Abstract
Silicon nanowire has been perceived as one of the most promising anodes in the next generation lithium-ion batteries (LIBs) due to its superior theoretical capacity. However, its high-cost and complicated fabrication process presents significant challenges for practical applications. Herein, we propose a simple scalable process, thermal-alkaline treatment followed by sputtering deposition, for preparing a unique self-standing anode of three-dimensional (3D) porous Si–TiO2 web-nanowired nanostructure for micro-LIBs. One-step thermal-alkaline synthesis of TiO2 nanowire scaffolds (TNS) with well-controlled thickness of 600–800 nm is reproducibly obtained onto Cu foils, achieving a 3D porous geometry for further growing Si active materials onto it to form 3D web-nanowired TiO2-Si composite material with interstitial voids. Profiting from the coverage of Si, direct contact of active materials on current collector, and the unique 3D web-nanowired structure, it exhibits high reversible volumetric charge capacity of 2296 mAh cm−3 with a coulombic efficiency of ∼95%, higher capacity retention, better capacity recovery ability and improved rate capability. Importantly, this work paves a simple way to directly build reliable 3D nanostructures or nanowired frameworks on selected current collectors as self-standing anodes for high volumetric capacity microbatteries; thus it is easy to scale up and beneficial for microelectronics industry.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
There is a strong demand in developing rechargeable lithium ion microbattery (denoted as Li-ion microbattery or micro-LIBs) with higher volumetric energy density that must feature small size, sufficient thinness, and easy integration onto a chip with other electronic components for applications in portable electric mobility and miniaturized electronic devices [1–4]. To meet such a strong demand, it urges the discovery of an effective active anode material with high volumetric capacity and rate capability directly grown onto selected current collectors without using any binder to provide system-level savings of volume and weight in anodes for micro-LIBs. Si nanowire anode has greatly attracted researchers' attention owing to its high theoretical capacity (∼4,200 mAh g−1 for Li22Si4), which is approximately 12 times higher than the standard commercialized graphite anode (372 mAh g−1), relative low working potential as an anode material (∼0.5 V versus Li/Li+), promising power density, shorter Li ions diffusion distances, accommodation for the large volume changes in Si, as well as environmentally benignity and abundance [5–10]. Great efforts have been dedicated to using Si nanowire based materials to design high performance anode materials for LIBs, including the Si nanowire array prepared by chemical vapor deposition (CVD) processing with anodic aluminum oxide (AAO) as the template [11], doped Si nanowires produced by a metal catalyzed electroless etching method [12], Si nanowire with atomic layer depositions (ALD) deposited TiN coatings by a gold-catalyzed vapour–liquid–solid (VLS) growth and magnetron sputtering [13], and Si nanoparticle-decorated Si nanowire networks prepared from a CVD method through the VLS mechanism [14]. However, even with improved designs, the Si nanowire based anodes still exhibit limited volumetric capacity which make them unsatisfactory for industrial demands. In addition, most of these production methods generally involve complex, time consuming and expensive steps such as extra clean process and etching with expensive templates, which will limit their potential in actual industrial applications.
Titanium oxide (TiO2), owing to its fast lithium insertion/extraction rate with small volumetric expansion (below 4%), good voltage plateau (above 1.7 V versus Li/Li+), and excellent overcharge protection than graphite, has been considered as an alternative for LIBs [15–18]. We previously achieved to directly grow porous TiO2 nano-scaffolds composed by the interconnected TiO2 nanowires with a 3D high-porosity cross-linked geometry on to the fluorine-doped tin oxide (FTO) substrates [19, 20], which can not only potentially be used as the host for other electrode material deposition but also can improve the wettability between the electrode and the electrolyte [21]. Nevertheless, its limited capacity would still hinder the actual applications for LIBs. Therefore, the construction of nanowire configuration by the integration of Si material with TiO2 will be an interesting challenge which may have major advantages. First, Si nanomaterial can provide high capacity. Second, the nanowired network design can provide enough free space around Si to accommodate the large volume expansion and can enhance the effective electrode–electrolyte interfacial area. Third, exist of TiO2 can also mitigate the volume change of Si. In this study therefore, we use a simple and scalable process of thermal-alkaline treatment followed with sputter deposition to build the self-standing anodes of 3D porous TiO2-Si web-nanowired framework on a Cu foil (denoted as TSi-WNW), which are fabricated through a one-step thermal-alkaline treatment for the direct formation of 3D TiO2 nano-scaffolds (TNS) constructed from cross-linked nanowires on the Cu foil followed by sputter-deposition of Si chain-like structures formed by overlapped Si nanogranular materials onto the surface of TNS. The 3D hierarchically porous TSi-WNW nanocomposite can be expected to enhance the electrolyte-accessible area to facilitate easy access of Li ions into the interior of the electrode and reduce the detrimental effects of volume variation, resulting in improved electrochemical performances. The highest volumetric specific capacity of around 3860 mAh cm−3 is achieved as the TSi-WNW nanocomposite anode with the largest Si-loading amount (weight ratio of TiO2/Si = 28/72 wt %). Besides, the resulting TSi-WNW anode possessing the coverage of Si to TiO2 with weight ratio of TiO2/Si = 37/63 wt % shows the best capacity performances as considering both aspects of high capacity and cycling stability, including high reversible volumetric charge capacities of 2296 mAh cm−3 with coulombic efficiency of ∼95% and good capacity retention of ∼81% for 50 cycles at 0.3 mA cm−2. Additionally, capacity recovery and good rate capability are demonstrated. Importantly, as for commercial application, the simplicity of the fabrication process can offer the scale-up potential to enable silicon nanowire based battery technology and other potential nanowired cathode and anode electrode systems without the usage of any binder and conductive agent, beneficial for commercial thin film micro-LIBs applications.
2. Experimental section
2.1. Preparation of 3D porous TiO2-Si web-nanowire framework on Cu foils
The synthetic process is similar with analogous procedure as described in our previous work [19]. Firstly, 50 nm-thick titanium film was coated onto the surface of Cu foil which was pre-adhesive to glasses surface (1.5*1.5 cm) to form a thin Ti-sputtered Cu foil through a magnetic sputter instrument (TVC-M8C8TV, Transvac co. Ltd) equipped with a Ti target at a sputter rate of 4 nm s−1 with an Ar flow rate of 300 sccm in the background pressure of 8.5 × 10−7 Torr and sputtering pressure of 1.2 × 10−3 Torr (target to substrate = 90 cm). Subsequently, a thermal-alkaline treatment was carried out in a 5 M NaOH (ACS reagent, ≥97.0%, Sigma-Aldrich) aqueous solution at 80 °C for 2 h, leading to the formation of 3D porous TiO2 nano-scaffolds (TNS) composed by interconnected nanowires, and subsequently washed by ethanol several times and then dried at 50 °C overnight. Second, the Si material was sputter-deposited at different deposition time of 1.5, 3.0, and 4.5 min onto the surface of TNS to form a 3D porous web-nanowired TiO2-Si architecture (TSi-WNW) by using an RF-magnetron sputtering system (TVC-MR6C4TV, Transvac co. Ltd) at a sputter rate of 1.8 nm s−1 with an Ar flow rate of 150 sccm in which the background pressure was 8 × 10−6 Torr and the sputtering pressure was 2.37 × 10−3 Torr. Then, the prepared films were rinsed and immersed in acetone for 24 h to remove the sample electrodes from the glass substrate. Finally, the samples were labelled as TSi-WNW-1.5, TSi-WNW-3.0, and TSi-WNW-4.5 according to the Si sputtering-deposition time, in which the content of silicon (Si wt%) in the designed TSi-WNW composite were 46%, 63%, and 72%, respectively. The Si wt% of the prepared TSi-WNW composite was calculated by the equations reported in the Supporting Information. Coulombic efficiency (CE %) is the ratio of discharge capacity and charge capacity (discharge/charge capacity)*100. Capacity retention is the ratio of the actual capacity to the initial reversible capacity.
2.2. Characterizations
The surface and cross section morphologies of the TNS samples before and after Si sputtering deposition were analyzed, using a Zeiss Ultra-Plus field emission scanning electron microscope (FESEM) with an accelerating voltage of 3 kV. Raman spectra were performed by a Raman microspectroscopy system of Nanofinder 30 (Tokyo Instruments, Inc.) with an excitation source of He–Ne laser (λex = 632.8 nm). The microstructure of the prepared TSi-WNW samples was also investigated using JEOL JEM-2100F transmission electron microscope (TEM) at 200 kV.
2.3. Electrochemical characterizations
Electrochemical measurements were evaluated with half cells (CR2032-type coin cell) which were assembled by sandwiching the separator (Celgard 2325 membrane with 19 mm) between the prepared 3D porous TSi-WNW based anode and a lithium metal within an argon-filled glove box (MBraun, Germany) with a moisture level below 1 ppm. The liquid electrolyte was 1 M LiPF6 in a mixture of ethylene carbonate (EC)/diethyl carbonate (DEC) (1:1, v/v) (Guangzhou Tinci Materials Technology Co., Ltd China). The electrochemical performances of the coin cells were conducted on a battery test system (Automatic battery cycles WBCS3000 M1) at room temperature in the voltage window of 0–3.0 V versus Li/Li+ at different current densities after 3 h rest. Cyclic voltammetry measurements were investigated through an electrochemical workstation (CHI 611E) between 0 V and 3.0 V versus Li/Li+ at a scan rate of 0.1 mV s−1.
3. Results and discussion
The structure of prepared TiO2-Si web-nanoriwed nanocomposites was confirmed by FESEM images, as shown in figures 1 and S1 (available online at stacks.iop.org/NANOX/1/030014/mmedia). It was clearly seen that a 3D porous nano-scaffold (TNS) with a thickness of approximately 636 nm composed of TiO2 cross-linked nanowires was directly grown onto the surface of current collector of Cu foil after the thermal-alkaline treatment. Specifically, the TNS can serve as preferential nucleation site to guide further silicon deposition and thereby avoid the formation of a uniform layer like a film deposition on a planar substrate. Thus after sputtering Si, chain-like nanowires formed by overlapped Si nanogranular materials distributed conformally and uniformly on topmost TNSs were exposed, proving the successful formation of TiO2-Si structures with a 3D porous web-nanowired framework. The cross-section images show the thickness for TSi-WNW-1.5 and TSi-WNW-3.0 are about 876 nm and 841 nm, respectively, in which the reduced thickness in TSi-WNW-3.0 sample could be ascribed to its increased loading amount compared to TSi-WNW-1.5. Owing to the high presence of pores and voids in architectures, the above TSi-WNW samples can lead to improved electrochemical performances due to the increased effective capability of electrolyte interaction with the active materials and the enough space of existing interstitial voids between nanowires to tolerate the volume expansion of Si. However, for the sample of TSi-WNW-4.5 with longer deposition time of 4.5 min, we could observe that the Si have been sputtered into the interspace between TiO2 nanowires and cover almost the whole voids (figure 1(d)), which would reduce electrolyte-accessible area and lower the volume alleviating effect of TiO2. In addition, for pure Si one without TNS as the scaffolds (figure S2), only Si aggregates forming denser films could be observed, which may confront severe problems of crack and pulverization, finally leading to rapid capacity decay for the performances of the cell. Considering both aspects of high capacity and cycling stability, the TSi-WNW-3.0 composite can be expected to exhibit better performances. Therefore, subsequent sample characterizations are performed on this sample.
Figure 1. SEM images for the as-prepared samples of (a) TNS, (b) TSi-NWW-1.5 composite, (c) TSi-NWW-3.0 composite, and (d) TSi-NWW-4.5 composite, respectively. Raman spectra of the (e) TNS anode and (f) TSi-NWW-3.0 composite anode, respectively.
Download figure:
Standard image High-resolution imageRaman spectra were collected for investigating the structure and electronic properties of prepared 3D porous TNS sample with and without Si coating. No obvious peak can be identified for pure Cu foil (figure S3). From the Raman spectra of the samples shown in figure 1(e), it can be observed that the TNS sample has a weak Raman band at approximately 278 cm−1 for Ti–O–Na and five Raman bands at 148 cm−1, 199 cm−1, 399 cm−1, 516 cm−1 and 613 cm−1 indexed as Eg, Eg, B1g, A1g and Eg modes of the crystalline anatase TiO2, respectively [22, 23]. For the TSi-WNW-3.0 sample (figure 1(f)), four Gaussian deconvoluted Raman peaks locating at 141, 281, 356, and 457 cm−1 corresponding to the transverse acoustic (TA) mode, longitudinal acoustic (LA) mode, longitudinal optic (LO) mode, and transverse optic (TO) mode of amorphous Si are obtained [24]. The results confirm the formation of amorphous Si onto the surface of crystalline anatase TiO2 with 3D porous nano-scaffolds. In addition, almost no obvious peaks of TiO2 was detected in this TSi-WNW-3.0 sample, which is because the surface of TNS was wrapped and covered by the amorphous Si nanogranulars after the Si coating.
Transmission electron microscopy (TEM) observations of the TSi-WNW-3.0 sample also unambiguously show the 3D porous web-nanowired structure of TiO2-Si composite, where the TiO2 nanowires are sequentially surrounded by the overlapped Si nanogranular materials with the size around 200 nm (figures 2(a) and S4), in accordance with the observations in SEM image and Raman data. The element mapping patterns for the prepared TSi-WNW-3.0 sample analyzed at low and high magnification were shown in the figures 2(b) to (d) and S5. It also confirmed the successful formation of the porous web-nanowired TiO2-Si nanocomposite, in which the Si owned the most amount homogeneously introduced to and covered on the surface of the 3D porous TNS architectures with a homogeneous distribution of elemental Ti and O. With such unique 3D porous framework, it can be supposed to be beneficial for buffering the volume variation of Si and providing fast electron transfer during the charge/discharge process.
Figure 2. Element mapping images of the prepared TSi-WNW-3.0 anode sample. (a) Corresponding TEM image, (b) Ti element, (c) O element, and (d) Si element.
Download figure:
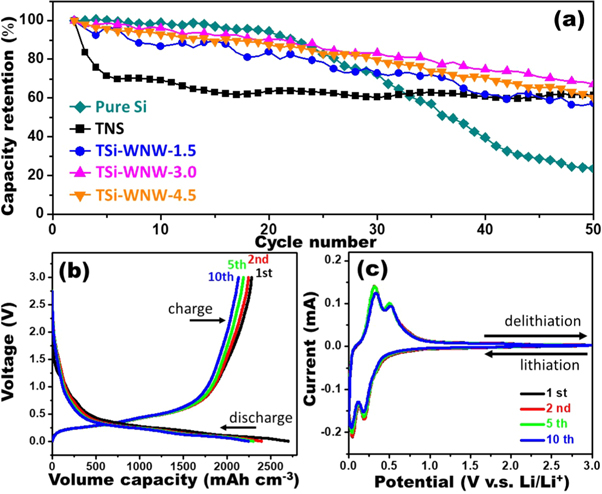
Standard image High-resolution imageFigure 3(a) demonstrates the cycling electrochemical performances of the as-prepared sample for the first 50 cycles at 0.015 mA/cm2. Not surprisingly, the bare Si electrode suffers a fast capacity decay as compared to other samples, in which the reversible charge capacity gradually reduces and shows a capacity retention rate of 93.8% of the second cycle at the 20th cycle and then sharply reduces to 23.6% after 50 cycles. This huge capacity fade can be ascribed to the rapid physical degradation of the electrode owing to the large volume change and pulverization in Si as repeatedly charging/discharging. For the pure TNS electrode, due to its structural stability associated with lithium to constrain the volume change, a good cycling stability with a capacity retention of around 70% of the second cycle even after 200 charging/discharging cycles can be observed (figure S6(b)). In TiO2-Si composite structures, the capacities are highly increased compared to the pure TNS; furthermore, they render much better cycling performances as compared to the pure Si one even their corresponding capacities fade gradually as charge/discharge cycles processing. For TSi-WNW-3.0 electrode, the capacity retention of ∼69% of the second cycle after 50 charge/discharge cycles is achieved; in contrast, the TSi-WNW-1.5 and TSi-WNW-4.5 samples with lower capacity retentions of 59.0% and 60.1% after 50 cycles are obtained, respectively. Therefore, the abovementioned data demonstrate that the TSi-WNW-3.0 electrode can provide more stable cycling behavior. However, it still subsequently suffers from fatal capacity decay with only around 40% retention after 100 cycles due to the volume expansion of Si from the high Si content of 63%. It should be noted that coatings on the surface of Si can guarantee to mitigate the impacts of volume expansion in Si, thus avoiding cracking of the anode structure [9, 25, 26]. Thus, design an effective protective coating on silicon to electrochemically protect the Si surface will be important. We have recently published the coating of tetrakis(4-carboxyphenyl)porphyrin on SiNWs surface, which can improve the electrode's rate capability and capacity recovery, but the cycling stability is still poor [27]. It indicates that achieving stable cycling performance of high-content Si nodes is very challenging. Thus, design an effective protection layer with optimal thickness and coating density on SiNWs surface for improving the cycling performance will be the focus of our future work in this system.
Figure 3. (a) Charge/discharge cycling electrochemical performances of the as-prepared sample for the first 50 cycles at a current density of 0.015 mA cm−2 in the cut-off voltage of 0–3.0 V versus Li/Li+. (b) Galvanostatic charge-discharge voltage profile and (c) typical cyclic voltammetry curve of the TSi-NWW-3.0 anode.
Download figure:
Standard image High-resolution imageThe detail charge and discharge curves were performed to illustrate the electrochemical behavior of the prepared TNS and TSi-WNW anodes in the voltage range of 0−3.0 V versus Li/Li+ at 0.015 mA cm−2 (j0/10, J0 = 0.15 mA cm−2) in the galvanostatic profile, as shown in figures 3(b) and S7. The TNS anode exhibited a slope profile of the voltage-capacity relationship during the charge and discharge process (figure S7(a)). The voltage-capacity profiles for TSi-WNW samples (figures S7(b)–(d)), apart from the TNS one and similar to the pure Si one (figure S7(e)), showed a long flat potential plateau below 1.0 V during charging/discharging which were attributed to the lithiation/delithiation of Si, in accordance with the electrochemical behavior of the typical Si based electrode [5, 28]. It is noted that no characteristic features of the TiO2 in high potential region (>1.0 V) can be found in these TSi-WNW samples, which is due to the low content of TiO2 and the large amount of coated Si. For the first discharge (lithiation) and charge (delithiation), the volumetric specific capacity for the TSi-WNW-3.0 anode reached 2698 mAh cm−3 and 2679 mAh cm−3 respectively, with a coulombic efficiency of 84.5%. The capacity drop after the 1st charge/discharge cycle was observed; however noticeably, the following charge/discharge cycles show stable CE values without significant capacity fade. The irreversible charge/discharge capacity in 1st cycle and the capacity drop can be attributed to the formation of solid electrolyte interphase (SEI) layer and the irreversible Li+ storage in active materials. As expected, the capacities of the TSi-WNW composites are highly improved compared to that of pure TNS one owing to the introduction of Si. Also, the TSi-WNW-3.0 composite exhibits improved capacitive performances compared to that of pure Si anode prepared at the same sputter-deposition time, indicating that the porous web-nanowired architecture can enhance the electrolyte-accessible surface area and facilitate easy access of electrolyte ions into the interior of the active materials. The Cyclic voltammetry (CV) is applied to further illustrate the corresponding lithiation and delithiation potentials of the samples. Figure 3(c) shows the CV curves at the of the 1st, 2nd, 5th, and 10th cycles for the TSi-WNW-3.0 anode at a scanning rate of 0.1 mV/s in the range of 0–3.0 V versus Li/Li+. Two prominent peak pairs located at 0.04 V/0.32 V and 0.21 V/0.50 V at low potentials (0.04–1.0 V) are displayed, which are associated with the lithiation/delithiation process of Li with active Si [29, 30]. In addition, in high potential region (>1.0 V), there are no distinguishable redox peaks that are usually observed in TiO2-Si based composite anodes resulted from the lithiation of TiO2 and delithiation of TiO2–Li alloys [31, 32], likely due to the low content of the TNS material and the Si-coating on the outer surface of TNSs, consistent with the voltage plateau shown in figure 3(b).
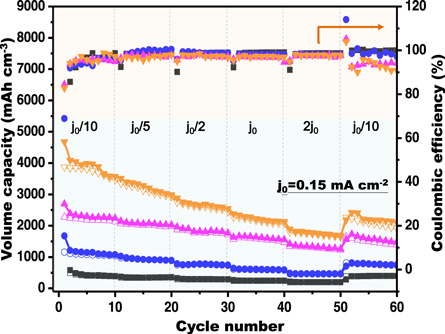
Figure 4 lays out the prepared TNS and TiO2-Si composites under different area current density varied from j0/10 to 2j0 (j0 = 0.15 mA cm−2) within a voltage window of 0–3.0 V versus Li/Li+ with the cell charged/discharged ten times and the corresponding capacity performances are summarized in table S1. Owing to the formation of the SEI layer and irreversible Li+ storage, irreversible capacity loss from the second cycle compared to the initial capacity can be observed for all samples, with a 27%, 32%, 15%, and 18% loss for respective TNS, TSi-WNW-1.5, TSi-WNW-3.0, and TSi-WNW-4.5. After the 1st cycle, the coulomb efficiency (CE) of charge and discharge is stable at >95% of all four samples at various area current density, even the capacity would decrease with the increasing area current density. The TNS electrode shows a low but good reversible capacity at different current density; as the current density increased and then reversed back to the initial low current density, it revealed the capacity retention of ∼90% of its second cycle capacitance. Highly improved capacities for all TSi-WNW anodes can be discovered as comparison to the pure TNS one, and the larger amount of Si loading onto the TNS surface gives the higher capacity. Therefore, the TSi-WNW-4.5 composite possesses the highest reversible volumetric capacities of 3860 mAh/cm−3 owing to its largest Si loading amount. It can be revealed that the TSi-WNW-3.0 anode held the best capacity performances as considerable both aspects of high capacity and cycling stability. Reversible discharge capacities of 2296, 2071, 1845, 1618, and 1330 mAh cm−3 are achieved from the current density of 0.015 mA cm−2 (j0/10) to 0.3 mA cm−2 (2j0), with the corresponding area capacity of 193, 157, 155, 136, and 111 μAh cm−2, respectively (figure S8). About 60% of the capacity at current density of 0.015 mA cm−2 (j0/10) remains while increasing current density by 20 times for the TSi-WNW-3.0 electrode, indicating its exceptional rate capability. Noticeably, the capacity recovers to ∼1580 mAh cm−3 reversibly after the current density returns to the low current density of 0.015 mA cm−2 (j0/10), which is about 69% of initial value. The improved electrochemical performances of TSi-WNW-3.0 composite can be understandably ascribed to the appropriate loading amount in Si coating for increased capacity and the steady 3D TNS framework for restricting Si volume expansion. At the same time, bare Si coating on Cu foil surface synthesized by sputtering was also tested as comparison sample. Only 27% retention of its initial capacity as going back with the rate to 0.015 mA cm−2 (j0/10) after cycling from j0/10 to 2j0, much lower than the returning capacity retention value for prepared TNS and TSi-WNW composites. It indicated that pure Si confronts crack and pulverization during lithiation/delithiation process, resulting in severe capacity fade.
Figure 4. Plots of the charge-discharge volumetric capacities and the corresponding Coulombic efficiencies of the TNS (black), TSi-NWW-1.5 (blue), TSi-NWW-3.0 (purple), and TSi-NWW-4.5 (orange) anodes performed at different current densities with the voltage window between 0 V to 3.0 V versus Li/Li+.
Download figure:
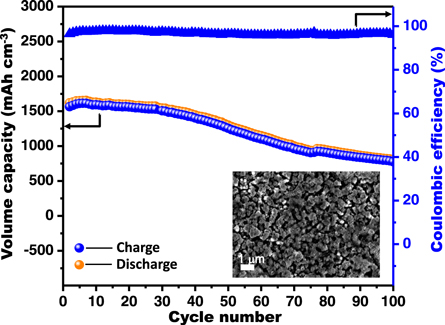
Standard image High-resolution imageThe cycle performance of the TSi-WNW-3.0 anode as the current density increased 20 times is shown in figure 5. A good reversible rate volumetric capability of ∼1650 mAh cm−3 and a retention capability of around 81% for 50 cycles can be achieved. The TSi-WNW-3.0 anode after 50 cycles is shown in the inset of figure 5. It can be revealed that the overlapped Si nanogranular materials of TSi-WNW-3.0 exhibit a deformation and aggregation due to the volume expansion in Si which lead to the capacity loss. However, the 3D porous configuration for the active materials still remains, ensuring the structural stability for the prepared materials in 50 cycles. In addition, this cycling life curve exhibits high Coulombic efficiency of approximately 97% for all 50 cycles, indicating its good rate capability.
Figure 5. The cycling performances of the TSi-NWW-3.0 composite anode at a current density of 0.3 mA cm−2. Inset: the SEM image of the electrode after 50 charge–discharge cycles.
Download figure:
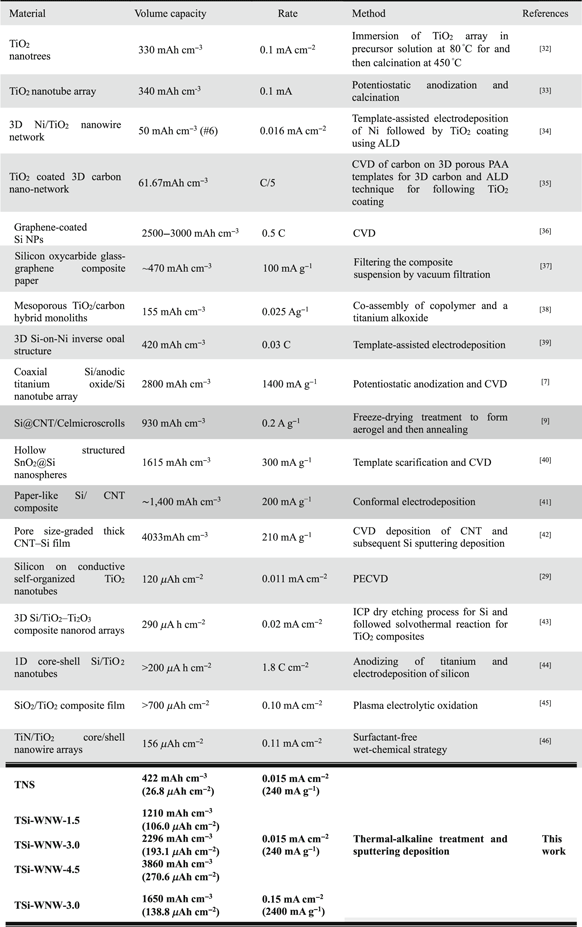
Standard image High-resolution imageSome other self-standing anodes based on Si-based and TiO2-based nanomaterials and their corresponding electrochemical performances in micro-LIBs have been summarized and compared with our experimental results, which summed up in table 1. It can be found that the as-prepared TSi-WNW nanocomposite anodes deliver a high volumetric specific capacities, good rate capacity, and favorable Coulombic efficiencies, competitive with those anodes used in micro-LIBs. The above good capacitive performances of the as-synthesized TSi-WNW can be ascribed to: (1) the direct contact of active materials onto current collectors to minimize the internal/interface resistance; (2) the coverage of Si onto TiO2 nanowires for improved capacity; (3) the 3D porous frameworks with interstitial voids for enhanced effective electrode–electrolyte interfacial area; (4) the structure advantage of TNS in which the large amount of free space in architecture can accommodate the large volume expansion from Si during Li+ insertion/extraction process. In addition, the developed fabrication process is simple and no special equipment is required (all commercial factory equipment used in this strategy), thus the preparation is easier and the costs are quite lower than CVD, template-assisted method, and etc, shown in the table 1.
Table 1. Comparison of micro-LIB anode performance of various Si-based and TiO2-based materials for binder-free self-standing anodes with this work.
|
4. Conclusion
In summary, a TiO2-Si web-nanowire with 3D hierarchical porous structure composed by cross-linked TiO2-Si nanowires have been produced directly onto the surface of a Cu foil current collector through a simple alkaline-thermal treatment followed by sputter-deposition process. Using as a self-standing anode material for micro-LIB applications, it exhibits a high volumetric reversible capacity of ∼3860 mAh cm−3 as the composite with the highest Si coverage of 72 wt% In the case of the composite with the most appropriate coverage of Si to TiO2 (Si wt% = 63 wt%), the best capacity performances as considering both aspects of high capacity and cycling stability are performed, showing a high reversible volumetric charge capacity of 2296 mAh cm−3, good coulombic efficiencies of ∼95% in all reversible charge/discharge process, a capacity retention of 60% of its initial capacity as the current density increased by a factor 20, and a good capacity retention of ∼70% for 50 cycles. In addition, capacity recovery ability and good rate capability are demonstrated. The abovementioned results are competitive with that of other reported anodes using Si or TiO2 as active materials in one or more categories for micro-LIBs. This unique 3D nanowired structure design will provide a new solution for electrochemical performance improvement for LIBs. Moreover, this simple but helpful preparation way to build the self-standing anodes with a reliable nanowired framework with high volumetric capacity, can be adapted to build the platform technology for efficient and fast fabricating other Li ion anodes in a large-scale-production process and provide a promising research platform for microbatteries.
5. Synopsis
A 3D porous Si–TiO2 web-nanowired framework directly fabricated onto a Cu foil for lithium ion microbatteries is obtained via a simple scalable process of thermal-alkaline treatment followed by sputtering deposition, adaptable for industrial fabrication of battery devices for microelectronics.
Acknowledgments
We acknowledge funding from the Ministry of Science and Technology of Taiwan (108-2113-M-005-002-; 108-2627-M-005-001-; 109-2113-M-005-010-; 109-2627-M-005-001-).