated their potential cytotoxic activity against HCT-116 colon-cancer cell line [96]. Plant mediated AgNPs using leaf extract of Plantago major, was reported for anti-cancer activity against human breast cancer cells (MCF-7) [97]. Kathiravan et al synthesized AgNPs from Melia dubai extract, and evaluated their anticancer properties against human breast cancer (KB) cells [98]. Our research groups, also synthesized BP-AgNPs by using aqueous leaf extract of Bryophyllum pinnatum, and found potential cytotoxicity against squamous cell carcinoma (A431) and melanoma (B16F10) cell lines [52].
Ag + released from AgNPs interferes with mitochondrial enzymes and interacts with –SH groups of proteins and glutathione (GSH), lowering GSH's ability to scavenge ROS and causing oxidative stress [99]. Thus, NPs increase the production of intracellular ROS, and activate the caspase-3 protein which in turn starts the apoptosis of the cell by capturing the G2/M phase. NP-generated ROS also trigger the damage of mitochondrial membrane. As a result, cell cycle is arrested and necrosis death occurs. [100].
4.8. As antioxidant agent
The antioxidant activity of AgNPs is well investigated by monitoring the scavenging of stable free radicals viz. 2, 2'-diphenyl-1-picryl hydrazyl (DPPH), and 2,2'-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS). Kharat and Mendhulkar synthesized AgNPs using aqueous leaf extract of Elephantopus scaber and evaluated significant antioxidant activity of Es-AgNPs, following DPPH assay [101]. Similarly, phytosynthesized AgNPs using root extract of Helicteres isora were investigated as better antioxidants than ascorbic acid and butylated hydroxytoluene (BHT), via DPPH, H2O2, and NO radical inhibition assays [102]. Ravichandran et al synthesized AgNPs using leaf extract of Artocarpus altilis and studied their antimicrobial and antioxidant activity [103].
AgNPs are recognized as antioxidant agents because silver favours two oxidation states (Ag+1 and Ag+2) that depend on the reaction conditions, thus, AgNPs may quench free radicals by accepting or donating electrons [104]. According to Bedlovikova et al the enhanced antioxidant potential of AgNPs than plant extract is due to the coating of phenolics, flavonoids, and terpenoids on surface which allow NPs to act as singlet oxygen quenchers, hydrogen donors, and reducing agents [105].
5. Characterization of AgNPs and analytical techniques
Characterization of the NPs is one of the crucial parts of the material science research, and without characterization we cannot be confirmed about the formation of nanostructures. Some basic analytical procedures like spectroscopy and microscopy are required to understand the synthesized material scientifically. Characterization includes methods for the exploring material properties and microscopic structures, i.e. processes involving material analyses as mechanical, thermal, and density analyses. Characterization helps to define the structure and composition of materials as well as enables us to evaluate whether or not the approach was successful. In this section, we described different characterization techniques such as Ultraviolet-Visible spectroscopy (UV–vis), Fourier Transform Infrared (FTIR) spectroscopy, Transmission Electron Microscopy (TEM), Scanning Electron Microscopy (SEM), x-ray Diffraction (XRD), and Zeta potential/particle analysis. Sophisticated instruments, used in such analyses are shown in figures 3 and 4.
Figure 3. A typical UV–visible Spectroscope (a), Fourier Transform Infrared (FTIR) Spectroscope (b), Transmission Electron Microscope (TEM) (c), and Scanning Electron Microscope (SEM) (d).
Download figure:
Standard image High-resolution imageFigure 4. A typical X-Ray Diffractometer (XRD)(a), and Zeta particle size analyzer (b).
Download figure:
Standard image High-resolution image5.1. UV–vis spectroscopy
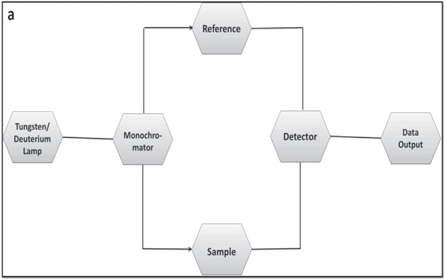
The most popular method for determining extracellular green synthesis of NPs in reacting solution is UV–vis spectroscopy. Besides estimating the concentration of a NP suspension, UV–vis spectroscope can also be used to determine the color absorption patterns of metallic-NPs (through surface Plasmon resonance), together with sorption, diffusion and release properties of nanostructures. A typical UV-visible spectroscope is comprised of a tungsten or deuterium lamp, a detector, and a monochromator for ultraviolet and visible region wavelengths (figure 5) [106]. The UV spectra are formed when the sample is exposed to UV light. Ultraviolet-visible (UV-Vis) spectrum is the count of minimization of light beams after passing through a sample or after refraction from the surface of the sample. Cuvettes are used for carrying a sample, and are kept on the lighting path within the instrument. The cuvettes are made up of plastic, glass, quartz, or silica. Plastic and glass cuvettes absorb wavelength below 310 nm and create interference, therefore, they are not appropriate for studies of absorption under UV light. Since quartz is transparent above 180 nm wavelength, therefore quartz cuvettes are used for ultraviolet absorption measurements. The reference beam is passed from the light source to the detector without interacting the sample. The wavelength of UV light changes constantly as the beam interacts with the sample. The electron moves to a higher orbital by absorbing energy from the light source and releasing the corresponding wavelength. The detector is used to record the intensity ratio between the reference and sample beams. According to Beer–Lambert's law, the absorption by the sample is directly proportional to the concentration of the sample and the length of the path. The equation of Beer–Lambert is mentioned below [107].

Where, A = absorbance, ε = absorptivity, c = concentration, and l = path length.
Figure 5. A schematic diagram of UV–vis spectrophotometer.
Download figure:
Standard image High-resolution imageThe formation of AgNPs in reacting solution is initially confirmed by employing UV-visible spectroscopy. Metallic NPs have characteristic bands in the visible range due to the SPR effect. Therefore, the formation of AgNPs is confirmed by characteristic peak of Ag0, displayed by UV–vis spectroscopy.
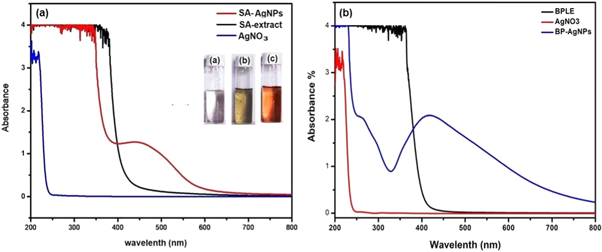
Chandraker et al synthesised AgNPs by using leaf extracts of Bryophyllum pinnatum and Sonchus arvensis. Although, change of reaction mixture from colorless to reddish-brown gave a clue of NP synthesis, the formation of BP-AgNPs and SA-AgNPs was confirmed by observing SPR peak within 400–460. On the other hand, no peaks were assigned with plant extract and solution of silver salt as shown in figure 6 [47, 52]. Moreover, H2O2 sensing, heavy-metal sensing, and CT-DNA binding are also determined by using UV–vis. spectroscopy.
Figure 6. UV-visible spectra of (a) SA-AgNPs and (b) BP-AgNPs (Images are taken from self publications 47, 52).
Download figure:
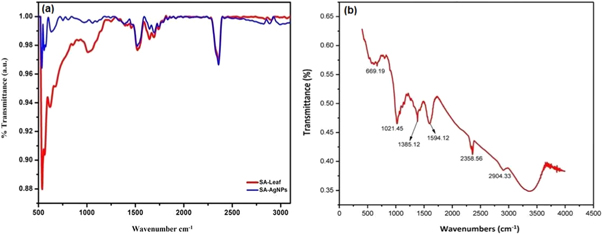
Standard image High-resolution imageFigure 7. FT-IR spectra of (a) SA-AgNPs and (b) BP-AgNPs (Images are taken from self publications 47, 52).
Download figure:
Standard image High-resolution image5.2. Fourier transform infrared (FTIR) spectroscopy
In FT-IR spectroscopy, an infrared spectrum is obtained from emission and absorption of samples like gas, liquid or solid. Each functional group and chemical bond can absorb a certain range of frequencies. Therefore, the characteristic peaks are observed for each functional group or part of the molecule. A typical FTIR spectroscope is consists of a source, detector, sample cell, A/D converter, amplifier, and a monitor. The radiation which is generated from the source reaches the detector from passing by an interferometer. The signal has been amplified by the A/D converter or deuterium lamp, a detector, and a monochromator, for ultraviolet and visible and amplifier, which then passes the signal to the computer where the fourier transforms. The absorbed radiation is converted to rotational or vibrational energy by the sample. The resulting signal is a spectrum of 4000 to 400 cm−1 typically reflecting the molecular fingerprint of the samples. Each molecule has a unique fingerprint that makes FTIR an invaluable tool to recognize phytochemicals [108]. Figures 7(a) and (b) represent signature peaks (transmittance) of certain phytomolecules (coated on the surface of SA-NPs, and BP-NPs, respectively) at defined wavenumbers [47, 52]. Figure 7(a) demonstrates the existence of similar functional groups in Sonchus arvensis leaf extract, indicating that they coat NPs as capping and reducing agents. The interpretation of FTIR data in figure 7(a) explains the attached functional groups on NP surface, i.e. wavenumbers 2358, 1694, 1520 indicate C–H, C=O, and O–C stretches, respectively; whereas, 637 and 534 indicate C–H bends. Figure 8(a) represents the schematic diagram of FTIR.
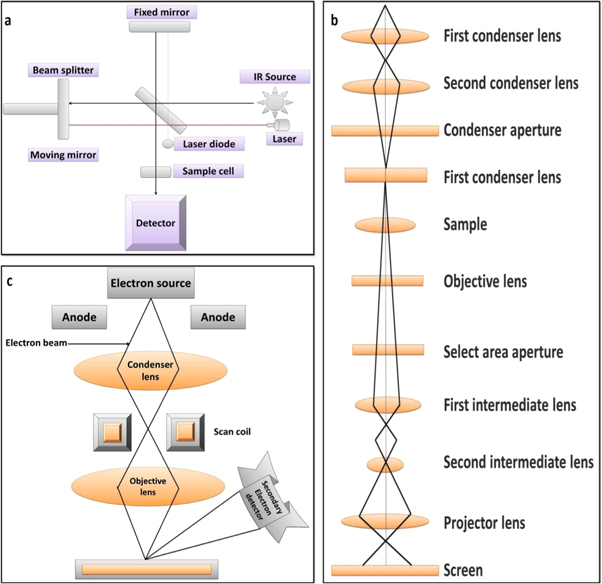
Figure 8. Schematic diagrams of FTIR (a), TEM (b) and SEM (c).
Download figure:
Standard image High-resolution image5.3. Transmission electron microscopy (TEM)
Transmission Electron Microscopy (TEM) is a technique in which beam of electrons are transferred through the sample and images are generated as a result of electronic interaction with the sample (figure 8(b)) [109]. The images are focused on the image detecting fluorescent screens, photographic film and charge-coupled device [110]. TEM is used in various research fields such as materials science, nanotechnology, cancer research, etc In nanotechnology research, TEM is used for the determination of size and surface morphology of the NPs. TEM can supply images with atomic lattice resolution. In TEM, the images are created because of the difference in the electron waves spread by thin samples.
Since the observational area in TEM is small, the analyzed region may not be representative of the entire sample. In the case of biological materials, the electron beam poses a risk of causing harm to the sample. Despite these limitations, TEM is a preferred technique for obtaining visual information on scale, shape, dispersion, and structure due to the atomic level resolution.
5.4. Scanning electron microscopy (SEM)
Scanning Electron Microscope (SEM), shown in figure 3(d), is a device that scans the surface of a sample with an electron beam, and creates images [111]. SEM can generate the surface topography and compositional information of the NPs by interacting the electrons and atoms of the NPs [112]. In field emission (FE) SEM, electrons are formed in an emission source and accelerated by strong electric fields. When an object is scanned, electromagnetic coils generate electrons that pass through a set of lenses to produce a focussed electron beam, and form secondary electrons after colliding with the surface of sample. These resulting electrons contain useful information that is used to rebuild a very detailed picture of the specimen surface topography. SEM is an excellent instrument for direct surface observations since it provides improved resolution and field depth as an electron microscope. The electron source and control convenience are two key components of SEM, shown in figure 8(c). The electron source consists of an electron piston and two or more lenses that affect the paths of electrons moving through an evacuated tube. The control convenience consists of an electron beam control unit, display screen, and monitor. The electron guns have the purpose of providing a stable electron beam. In general, the electron gun is made by the tungsten or lanthanum hexaboride (LaB6). The filament is heated to a resistant current to achieve a temperature between 2000–2700 K. The electron gun emits and speeds up the electrons in the 0.1–30 keV range towards the sample.
5.5. X-ray diffraction (XRD)
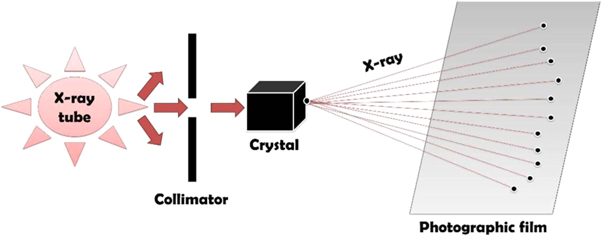
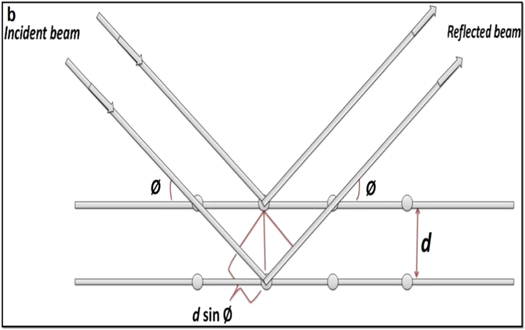
X-ray diffraction is a widely used technique for the structural characterization of AgNPs. This technique is also useful to calculate the average particle size. As monochromatic X-rays occur on a crystal, they are scattered through the atoms and these scattered rays interact constructively to give a diffracted ray as shown in figure 9. The diffraction usually happens when the path difference is several wavelengths between the rays, scattered from successive planes. Every crystalline material has a unique atomic structure, and thus diffracts the x-rays in a separate pattern. The diffraction angle is measured by Bragg's equation [113], as mentioned below.

where, d = pacing between the plane, θ = angle of incidence, n = integer and λ = beam wavelength The crystalline size of the NPs is calculated from the Scherer equation by using XRD data. The Scherer equation is represented as follows.

where, K = 0.9, D = Crystal size (Å), λ = Wavelength of Cu-Kα radiation, and β = Corrected half-width of the diffraction peak.
Figure 9. X-ray diffraction pattern in XRD analysis.
Download figure:
Standard image High-resolution imageThe fundamental part of the geometry of this system is the source of radiation and the x-ray detector positioned around the graduated circle, which focuses on the powder specimen. Figure 10 shows the schematic diagram of XRD. Divergent slit lies between the sample and the ray source, and the detector and the sample. That reduces the background noise, helps to limit dispersed radiation, and there is collimated radiation.
Figure 10. A schematic diagram of XRD.
Download figure:
Standard image High-resolution image5.6. Particle size and zeta potential analysis
Zeta particle analyzer is being employed to determine average size of NPs and their dispersion (figure 4(b)). The distribution of NPs and zeta potential are also studied via 'dynamic light scattering' and 'laser doppler electrophoresis', respectively. Zeta potential magnitude provides details on the stability of the particles. Greater the magnitude, the more stable is NP due to increased electrostatic repulsion. The magnitudes like 0–5 mV, 5–20 mV, 20–40 mV, and above 40 mV indicate the aggregate, minimum stable, moderate stable and highly stable NPs, respectively. The pH of the solution is another important factor that can affect the magnitude of the charge on the surface of NPs. The surface charge, known as the isoelectric point, may be zero at a particular pH [114, 115].
6. Comprehensive review
As discussed in the preceding sections, phytosynthesis of AgNPs have been given major attention due to their various properties. Some of the recent research outcomes on plant-mediated green synthesis of AgNPs, botanical and common names of synthesizing plants, belonging families, growth forms (habit), part used, shape-size of NPs, and their explored applications are mentioned in table 2.
Table 2. AgNPs synthesized using plant extracts (with names of the plants, their habit, part used, size and shape of NPs and their proposed applications).
| S. No. | Plants used | Common/vernacular name of the plants | Plant family | Plant habit | Plant part used | Size of AgNPs (nm) | Shape of AgNPs | Applications | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Abutilon indicum | Atibalaa | Malvaceae | Shrub | Leaf | TEM (5–25) | Spherical | Antioxidant, Antibacterial and Cytotoxic effects | [116] |
| 2 | Acacia nilotica | Babul | Fabaceae | Tree | Leaf | HRTEM (20–30) | Distorted spherical | Reduction of benzyl chloride | [117] |
| 3 | Acacia senegal | Kher/Gum Arebic | Leguminosae | Tree | Gum | AFM (81.45 ± 2.07) | Spherical | Antiarthritic | [118] |
| 4 | Acacia senegal | Kher/Gum Arebic | Leguminosae | Tree | Gum | TEM (1–20) | Cubic | Antibacterial | [119] |
| 5 | Acacia seyal | Shittah | Fabaceae | Tree | Gum | AFM (155.50 ± 1.56) | Spherical | Antiarthritic | [118] |
| 6 | Allium cepa | Onion | Liliaceae | Herb | Leaf | XRD (28.41–56.82) | Spherical | Antimicrobial | [120] |
| 7 | Allium cepa | Onion | Liliaceae | Herb | Fruit | XRD, TEM (~ 6) | Spherical - | Ascorbic acid detection | [121] |
| 8 | Allium cepa | Onion | Liliaceae | Herb | Leaf | TEM (~10) | Spherical | Antimicrobial | [122] |
| 9 | Allium sativa | Garlic | Liliaceae | Herb | Leaf | XRD (22.73–60.61) | Spherical | Antimicrobial | [120] |
| 10 | Allium sativa | Garlic | Liliaceae | Herb | Fruit | TEM (7.4 ± 3) | — | Antioxidant | [123] |
| 11 | Allium sativa | Garlic | Liliaceae | Herb | Fruit | TEM (3–32) | Spherical | Spherical | [124] |
| 12 | Acalypha hispida | Red hot and fox tail | Euphorbiaceae | Shurb | Leaf | TEM (20–50) | Spherical | Heavy metal sensing (Detection of Mn(II) ions) | [125] |
| 13 | Adansonia digitata | Baobab | Malvaceae | Tree | Leaf | TEM (5–64) | Spherical | Antimicrobial | [126] |
| 14 | Adansonia digitata | Baobab | Malvaceae | Tree | Fruit pulp | TEM (3–7) | Spherical | Antimicrobial | [127] |
| 15 | Aloe vera | Ghritkumari | Asphodelaceae | Herb | Leaf | SEM (70) | Spherical, rectangular, Cubical and triangular | Antifungal | [128] |
| 16 | Aloe vera | Ghritkumari | Asphodelaceae | Herb | Leaf | TEM (15.2 ± 4.2) | Spherical | — | [129] |
| 17 | Annona squamosa | Seetafal/ Sharifa | Annonaceae | Tree | Leaf | TEM (20–100) | Spherical | In-vitro cytotoxic effect | [130] |
| 18 | Alternanthera dentate | Joseph's coat | Amaranthaceae | Herb | Leaf | TEM (50–100) | Spherical | Antimicrobial | [131] |
| 19 | Alpinia katsumadae | — | Zingiberaceae | Herb | Seed | FETEM (12.6) | Spherical | Antioxidant, Cytotoxicity, and Antibacterial | [132] |
| 20 | Azadirachta indica | Neem | Meliaceae | Tree | Leaf | TEM (34) | Spherical and irregular | Antimicrobial | [133] |
| 21 | Achillea bieberstennii | — | Asteraceae | Shurb | Flower | TEM (12 ± 2) | Hexagonal, pentagonal and spherical | Anti-angiogenic | [134] |
| 22 | Ageratum conyzoides | Kubbi | Asteraceae | Herb | Leaf | TEM (14–48) | Spherical | DNA-binding, Antioxidant, H2O2 sensing and Photocatalytic properties | [135] |
| 23 | Artemisia turcomanica | — | Asteraceae | Herb | Leaf | TEM (~20) | — | Anticancer and Apoptosis induction | [136] |
| 24 | Berberis vulgaris | Barberry | Berberidaceae | Shurb | leaf and root | TEM (30–70) | Spherical | Antibacterial | [137] |
| 25 | Brassica rapa | Sarson | Brassicaceae | Herb | Leaf | TEM (30–70) | Spherical | Antifungal | [138] |
| 26 | Cinnamomum camphora | Camphorwood | Lauraceae | Tree | Leaf | TEM (55−80) | Spherical | — | [139] |
| 27 | Calatropis procera | Akwan | Apocynaceae | Shurb | Latex | TEM (12.33) | Spherical | Antimicrobial | [140] |
| 28 | Capparis decidua | Karira | Capparaceae | Shurb | Stem | TEM (1−19) | Spherical | — | [141] |
| 29 | Capsicum frutescens | Mirch | Solanaceae | Herb | Fruit | TEM (3−18) | Spherical | Antibacterial and Antioxidant | [124] |
| 30 | Cyperus rotundus | Motha | Cyperaceae | Herb | Whole plant | FESEM (20.5 ± 9.6) | Spherical | Antibacterial | [142] |
| 31 | Clerodendrum phlomidis | Urni, Arna, Aarni | Lamiaceae | Herb | Leaf | TEM (23–42) | Spherical | Anticancer and Antioxidant | [143] |
| 32 | Catharanthus roseus | Periwincle | Apocynaceae | Herb | Lzeaf | SEM (35–55) | — | Antiplasmodial | [144] |
| 33 | Carum carvi | Meridian fenne | Apiaceae | Herb | Leaf | SEM (10) | — | Anti-yeast | [145] |
| 34 | Cassia auriculata | Matura tea tree, Ranawara or Avaram | Fabaceae | Shurb | Flower | HRTEM (10–35) | Spherical and Triangle shapes | Photocatalytic activity | [146] |
| 35 | Cassia auriculata | Matura tea tree, Ranawara or Avaram | Fabaceae | Shurb | Leaf | SEM (50–100) | Randomized biscuit like crystal | Antibacterial and Antifungal | [147] |
| 36 | Citrus sinensis | Orange | Rutaceae | Tree | Leaf | AFM (65) | — | Antimicrobial | [148] |
| 37 | Centella asiatica | Gotu Kola | Apiaceae | Herb | Leaf | AFM (26.4) | — | Antimicrobial | [148] |
| 38 | Coriandrum sativum | Dhaniya | Apiaceae | Herb | Seed | TEM (13.09) | Spherical | Antimicrobial | [149] |
| 39 | Coriandrum sativum | Dhaniya | Apiaceae | Herb | Leaf | TEM (~26) | — | Nonlinear optics | [150] |
| 40 | Clidemia hirta | Soapbush or Koster's curse | Melastomataceae | Shrub | Whole plant | FESEM (57.4 ± 24.2) | Irregular shape | Antibacterial | [142] |
| 41 | Datura stramonium | Datura | Solanaceae | Shurb | Leaf | TEM (0.23) | Spherical | Antibacterial | [151] |
| 42 | Daucus carota | Wild carrot | Apiaceae | Herb | Tuber | TEM (20) | Spherical | — | [152] |
| 43 | Eucalyptus globulus | Neelgiri and Southern blue gum | Myrtaceae | Tree | Leaf | TEM (1.9–4.3, 5–23) | — | Antibacterial and Antibiofilm | [153] |
| 44 | Eclipta prostrata | False daisy or Bhringraj | Asteraceae | Herb | Leaf | TEM (35–60) | Spherical | Antilarvacidal and Antifilarisis | [154] |
| 45 | Euphorbia hirta | Asthma-plant | Euphorbiaceae | Herb | Leaf | FESEM (56.25 ± 21.8) | Irregular shape | Antibacterial | [142] |
| 46 | Eleusin indica | Indian goosegrass, Yard-grass | Poaceae | Grass | Hole plant | FESEM (55.0 ± 24.1) | Spherical | Antibacterial | [142] |
| 47 | Eriobotrya japonica | Lokat | Rosaceae | Tree | Leaf | HRTEM (9–17) | Spherical | Catalytic degradation of reactive dyes | [155] |
| 48 | Fagonia cretica | Virgin's Mantle | Zygophyllaceae | Herb | Whole plant | TEM (16) | Spherical | Antimicrobial | [156] |
| 49 | Foeniculum vulgare | Fennel | Apiaceae | Herb | Seed | SEM (11–25) | Spherical | Antimicrobial | [157] |
| 5052 | Ficus benghalensis | Banyan | Moraceae | Tree | Leaf | TEM (~16) | Spherical | Antibacterial | [158] |
| 53 | Ficus carica | Anjeer | Moraceae | Tree | Fruit | TEM (10–30) | Spherical | Antioxidant | [159] |
| 54 | Garcinia mangostana | Purple mangosteen | Clusiaceae | Tree | Stem | SEM (30) | Spherical | Antimicrobial | [160] |
| 55 | Glycyrrhiza uralensis | Chinese liquorice | Fabaceae | Herb | Root | XRD (8.01) | Spherical | Antimicrobial, dye degradation, Antioxidant and Anticancer | [161] |
| 56 | Glycyrrhiza glabra | Liquorice | Fabaceae | Herb | Root | TEM (~19) | Spherical | Treatment of gastric ulcer | [162] |
| 57 | Helicteres isora | Indian screw tree | Malvaceae | Tree | Root | TEM (30–40) | Crystalline and spherical | Antimicrobial and Antioxidant | [102] |
| 58 | Hibiscus rosa-sinensis | Rose mallow | Malvaceae | Shurb | Leaf | TEM (~13) | Spherical | — | [163] |
| 59 | Hibiscus sabdariffa | Rosella | Malvaceae | Shurb | Flower | TEM (5–60) | Spherical, triangular and hexagonal | Catalytic reduction of 4-nitrophenol | [164] |
| 60 | Hibiscus cannabinus | Kenaf | Malvaceae | Shurb | Leaf | TEM (~15) | Spherical | Antimicrobial | [165] |
| 61 | Holarrhena antidysenterica | Coral swirl or Kutaj | Apocynaceae | Tree | Bark | SEM (32) | Spherical | Antilarvacidal and Antifilarisis | [166] |
| 62 | Justica adhatoda | Malabar nut, Adusa | Acanthaceae | Shurb | Leaf | TEM (11–20) | Smooth and spherical | Antibacterial and Anticancer | [167] |
| 63 | Justica adhatoda | Malabar nut, Adusa | Acanthaceae | Shurb | Leaf | TEM (2.0, 26.7 and 47.9) | spherical and irregular | Photocatalytic and Anticancer activity | [168] |
| 64 | Jatropha curcas | Ratan jot | Euphorbiaceae | Shrub | Seed | HRTEM (15–50) | Spherical | — | [169] |
| 65 | Jatropha curcas | Ratan jot | Euphorbiaceae | Shrub | Leaf | TEM (100) | Spherical and irregular | Antibacterial | [170] |
| 66 | Jatropha curcas | Ratan jot | Euphorbiaceae | Shrub | Latex | TEM (10–20) | Spherical | — | [171] |
| 67 | Kigelia Africana | Sausage tree | Bignoniaceae | Tree | Fruit | TEM (10) | — | Antimicrobial | [172] |
| 68 | Lantana camara | wild/red sage | Verbenaceae | Shurb | Leaf | TEM (14–27) | Spherical | Antibacterial | [173] |
| 69 | Lawsonia inermis | Heena | Lythraceae | Shurb | Leaf | TEM (5–45) | Spherical | Antibacterial and Antifungal | [174] |
| 70 | Lippia nodiflora | Bukun boot | Verbenaceae | Herb | Leaf | TEM (20) | Spherical | Antioxidant, Antibacterial and Cytotoxic effect | [175] |
| 71 | Litchi chinensis | Lychee | Sapindaceae | Tree | Leaf | — | — | Muscle relaxant, Analgesic and Anti-inflammatory | [176] |
| 72 | Lens culinaris | Lentil | Fabaceae | Herb | Seed | TEM (13) | Crystalline and Spherical | — | [177] |
| 73 | Mimusops elengi | Spanish cherry | Sapotaceae | Tree | TEM (12.8–30.48) | Spherical | Antimicrobial and Antioxidant | [178] | |
| 74 | Memecylon edule | Delek bangas | Melastomataceae | Tree | Leaf | TEM (50–90) | Square | — | [179] |
| 75 | Melastoma malabathricum | Malabar melastome | Melastomataceae | Herb | Hole plant | FESEM (108.35 ± 36.3) | Irregular shape | Antibacterial | [142] |
| 76 | Melia dubia | Malai vembu | Meliaceae | Tree | Leaf | XRD (average 7.3) | Irregular, but mostly Spherical | Anticancer activity | [98] |
| 77 | Moringa oleifera | Drumstick tree | Moringaceae | Tree | Bark | HRTEM (average size 40) | Spherical and Pentagonal | Anticancer activity | [180] |
| 78 | Mukia maderaspatana | Madras pea pumpkin | Cucurbitaceae | Herb | Leaf | FESEM (13–34) | Spherical | Anti larvicidal | [181] |
| 79 | Musa acuminata | Banana | Musaceae | Herb | Leaf | TEM (~30) | Spherical | Antimicrobial | [122] |
| 80 | Nelumbo nucifera | Sacred lotus | Nelumbonaceae | Herb | Leaf | TEM ( ~45) | Spherical (TEM), triangular (SEM) | Anti-malaria and Anti filariasis vectors | [182] |
| 81 | Nigella sativa | Black cumin | Ranunculaceae | Herb | Seed | TEM (10–20) | Spherical | Anticancer activity | [183] |
| 82 | Ocimum tenuiflorum | Tulsi | Lamiaceae | Herb | Leaf | AFM (28) | — | Antimicrobial | [148] |
| 83 | Origanum vulgare | Oregano | Lamiaceae | Herb | Leaf | FESEM (63–85 nm), DLS (136 ± 10.0) | Spherical | Antibacterial and anticancer activity | [184] |
| 84 | Olea europaea L | Olive | Oleaceae | Tree | Leaf | TEM (~20–25) | Spherical | Antibacterial | [185] |
| 85 | Pterocarpus santalinus | Sandal wood | Fabaceae | Tree | Leaf | SEM (~20 nm), AFM (41) | Spherical | Antibacterial | [186] |
| 86 | Piper longum | Pipli | Piperaceae | Herb | Fruit | DLS (~46 nm) | Spherical | Antioxidant, Antibacterial and Cytotoxic effects | [187] |
| 87 | Piper nigrum | Black pepper | Piperaceae | Herb | Leaf | TEM (90) | Spherical | Histopathological study on fish (Labeo rohita) | [188] |
| 88 | Phyllanthus amarus | Bhui Aamla | Phyllanthaceae | Herb | Leaf | TEM (36) | Flower-like structure | Antimicrobial and Catalytic activity | [189] |
| 89 | Phyllanthus amarus | Bhui Aamla | Phyllanthaceae | Herb | Hole plant | TEM (51 ± 28) | — | Antimicrobial | [190] |
| 90 | Pachyrhizus erosus | Jicama | Fabaceae | Vine | Hole plant | FESEM (40.6 ± 10.8) | Spherical | Antibacterial | [142] |
| 91 | Solanum tricobatum | Achuda and Alarka | Solanaceae | Herb | Leaf | AFM (22.3) | Spherical | Antimicrobial | [191] |
| 92 | Sonchus arvensis | — | Asteraceae | Herb | Leaf | TEM (25 ± 5) | Spherical | Heavy metal sensing | [47] |
| 93 | Sesbania grandiflora | Hummingbird tree | Fabaceae | Tree | Leaf | TEM (~24.1), XRD (18.52) | Spherical | Photothermal therapy | [192] |
| 94 | Sesuvium portulacastrum | Salt Marsh | Aizoaceae | Herb | Callus and leaf | TEM (5–20) | Spherical | Antimicrobial | [193] |
| 95 | Sambucus nigra | European black elderberry | Adoxaceae | Shurb | Frozen fruit | TEM (20–80) | Spherical | Anti-inflammatory | [194] |
| 96 | Tamarindus indica | Tamarind | Fabaceae | Tree | Fruit | TEM (10) | Spherical | Antibacterial | [195] |
| 97 | Tribulus terrestris | Land caltrops, Puncture vine | Zygophyllaceae | Herb | Fruit | TEM (16–28) | Spherical | Antibacterial | [196] |
| 98 | Tanacetum vulgare | Tansy | Asteraceae | Herb | Fruit | TEM (16) | Triangular and spherical | — | [65] |
| 99 | Tagetes erecta | Marigold | Asteraceae | Herb | Flower | TEM (10–90) | Irregular, hexagonal and Spherical | Antibacterial | [197] |
| 100 | Tephrosia tinctoria | Alu pila | Fabaceae | Herb | Stem | TEM (73) | Spherical | Antidiabetic | [198] |
| 101 | Tinospora cordifolia | Giloye | Menispermaceae | Herb | Hole plant | TEM (53 ± 31) | — | Antimicrobial | [190] |
| 102 | Vitis vinifera | Grape | Vitaceae | Herb | Fruit | TEM (19) | Spherical | Antibacterial | [199] |
| 103 | Vitex negundo | Chinese chaste tree | Lamiaceae | Tree | Leaf | TEM (20) | Spherical | — | [200] |
| 104 | Vitex negundo | Chinese chaste tree | Lamiaceae | Tree | Leaf | TEM (~ 18.2 nm) | Spherical | Antibacterial | [201] |
| 105 | Withania somnifera | Ashwagandha and Indian ginseng | Solanaceae | Shurb | Leaf | TEM (70–110) | — | Antimicrobial | [202] |
| 106 | Withania somnifera | Ashwagandha and Indian ginseng | Solanaceae | Shurb | Leaf | TEM (12–36) | — | Antimicrobial | [203] |
| 107 | Zingiber officinale | Ginger | Zingiberaceae | Herb | Rhizome | TEM (3–22) | Spherical | Antibacterial and Antioxidant | [124] |
| 108 | Ziziphora tenuior | Lamiaceae | Lamiaceae | Herb | Leaf | TEM (8–40) | Spherical | — | [204] |
7. Conclusion
Plant-mediated green synthesized AgNPs have incredible physico-chemical properties compared to other metal-NPs, thus make them most suitable agents in nanobiotechnology research. Plant-mediated AgNPs have been explored for diverse biomedical applications viz. antioxidant, anticancer, antimicrobial, photocatalysis, antimalarial, biosensors, etc. Growing knowledge of green chemistry, which aims to reduce the use of hazardous chemicals or radiations, as well as the release of toxic wastes and byproducts in physical and chemical approaches, has encouraged researchers to focus on plant-mediated green synthesis protocols, which are a one-step method that is easy, safe, quick, energy efficient, dependable, cost-effective, and environmentally friendly. In the field of nanomedicines, green synthesized AgNPs have extraordinary significance to function as therapeutics with wide range of proven clinical and pharmacological properties. Recent research findings on phytosynthesis of AgNPs are documented in this topical review. Significant variations in the size, shape, and applications of AgNPs have been identified, owing to the presence of different phytochemicals in different plant extracts, even from the same species collected from different locations. This report will aid researchers involved in the finding of novel AgNP-based nanomaterials and their use in green chemistry applications.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Author contributions
The Data was collected by SKC and manuscript was written and reviewed by SKC, MKG, ML, and RS. All authors contributed to the article and approved the submitted version Author contributions. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
No funding to declare.
Conflict of interest
All authors have no conflict of interest to report.