Abstract
With estimated worldwide cost over $1 trillion just for dementia, diseases of the central nervous system pose a major problem to health and healthcare systems, with significant socio-economic implications for sufferers and society at large. In the last two decades, numerous strategies and technologies have been developed and adapted to achieve drug penetration into the brain, evolving alongside our understanding of the physiological barriers between the brain and surrounding tissues. The blood brain barrier (BBB) has been known as the major barrier for drug delivery to the brain. Both invasive and minimally-invasive approaches have been investigated extensively, with the minimally-invasive approaches to drug delivery being more suitable. Peptide based brain targeting has been explored extensively in the last two decades. In this review paper, we focused on self-assembled peptides, shuttle peptides and nanoparticles drug delivery systems decorated/conjugated with peptides for brain penetration.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Abbreviations
| α-Syn | α-synuclein |
| ABCB1 | ATP-binding cassette sub-family B member 1 (ABCB1) |
| AC | Astrocyte |
| AChR | Acetylcholine receptor |
| AD | Alzheimer's disease |
| AF6 | lL1-fused gene from chromosome 6 protein |
| AFM | Atomic force microscopy |
| AMT | Adsorptive-mediated transport |
| ANG | Angiopep |
| ApoB | Apolipoprotein B |
| ApoE | Apolipoprotein E |
| AuNP | Gold nanoparticle |
| ASNP | Alginate-stearic acid nanoparticles |
| B6 | CGHKAKGPRK peptide |
| BBB | Blood-brain barrier |
| BCSFB | Blood-cerebrospinal fluid barrier |
| BSA | Bovine serum albumin |
| CNT | Carbon nanotubes |
| CMC | Critical micelle concentration |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| DLS | Dynamic light scattering |
| EAE | Experimental autoimmune encephalomyelitis |
| ECs | Endothelial cells |
| FBS | foetal bovine serum |
| FITC | Fluorescein isothiocyanate |
| g7 | 7-amino acid glycoprotein, GFtGPLS (O-β-d-Glucoseglucose)CONH2 |
| GE11 | CYHWYGYTPQNVI peptide |
| GSH | Glutathione |
| HD | Huntington's disease |
| HIFU | High-intensity focused ultrasound |
| HuHtt | Human huntingtin exon 1 |
| IFN-α | Interferon-α |
| IFN- γ | Interferon gamma |
| i.v. | Intravenous |
| Lamp2b | Lysosome-associated membrane protein 2b |
| LDLR | Low-density lipoprotein receptor |
| LRP-1 | lipoprotein receptor-related protein 1 |
| MCAO | Middle cerebral artery occlusion |
| miniAp-4 | H-DapKAPETALD-NH2 peptide |
| MMP | Matrix metalloproteinase |
| MND | Motor neurone disease |
| MOR | Opioid receptor mu |
| MS | Multiple sclerosis |
| MSC | Mesenchymal stem/stromal cell |
| MWCNT | Multi wall carbon nanotubes |
| nAChR | Nicotinic acetylcholine receptor |
| ND | Neurodegenerative Disease |
| NP | Nanoparticle |
| NIR | Near infrared |
| NVUs | Neurovascular Units |
| NW | Nanowire |
| PAH | Poly allylamine hydrochloride |
| PD | Parkinson's Disease |
| PEG | Polyethylene glycol |
| PepH3 | AGILKRW peptide |
| PLA | Poly(lactic acid) |
| PMNP | Polymeric nanoparticles |
| pSiNPs | Porous silica nanoparticles |
| RES | Reticuloendothelial system |
| ROS | Reactive oxygen species |
| RVG | Rabies virus glycoprotein |
| RVG-29 | YTIWMPENPRPGTPCDIFTNSRGKRASNG |
| SWCNT | Single wall carbon nanotubes |
| SE | Status epilepticus |
| SEM | Scanning electron microscopy |
| siRNA | Small interfering RNA |
| SNALP | Stable nucleic acid lipid particle |
| SPION | Superparamagnetic iron oxide nanoparticle |
| t-MCAO | transient middle cerebral artery occlusion |
| TAT | Trans-activating transcriptional activator |
| TEM | Transmission electron microscopy |
| Tf | Transferrin |
| TfR | Transferrin receptor |
| TJ | Tight junction |
| TNF-α | Tumor necrosis factor-α |
| WHO | World Health Organisation |
| ZO | Zonula occludens (a.k.a. tight junction protein) |
1. Introduction
The central nervous system (CNS) comprises the brain and the spinal cord. Any injury or damage to the CNS affects its normal functioning and may lead to permanent disability in many cases, due to a largely limited ability for neural tissue regeneration in humans [1, 2]. The broad term 'Neurodegenerative Diseases' (NDs) covers a range of pathologies, principally affecting neurons in the brain and causing significant neuronal dysfunction, neuronal death and neuronal loss. NDs once established are irreversible and sapping conditions resulting in progressive degeneration of neuronal cells [3]. The signs and symptoms are diverse in range, depending on the affected part of the brain. The cause of an ND is often unknown but can involve a complex convergence of multiple molecular mechanisms; and disease progression is usually unpredictable. NDs include a number of conditions: Alzheimer's disease (AD) and other forms of primary dementias, Multiple Sclerosis (MS) and other forms of chronic inflammatory neurological disease, Parkinson's disease (PD), Motor Neurone Disease (MND), Huntington's disease (HD) and ataxias [4]. The World Health Organisation (WHO) reported that NDs affect around 0.1 billion individuals (24 million individuals suffer from AD and other dementias) [5] all over the world, and the incidence is on the rise as average life expectancy is increasing. Around 850,000 people in the UK are affected by dementia, costing the healthcare system over £26 billion a year [6]. In the US nearly 100 million people are affected by NDs costing around $724 billion in 2014 [7]. It is estimated that the cost of AD would be over 1 trillion dollars worldwide [8]; and the estimated number of people with dementia will reach 131.5 million by 2050 [9] in the absence of effective therapies. Just in Europe, the annual cost of neurological disease reaches 800 billion Euros per year, with a majority attributed to direct costs [10].
The brain is one of the most vital and sensitive organs in the body, which, to perform its functions in an appropriate way, needs nutrients and gases [11]. Due to its pivotal role and functions, it is protected in a number of ways, including by the skull, the outer skin, three layers of meninges and the blood-brain barrier (BBB) [12]. The BBB is a layer of endothelial cells (ECs) associated with pericytes (PCs) and astrocytes (ACs) and acts as a separator of the blood from parenchymal cells, thus preventing penetration of drugs into the CNS. It therefore protects the brain from overexposure to substances such as potassium, glycine and glutamate, which, in high levels such as found in pathological conditions, are neurotoxic [13, 14].
Despite many advances in drug delivery systems that target the brain, it is still a challenging area. The failure of therapies administered via an intravenous (i.v.) or an oral route is often due to their inability to cross/penetrate the brain parenchyma. The use of peptides for drug delivery to the brain has been extensively explored in the last decade. Self-assembled peptides, shuttle peptides and peptide-decorated nanoparticles have been reported to effectively deliver drugs in the brain. This review covers peptide based drug delivery systems for the brain and future prospects.
2. Blood-brain barrier
Figure 1 is the schematic representation of healthy and diseased BBB. Numerous gateways have been reported to provide access the brain; the most significant are through blood stream or by getting access to the cerebrospinal fluid (CSF) circulation. Penetration of any molecules administered via the parenteral route is controlled by the BBB, the blood–cerebrospinal fluid barrier (BCSFB), arachnoid barrier and circumventricular organ barrier. However, drug molecules up taken by the brain are flushed back towards the blood through the return of the CSF to the blood or transporters on the BBB [15]. The BBB acts as a guard filter that prevents the uptake of large-molecules and more than 98% of pharmaceuticals [12, 16] and small-molecule drugs [17]. Small molecules that are lipid soluble, electrically neutral and weak bases may be able to diffuse passively across the BBB.
Figure 1. BBB composition and pathological conditions. (A) In normal states, the BBB comprises vascular endothelial cells connected with TJs and the PCs layer. A basement membrane linked with AC end-feet surrounds the endothelium. (B) Increased permeability of the BBB in pathological conditions results from high matrix metalloproteinase (MMP) activity and increased reactive oxygen species (ROS) and nitric oxide (NO) levels. Cytokines and chemokines are released and then activate microglia/macrophages, leading to basement membrane degradation, TJs disruption and an inflammatory response.
Download figure:
Standard image High-resolution imageThus, the BBB, with its extensive blood capillary network, is considered the most important barrier that controls a molecule's access to the brain parenchyma. Neurovascular units (NVUs) comprising endothelial cells, extracellular base membrane, adjoining pericytes, astrocytes, and microglia (although not a structural component of the BBB, are often included in the NVU as they influence barrier function in response to injury and disease [18] are integral parts of the BBB supporting system [19]. NVUs collect signals from the adjacent cells and generate functional responses that are crucial for appropriate CNS function [20, 21]. Both tight intracellular junctions (i.e. zona occludens, characteristic of the BBB) and the absence of fenestrations limit the permeability of drug molecules [22].
Various transport routes have been reported by which solutes and drug molecules can cross the BBB,[23, 24] as shown in figure 2. Diffusion of substances across the BBB can be generally categorised into paracellular (namely the transfer of nutrients/drugs across an epithelium by passing through the intercellular space between the cells) and transcellular (namely the movements of solutes through a cell). In order to cross the BBB by passive diffusion, various parameters play pivotal roles. Molecular mass is an important factor and the ideal molecular weight reported to be suitable for passive diffusion is <400 Da [25]. A value of between 5.0 and 6.0 for the log of the octanol-water partition coefficient (logPo/w), a measure of lipophilicity, is suitable for passive diffusion [26].
Figure 2. Transport routes across the BBB. Solute molecules follow from 'a' to 'f' pathways and the route 'g' involves monocytes, macrophages and NPs (NPs).
Download figure:
Standard image High-resolution imageCompounds that are lipophilic, neutral or uncharged at pH 7.4 and have less than 8 hydrogen bonding groups are more suitable to cross the BBB [27]. In another study, reported by Partridge in 2012, [28] it was found that small drug molecules can cross the BBB if their molecular mass is less than 400 and they have the ability to form 8–10 hydrogen bonds. Unfortunately, it has been reported that more than 98% of drugs for the CNS are unable to cross the BBB adequately to attain the minimum therapeutic concentration [12]. Several invasive and non-invasive approaches have been anticipated to evade the BBB and enhance drug delivery to the CNS.
3. Novel shuttle peptides
Shuttle peptides facilitate the influx of a diverse range of small molecule cargoes across the BBB. The concept of shuttle peptides for BBB was coined by William M Pardridge in the mid-1980s [29]. Small synthetic peptide shuttles (comprising natural amino acids) have been reported to cross the BBB. For example, the short rabies virus glycoprotein (RVG), RVG-29 (YTIWMPENPRPGTPCDIFTNSRGKRASNG), binds exclusively to the nicotinic acetylcholine (nAChR) receptor found on neuronal cells and on the endothelial cell lining of the BBB, making it possible for peptide carriers to penetrate [30]. Javed et al (2016) used C2-9r (H2N-CDIFTNSRGKRAGGGGrrrrrrrrr, where r is D-arginine) to deliver siRNA for suppressing the α-synuclein (α-Syn) gene, implicated in the development of PD. CDIFTNSRGKRA is a shorter version of RVG, linked with four extra glycine acting as a spacer and positively charged arginine (R), which at the end of the C-terminus bind with negatively-charged siRNA. It was reported that this delivery system (peptide-based) not only crosses the BBB, but also stabilizes the siRNA that supresses the α-Syn protein, thus mitigating PD-like symptoms [31]. Although this delivery system has been derived from the rabies virus, it was reported to be non-toxic to neuronal cells.
Venom-derived, peptide-based shuttles have been reported to cross the BBB and to be able to deliver drugs to the desired site. Oller-Salvia et al (2016) have demonstrated that miniAp-4 (H-DapKAPETALD-NH2) derived from Apamin (a neurological toxin from bee venom) is able to cross the BBB and can deliver gold nanoparticles (NPs), showing proof of concept for drug delivery [32]. PepH3 (AGILKRW) has shown greater penetration upon i.v. administration in CD1 mice and bio-distribution was measured in mice sacrificed 5 min and 1 h after administration. Furthermore, its clearance and excretion is relatively fast, making it a good candidate for a shuttle carrier [33]. Spontaneous internalisation of nanowires (NW), linked with a cell penetrating peptide: the trans-activating transcriptional activator (TAT) from human immunodeficiency virus 1, has also been reported [34]. Two other shuttle peptides PWVPSWMPPRHT and GPWVPSWMPPRHT (composed of D-amino acids) have been found to cross the BBB and are able to transport drug molecules or diagnostic substances into the CNS. These peptides have been reported to be biocompatible and non-toxic (as they were made up of amino acids) [35]. In recent decades, a number of BBB shuttle peptides with improved efficiency have been reported (table 1). Apolipoprotein (Apo) derivative peptides have been shown to cross the BBB (in in vitro and in vivo experiments) [36, 37]. Whilst numerous studies have demonstrated that Apolipoprotein B (ApoB) (SSVIDALQYKLEGTTRLTRKRGLKLATALSLSNKFVEGS) and Apolipoprotein E (ApoE) (LRKLRKRLL)2 analogues are able to cross the BBB [38–40]. Gao et al (2012) reported the use PEG-(poly(ε-caprolactone)) NPs (prepared by emulsion solvent evaporation) for brain drug delivery, and contained docetaxel, a widely used drug in the treatment of several malignancies including brain tumours. They successfully conjugated a phage displayed TGN (table 1) peptide and an AS1411 aptamer, which specifically targets the ligands on the BBB and cancer cells respectively. In vitro experiments showed excellent permeability across the BBB along with suitable endothelial monolayer targeting. In vivo imaging showed that unmodified NPs hardly distributed in the brain while AsNPs (AS11411 conjugated NPs) accumulated slightly in the brain. However, the accumulation of TGN conjugated NPs in the brain significantly increased and the brain distribution achieved the highest intensity at 12 h [41]. GRN1005 a peptide-drug conjugate (taxane paclitaxel and angiopep-2 (ANG = TFFYGGSRGKRNNFKTEEY)) that interacts with lipoprotein receptor-related protein 1 (LRP1) has shown excellent permeability across the BBB. Phase I and II clinical trials suggested that GRN1005 was able to cross the BBB and limit tumour growth [42, 43]. Similarly, Li et al (2016) used a combination of two peptides (ANG and TAT) conjugated with paclitaxel to deliver the drug across the BBB [44]. Zou et al (2019) used a 16 lysine (K16) residue-linked low-density lipoprotein receptor-related protein (LDLR)-binding amino acid segment of apolipoprotein E (K16APoE) to deliver a therapeutic peptide (HAYED) into an AD mouse model brain leading to reduced the necrosis [45]. Numerous shuttle peptides have been investigated for drug delivery to the brain but there is still a need to find magical combination. In another study, Sonoda et al (2018) formulated a BBB penetrant protein conjugate (JR-141), comprising an anti-human transferrin receptor (hTfR) antibody and human iduronate-2-sulfatase (hIDS) to treat mucopolysaccharidosis II (MPS II, caused by accumulation of glycosaminoglycans) [46]. Upon i.v. administration, JR-141 was detected in the brain but hIDS alone failed to penetrate into the brain. In addition, ostensibly therapeutic outcomes were observed, with a lower accumulation of glycosaminoglycans measured in brain and peripheral tissues [46]. Self-assembled peptide nanoligand derived from phage display library was used to down regulate the BACE1 without toxicity and inflammation [47] .
Table 1. A list of shuttle peptides that can target the BBB.
| Peptide | Typical Sequence | Origin | Transport Mechanism | References |
|---|---|---|---|---|
| g7 | GFtGPLS (O-β-d-glucose)CONH2 | Enkephalin analogues/opioid | RMT | [48–51] |
| Apamin | H-CNCKAPETALCARRCQQH-NH2 | Venom neurotoxin | Unknown | [32] |
| MiniAp-4 | [Dap]KAPETALD | Venom neurotoxin | Unknown | [32] |
| Regulon polypeptides | PTVIHGKREVTLHL | Neurotropic endogenous Protein | LDLR | [52] |
| RAP | ELKHFEAKIEKHNHYQKQLE | Neurotropic endogenous Protein | LDLR | [52] |
| Angiopep-2 | TFFYGGSRGKRNNFKTEEY | Neurotropic endogenous Protein | LRP1 | [53, 54] |
| TAT (47-57) | GGGGYGRKKRRQRRR | HIV Protein | CD4 + T lymphocytes | [55] |
| PhPro | [Phenyl-Proline]4 | Chiral library design | Passive transport (paracellular and transcellular) | [56] |
| RI-OR2-TAT | Ac-rGffvlkGrrrrqrrkkrGy-NH2 | HIV Protein and Amyloid beta | Aβ peptide binding | [57] |
| SynB1 | RGGRLSYSRRRFSTSTGR | Protegrins | AMT | [58] |
| Pep 22 | Ac-[cMPRLRGC]c-NH2 | Phage display (receptor) | LDLR | [59] |
| Leptin 30 | YQQVLTSLPSQNVLQIANDLENLRDLLHLLC | Leptin | RMT | [60] |
| TGN | TGNYKALHPHNG | Phage display | Unknown | [61, 62] |
| CNG-QSH | (d-CGNHPHLAKYNGT) (d-QSHYRHISPAQVC) | Phage display | Unknown/Aβ peptide binding | [63] |
| LNP | KKRTLRKNDRKKRC | the nucleolar translocation signal sequence of the LIM Kinase 2 protein | Caveolae-mediated endocytosis and macropinocytosis | [64] |
| ApoE (157-167) | (LRKLRKRLLR)2 | Apolipoprotein E | LRP1 | [38, 39, 65] |
| ApoB | SSVIDALQYKLEGTTRLTRKRGLKLATALSLSNKFVEGS | Apolipoprotein B | LRP2 | [40] |
| RVG-29 | YTIWMPENPRPGTPCDIFTNSRGKRASNG | Rabies Virus Glycoprotein | nAChR | [30] |
| G23 | HLNILSTLWKYRC | Phage display | GM1 and GT1b | [66, 67] |
| T7 | HAIYPRH | Phage display | hTfR | [68–71] |
| THR | THRPPMWSPVWP | Phage display | hTfR | [35, 72–74] |
| THRre | pwvpswmpprht (retro-enantio version of THR) | Phage display | hTfR | |
| THRre_2f | (pwvpswmpprht)2KKGK(CF)G | Branched - Phage display | hTfR | [75] |
| DKP | Phe(p-NH-Dhp)-L-N-Me[Cha]/[2Nal] | Unknown | Passive diffusion | [76] |
| GSH-PEG | GSH[PEG] | Endogenous tripeptide | Glutathione | [77–79] |
| CDX | D-[FKESWREARGTRIERG] | Structure-guided design | nAchR | [80, 81] |
| CRT | CRTIGPSVC | Phage display | TfR | [82] |
| T7 - #2077 | RLSSVDSDLSGC | Phage display | RMT | [83] |
| CAQK | CAQK | Phage display | Proteoglycan complex | [84] |
Datta et al (2000) used a receptor binding domain peptide derived from human apolipoprotein E (hApoE), LRKLRKRLLR [hApoE (141–150)] as a vehicle to cross the BBB. They fused hApoE (141–150) with 18A (DWLKAFYDKVAEKLKEAF) [Ac-He18a-NH2], a high affinity lipid-associated peptide to assess the uptake and degradation of low-density lipoprotein (LDL) in murine embryonic fibroblast (MEF1). In addition, four analogues were prepared, of which, Ac-LRRLRRRLLR-18A-NH2 [Ac-hE(R)18A-NH2] and Ac-LRKMRKRLMR-18A-NH2 (Ac-mE18A-NH2) have an extended hydrophobic moiety, including the receptor binding region. Control peptides were Ac-LRLLRKLKRR-18A-NH2 [Ac-hE(Sc)18A-NH2], which has amino acid residues of the ApoE to disrupt the hydrophobic face, and Ac-RRRRRRRRRR-18A-NH2 (Ac-R1018A-NH2), which has only positively charged arginine (R) as the receptor binding domain. Increased internalisation of LDL was observed by 3-, 5- and 7-fold by Ac-mE18A-NH2, Ac-hE18A-NH2, and Ac-hE(R)18A-NH2, respectively, whereas the control peptides had no significant biological activity as illustrated in figure 3 [38]. Wang et al (2013) used a receptor binding peptide of ApoE (residues 159–167 [monomer: LAVYQAGAR], but the peptide had 18 amino acids, 2×monomer) fused to IDUA (a lysosomal enzyme, α-L-iduronidase) [IDUAe1] to deliver across the BBB by targeting the LRP1, for the treatment of mucopolysaccharidosis (MPS) type I [39]. Zhang et al (2018) used BBB shuttle peptides to enhance the brain transduction of AAV8 after systemic administration. THR (THRPPMWSPVWP-NH2), a shuttle peptide that binds specifically to TfR1 was used to promote the internalization and transduction of AAV8 in a dose dependent manner [85].
Figure 3. Schematic presentation of high affinity lipid peptide linked with poly-arginine and ApoE. Peptide conjugated with poly-arginine served as control and no permeation was observed, while conjugated ApoE showed improved internalization into cells.
Download figure:
Standard image High-resolution image4. Novel nanotechnology for brain drug delivery
NPs are carriers composed of natural (e.g. lipidic) or synthetic (e.g. polymeric) materials ranging from 1–500 nm in size. NPs are able to encapsulate, adsorb, or conjugate drugs or diagnostics and release the payload at a specific rate in the human body [86]. The physicochemical properties of NPs such as size, surface charge (zeta potential), morphology and composition are important factors deciding the fate of NPs, such as passage across the BBB, biological activity, release profile and biocompatibility [87]. A list of NPs used for brain drug delivery are summarised in table 2.
Table 2. A summary of formulations (NPs) targeting the BBB.
| Formulation/Polymer | Drug | Disease | Method used for NP preparation | Mechanism for BBB crossing | Key findings | References |
|---|---|---|---|---|---|---|
| g7-PLGA-NPs (NPs of less than 300 nm) | FITC-albumin | MPS I and MPS II | Double emulsion technique | RMT | The C57BL/6 Idua knockout and C57BL/6 Ids knockout mice were used. High MW molecule delivery across the BBB achieved | [88] |
| Functionalized solid lipid NPs with apolipoprotein E, (SLN-DSPE-ApoE) (Average size was less than 200 nm with zeta potential of –10–15 mV) | Resveratrol | Neuroprotective | High shear homogenization | LDLR | In vitro cytotoxicity evaluation via MTT and LDH using hCMEC/D3 cell line showed that SLNs affected neither the metabolic activity of the cells nor the membrane integrity at concentrations less than 1500 μg ml−1. hCMEC/D3 monolayers in transwell devices showed SLN-DSPE-ApoE, permeabilities 1.5-fold higher than for non-functionalized SLNs | [89] |
| Bovine Serum Albumin NPs with LMWP cell penetrating peptide (LMWP-albumin) [LMWP: CVSRRRRRRGGRRRR] (Particle size less than 200 nm,) | PTX and 4-HPR | Brain cancer | Self-assembly | Brain penetration mainly by EPR, but also through SPARC and gp60 albumin binding proteins overexpressed in glioma tissues | FACS showed in vitro cellular uptake of the NPs. bEnd.3 cell line showed BBB penetration of the NPs U87 cells showed cytotoxicity of NPs. The NPs were administered by i.v. injection to orthotopic glioma (Luc-U87) mouse model (bearing intracranial tumor). The mice received the NPs (LMWP-modified bovine serum albumin (BSA) NPs containing PTX and 4-HPR) showed the longest survival time | [90] |
| PEG–PLA-penetratin (RQIKIWFQNRRMKWKK) (Particle size 100 nm, zeta potential −4.42 mV) | Coumarin-6 | CNS disorders | Emulsion/solvent evaporation technique | AMT/RMT | MDCK-MDR cell model showed enhanced accumulation via both lipid raft-mediated endocytosis and direct translocation. In vivo administration showed significant brain uptake with less deposition in non-target tissues | [91] |
| Angiopep conjugated with poly(ethylene glycol)-co-poly(ε-caprolactone): ANG-PEG– poly(ε-caprolactone) (Particle size was less than 100 nm with zeta potential of 3.28 ± 0.75 mV) | Paclitaxel | Glioblastoma multiforme | Sonication | LDLR | U87 MG glioma cells indicated the ANG-PEG- poly(ε-caprolactone) NPs uptake via LDLR (Angiopep-2 and Aprotinin significantly reduced the cellular uptake of the NPs). Real time fluorescence imaging showed accumulation of ANG-NPs in the brain of intracranial U87 MG glioma tumor-bearing nude mice after i.v. injection. | [92] |
| TAT-poly(ethylene glycol) (PEG)-b-cholesterol: TAT–PEG-b-Chol (Particle size less than 200 nm) | Ciprofloxacin | Encephalitis | Self-assembly | AMT | Enhanced in vitro cellular (ACBRI 376) uptake. NPs crossed the BBB and located around the cell nucleus of neurons (SD adult rats) following i.v. injection | [93] |
| RVG-29-PEG-PLGA/DTX-NPs (Particle size was around 110 nm) | Docetaxel | Gliomas | Nanoprecipitation | nAchR | In vitro bEnd3 cells showed ermeability across the BBB. RVG-29-PEG-PLGA/DTX-NPs had a stronger inhibitory effect on C6 cell proliferation than free DTX. In vivo experiments confirmed selective accumulation of NPs in intracranial glioma tissues following i.v. injection. | [94] |
| PEG-Poly(ε-caprolactone)-CH2R4H2C/Stearate- CH2R4H2C (CH2R4H2C: CHHRRRRHHC peptide) (Particle size was in the range of 50–100 nm with zeta potential of 15–20 mV) | Dextran (as model drug) | CNS disorders | Self-assembly | Olfactory nerve channels | Hydrophobic carrier is more suitable for the delivery of drug in forebrain, while hydrophilic carrier is suitable for hindbrain (brainstem). | [95] |
| g7- PLGA-Np (Particle size was in the range of 155 ± 26 nm with zeta potential of −15 ± 5.6 mV) | Loperamide | CNS disorders | Nanoprecipitation | AMT | Long term in vitro release over 192 h and 20% in 2 h. In vivo experiments showed excellent bio-distribution in brain. | [96, 97] |
| mPEG−PLGA-RVG (Particle size was in the range of 168.8 ± 1.9 nm with zeta potential of −27.40 ± 0.71 mV) | Deferoxamine | PD | Double emulsion technique | nAchR | In vivo administration reduced the oxidative stress and iron contents in the substantia nigra and striatum of PD mice. | [98] |
| siRNA/TMC–PEG-RVG (Particle size was in the range of 207 ± 2 nm with zeta potential of 9 ± 2.5 mV) | siRNA | AD | — | nAchR | In vitro and in vivo experiment showed excellent penetration into brain with low toxicity and higher serum stability. | [99] |
| AuNCs-RDP (Particle size was in the range of 10 ± 2.85 nm with zeta potential of −5.92 ± 3.16 mV) | Carboxyfluorescein | Neural cell imaging | Green synthetic route | RMT | In vitro and in vivo results suggested the effective internalization in the brain cells. | [100] |
4.1. Polymeric NPs (PMNPs)
Polymeric NPs (PMNPs) are most extensively studied for the purpose of drug delivery. These NPs can not only deliver small drug molecules but can also be used for the delivery of genes and proteins [101]. PMNPs can have good penetration through cell membranes, serum stability, and can be easily manufactured. Furthermore, the surface of NPs can be modified for various medical applications. For brain drug delivery, PMNPs are made up of proteins, amino acids, polysaccharides and polyesters. Different mechanisms can be adapted by the PMNPs to cross the BBB. They can cross the BBB either by transcytosis through endothelial cells, mucoadhesion, or by disturbing the TJ in the brain capillaries [102]. On the other hand, PMNPs can be identified upon i.v. injection by the reticuloendothelial system (RES), leading to wide distribution to liver, spleen and bone marrow, resulting in elimination or very short half-lives [103]. Tf and poly-L-arginine (cell penetrating peptide) linked with 1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol) (DSPE-PEG) liposomes were developed for brain delivery of imaging agents and DNA [104]. B6 (CGHKAKGPRK), a TfR-specific peptide, and GE11 (CYHWYGYTPQNVI), a peptide specific for endothelial growth factor receptor (EGFR) overexpressed on cancer cells, were linked with poly(amido)amine-PEG (PAMAM-PEG) based dendriplexes for siRNA delivery [105].
PLGA-NPs modified with 7-amino acid glycopeptide (g7) have been shown to deliver small drug molecules across the BBB in rodents. Furthermore, g7-NPs successfully crossed the BBB with model drug (fluorescein isothiocyanate (FITC)-albumin). Injection in wild-type and knockout mice clearly showed penetration into the brain [88]. Luo et al (2017) developed high-intensity focused ultrasound (HIFU) responsive angiopep-2-decorated poly(lactic-co-glycolic acid) (PLGA) hybrid NPs able to transport doxorubicin/perfluorooctyl bromide (ANP-D/P). Decorated-NPs showed 17-fold increased accumulation in glioblastoma and 13.4 fold higher than unmodified NPs. Significant amount (47%) of drug released within two minutes after HIFU irradiation, causing apoptosis of tumour cells [106]. Methoxypolyethylene glycol (MPEG) and methoxypoly(ethylene glycol)-b-polycaprolactone (PCL) NPs, conjugated with angiopep-2 (CTFFYGGSRGKRNNFKTKRY) peptide with encapsulation efficiency of more than 95% showed higher in vivo accumulation in the brain [107].
Di Mauro et al (2018) developed novel biodegradable block co-polymeric NPs, functionalized with two different peptides AGBBB015F (CGGKTFFYGGSRGKRNNFKTEEY) and Regulon (HKKWQFNSPFVPRADEPARKGKVHIPFPLDNITCRVPMAREPTVIHGKREVTLHLHPDH). These peptide functionalized NPs showed higher brain permeability than non-functionalized in U-87 MG cell line [108]. K16ApoE decorated PLGA-NPs have shown better accumulation in the cerebral vasculature. These NPs showed higher uptake into brain and provided better MRI contrast for diagnostic purpose [109].
4.2. Metallic NPs
Metallic NPs for brain delivery have been under investigation due to their serum stability and long half-life. Ghorbani et al (2018) reported the use of gold-iron nanocomposites encapsulated with curcumin-lipoic acid, a pH-sensitive delivery system for the brain. GSH is used as targeting ligand, leading to 2-fold increases in cellular uptake [110]. Nosrati et al (2019) reported the use for glutathione (GSH) decorated iron NPs (GSHIONPs) for brain drug delivery. IONPs@Asp-PTX-PEG-GSH are stable, non-toxic and enhance MRI contrast for diagnostic purpose [111].
In a comparative study conducted by Wang et al (2019) reported the peptide functionalized polyethylene glycol and maleic anhydride‐coated superparamagnetic iron oxide nanoparticles (Mal‐SPIONs) showed better diffusion to the thalamus, frontal cortex and temporal lobe than bovine serum albumin (BSA) conjugated NPs [112]. In another study, Albertini et al (2019) used AUNPs decorated with RGD like peptides (GRGDG-NH2, GRGDS) for drug delivery to brain tumour. Two hours after injection, the concentrations of NPs were 1.5 and 5 fold higher than undecorated NPs and PEGylated NPs [113]. TAT-conjugated gold NPs have been employed for brain drug delivery. The cellular uptake of AuNPs-TAT was 7.4% compared to 0.03% of AuNPs-PEG [114]. Chlorotoxin (CTX), a glioma specific peptide conjugated with polyethylenimine-entrapped gold nanoparticles (Au PENPs) showed excellent penetration into brain [115]. Ivask et al (2018) evaluated the uptake of iron oxide NPs conjugated with biomimetic phosphorylcholine brushes in an in vitro BBB model system. They reported that after 24 h, 78% of the formulation crossed the BBB via adsorption mediated transport (AMT) [116]. This ability of iron oxide NPs has provided the opportunity of delivering therapeutic peptides to the brain by conjugating the peptide to the surface of iron-oxide NPs (5 nm diameter) [117]. Tf-conjugated magnetic dextran‐spermine NPs (DS‐NPs) have also demonstrated excellent penetration across the BBB [118].
Kang et al (2016) reported a single-step procedure to simultaneously load porous silicon NPs with high concentrations of siRNA and protecting them by formation of Ca2SiO4 at the surface of NPs (pSiNPs). These core–shell NPs had the size of 180 ± 20 nm. Then pSiNPs were surface functionalised with RVG peptide (cell targeting ligand) and a cell penetrating peptide (myr-GWTLNSAGYLLGKINLKALAALAKKIL(GGCC), a myristoylated transportan) to deliver the siRNA across the BBB. Addition of these peptides increased the size of pSiNPs to 220 nm. The pSiNPs were administered intravenously to mice with brain injury, and a significant amounts of siRNA were accumulated at the site of injury [119]. Similarly, Lee et al (2017) reported the use of rabies virus‐mimetic silica‐coated gold nanorods to treat brain gliomas. The nanorods were prepared by converting spherical gold NPs to gold nanorods. Then coating the gold nanorods with SiO2. This was to adjust the size of the nanorods to the size of rabies virus as much as possible. This was followed by coating the resulting Au-SiO2 nanorods by PEG and RVG-29. The nanorods (RVG-PEG-Au@SiO2) had the length of 117.7 ± 7.3 nm and width of 50.3 ± 3.1 nm. The RVG-PEG-Au@SiO2 nanorods were administered intravenously to orthotopic glioma-bearing mice, which in vivo fluorescence imaging indicated the accumulation of RVG-PEG-Au@SiO2 nanorods in the mouse brains. The mice were subjected to photothermal therapy using near infrared (NIR) laser. The temperature changes (up to 60 °C) caused by the laser therapy (localized surface plasmon resonance) of gold nanorods resulted in irreversible damages to or death of tumor cells. Tumor volumes in mice treated with RVG-PEG-AuNRs@SiO2 nanorods and applying NIR laser were considerably smaller than those of mice treated with PEG-AuNRs@SiO2 nanorods or control saline (124.8 ± 147.5, 1067.4 ± 295.4, and 2323.2 ± 436.3 mm3 , respectively) at 7 d after the treatment. Even, the tumors of two mice treated with RVG-PEG-AuNRs@SiO2 nanorods nearly vanished. This therapy caused slight skin damage by 808 nm laser irradiation, which was healed after 13 days [120]. This study indicates that even the EPR of the brain tumors was not sufficient to allow accumulation of PEG-AuNRs@SiO2 nanorods in the tumors and use of RVG-29 cell targeting peptide was necessary to achieve desired therapeutic outcomes. In addition, the size of RVG-PEG-AuNRs@SiO2 nanorods could be part of the successful application of these NPs.
Numerous factors control the systemic circulation, cell penetration and BBB passage of NPs. Particle size is one of the important factors controlling the access of NPs across the BBB. Studies conducted in animal models of AD, PD and stroke have used NPs of 50–100 nm [121–126]. Several techniques, such as dynamic light scattering (DLS), atomic force microscopy (AFM), TEM and scanning electron microscopy (SEM) are used to characterise NPs [127]. Several factors control the particle size, such as the polymers used, drug loading, drug/polymer ratio and hydrophilic/lipophilic ratio. Previous studies have reported an increase in particle size after drug loading [128, 129]. On the other hand, Lopalco et al (2015) have reported no changes in the size of NPs made up of PLGA, PLGA-d-α-tocopheryl polyethylene glycol 1000 succinate (TGPS) and Resomer RGPd5055 pre- and post-loading of drugs (oxcarbazepine and coumarin-6) [130].
4.3. Exosomes
Exosomes are comprised of natural lipid bilayers with an abundance of adhesive proteins that readily interact with cellular membranes. These are small extracellular nanovesicles secreted by numerous cell [131, 132]. Naturally-occurring extracellular vesicles such as exosomes traffic endogenous small molecules, proteins and nucleic acids between cells,[133, 134] and they have shown considerable promise for the delivery of exogenous drugs or biological therapeutics,[135–138] including to the brain [139, 140]. Exosomes have several advantages over synthetic NPs in that their biocompatibility confers upon them an inherent non-immunogenicity and long circulation times, however surface-functionalisation (e.g. for targeted delivery) and synthetic analogues of 'natural' exosomes have also proven to be successful therapeutic strategies [141–143]. Drugs delivered by means of an exosomal vector often show enhanced efficacy and fewer adverse effects. Enhancing and exploiting the innate drug-delivery capabilities of exosomes make for a highly attractive therapeutic approach.
Alvarez-Erviti et al (2011) used exosomes (obtained from self-derived dendritic cells) decorated to express Lysosome-associated membrane protein 2b (Lamp2b) and fused with neuron-specific RVG peptide to deliver siRNA into mouse brains [144]. They also compared the immune response of siRNA-RVG exosomes and siRNA-RVG-9R in vivo by measuring the interleukin (IL)−6, interferon gamma-induced protein (IP)−10, tumor necrosis factor (TNF)-α and interferon (IFN)-α serum levels. They found non-substantial changes in all cytokines compared to siRNA-RVG-9R [144]. Although, IFN-α and IP-10 increased in average for mice injected with siRNA-RVG exosomes compared to control mice [144].
Curcumin-loaded exosomes tagged with cyclo(Arg-Gly-Asp-D-Tyr-Lys) peptide [c(RGDyK)] were used to target the lesion region of the ischemic brain in a transient middle cerebral artery occlusion (tMCAO) mouse model [145]. Alvarez-Erviti et al (2011) used RVG decorated exosomes to deliver siRNA to the mouse brain [144]. Long et al (2017) used A-1 exosomes (derived from human bone marrow mesenchymal stem/stromal cells (MSCs)) for the rectification of pilocarpine-induced status epilepticus (SE) [146]. Exo-JSI124 exosomes derived from EL-4 cells (a mouse lymphoma cell line) were used to deliver an encapsulated anti-inflammatory drug in experimental autoimmune encephalomyelitis (EAE) mice via an intranasal route, modulating inflammation [147]. Exosomes derived from dendritic cell cultures treated with interferon-γ were found to increase myelination in rats upon intranasal administration, possibly by delivery of miR-219 [148]. Exosomes loaded with superparamagnetic iron oxide NPs (SPIONs) and curcumin and conjugated with neuroleptin-1-targeted peptide (RGERPRR) crossed the BBB and were used for imaging and treatment of glioma [149]. Iraci et al (2017) revealed the unexpected ability of stem cell exosomes to harbour and deliver functional enzymes (e.g. Asparaginase-like 1) extracellularly, thus behaving as fully independent small metabolic units with exciting therapeutic implications [150].
Cooper et al (2014) described the use of exosomes derived from murine bone marrow dendritic cells to block the aggregation of α-Syn, a pathological process implicated in PD progression. siRNA-loaded exosomes decorated with RVG (targeting ligand) effectively reduced the α-Syn aggregation in normal mice and transgenic mice expressing the human phosphorylation-mimic S129D α-Syn [151]. Dopamine-loaded exosomes derived from the blood of mice were used to deliver drugs across the BBB with lower systemic toxicity compared to i.v. administration of naked dopamine [152]. As an alternative approach, Haney et al (2015) circumvented the BBB, using intranasal delivery to successfully administer the catalase-loaded macrophage-derived exosomes to the brain of mice with a model of PD, resulting in significant neuroprotective effects [131]. Conversely, a potential role of exosomes in diagnosing neurodegenerative conditions was highlighted by Gui et al (2015) who developed a microRNA-profiling strategy for the early detection of PD. They used exosomes isolated from the CSF of PD and AD patients, reporting sixteen miRNAs upregulated and 11 miRNAs under regulated in PD [153].
Liu et al (2015) successfully deployed exosomes expressing RVG on the surface loaded with opioid receptor mu (MOR) siRNA into the brain for the treatment of morphine addiction [154]. Wu et al (2018) also used RVG decorated exosomes for brain drug delivery. They encapsulated siRNA targeting human huntingtin exon 1 (HuHtt) transcript. HuHtt-siRNA loaded RVG-exosomes were then administered intravenously to normal mice and BACHD and N171–82Q transgenic (Huntington's Disease-model) mice at 10 mg kg−1 every two days for 2 weeks. siRNA-loaded RVG exosomes significantly reduced HuHtt mRNA and protein levels up to 46% and 54%, respectively, in transgenic animals [155].
4.4. Liposomes for brain drug delivery
Liposomes are self-assembled NPs made up of phospholipid bilayer membrane. Phospholipids are heterogeneous molecules containing phosphate residues, polar head groups, and non-polar alkyl chains [156] that self-assemble (according to the fluid mosaic model) into biological membranes. Liposomes for brain drug delivery have been studied extensively in the last two decades.
Pulford et al (2010) formulated liposomes (178 ± 20 nm) containing cationic lipid octadecenolyoxy[ethyl-2-heptadecenyl-3 hydroxyethyl] imidazolinium chloride to deliver siRNA into the brain of mice following i.v. injection. The cationic liposome-siRNA-peptide (RVG-9r) penetrates the BBB, with the peptide moiety binding to nAChRs [157]. Bender et al (2016) used two liposomal systems for the delivery of prion protein siRNA to the brain of mice following i.v. injection. One of the liposome formulations was cationic liposomes containing 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), which formed a complex with siRNA and RVG peptide. The other liposomal system contained DOTAP or 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) to encapsulate the siRNA. Both systems decreased the prion protein expression of neurons in the CNS [158]. Grinberg et al (2005) reported novel cationic amphiphilic compounds synthesised from vernonia oil. The quaternary methyl ester derivative of methyl vernolate self-assembled into vesicles (in the presence of cholesterol 1:1) with the size of 50–200 nm in diameter [159]. Vesicles made from the quaternary vernonia oil derivative (triple-headed amphiphile) were found to be efficient in transfection of cDNA encoding for GFP into cultured COS-7 cells [159]. These vesicles were employed to deliver analgesic peptides (kyotorphin or leu-enkephalin) to the brain of male ICR mice following i.v. injection [160].
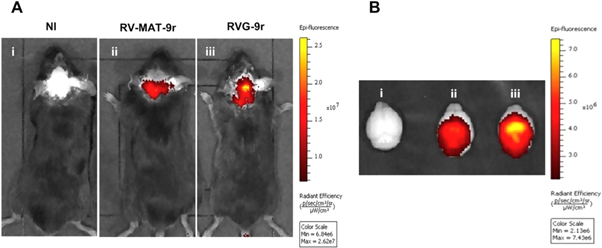
Moreover, Conceicao et al (2016) reported that the RVG-9r peptide decorated liposomes (also referred as stable nucleic acid lipid particles [SNALPs]) were able to cross the BBB and deliver siRNA, which can target mutant ataxin-3 in the brain of Machado-Joseph disease mouse models. These SNALPs offered high encapsulation of siRNA, optimum particle size and almost no toxicity. In vivo experiments showed the ability of SNALPs to accumulate in the brain and silence the mutant ataxin-3 upon i.v. injection as shown in figure 4 [161].
Figure 4. In vivo images showing the uptake of the RVG-9r decorated SNALPs in mice (C57 BL/6 ataxin-3 [Q69]-transgenic) after i.v. injection reproduced from [161] after permission (NI: non-injected animal, RV-MAT-9r: non-targeted liposomes, RVG-9r: targeted-liposomes). Reprinted from [161], Copyright (2016), with permission from Elsevier.
Download figure:
Standard image High-resolution image4.5. Dendrimers for brain drug delivery
Dendrimers are chemically synthesised polymeric particles with defined shapes (due to monodispersity). Dendrimers have been investigated for brain drug delivery. It has been reported, apolipoprotein A-I (ApoA-I) and NL4-peptide dual modified dendrimer NPs were efficient carriers for siRNA delivery to PC12 cells and efficiently penetrate through a bEnd.3 monolayer via LDLR [162]. KE et al (2009) used PAMAM–PEG–Angiopep/DNA-NPs to deliver plasmid DNA across the BBB. The PAMAM was fifth generation with 128 surface primary amino groups. In vitro BBB model showed clathrin and caveolae-mediated endocytosis (also partly through marcopinocytosis) of the nanocarriers containing Angiopep peptide [TFFYGGSRGKRNNFKTEEYC]. PAMAM–PEG–Angiopep dendrimers were loaded with pEGFP plasmid; and the NPs were administered intravenously to mice. Gene expression was observed in all four regions of the mouse brain for the PAMAM–PEG–Angiopep/DNA NPs, which was much higher than those for the PAMAM/DNA NPs [163]. In another study, low generation lysine dendrons (G0 and G1) conjugated with ApoE derived peptide (LRKLRKRLLR) were reported to cross the BBB efficiently with no cytotoxicity up to 400 μm [164]. It should be noted that PAMAM/siRNA complexes appear to show significant cell toxicity even at low concentrations such as 20 μg ml−1 [165]. As it would be expected, the cationic dendrimers show haemolytic activity. However, increasing the dendrimer generation decreases the haemolytic activity. For example, G2 dendrimers showed 100% haemolysis at 1 mg ml−1 concentration after 24 h incubation with RBCs, while G5 dendrimers showed no haemolysis (comparable to negative control) at the same concentration and incubation period [166]. Dynamic light scattering (DLS) studies showed that PAMAM/siRNA complexes had sizes in the range of 150–200 nm, while TEM results indicated a wider size distribution with majority in the range of 30–45 nm for G7 PAMAM/siRNA with N/P ration of 10 [167].
4.6. Carbon nanotubes
Carbon nanotubes (CNT) are cylindrical molecules that consist of rolled-up sheets of single-layer carbon atoms. Distinctive properties of CNT such as good electronic properties, excellent penetration into cell membrane, high loading capacity, pH-dependent unloading, greater surface area and ease of modification make them one of the suitable drug delivery system for the brain [168, 169]. CNT have been extensively investigated as a drug carrier to the brain in past few years. Functionalized CNT can potentially be used as a carrier for drugs that have poor permeability across the BBB and also can be used for diagnostic and for the treatment of brain disorders [170].
CNT can be synthesized electric arc discharge and laser ablation using vaporisation of graphite target [171] or by chemical vapour deposition [172]. CNT can be grouped into single wall carbon nanotubes (SWCNT) or multi wall carbon nanotubes (MWCNT) depending on the number of layers that constitute a CNT. CNT size ranges from 0.4nm to 100nm depending on the layers. CNT can be functionalized covalently or non-covalently [173].
Ren et al (2012) developed PEGylated oxidized multi-walled carbon nanotubes (O-MWNTs) modified with angiopep-2 (O-MWNTs-PEG-ANG) to treat brain glioma. They reported the high uptake and accumulation of CNT in the desired area with excellent loading capacity. Angiopep-2 specifically binds to LDLR and promotes the internalization. Doxorubicin loaded CNT were found to have better anti-glioma effects than naked doxorubicin [174]. In another study, ANG functionalized radiolabelled CNT were employed to deliver drug across the BBB. In vitro experiments suggested higher penetration of ANG-CNT than chemically functionalized CNT. Enhanced localization of ANG-CNT was reported upon in vivo injection and 2% of the injected dose was accumulated in the brain within the first hour post-injection [175, 176]. TAT (YGRKKRRQRRR) conjugated CNT were reported to have excellent BBB penetration and anticancer activity through increased ROS production [177].
4.7. Parameters affecting the BBB transport
4.7.1. Size, morphology and surface zeta potential
NPs in the range of 120–180 nm after crossing the BBB may be entrapped in the BL [178]. However, NPs with the size in the range of 16–24 nm are able to diffuse in the brain parenchyma [178]. These observations indicate that NPs should be less than 120 nm such as exosomes in order to diffuse in the brain parenchyma, otherwise they will remain trapped in the BL following crossing the BBB.
The morphology of NPs affects their bio-distribution and cellular uptake. NPs could be spherical, cubic, tubular or rod-like in shape [179, 180]. A majority of the particles reported for brain delivery are roughly spherical in shape. Zeta potential or surface charge of NPs is another factor that controls the diffusion across the BBB. It has been reported that a high (positive) zeta potential causes toxicity to the BBB [181, 182]. Rassu et al (2017) reported that a positive surface charge on NPs ensures their mucoadhesion [183]. On the other hand, NP formulations have been reported for brain delivery with zeta potentials between −1 and −45 mV [184–186]. Different shapes of NPs are shown in figure 5.
Figure 5. Different morphologies and shapes of NPs used for brain drug delivery.
Download figure:
Standard image High-resolution image4.7.2. Critical micelle concentration (CMC)
CMC is the minimum concentration of a compound at which it forms micelles. CMC plays a major role in the stability of micelles/NPs due to excessive dilution in the blood, upon i.v. injection. If the concentration in systemic circulation drops below the CMC, then it releases the payload in the blood stream before getting to its target.
CMC can be determined by using set concentrations of a pyrene probe with serial dilution of copolymer solution [187, 188]. Ruan et al (2018) used RAP12 peptide (a part of the receptor associated protein that binds to LRP1) and decorated PEG-poly(lactic acid) (PLA) micelles to deliver drug (paclitaxel) across the BBB [189]. Liu et al (2009) reported CG3R6TAT (CGGGRRRRRRYGRKKRRQRRR), a self-assembled cationic antimicrobial peptide able to cross the BBB. They measured the CMC by using the pyrene as a probe and found to be 31.6 mg l−1 (10.1 μm) in deionized water [187]. Micelles and PMNPs both can target the brain and cross the BBB. Efficacy and efficiency of crossing the BBB are dependent on targeting via the surface of the nanocarriers.
4.7.3. Protein corona
NPs, upon contact with biological fluids, are surrounded by a protein layer that is called protein corona [190–193]. The first layer of protein corona is bound tightly on the surface (primary contact with NPs), which is referred as 'hard' corona. Usually, another layer is loosely bound on the first layer, which is referred as 'soft' corona; and that consists of serum proteins, mainly comprising albumin and its derivatives [194–196]. This surface adsorption of protein can alter the physiological response [195]. The adsorption of proteins on NPs mostly has undesirable effects such as prompt clearance from blood stream, compromised targeting capacity [197] and toxicity [198, 199]. Proteins bound to a NP surface may rearrange their structure and shape according to NP surface and environment, this is known as 'conformational change'. Conformational change accompanied with the modification of secondary or tertiary protein structure. Proteins are supposed to interact with other biomolecules to initiate biological responses, hence a small modification in protein structure has huge impact on their pharmacological activities [200].
Several factors dictate the nature of adsorbed proteins. Particle size plays an important role in protein adsorption. As NPs are bigger than proteins, NPs make proteins to adapt the NPs' surface. Smaller NPs has less interaction with proteins [201]. Surface charge of the NPs affects the secondary structure of proteins. Huhn et al (2014) reported that gold NPs with different surface charge (positive [+9.7 ± 8.9 mV] or negative [−39.8 ± 10.0 mV]), but similar sizes adsorbed comparable amounts of HSA. Whereas, positively charged NPs showed higher cellular uptake than negatively charged NPs. This change in the activity can be due to conformation changes in protein structure due to surface charge [202]. Fleischer and Payne (2014) observed that similar NPs with identical protein corona compositions bind to different cellular receptors, suggesting that a difference in the structure of the adsorbed protein may be responsible for the differences in cellular binding of the protein–NP complexes. These authors also found that cationic polystyrene NPs showed improved cellular binding to monkey kidney epithelial cells compared to negatively charged NPs in the presence of fetal bovine serum (FBS). It should be noted that in both cases, the NPs formed protein–NP complexes immediately following exposure to FBS [199].
Media composition affects the protein corona. Silica NPs in the presence of serum proteins showed less uptake compared to serum free media [203]. Gold NPs incubated with Dulbecco's Modified Eagle's Medium (DMEM) media for 48 h showed higher protein adsorption than Roswell Park Memorial Institute media (RPMI), but same amount after 1 h incubation [204]. Protein concentration in media affects the protein corona. Silica NPs incubated with 3%, 20% and 80% plasma exhibited different protein patterns. Changes in primary protein band was observed with increasing plasma concentration. Lower amounts of proteins were measured on silica NPs compared to sulfonated polystyrene (PSOSO3) NPs with increased plasma concentrations [205]. Exposure time affects the protein corona. Protein corona forms immediately as soon as the NPs come into contact with human plasma. Tenzer et al (2013) reported complex protein corona (formed of 300 proteins) just after 30 s [206]. In addition, temperature plays an important role in protein corona formation. Cu-NPs showed higher protein adsorption when incubated by increasing temperature from 15 °C, 27 °C, and 37 °C to 42 °C [207].
A decline (from 76% to 26%) in the cellular uptake of cRGD decorated NPs was reported by Su et al (2018) in protein bound NPs compared to non-protein bound NPs. They found that even the targeting ability was not affected but cellular uptake was compromised [208]. Tf decorated NPs were reported to lose their targeting ability in the biological medium. Proteins in the medium are reported to shield the NPs and hence results in disappearance of targeting ability. However NPs can enter the cells but the targeting capacity is lost [209]. Aptamer functionalized AuNPs lost the targeting ability due to protein corona blocking after serum exposure. Immune related proteins were found on the surface of aptamer that can induce immune reaction and clearance eventually [210].
4.7.4. Stability of NPs
The stability of NPs can be categorised into two, shelf stability and serum stability. NPs should be stable enough to retain their therapeutic effects for a specific time when stored or administered to the body. Oller‐Salvia et al (2016) tested the serum stability of peptide NPs in human serum. They found that switching from linear to monocyclic analogue didn't affect the permeability but showed 30-fold enhanced stability than linear peptide analogue [32]. In addition, upon switching disulphide to a lactam bridge in Miniap-4 shuttle peptide, they found 50% higher permeability with better resistance to proteases [32]. El-Marakby et al (2017) assessed the serum stability of chitosan NPs in rat serum. They reported a sharp reduction in particle size (up to 62% of original size) prepared from the native chitosan, whereas modified chitosan showed slight increase in the size from 87.39 ± 1.56 nm to 122.33 ± 1.95 nm after 2 h incubation with the serum. After 24 h incubation no significant changes were noticed [211]. Oliveira et al (2017), tested uncoated and poly allylamine hydrochloride (PAH)-coated PLGA-NPs in biological environments: BSA solution, mouse and human plasma. Both formulations were reported stable in BSA and mouse plasma on incubation, but surprisingly not stable in human plasma (formed aggregates greater than 1 μm). They also studied protein corona in all solutions. In mouse plasma uncoated NPs showed protein concentration of 4.1 ± 2.6 μg ml−1, which was much greater than incubating these NPs in BSA solution. Surprisingly, in human plasma it was 2.5-fold higher (10.4 ± 3.0 μg ml−1) than mouse plasma. Similarly PAH-coated PLGA-NPs showed higher protein adsorption after incubation with human plasma than BSA solution and mouse plasma [212].
Uncoated chitosan NPs were to increase in size by storage at 25 °C for 3 m in 10% glucose solution [213]. This alteration in size results in modified physicochemical, pharmacodynamic and pharmacokinetic properties of the PMNPs. Lyophilisation with cryoprotectants is reported to enhance the stability and to stop contents leaking from the NPs [214–216]. Cryoprotectants such as glucose, sucrose, mannitol and trehalose are most commonly used because of their low toxicity [214, 217].
5. Conclusion
Peptide based drug delivery systems have been studied extensively in the last two decades to overcome the BBB. Peptide based formulations come with its advantages (less toxicity, low alteration in the BBB integrity and specific targeting) and disadvantages (serum stability). Shuttle peptides, exosomes, liposomes, NPs and dendrimers decorated with peptides have shown much improved permeability across the BBB. Targeting and crossing the BBB is an ever expanding and challenging yet promising field. To design and develop a CNS drug that can target the BBB requires a detailed understanding of both the BBB at a molecular level and drug properties (pharmacokinetics and pharmacodynamics). Despite many advances in drug delivery systems, there is still an essential need for research aimed at attaining improved delivery systems with fewer limitations. Peptide based delivery systems along with pro and cons need further optimization and high specificity in brain targeting.
6. Future direction
Despite extensive research in the use of peptides in nanoparticles for drug delivery to the brain, yet there is no clinical trial of them. Then, the next steps would be developing scalable and reproducible brain targeting nanoparticle delivery system using peptides as targeting ligands. Peptide based NPs provide the opportunity of formulating enzyme responsive or biodegradable delivery systems, which may offer less toxicity and immunogenicity, and improved efficacy. Peptide based nanoparticles should be able to deliver/encapsulate suitable amounts of drug to the brain; and these should protect the drug from enzymes in the blood.
Conflict of interest
The authors declare no conflict of interest.