Abstract
Zinc oxide nanoparticles (ZnO–NPs) were synthesized and decorated simultaneously onto the glass fiber pre-filter paper (GF paper) by the sonochemical method without using any additional reagents (a 'Green' synthesis approach). ZnO–NPs decorated GF paper was characterized by electron, confocal laser scanning and atomic force microscopy, fourier transform infrared and atomic emission spectroscopy, X-ray diffraction, and thermogravimetric analysis etc. Due to the massive void volume space, exceptional dimensional stability, large thickness (790 μm) of the GF paper (unlike other paper materials) and ultrasonic irradiation effects, ZnO–NPs were decorated in the enormous amount (96 mg per paper) without causing any adverse effects on the GF paper. Such a huge amount decoration onto GF paper makes it multifunctional, fluorescencet (orange-pink color, 535–624 nm) under ultra-violet light (360 nm) and antibacterial. The antibacterial activity of the ZnO–NPs decorated GF paper was examined against Gram-positive bacteria Bacillus subtilis 168 and Staphylococcus aureus (MCC 2043, pathogenic). The outcomes from the antibacterial experiments revealed ∼99% (2 log) reduction in the survival of the filtered bacteria (B. subtilis) on the ZnO–NPs decorated GF paper due to the toxicity of ZnO–NPs on bacterial cells like cell shrinkage, cytoplasmic leakage, cell burst, etc. Multifunctional, ZnO–NPs decorated GF paper could be used for fluorescencet and antibacterial paper-based applications.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Paper as a derived and processed product from natural or synthetic resources is a very versatile, attractive, inexpensive, biodegradable, easily available, lightweight, abundant and the most applicable material existing in all the discipline [1]. Paper products can be made from organic or inorganic materials or from the mixture of both [2]. Most of the paper products are derived from organic resources like cellulose, a natural polymer and others of synthetic origin like nylon, polyvinylidene fluoride (PVDF), polytetrafluoroethylene (PTFE), polyamide (PI), polycarbonates, polyethersulfone (PES) etc [2]. Glass filter papers are made up of inorganic material, borosilicate microfibers [3]. Application of the papers vary depending on the nature of the material, strength, chemical properties, pore size, void volume space, thickness, flow rate, durability etc [1, 2]. The most common applications of the paper are value representative (money, ticket etc), information storage (newspapers, books etc), writing, printing, packaging, cleaning, laboratory filtration etc [4]. The physical and chemical modification of the existing papers can result into other advanced applications like electronic, piezoelectric and energy devices, microfluidic based biosensors, chemical and physical sensors, heavy metal ion filtration and bio-diagnostic devices, and antibacterial materials etc [1–16]. Nanomaterial modified papers or membranes as an antibacterial material can be used for water filtration, etc [2, 15]. There are several paper coating techniques like strip coaters, print, cast, spray, extruded, spin coating, rolling, brush, bench coating, mechanical blade, dip etc [15]. Most of the mechanical techniques have drawbacks on account of their harmful effect on the fiber as well as physiochemical properties of the paper. The downsides of some other coating techniques are its expensiveness, inefficient in throughout three dimensional (3D) coating, requirement of extra reagents etc [15]. Thus, appropriate paper material as well as coating technique is required to fabricate cost-effective and novel multifunctional paper. To the best of our information, fewer reports are available on glass filter papers. Through this study we have tried to explore the properties and possible applications of existing glass fiber pre-filter paper (GF paper) through decoration with the zinc oxide nanoparticles (ZnO–NPs). GF paper is made up of borosilicate microfibers, silica (SiO2) as a main component and some quantity of boron oxide (B2O3), aluminium oxide (Al2O3), sodium oxide (Na2O) and potassium oxide (K2O) [17]. Glass fibers have some exceptional properties which make them very different from natural fibers such as excellent strength, heat and chemical resistant but not to strongly alkaline and acidic (hydrofluoric acid) environment [17]. Fibers of the GF paper are thinner in diameter (100 nm to several μm) and uniform in contrast to the fibers of the other papers because of which GF paper have massive void volume space and large surface area [17]. Thickness of the GF paper is large (790 μm), so all these properties make it appropriate for filtering heavily contaminated liquids. Resin bonded and unbounded GF papers are available for the wide application in the filtration technique. Acrylic resin-bonded GF papers have more rigidity, dimensional stability and strength in comparison to the unbounded one as well as cellulose papers too [17]. Properties such as thin fiber, more void volume space, high surface area, dimensional stability, large thickness and excellent strength make it suitable for the ZnO–NPs decoration through sonochemical method.
Zinc oxide (ZnO) as an inorganic material is well known because of its various nanostructures, physical and biochemical properties and extensive applications [18]. The most common and thermodynamically stable crystal structure of ZnO is wurtzite [18–20]. The direct band gap energy of 3.4 eV and some crystal defects make ZnO–NPs optically active in Ultra-Violet (UV) region of the visible spectrum for which it is used as UV protective agent [18–22]. Intrinsic defects like oxygen vacancies (Vo), zinc Interstitials (Zni), oxygen interstitials (Oi), zinc vacancies (Vzn), & oxygen antisite (Ozn), are responsible for the broad fluorescence emission of ZnO–NPs in the visible region with stronger emission between 550 to 650 nm [18–22]. The antimicrobial activity of ZnO–NPs arises from the capability to generate hydrogen peroxide (H2O2), reactive oxygen species (ROSs) and release of metal ion (Zn+2) from the surface. This activity depends on the micro to nanostructure, porosity, size, morphology, crystal defects, surface area, environment, etc [18–22]. There are various methods existing for the synthesis of ZnO–NPs like hydrothermal, electrochemical, sol-gel, solvothermal, micro-emulsion, precipitation sonochemical, etc [18–22]. Among all, sonochemical method has gained huge attention because of simple, large-scale production capability and environmentally friendly approach [18, 23]. In this method (bath and probe sonication, etc.), ultrasound wave of 20 to 100 kHz frequency is used. These waves in the liquid (cyclic succession of the compression and rarefaction wave) create microbubbles which rapidly collapse in microseconds and increase the local temperature and pressure upto 5000 K and 500 atm respectively. This phenomenon is known as cavitation and the increase in the local temperature and pressure transforms the mechanical energy into chemical energy by sonolysis of water (H2O) and oxygen (O2) molecules into free radicals like OH·, H·, and O·, etc [18, 23]. These reactive free radicals assist the chemical reactions without any requirement of extra reagents due to which sonochemical method is known for its 'Green' synthesis route [18, 23]. In this method, microjets and shock waves are produced in the liquid due to the propagation of ultrasonic waves (collapse of microbubbles). These microjets and shockwaves help in the physical coating of nanomaterials on the desired substrate without needing any extra reagents ('Green' approach for physical coating) [24]. To the best of our information, we are first to report ZnO–NPs decoration onto GF paper by green route (sonochemical method). Using this method, ZnO–NPs were synthesized and decorated simultaneously in the huge amount (95 mg) onto the GF paper. This turns the existing paper as multifunctional, fluorescent under UV light and antibacterial against various pathogens [Bacillus subtilis (168) and Staphylococcus aureus (MCC 2043, disease-causing strain)].
2. Experimental section
2.1. Materials and instrument
All the chemicals and bacterial culture media reported in this study were used in the pure form without any further modification or purification. GF paper (AP1504200) was purchased from Merck Millipore. Active culture of Gram-positive, diseases causing bacteria S. aureus (MCC 2043) and B. Subtilis (strain 168) was obtained from National Centre for Cell Science, Pune, Maharashtra, India. Bath sonicator (LA-10L, Limplus, Beijing, China) of configuration 33 ± 3 kHz frequency and 200 watt-power was used in this study for the purpose of sonication.
2.2. ZnO–NPs decoration onto the GF paper
For ZnO–NPs decoration onto the GF paper, 5 pieces of GF paper were submerged in the beaker containing 150 ml of 55 mM zinc acetate dihydrate [Zn(CH3COO)2·2H2O] aqueous solution. After 10 min of magnetic stirring, 1 M aqueous solution of sodium hydroxide (NaOH) was added dropwise to the zinc acetate dihydrate solution containing GF paper till its pH reaches to 10. After pH adjustment, the reaction mixture containing GF paper was placed in the bath sonicator for the ultrasonic irradiation for 1 h. At the end of the reaction, the color of the solution turned to milky white due to ZnO–NPs formation. After the completion of the reaction, GF paper was washed five times with fresh MQ water, dried in the oven at 60 °C for 30 min and stored at room temperature for further characterizations.
2.3. Characterization
Morphology of the ZnO–NPs and its decoration on the GF paper was characterized by the Field Emission Gun-Scanning Electron Microscope (FEG-SEM, JSM-7600F, Tokyo, Japan). ZnO–NPs decorated and blank GF papers were cut into small pieces (3 mm), sputter coated with the platinum target and placed on the sticky carbon tape for imaging. The accelerating voltage was fixed at 5 kV and the working distance between 4 to 7 mm was maintained during all imaging. For the elemental analysis, Energy-Dispersive X-ray spectroscopy (EDX) and elemental mapping were performed while FEG-SEM imaging. Size of the ZnO–NPs on the ZnO–NPs decorated GF paper was calculated by measuring the length of the ZnO–NPs manually using ImageJ software (version 1.51j8). Amount of ZnO–NPs on the GF paper and its released amount in the filtrate were quantified by the Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES, ARCOS Simultaneous ICP Spectrometer, SPECTRO Analytical Instruments GmbH, Germany). Fluorescence property of the ZnO–NPs decorated GF paper was characterized by the Confocal Laser Scanning Microscope (CLSM, LSM 780, AxioObserver), using Plan-Apochromat 63×/1.40 Oil DIC M27 objective. A small piece of the GF paper was excited with a multiphoton laser source at 360 nm, and the fluorescence emission was observed between 535–624 nm. For the naked eye fluorescence of the ZnO–NPs decorated GF paper, it was exposed to the UV light (360 nm) inside the UV transilluminator (Geliance 200 2D Imaging System). In order to examine the crystalline nature, X-ray diffraction analysis of the ZnO–NPs decorated and blank GF paper was performed on the Smartlab Rigaku High-resolution Diffractometer (HR-XRD) at 40 kV in the continuous scanning step-scan mode. The diffractograms were recorded as a function of angle 2θ, range between 10°–90°, using Ni-filtered Cu-Kα (λ = 1.54 Å) monochromatic x-ray beam with scanning speed of 0.1 s/step with the step size of 0.01°. For the compositional or functional group analysis, Fourier Transform Infrared Spectroscopy (FTIR) of ZnO–NPs decorated and blank GF paper was performed as a function of % transmittance using 3000 Hyperion Microscope with vertex 80 FTIR system, having 0.20 cm−1 of spectral resolution and 65 spectra/s of scan speed at 16 cm−1. For the thermal analysis, Thermogravimetric Analysis (TGA) was performed using Diamond TG/DTA apparatus (Perkin Elmer make, USA). For TGA, the samples were heated from 33 to 500 °C at the rate of 10 °C min−1 in the inert atmosphere and change in the mass was observed. For three-dimensional analysis of the ZnO–NPs decoration, the topography of the ZnO–NPs decorated and blank GF paper surfaces was measured by the Atomic Force Microscopy (AFM, MFP3D Asylum Research, CA, USA) using pyramidal silicon nitride probe of 70 kHz (Olympus, Tokyo, Japan).
2.4. Quantification of the decorated and leached ZnO–NPs of the ZnO–NPs decorated GF paper
The amount of ZnO–NPs present on the decorated GF paper and leached out while filtration will play important role in its application. The best method is ICP-AES due to its high sensitivity to quantify the amount present. ZnO–NPs present on the GF paper should be dissolved in a solution to know the amount. To achieve this, ZnO–NPs decorated GF paper was submerged in the glass bottle containing 50 ml of diluted aqua regia solution and kept on shaking for 24 h to ensure digestion of metal. After 24 h, 1 ml of aqua regia containing digested zinc metal (Zn) was diluted with Milli-Q (MQ) water and the concentration of zinc in the solution was determined by the instrument (ICP-AES) in the parts per million (ppm). For the quantification of leached out ZnO–NPs while filtration and measurement of flow rate, the single experiment was performed on the Franz diffusion cell setup (detail of the setup is described in the antibacterial experiment section). 10 ml of the MQ water was filtered separately through ZnO–NPs decorated and blank GF and the flow rate was calculated by the time required to pass the water through the unit area. 1 ml of the filtrate (water) from ZnO–NPs decorated GF paper was used to calculate the amount of ZnO–NPs leached out by ICP-AES as described before. All the experiments were performed in the triplicate, and the average values are mentioned in the manuscript.
2.5. Antibacterial activity of the ZnO–NPs decorated GF paper
To examine the antibacterial activity of the ZnO–NPs decorated GF paper, filtration based experiment was performed using Franz diffusion cell setup. All the glassware including Franz diffusion cells and media were autoclaved before the use. All the experiments were performed in the laminar air flow. The freshly grown culture of the bacteria B. subtilis in the Luria Broth (LB) was diluted to the concentration of 105 cells ml−1. ZnO–NPs decorated and blank GF papers were exposed to UV light for the sterilization till 30 min. In between the donor and receiver compartment of the Franz diffusion cell, sterile ZnO–NPs decorated and blank GF paper were kept separately (test and control respectively) and fixed with the plastic clamps which were also exposed to UV light for 30 min. 5 ml of the media containing bacterial culture (105 cells ml−1) was poured in the donor compartment of the each Franz diffusion cell setup. After the media get filtered, 100 μl of the filtrate from both test and control was spread on the sterile nutrient agar plates. After the filtration, GF papers of the test and control was transferred to the sterile nutrient agar plate, position of the GF paper on the plate was kept same upright as it was on the Franz diffusion cell and incubated at 37 °C. After 24 h of the incubation, GF paper of both test and control were immersed separately in the sterile falcon tube containing 20 ml LB media and vortexed at 3000 rpm for 5 min to separate the bacterial cells from the paper. LB containing separated bacteria from test and control were serially diluted (105 times) with the fresh LB, 100 μl of it was spread on the nutrient agar plate and incubated at 37 °C. After 24 h of the incubation, bacteria on the plates were counted for the Colony Forming Unit (CFU) analysis. For the visual inspection of the bacterial survival on the GF papers (test and blank), electron microscopy (FEG-SEM) was performed. After 24 h of the incubation of GF papers containing filtered bacteria on the agar plates, they (test and control both) were cut into small pieces, sputter coated with the platinum target and imaging was performed using FEG-SEM in the cryo mode. For seeing the effects of ZnO–NPs on the microstructure of the bacteria, 10 ml of the separated bacteria in the falcon tube containing LB (as discussed earlier) was centrifuged at 5000 rpm for 7 min, pellet was washed 3 times with sterile phosphate buffer saline (PBS) and finally suspended in the 4% glutaraldehyde solution for 30 min for fixation of the bacterial cells. After fixing, again they were centrifuged, washed three times and finally suspended in the 2 ml PBS. 10 μl of the fixed cells (test and blank) were sputter coated with the platinum target, and FEG-SEM imaging was performed. The antibacterial activity of the ZnO–NPs decorated GF paper was also examined against diseases causing Gram-positive bacteria S. aureus. Tryptone Soya Yeast Extract Broth (TSYEB) and Tryptone Soya Yeast Extract Agar (TSYEA) (HIMEDIA, India) were used in place of LB and nutrient agar as a selective media for the proper growth of S. aureus. To explore the possible antibacterial methods, experiment was performed in a different way, ZnO–NPs decorated and blank GF paper were placed on the TSYEA plates separately, then both were inoculated (moistened) with 1 ml of TSYEB media containing S. aureus (105 cells/mL) cells and incubated at 37 °C. After 24 h of the incubation, GF papers were cut into small pieces, sputter coated with the platinum target and surface imaging was performed in the cryo mode of the FEG-SEM to visualize the bacterial survival.
3. Results and discussion
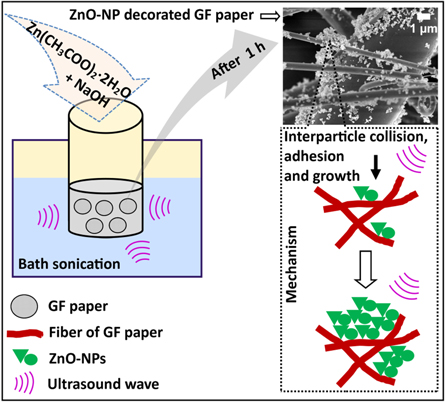
As shown in the figure 1, ZnO–NPs were decorated onto the GF paper by synthesizing it in the presence of GF through sonochemical method. The mechanism behind the synthesis and morphological evolution of ZnO–NPs through this method is briefly described in our previous report [18]. Reactive free radical species generated due to the sonolysis of water molecule (OH· etc) assist in the synthesis of ZnO–NPs through the intermediate formation [18]. Due to the submerged coating procedure, reaction mixture is absorbed by the fibers of the GF paper. This results in the formation of ZnO–NPs in the solution as well as on the surface of the fibers too. Hydroxyl group (OH), a chemical moiety present on the ZnO–NPs may facilitate its adsorption on the borosilicate fibers through hydrogen bonding [18]. Due to the rapid collapse of microbubbles, microjets and shockwaves are produced in the solution which provided huge velocity to the ZnO–NPs, resulting in the high-speed inter-particle collision between glass fibers and nanoparticles. The continuous inter-particle collision and intra-particle collision (between ZnO–NPs) resulted in the adherence of it onto the borosilicate microfiber surfaces, growth and finally its decoration throughout the GF paper after 1 h of ultrasonic irradiation [24]. Efficient decoration of the ZnO–NPs in the massive quantity on the GF paper is achieved with this method. This is due to the sonochemical effects along with the unique physical properties of the GF paper (table 1) like small fiber diameter, large void volume space, high surface area, large thickness, excellent strength and dimensional stability (acrylic resin-bonded glass fibers). All these properties of the GF paper provide dense network of fibers with sufficient void spaces and chemically active surface to decorate ZnO–NPs effciently with sonochemical methd. The presence of sufficient oxygen molecule or atom on the fibers of the GF paper may faciliate ZnO–NPs adherence and decoration. The advantage of the sonochemical approach for the ZnO–NPs decoration is saving of the time and effort, no demand of any extra reagents and equipment. Large-scale production of the ZnO–NPs decorated GF papers is possible with this method only in few hours due to the high volume capacity of the industrial bath sonicators (∼100 L).
Figure 1. Schematic representation of the method and mechanism for the ZnO–NPs decoration on the GF paper. Cavitation, microjets, shockwaves and functional groups (OH, Si-O etc.) are the driving energy for the assistance in ZnO–NPs synthesis and its decoration on GF paper.
Download figure:
Standard image High-resolution imageTable 1. Physiochemical specification of the GF paper.
| 1. Catalogue Number | AP1504200 | 2. Pore size | 1 μm |
| 3. Description | Glass fiber filter with acrylic binder | 4. Thickness | 790 μm |
| 5. Chemistry | Borosilicate | 6. Filter diameter | 4.20 cm |
| 7. Wettability | Hydrophilic | 8. Water flow rate | 1.60 ml min−1 × cm-2 |
| 9. Maximum Operating Temperature | 100°C | 10. Application | Pre-filter paper for 0.90 to 8 μm filters, especially for heavily contaminated liquids. |
3.1. Morphology and elemental analysis
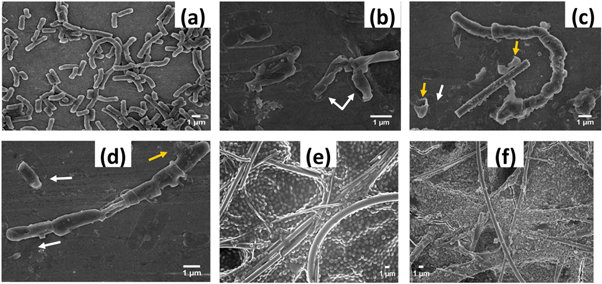
Figure 2 shows the top view FEG-SEM images of the ZnO–NPs decorated and blank GF paper at various magnifications (low and high) to examine the morphology of the ZnO–NPs, its distribution and any unfavorable effect on the GF paper.
Figure 2. Top view FEG-SEM images of the blank GF paper (a)–(c) and ZnO–NPs decorated GF paper (d)–(f). No adverse effect on the fibers and its 3D network is seen due to ultrasonic irradiation, high pH, reaction time etc. The dense population of the ZnO–NPs is seen at some places (d) without much affecting the void volume space (e). Size of ZnO–NPs is in the range of 70 to 280 nm.
Download figure:
Standard image High-resolution imageIt is obvious from figure 2 that ultrasonic irradiation has no adverse effect on the pore size, structure and network of the fibers due to the excellent strength of the GF. The rigidity and dimensional stability of the GF paper were not affected by the ultrasonic irradiation due to the strength provided by the acrylic resin-bonded glass fibers. GF paper differs from the other filter papers of same pore size (1 μm) due to the thin fibers and its dense network. This is the reason why GF paper has large void volume space and large surface area, which can be seen in the figures 2(a)–(c). As seen in the figure 2(d), such huge amount decoration of ZnO–NPs is possible because of large void volume space available (figure 2(a)). Such huge population of ZnO–NPs may affect the number of pores insignificantly (figure 2(e)). But from application point of view, very beneficial in providing the appropriate interaction with bacterial cells leading to enhanced antibacterial activity. Most of the ZnO–NPs morphology is like broken nanoflowers and few of irregular shape (figure 2(f)). To measure the size of the ZnO–NPs, higher magnified FEG-SEM image was used. Manually, the size was measured by using ImageJ software which shows range between 70 to 280 nm and average size 163.64 nm, as shown in the figure S1 is available online at stacks.iop.org/NANOX/1/010048/mmedia.
EDX analysis revealed the elemental composition of the blank and ZnO–NPs decorated GF paper, as shown in the figure S2. Presence of the elements Si, O, Al, K, and B shows the intrinsic borosilicate composition of the GF paper. (3) The high-intensity peak of Zn in the EDX spectra of ZnO–NP decorated GF paper reveals the abundance of the ZnO–NPs. EDX elemental mapping of the ZnO–NPs decorated GF paper (figure 3) shows the elements distribution throughout the selected area. Figures 3(a)–(f) are the area of less populated ZnO–NPs, here pores are not compromised along with efficient distribution. Figures 3(g)–(h) shows the densely populated area where pores are compromised but this may function as an antibacterial barrier during filtration.
Figure 3. Elemental mapping of ZnO–NPs decorated GF paper of low (a)–(f) and high density region of ZnO–NPs (g)–(h). No negative impact can be seen on the inherent chemical composition due to all experimental conditions. The variations in ZnO–NPs density are responsible for multi-functionality of the paper. Element's symbols are denoted on respective maps.
Download figure:
Standard image High-resolution image3.2. ICP-AES results and flow rate analysis
Presence of huge amount of ZnO–NPs on the each ZnO–NP decorated GF paper (96 mg) and very less leaching during the filtration (0.77 μg ml−1) is revealed by ICP-AES results, as listed in the table 2. Loosely adhered nanoparticles on the fibers of the GF paper are responsible for such small amount leaching during filtration. Presence of large quantity of the ZnO–NPs added a new property (fluorescence) and activity (antibacterial) to the existing GF paper which is discussed in the later sections. The decrease in the flow rate of water through ZnO–NPs decorated GF paper (table 2) is because of an insignificant decrease in void volume space due to the huge amount decoration. Such decrease in the flow rate may not have any significant effects on the application based on a new property (fluorescence) and activity (antibacterial).
Table 2. Effect of ZnO–NPs decoration on the flow rate. The decorated and leached amount of ZnO–NPs whille filtration.
| Analysis | Zn-NPs decorated GF paper | Blank GF paper |
|---|---|---|
| 1. Flow rate of the water | 1 ml min−1 × cm-2 | 1.27 ml min−1 × cm-2 |
| 2. Amount of ZnO–NPs present | 96 mg | — |
| 3. Amount of ZnO–NPs leached out while filtration | 0.77 μg ml−1 | — |
3.3. Fluorescence property of the ZnO–NPs decorated GF paper
As mentioned in our reported work, aqueous solution of ZnO–NPs exhibit broad fluorescence emission in the visible region after excitation at 360 nm [18]. Due to the decoration of ZnO–NPs in huge amount throughout the GF paper, CLSM imaging results of ZnO–NPs decorated GF paper shows stronger fluorescence emission in the 535–624 nm (pink color) regions of the visible spectrum, as shown in the figure 4.
Figure 4. CLSM images of the ZnO–NPs decorated GF paper, showing the fluorescent image (a), Differential Interference Contrast (DIC) image (b) and merge of both (c). Selection of suitable paper substrate and appropriate experimental conditions leads to the origin of fluorescence emissions (535–624 nm, pink color) in the decorated GF paper.
Download figure:
Standard image High-resolution imageAs shown in the figure 5(a) and its inset photograph, decoration of the ZnO–NPs in the huge amount throughout the GF paper makes it naked eye fluorescent (pink-orange color) as compared to the blank GF paper (violet) under exposure of UV light (360 nm). Using this technique, ZnO–NPs decorated glass fibers paper can be used in the printing industries for the UV light based authentication of the documents. Through chemical modification of the ZnO–NPs present on the decorated GF paper with appropriate reagents, it can be used in the various sensing applications, affinity-based filtrations, etc.
Figure 5. Digital photograph (a), showing a naked eye fluorescence of the ZnO–NPs decorated GF paper (96 mg per paper) in the alphabetical shape and ZnO–NPs decorated (pink-orange) and blank GF paper (violet) exposed under UV light (360 nm), inset photograph (a). XRD (b), FTIR (c), and TGA spectra (d) of the ZnO–NPs decorated and blank GF paper. Results are showing broad and sharp XRD peaks due to the borosilicate nature and ZnO–NPs respectively (b), infrared absorption peaks related to the borosilicate composition and ZnO–NPs (c) and retaining of the thermal stability in the ZnO–NPs decorated GF paper due to appropriate materials and approaches (d).
Download figure:
Standard image High-resolution image3.4. XRD, FTIR and TGA analysis
XRD was performed to analyze the structural properties of the ZnO–NPs decorated and blank GF paper. Result of the blank GF paper is showing strong and broad diffraction in the range of 2θ = 15°–30° (figure 5(b)), which can be assigned to the amorphous nature of the borosilicate glass fibers [25–28]. Obtained diffraction peaks of the ZnO–NPs decorated GF paper at 2θ = 31.92°, 34.62°, 36.44°, 47.80°, 56.89°, 63.23°, 66.73°, 68.33°, 69.47°, 73.04°, 77.41°, 81.93°, 89.99° matches with the crystal lattice plane (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2), (2 0 1), (0 0 4), (2 0 2), (1 0 4), and (2 0 3) respectively of the ZnO (figure 5(b)). These 2θ and (hkl) values match well with the standard data of the PDF card number 01-074-9940 (International Centre for Diffraction Data, 2013). Crystal parameters and peak positions confirmed the wurtzite hexagonal lattice arrangement of the ZnO–NPs [18]. The obtained diffractogram of the ZnO–NPs decorated GF paper justify the presence of the perfectly crystallized ZnO–NPs. Because of the high-intensity ZnO peaks in the ZnO–NPs decorated GF paper, the peak intensity due the borosilicate glass is low. No, any other diffraction peak is observed other than ZnO and borosilicate which confirms the purity of the ZnO–NPs decorated GF paper. To reveal the functional groups present, its interactions and fingerprint of chemical composition of the ZnO–NPs decorated and blank GF paper, FTIR spectroscopy was used. Figure 5(c) shows the FTIR spectra of both samples, the peak at ∼470 cm−1 is due to the O-Si-O bending vibration and peaks at ∼780 and ∼1010 cm−1 are due to the symmetric and asymmetric stretching vibration of the Si–O–Si linkage respectively. The peak at ∼670 cm−1 can be assigned to the Si-O-B linkage [25–32].. The peak at ∼428 cm−1 in the FTIR spectra of the ZnO–NPs decorated GF paper is due to the stretching mode of the Zn–O linkage [33–35]. The tractable change observed in the FTIR data is the shift in the exact peak position from 1013.38 (blank) to 1016.55 (decorated) of the Si–O–Si band probably due to the hydrogen bonding between ZnO–NPs (OH) and Si–O–Si group. For comparative thermal stability analysis of the ZnO–NPs decorated and blank GF paper, TGA analysis was performed. Its spectra (figure 5(d)) of both samples exhibited no any considerable weight loss till 500°C. The ∼4% weight loss in the blank GF paper could be attributed to the loss of physically adsorbed water molecules [36–38]. The ∼8% weight loss in the ZnO–NP decorated GF paper could be due to the decomposition of the hydroxyl groups on the ZnO–NPs and loss of physically adsorbed water molecule on the both ZnO–NPs as well as GF paper [18, 38]. TGA spectra of the ZnO–NPs decorated and blank GF paper reveals no any adverse effect on thermal stability due to the ultrasonic irradiation and huge amount of ZnO–NPs.
3.5. Topography of the ZnO–NPs decorated GF paper
AFM was used to analyze the topological properties of the ZnO–NPs decorated and blank GF paper. 3D distributions of the nanoparticles can be seen on the ZnO–NPs decorated GF paper (figure 6). Both FEG-SEM (figures 2(a)–(c)) and AFM images (figures 6(a) and (c)) of the blank GF paper reveals the dense 3D network of the glass fibers due to its thinner nature. Due to this, efficient 3D decoration of the ZnO–NPs throughout the GF paper (figures 6(b) and (d)) is achieved.
Figure 6. AFM images, showing the topography of the blank ((a) and its 3D view (c)) and ZnO–NPs decorated GF paper ((b) and its 3D view (d)). 3D distributions of the ZnO–NPs (arrowheads) throughout the paper are possible due to submerged sonochemical coating and excellent structural and mechanical property of the GF paper.
Download figure:
Standard image High-resolution image3.6. Antibacterial activity of ZnO–NPs decorated GF paper: antibacterial filtration (Bacillus subtilis)
As we have already discussed, the GF paper has 1 μm of pore size, large void volume, more surface area and large thickness due to which it is widely used to pre-filter heavily contaminated liquids to save the membranes (0.20 μm pore size, etc) life. If we consider pre-filtration of the bacterial contaminated liquid, the continuous multiplication and growth of the bacteria on the GF paper will affect the whole filtration process. Through our work we have revealed huge reduction in the bacterial growth on the ZnO–NPs decorated GF paper. Such antibacterial modification of the glass fibers (GF paper) through green strategy can be done at industrial level (large-scale) to treat bacterial contaminated or polluted water as an effective water filtration system. As shown in the scheme (figure 7), Franz diffusion cell setup is used for the filtration experiment to avoid any contamination during the experiments because it is autoclavable and the whole set of the experiment can be performed in the laminar hood (sterile zone). After filtration, the GF papers were transferred on the bacterial culture plate to ensure that reduction in the bacterial survival is due to the toxicity of ZnO–NPs towards bacteria not because of the depletion of the media (food of the bacteria). Quantitative as well as qualitative antibacterial activity was examined through CFU analysis and electron microscopy. To justify that the ZnO–NPs decorated GF paper can be used in the pre-filtration of bacterial contaminated waste or polluted water, the antibacterial activity was investigated against model organism B. subtilis. B. subtilis is a spore-forming, rod-shaped, usually non-pathogenic, and are about 4–10 μm long and 0.20–1 μm diameter gram-positive bacterium commonly found in the soil and gastrointestinal tract of human and ruminants [39, 40]. It is extensively used as a model organism to study cell differentiation, chromosome replication and enzyme production in biotechnological companies [39, 40]. Some Bacillus spp. Like B. cereus, B. licheniformis and B. Pumilus are cytotoxic, found in the surface waters and are associated with foodborne diseases [39, 40].
Figure 7. Schematic representation, describing the experimental workflow for the evaluation or screening of the antibacterial activity of the ZnO–NPs decorated GF paper. Survivability of the filtered bacteria is evaluated quantitatively as well as qualitatively.
Download figure:
Standard image High-resolution image3.7. Quantitative results of the antibacterial activity of ZnO–NPs decorated GF paper (CFU analysis)
Through high-speed vortexing, the bacteria (B. subtilis) on the blank (control) and ZnO–NP decorated GF paper (test) were separated out in the sterile media for the quantification. Through serial dilution and plating of the separated bacteria, colonies on the plate were counted for the CFU analysis. As shown in the figures 8(a) and (b), the absence of the bacterial colonies on the culture plates reveals the nonappearance of bacteria in the filtrates of the blank and test respectively. Absence of the bacteria in the filtrate is the intrinsic property of the GF paper and it is retained in the ZnO–NPs decorated GF paper too. This Justify that the sonochemical method of the ZnO–NPs decoration has no adverse effect on the intrinsic physical properties (particle retention, pore size, etc) of the GF paper. As shown in the figure 8(d), the huge reduction in the bacterial colonies on the culture plate justify the enormous reduction in the survival of the bacterial population on the ZnO–NPs decorated GF paper as compared on the blank GF paper (figure 8(c)). CFU/mL, percentage, and log reduction were calculated using equations (1)–(3) respectively [41]. Results of the CFU analysis are listed in table 3, which shows ∼99% or 2 log reduction of the bacteria on the ZnO–NPs decorated GF paper as compared on blank GF paper in 24 h. During the experiment performed on the Franz diffusion cell, media containing bacteria was passed through less than one fourth area (22.60%) of the ZnO–NPs decorated GF paper due to the apparatus design. So, if the filtration would be performed through the whole area of the ZnO–NP decorated GF paper (13.80 cm2), the percentage reduction in the bacterial survival would increase due to the increase in the exposure of bacterial population with ZnO–NPs.
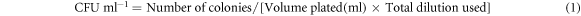
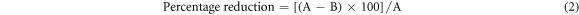

Where, A and B in equations (2) and (3) are the number of viable bacteria on the blank and ZnO–NPs decorated GF paper respectively.
Figure 8. Bacterial colonies (B. subtilis) grown on the nutrient agar plates representing the bacterial population present in the filtrate, of the blank (a) and ZnO–NPs decorated GF paper (b) and survived, on the blank (c), and ZnO–NPs decorated GF paper (d). The essential feature of the GF paper (pre-filtration) is unaffected and new property (antibacterial) added due to both, low and high density distribution of ZnO–NPs.
Download figure:
Standard image High-resolution imageTable 3. CFU analysis, showing ∼99% reduction in the survival of the bacteria (B. subtilis) on the ZnO–NPs decorated GF paper as compared on control after 24 h.
| Sample | CFU ml−1 (B. subtilis) |
|---|---|
| Filtrate of the blank GF paper | 0 |
| Filtrate of the ZnO–NPs decorated GF paper | 0 |
| On blank GF paper | ∼3.80 × 109 |
| On ZnO–NPs decorated GF paper | ∼3.40 × 107 |
3.8. Qualitative results of the antibacterial activity of ZnO–NP decorated GF paper (scanning electron microscopy)
It is needed to perform the both quantitative as well as qualitative antibacterial activity experiments to establish the complete study of any newly introduced product. So, to complete this study, a visual picture of the reduction in the bacterial survival on the ZnO–NPs decorated GF paper as well as the effect of ZnO–NPs on the microstructure of the bacteria was examined through FEG-SEM imaging. As we can see in the figures 9(a) and (b), filtered bacterial cells (B. subtilis) survived on the blank GF paper and gone through rapid multiplication which resulted in the massive growth of the cells throughout the paper. The microstructure of the bacterial cells on the blank GF paper is intact. The absence of the bacterial population (B. subtilis) on the uppermost part of ZnO–NPs decorated GF paper (figures 9(c) and (d)) shows a huge reduction in the survival of the filtered bacterial cells due to the toxicity of ZnO–NPs towards them. From these results, it seems that the bacterial cells which survived on the ZnO–NPs decorated GF paper are present at places where there is depletion of ZnO–NPs or very less exposure between ZnO–NPs and cells.
Figure 9. FEG-SEM images, showing survival and massive growth of the filtered bacterial cells (B. subtilis) on blank GF paper (a) and (b), arrows are indicating the population of the cells. The absence of the bacterial population on the ZnO–NPs decorated GF paper (c) and (d) shows reduction in the survival of the filtered bacterial cells. 3D distribution of the ZnO–NPs in sufficient amount due to the fiber architecture leading to proper interaction between bacteria and ZnO–NPs is responsible for such reduction.
Download figure:
Standard image High-resolution imageRather than scraping the upper part of the ZnO–NPs decorated GF paper and searching for the visibility of the bacterial cells while FEG-SEM imaging, an alternate and correct method was used to analyze this. FEG-SEM imaging of the separated bacterial cells from the blank and ZnO–NPs decorated GF paper was performed to study the effects of ZnO–NPs on the microstructure of the cells. The results showed that bacterial cells from the blank GF are intact (figure 10(a)), whereas, from the ZnO–NPs decorated GF paper showed morphological changes associated to the necrosis (pre-mature death due to the external stimuli) like cell shrinkage, leakage of the cytoplasmic content, cell burst, etc (figures 10(b)–(d)) which is the biological indication of the cellular death or leading toward death [41]. The FEG-SEM images of figures 9(c), (d) along with figures 10(b)–(d) justify the huge reduction in the bacterial survival on the ZnO–NPs decorated GF paper because of the premature death of the bacterial cells due to the toxicity of ZnO–NPs [41].
Figure 10. FEG-SEM images after 24 h on plate incubations, separated bacterial cells (B. subtilis) after from the blank (a) and ZnO–NPs decorated GF paper (b)–(d). Toxic effect of ZnO–NPs on the microstructure of the bacteria like cell shrinkage [white arrows (b)–(d)], cytoplasmic leakage and cell burst [yellow arrows (c) and (d)] due to the large amount decoration (96 mg) throughout the paper. Blank GF paper showing the survival of the inoculated bacteria S. aureus and its massive growth (e) whereas the absence of this on the ZnO–NPs decorated GF paper (f). The antibacterial activity of the decorated paper is not limited to the pathogenicity and shape/sizes of the bacteria.
Download figure:
Standard image High-resolution image3.9. Antibacterial activity of ZnO–NPs decorated GF paper against the disease-causing pathogen
As B. subtilis 168 is a non-pathogenic bacterium, the antibacterial activity of the ZnO–NPs decorated GF paper was also tested against Gram-positive disease-causing bacteria S. aureus to validate its broad-spectrum antibacterial property. S. aureus is round-shaped bacteria, ∼1 μm in diameter and commonly found as normal flora in the respiratory tract, nose and on human skin [18]. Some common infections and diseases associated with the pathogenic strain of S. aureus are wound infection, sinusitis, respiratory infection, skin infection, osteomyelitis, meningitis, pneumonia, etc [18]. Due to the temporary residence of this bacterium within the hospital, they get enter into the environment through sewage treatment of the hospital wastewater [42, 43]. To examine the antibacterial activity of the ZnO–NPs decorated GF paper against S. aureus, the experiment was performed through the different method as mentioned in the experimental section. The survival of the bacterial population on the blank and ZnO–NPs decorated GF paper was analyzed through FEG-SEM imaging of the uppermost part of the GF papers through scanning at various places. The inoculated bacterial cells on the blank GF paper get survived in undamaged condition leading to the massive growth, as shown in the figure 10(e). Whereas, the absence of bacterial population on the ZnO–NPs decorated GF paper shows reduction in the survival of the inoculated bacterial cells (figure 10(f)). This result is qualitative but strongly supports the broad spectrum antibacterial activity of the ZnO–NPs decorated GF paper which manifests that ZnO–NPs decoration of the industrial grade GF paper can be very promising in the pre-filtration of the various kind of bacterial contaminated wastewater. Advance nanocomposites or nanomaterial modified compounds have got huge attention in the past few years due to its remarkable properties and applications. Some examples are zeolite/zinc oxide-copper oxide nanocomposite, copper oxide loaded zeolite nanoparticles, pseudobactins bounded iron nanoparticles for extraordinary antibacterial properties [44–46]. Few others are, silica/carbon nanotubes and silica activated carbon, bimetallic nanoparticles loaded on activated carbon, etc for water and petroleum product purifications [47–51].
3.10. Proposed mechanism of antibacterial activity of the ZnO–NPs decorated GF paper
ZnO–NPs as a semiconductor material consists of the conduction band (CB) and valence band (VB). Incident light of photon energy more than 3.30 eV is absorbed, leading to the transfer of the electrons from VB to CB, leaving positive holes in the VB and free electron in the CB. Based on the well-known mechanisms of the antibacterial activity of ZnO–NPs, the hole and electron will react with water (H2O) and oxygen (O2) molecules present on the ZnO–NP decorated GF paper (glass fibers will absorb water from the nutrient agar plate) and produce hydrogen peroxide (H2O2) through various intermediates formation [18, 22]. H2O2 is a strong oxidizing agent, capable of entering into a bacterial cell, subsequently leading to the demolition of the cellular components and finally cell death [18, 22]. Another possible mechanism is the release of metal ions (Zn+2) from the surface of ZnO–NPs in the water present on the ZnO–NPs decorated GF paper. These metal ions Zn+2 can enter into bacterial cells through biosorption leading to the destruction of the cellular component and finally cell death [18–22]. From the analysis of the obtained results we can say that the antibacterial activity of the ZnO–NPs decorated GF paper is determined by three important factors; exposure between the ZnO–NPs and bacterial cells, amount of water present and surface area of the filter paper. Sufficient amount of water and more exposure between ZnO–NPs and the bacterial cell will facilitate the release and uptake of the H2O2 molecule and Zn+2 ions into the bacterial cell. Larger the surface area of the filter paper more will be the amount of ZnO–NPs present, thus more will be release of H2O2 molecule and Zn+2 ions. This will increase the exposure between ZnO–NPs and the bacterial cell which may increase the efficiency of the antibacterial activity of ZnO–NPs decorated GF paper.
4. Conclusion
In summary, ZnO–NP decoration onto underexplored GF paper is reported for first time. Sonochemical method (bath sonication); a green approach is used to efficiently decorate the ZnO–NPs. The characterization results revealed effective 3D decoration (96 mg/paper) throughout the paper, less leaching of ZnO–NPs while filtration and not much effect on the physiochemical properties like flow rate, structure, thermal stability etc. Presence of ZnO–NPs in the huge amount (96 mg/paper) throughout the GF paper makes it multifunctional; UV fluorescent (naked eye and microscopy both) and antibacterial. The quantitative, as well as qualitative antibacterial activities of the ZnO–NPs decorated GF paper were investigated. Which showed ∼99% (2 log) reduction of the bacterial population (B. subtilis) due to the toxicity of ZnO–NPs towards bacteria cells (cell shrinkage, cell burst, cytoplasmic leakage, etc). Based on the biochemical properties of the ZnO–NPs, its decorated GF paper can be explored for various applications.
Acknowledgments
All the authors of the manuscript are thankful to the School of Biochemical Engineering, IIT (BHU), Varanasi for their technical support. Author (AG) would like to thanks Prof. Rohit Srivastava and department BSBE, IIT Bombay for their support.
Appendix A.: Supplementary data
Supplementary data associated with this article can be found, in the online version, at