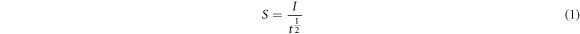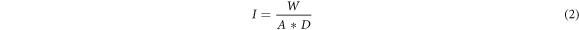Abstract
The reduction of the carbon footprint and the preservation of natural raw materials in the construction industry is crucial for the improvement of the environment. Geopolymer is a novel approach that has recently come to the limelight for sustainable and greener construction development due to its lower greenhouse gas emissions. This study focuses on utilizing industrial waste products like Ground granulated blast furnace slag (GGBS), Sugarcane bagasse ash (SBA), and Micronized biomass silica (MBS) for the production of geopolymer concrete. The geopolymer concrete's performance is assessed by testing various combinations of materials at different percentages to measure compressive and flexural strength, water absorption, water sorptivity, Rapid Chloride ion Penetration test (RCPT), carbonation, resistance to acid attack, and characteristics studies. The results revealed that the combination of 10% of SBA and 8% of MBS on GGBS-based geopolymer improves strength and durability compared to the control mix. The role of MBS has been highlighted, which serves its purpose by densifying the matrix by extending C-S-H gel formation and filling voids caused by incorporating SBA, which is validated through characterization studies—feasibility of agro waste in the geopolymer production.
1. Introduction
The population explosion surged the demand for infrastructure development, enhancing the demand for building materials globally and hence the requirement of Ordinary Portland Cement (OPC). The need for cement will increase by around 8% in the coming fiscal year [1]. Cement production is a highly energy-consuming process with colossal greenhouse gas emissions. The CO2 emission has grown by 321 metric tons or 0.9% compared to previous years (International Energy Agency - IEA,2022) [2]. The prime contribution to the CO2 release is the combustion process. The construction industry has been growing rapidly, with a growth rate of 5.65% from the fiscal year 2015 to 2020, compared to 2.95% from the financial year 2010 to 2015 [3]. This growth has led to an increased demand for cement for building structures. However, as Portland cement is not environmentally sustainable, it is important to find alternative materials that can perform as well as concrete. One solution is to partially replace cement with supplementary cementitious materials to help conserve natural resources for future generations. These materials can enhance the performance of concrete in its fresh and hardened states, leading to increased durability and strength when used as partial replacements for cement.
Researchers have developed Geopolymer Concrete (GPC) to overcome the environmental burdens caused by the concrete and cement industry. GPC use industrial waste like fly ash, slag, and silica fume reduces CO2 emissions and benefits the environment [4, 5]. GPC is casted and cured at different temperature conditions and with various chemical combinations. According to the chemical combination and curing methods, the strength and durability performance change. Agricultural wastes, which are difficult to dispose of and will pose a severe threat to the environment. So, using these materials in concrete will increase concrete performance and create a sustainable environment [6].
Sugarcane bagasse ash is a grey color powder obtained by burning the sugarcane biomass (straw and bagasse). A total of 2.1 billion metric tons of sugarcane are produced annually worldwide from commercial cultivation in more than 100 countries. Furthermore, estimates for the amount of SBA produced globally each year in 2021 range from 21 to 31.5 million metrics [6], The SBA, a byproduct of sugar mills, is commonly used as fertilizer for sugarcane crops. However, this practice raises environmental concerns as the material can lead to river siltation, groundwater contamination, soil sealing, and may be ineffective due to the lack of necessary minerals [7]. With more than 45 million sugarcane growers and approximately 65% of India's rural population relying on this agro-based business, the disposal of SBA poses a significant environmental challenge for the country, which stands as the second-largest producer of sugarcane [8].
This waste generated from sugar industries can be effectively made use in developing a binding material in construction field. Granulated blast furnace slag precursor has already been partially replaced by the SBA in studies conducted by [9]. Singh (2021) conducted research on substituting part of metakaolin with SBA [10]. Tchakouté et al (2017) investigated the utilization of sodium waterglass from SBA as a catalyst for creating metakaolin-based geopolymer [11]. Sousa et al (2022) studied the application of sodium waterglass from SBA as a catalyst for creating metakaolin-based geopolymer [12]. Sugarcane bagasse ash and marble waste were used to develop an alkali activated binder; specimens cured at ambient temperatures with combined bagasse ash exhibited higher strength compared with heat cured specimens [13]. The ideal percentage for achieving the greatest mechanical and durability results for Metakaolin geopolymer concrete was found to be 10% replacement of sugarcane bagasse ash [10]. Based on earlier research, it has been noted that substituting 5% to 15% of cement with SBA proves to be an efficient supply chain management solution and could help address waste disposal issues [14]. Studies shown that the SBA blended concrete showed improved strength and durability properties due to high silica content, high degree of reactivity, and binding properties [15].
Micronized Biomass silica is a very fine powder obtained by burning and grinding the rice husk ash in a rotary furnace. Rice husk is a byproduct of processing paddy, produced in large amounts worldwide. In India, rice husk is typically discarded or used for cattle feed, landfilling, and partition board manufacturing. Obtaining rice husk ash (RHA) involves controlled burning of agricultural waste [9]. India produces the second-largest amount of rice in the world, resulting in approximately 200 kg of RHA for every ton of rice, according to a market study [16], and approximately 130 MT of RHA produced around the world per year [17]. The magnitude of these industrial by-products has made their disposal a major problem [18]. This problem can be effectively addressed by incorporating these by-products in the construction field. The RHA basically consist of 85% - 90% of amorphous silica [18]. The RHA affects the durability and mechanical properties of concrete, and also observed that incorporating ultrafine rice husk ash into the cement matrix reduced water permeability while improving workability and compressive strength [18]. The Geopolymer pozzolanic reaction properties of red mud and RHA mixed in various ratios were studied and it was observed that long-term curing produced notable increases in compressive strength and Young's modulus at the expense of diminished ductility [19]. The combination of RHA and fly ash geopolymer achieved a compressive strength of 75 MPa [17]. The study's primary conclusions made it abundantly evident that MBS may be used as a binder while making GPC [20]. The results of the test indicated that an optimal blend of MBS is 20%, leading to a 37% reduction in sorptivity compared to the control specimens. The concrete samples exhibited significant resistance to chloride permeability at a 10% concentration of MBS, the level of chloride permeability was low [20].
MBS is an emerging SCM and only a limited amount of research work has been carried out till now. From past studies, it has been observed that blending of MBS enhanced the strength and durability properties of concrete in the binary form. This is a result of its extremely small particle size and substantial levels of non-crystalline silica [15]. MBS serves as a filler substance in concrete, filling the empty spaces and promoting the formation of C-A-S-H, contributing to enhanced strength and decreased porosity [16].
Geopolymer concrete exhibits potential as a substitute for conventional OPC concrete, offering the possibility of decreasing the environmental footprint of the concrete industry [21, 22]. The conversion of industrial and agricultural waste into a valuable building material for infrastructure development is under exploration. Current research is focusing on optimizing various factors such as curing methods, mixture designs, and material selections to improve the properties of these sustainable options. Two areas of particular focus are ambient-cured geopolymer concrete blends and agricultural waste materials as SCMs. By further exploring and adopting these technologies, it is possible to work towards making concrete more sustainable on an industrial scale. This will help reduce emissions and energy consumption during cement production while benefiting waste disposal. The growth of the construction industry provides both the necessity and opportunity to implement greener advancements in this critical building material. Additional research and collaboration within the industry can facilitate this transition towards a more environmentally responsible future for concrete.
The objective of this study is to address a gap in the current body of research by investigating the engineering characteristics of ternary blended geopolymers created from GGBS, SBA, and MBS. Previous studies on GPC have primarily concentrated on using fly ash as the primary binding material in geopolymerization. However, this study utilizes iron and steel industry residue (GGBS) and agro-industry waste (SBA and MBS), which is produced from sugar cane and rice husk to develop and optimize a ternary blended geopolymer mortar with NaOH and Na2SiO3 as an alkaline activating agent cured under ambient condition. The study examined the strength, durability, and microstructural properties of the ternary blended geopolymer concrete to assess its viability as a sustainable alternative. Chemical, physical, mineralogical, and morphological study of each material has done. The reference mix with 100% GGBS is compared with the binary blended mix with a replacement of 5 to 15% at 5% offset with SBA. The optimum binary mix is replaced with MBS at 4,8, and 12% to study strength, durability and characterization studies to obtain the best ternary mix.
2. Materials and methodology
The GGBS residue from the iron industry, provided by Astra Chemicals, serves as the primary material in the current study. Additionally, it is utilized for creating the reference mixture.
The geopolymer is created using Sugarcane Bagasse Ash (SBA) and Micro ionized biomass silica (MBS) in addition to a solution of alkali activators made from a 4 M sodium hydroxide solution and sodium silicate solution. A solution of 4 M sodium hydroxide was prepared a day before casting using sodium hydroxide flakes. The liquid/binder (l/b) ratio was maintained at 0.45, and the ratio of SS/SH was set at 2.5 based on the initial investigation. SBA is an agro waste from the sugar cane industry which is rich in silica content collected from N. Mathi sugarcane industry, Salem, Tamil Nadu. MBS formed as a result of the combustion of the crushed rice husk ash is rich in amorphous silica. MBS of particle size less than 45 μm is obtained from Astra Chemicals, a chemical manufacturing company in Chennai. The chemical characteristics of the precursor were determined using the x-ray fluorescent (XRF) test on the Bruker model S8 Tiger, which comes equipped with a goniometer 4 kW Rh x-ray tube and 0.23°, 0.46°, 1°, and 2° collimators. Analysis of the elements was carried out using the SPECTRA Plus software. The chemical composition of GGBS, SBA, and MBS is detailed in table 1.
Table 1. Chemical composition of precursors.
| Oxide comp (%) | GGBS | SBA | MBS |
|---|---|---|---|
| CaO | 43.9 | 4.09 | 1.23 |
| SiO2 | 30.2 | 78.28 | 87.79 |
| Al2O3 | 13.9 | 5.78 | 0.43 |
| Fe2O3 | 4.5 | 4.21 | 0.54 |
| MgO | 7.2 | 2.69 | 0.97 |
The precursors, Fine aggregate (FA), and coarse aggregate (CA) utilized in this investigation comply with the regulations in standard IS4031 part11. The water absorption of CA and FA adheres to the specifications laid out in IS383. The physical characteristics of the materials used in the current research are outlined in table 2.
Table 2. Physical properties of materials used.
| GGBS | SBA | MBS | FA | CA | |
|---|---|---|---|---|---|
| Specific gravity | 2.8 | 2.1 | 2.2 | 2.69 | 2.73 |
| Water absorption (%) | — | — | — | 1.26 | 0.42 |
3. XRD of precursors
The analysis using x-ray Diffraction (XRD) was conducted with a D8 advanced Bruker instrument, utilizing Cu-Ka radiation at 30 kV and 40 mA. Subsequently, the X'pert high score software was used to analyze the phases. Analysis of GGBS using XRD offers valuable information about its mineral composition and crystalline structure.
The XRD for GGBS material is as shown in figure 1(a). In GGBS XRD results, diffraction patterns are obtained when x-rays interact with the crystal lattice of the slag's components. These patterns help identify the minerals present in GGBS, which are typically a combination of glassy and crystalline phases. Common phrases found in GGBS include amorphous materials like vitreous silica which contribute to the material's cementitious properties. This amorphous hump is exhibited by GGBS between the angle 20.27 to 37.03° 2θ represented by AH as observed by [23]. The amorphous hump at 20–30° in GGBS shows its high reactivity.
Figure 1. XRD of precursors.
Download figure:
Standard image High-resolution imageIn XRD analysis of SBA, diffraction patterns are obtained when x-rays interact with the crystal lattice of the ash's components. These patterns help identify the minerals and compounds present in the ash. SBAs include quartz (SiO2) at 20.11, 26.82 35.54, 50.11, and 68.23° 2θ, the mineral cristobalite (SiO2) also exhibited by the SBA at 9.17 and 27.87 2θ as shown in figure 1(b) as observed by [24]. The amorphous (non-crystalline) portion of silica in SBA can be identified which is residing at 10.59 to 30.21° 2θ in the form of a hump, this is well documented in [25]. Crystalline phases can indicate the potential reactivity of the ash when used in applications such as cement or concrete production, soil improvement, or waste remediation. In the context of micronized biomass silica, XRD results would reveal information about the arrangement of atoms within the material's crystal lattice, providing insights into its composition, crystallinity, and potentially its functional properties. The XRD of MBS showed the presence of Cristobalite and Quartz minerals. The cristobalite marked its presence at an angle of 20.50, 54.64° 2θ degree. The quartz which is a very commonly occurring mineral present at 26.48, 36.37, 49.96 59.82, and 67.67° 2θ degrees as shown in figure 1(c).
4. Morphological properties of the precursors
Morphological research such as SEM and EDS was carried out on a CARL ZEISS make EVO 18 Research. This instrument is equipped has an acceleration voltage range of 0.2 to 30 kV, Probe Current 0.5 pA to 5 μA, Pressure Range 10–400 Pa, and Field of View 6 mm at the Analytical Working Distance (AWD) under high vacuum conditions, it offers resolution of 4 nm @ 30 kV VP mode. Additionally, it incorporates the use of energy dispersive spectroscopy (EDS) for elemental analysis. Before analysis, the samples were prepared by crushing sample chips from the core and storing them in acetone for 24 h before oven-drying them for the same amount of time at 105 °C.
4.1. FESEM of precursors
The morphology of GGBS can provide information about its physical structure when seen under a scanning electron microscope (SEM) as shown in figure 2(a). This information is essential for comprehending the material's characteristics and possible uses. Under a SEM, GGBS usually shows an amorphous or glassy structure. This structure is the result of the slag components' not crystallizing due to the quick cooling method used during manufacture. The form and distribution of GGBS particle sizes can be seen in SEM pictures. The irregular morphologies of the particles might vary from angular to more irregular shapes with sharp edges, contingent on production-related and post-processing parameters including the rate of cooling.
Figure 2. SEM images of precursors (a) GGBS (b) SBA (c) MBS.
Download figure:
Standard image High-resolution imageThe morphology of SBA particles can differ, ranging from asymmetrical to elongated shapes. The size and form of SBA particles usually fluctuate, as shown in figure 2(b). Pores or cavities inside SBA particles can be seen using SEM imaging. Properties like permeability and water absorption capacity can be impacted by variations in the size and distribution of these pores. Factors like the degree of ash treatment and the combustion process have an impact on the porosity of SBA particles. For sugarcane bagasse ash to be optimally utilized as a supplemental cementitious material in concrete and other building applications, its shape must be comprehended through SEM examination.
The SEM images of MBS shows that the particles are typically in the micron range, with diameters varying from a few micrometres to tens of micrometres as shown in figure 2(c). Different shapes, such as spherical, angular, or irregular forms, may be displayed by the particles. The kind of biomass source, the parameters of the micronization process, and any further treatments or adjustments all affect the distribution of particle size and form. The pore structure and porosity of micronized biomass silica particles can be understood using SEM investigation.
5. Mix design and experimental studies
5.1. Mix design
A total of 7 mixes are considered for the study and each mix is represented as M1 to M7. The mix details are given in table 3 and the reference mix contains 100% GGBS. The GGBS is initially replaced by SBA from 5 to 10% at 5% offset. The optimum mix is found as 10% GGBS replacement by SBA and the further replacement with MBS is done at GGBS-BA mix with 10% replacement. The MBS is added from 4 to 12% at 4% offset for further studies.
Table 3. Mix designation of the mixes.
| Mix ID | Mixture type | GGBS (Kg/m3) | Sodium hydroxide solution (lit/m3) | Sodium Silicate Solution(lit/m3) | FA (Kg/m3) | CA (Kg/m3) | SBA (Kg/m3) | MBS (Kg/m3) |
|---|---|---|---|---|---|---|---|---|
| M1 | SBA0&MBS0 | 413.33 | 74.4 | 111.6 | 793 | 1054 | — | — |
| M2 | SBA5&MBS0 | 392.66 | 74.4 | 111.6 | 789 | 1049 | 20.67 | — |
| M3 | SBA10&MBS0 | 372 | 74.4 | 111.6 | 785 | 1044 | 41.33 | — |
| M4 | SBA15&MBS0 | 351.33 | 74.4 | 111.6 | 782 | 1039 | 62 | — |
| M5 | SBA10&MBS4 | 359.59 | 74.4 | 111.6 | 784 | 1042 | 20.67 | 33.06 |
| M6 | SBA10&MBS8 | 338.93 | 74.4 | 111.6 | 780 | 1037 | 41.33 | 33.06 |
| M7 | SBA10&MBS12 | 318.26 | 74.4 | 111.6 | 777 | 1032 | 62 | 33.06 |
5.2. Experimental methods
The compressive strength and flexural strength of specimens were examined to analyze the durability of the geopolymer concrete cubes after 7 and 28 days of curing, following the IS: 516–1959 standard [26]. The geopolymer concrete was cured using the ambient curing method. Compressive strength tests were conducted on concrete cubes measuring 100 mm × 100 mm × 100 mm using a digital compression testing machine with a 2000 kN capacity at a consistent load rate of 25 N mm−2/min. Additionally, the flexural strength of 500 mm × 100 mm × 100 mm prisms was also determined.
A research was conducted to measure the water absorption and sorptivity of geopolymer concrete using 100 mm × 100 mm × 100 mm cubes, following the guidelines outlined in ASTM C-64213 [27]. The geopolymer concrete specimens were placed immersed in normal water up to 3 mm depth. The gain in mass is evaluated to an accuracy of 0.01 g in at stipulated intervals. The sorptivity index was calculated as in equation (1). The capillary rise versus square root of time graph has been drawn and the sorptivity index was measured by normalizing the slope. From the regression equation, primary and secondary sorptivity were derived.
Where, I - The amount of water retained per unit area of the inflow surface as shown in equation (2)
S - Sorptivity (mm/min1/2);
t - time elapsed (min).
W - change in weight
A - surface area of specimen through which water penetrated (mm2)
D - Density of Water
The carbonation of the samples was carried in accordance with RILEM TC056-CPC-18 refers to the Technical Committee 056 of RILEM (International Union of Laboratories and Experts in Construction Materials, Systems and Structures) which deals with Carbonation of Concrete. Allowing the specimens to cure under controlled conditions until they reach the desired age 28 days. Exposing the specimens to a controlled environment with a known concentration of CO2 (4%) with 70% relative humidity for a period of 22 days of exposure. This can be done in a carbonation chamber where the CO2 concentration, temperature, and humidity are regulated according to the requirement.
Specimens in the shape of cubes, with dimensions of 150 mm × 150 mm × 150 mm, were placed in sulfuric acid solutions with a pH of 0.8 in order to investigate the durability of materials against acid corrosion. The acid solutions and their concentrations were selected based on the practical application of concrete in industries such as sewage pipes, mining, and food processing. The pH value was used to verify that fresh solutions replaced the acid. To confirm the deterioration of the specimens, optical microscopic images were utilized, and the compressive strength was assessed after 28 and 90 days of exposure.
6. Results and discussions
6.1. Compressive strength
The geopolymer concrete specimens achieved a compressive strength of 38.23 after 7 days, and 43.21 after 28 days, as illustrated in figure 3. The strength gain rate was approximately 88.47%, achieved within 7 days, which may be attributed to the intense geopolymer reaction during the early stages [28]. The control mix only with GGBS shown a 28 days strength of 43.21 MPa. This my be attributed to tiny and angular structure of GGBS particles, rich calcium content and the rapid reaction it also (GGBS) lessen the need for an elevated curing temperature [29]. The primary outcome of the reaction is the formation of C-A-S-H gel, a mineral that contributes to strength, as evidenced by the SEM images.
Figure 3. Compressive strength of geopolymer concrete.
Download figure:
Standard image High-resolution imageThe mix with SBA of more than 10% showed a reduced strength this may be due to the reduction in the GGBS content and increase SBA which exhibits low reactivity [30]. It can be attributed to the fact that the alkaline-activated solution did not dissolve the SBA, thereby preventing it from contributing to the polymerization process. Even though there was unreacted SBA remaining in the geopolymer matrix, the unreacted SBA particles did not have a significant impact on strengthening the material [31].
The geopolymer concrete with 10% SBA and 8% MBS content exhibited better compressive strength than the reference mix M1. Maximum compressive strength is observed for the mix with 10% SBA and 8% MBS and is 45.98 MPa, which is around 6.41% more compared to the reference mix. This may be attributed to the compact and dense microstructure developed due to the incorporation of the MBS content with SBA [20]. Similar to SBA an addition of MBS beyond 8% significantly reduced the compressive strength. The fineness of SBA and MBS tends to absorb the free water available in the mix. As the percentage of SBA and MBS increases the water-to-binder ratio decreases and it affects the compressive strength.
It has been discovered that when unreacted silica accumulates in SBCA, it results in SiO2. Additionally, the levels of Al2O3 increase, which has an impact on the compressive strength. Nevertheless, the MBS demonstrated greater strength than anticipated, reaching 63.48 MPa with 10% replacement and 45.98 MPa for M6. This increased strength of MBS is attributed to the higher presence of calcium silicate hydrate gel and primary alumina silicate gel. As the volume of MBS increases, the workability of the concrete decreases. The desired consistency of the concrete can be achieved by adding an extra alkali-activated solution. The capacity of tiny shapeless MBS particles to absorb also requires a more alkaline solution [20].
6.2. Flexural strength
The data shown in figure 4 illustrates the bending strength of the standard concrete and the geopolymer concrete. When the SBA content exceeds 10%, flexural strength decreases. After 28 days of curing, the mix with 10% SBA (M3) exhibited the highest flexural strength, 11.32% lower than the unblended mix. The increase in MBS content at 8% enhanced flexural strength. The reduction in strength diminished beyond 8% blending MBS. When the percentage of SBA increased beyond 10% and MBS more than 8% the strength reduced due to the weaker bond strength. Maximum flexural strength is obtained for the geopolymer with the ternary blend of 10% SBA and 8% MBS (M6) and has a 7.54% increase in strength compared to the reference mix (M1) for 28 days of curing. The presence of an additional calcium silicate hydrate phase led to a significant rise in flexural strength, which is influenced by the degree of aluminosilicate polymerization. The molar concentration, type of alkali activation solution (sodium hydroxide and silicate), crystallinity of the materials, and Si/Al ratio all affect the degree of aluminosilicate polymerization [32]. Increasing the MBS content even more led to a decrease in flexural strength. The flexural strength was reduced when MBS was added beyond its limit, as shown in a previous study [33].
Figure 4. Flexural strength of geopolymer concrete.
Download figure:
Standard image High-resolution image7. Durability tests
7.1. Water absorption
The figure 5 shows the water absorption results. The control mix M1 generate more heat attributed to the GGBS induced polymer reaction which results in the cracks as shown in SEM and EDS images which results int more water absorption [34]. The SBA nullify the thermal effect of GGBS as a result less heat paved the way for dense matrix [35]. The water absorption has reduced as the percentage of SBA increased to 10%. Compared to the reference mix M1 the water absorption has reduced to 4.62%. Similarly, the increase in MBS up to 8% tends to decrease in water absorption compared to reference mix since the pores are occupied by the C-A-S-H gel [36]. However, increasing the percentage of SBA beyond 12% resulted in a non-homogenous structure due to the occupation of more spherical particles in the pores and resulted in increased water absorption. The lowest water absorption rate is 25.49% and is observed for the geopolymer concrete with 10% SBA and 8% MBS which supports the compressive strength values. This can be due to the fact that fine MBS particles obstruct the pores due to the micro filler effect, resulting in solid concrete structures with less ability for liquid penetration .The increase in MBS content beyond 8% range however increased the water absorption which is well supported by the reduction in strength this can be attributed to the hindrance action shown by the MBS on the polymeric aluminosilicate gel which affects the compact and denseness of the geopolymer matrix [20].
Figure 5. Water absorption of geopolymer concrete.
Download figure:
Standard image High-resolution image7.2. Water sorptivity
Figure 6 shows the Initial sorptivity versus mix designation. In terms of absorption, micro/nano silica performed marginally better than SBA [37]. The sorptivity value was reduced by around 3.07, 9.23, 3.58% less compared to control mix when SBA was introduced in the mix at 5, 10 and 5% respectively. M3 mix with 10% of SBA shown better results in terms of capillary absorption this may be attributed to blocking of pores by the filler effect of ground SBA [38]. Increase in the SBA beyond 10% resulted in the increase of pores and adverse effect on capillary absorption. The MBS incorporation into the mix along with SBA produced positive effect on sorptivity by reduced water absorption for M6 mix which consist of SBA (10%) and MBS (8%) due to the fine particle occupied in the pores. The incorporation of only SBA may lead to the more pores but when it is combined with MBS it leads to the denser matrix reducing the pores. The strong bridging and filling effects of micro/nanomaterials may cause a decrease in permeable gel pores when the curing time and proportion of micro/nanoparticles are increased [37]. The sorptivity reduces to 27.03% compared to conventional concrete for an optimum mix M6 as shown in figure 6. The incorporation of excess of MBS into the matrix creates an effect on packed aluminosilicate polymer gel's ability to form was likely impeded by the high MBS content, which had a negative impact on the GPC's structural suitability. The capillary suction increased as a consequence [20].
Figure 6. Sorptivity of geopolymer concrete.
Download figure:
Standard image High-resolution image7.3. Rapid chloride ion penetration test (RCPT)
Table 4 presents the data from the RCPT test for the GPC mixes on the 28th day. Concrete's pore structure and connectivity are essential in determining how aggressive ions can enter the material if exposed to harsh conditions, such as in a sea environment. Concrete offers increased resistance to water absorption when its sorptivity is reduced. A high sorptivity coefficient indicates the pore network has minimal tortuosity or a well-connected porous structure. Electric charges transmitted through specimens with varying compositions - including 100% GGBS, 5, 10, and 15% of SBA were measured at 1762, 1989, 2339, and 2787 Coulombs, respectively. A decrease in the charge received was observed for the M6 mix, which contained 10% SBA and 8% MBS, registering 1713 Coulombs. The charge transmitted through the control specimen was similar to that of the M6 mix. Here is the reworded text: According to ASTM C 1202, the electric charge passing through the specimen is utilized to assess the low chloride penetrability of sample M6. A moderate level of chloride permeability was observed for SBA replacement at 10% and 15%. The pore structure plays a crucial role in determining the permeability of concrete. The impact of MBS particles can be characterized as a decrease in the penetration of chloride ions when MBS is present in optimal proportions. The arrangement of pores is a crucial factor in determining the permeability of concrete. The impact of MBS particles can be characterized as a decrease in the penetration of chloride ions when MBS is used in the right amounts. Because it is porous and has high density, concrete has low permeability to liquids, resulting in MBS-filled concrete absorbing less water. However, if an excessive quantity of MBS rich in silica is introduced, it disrupts the SiO2 to Al2O3 ratio, impacting the formation of the geopolymer and causing increased ion transfer in the concrete. Excess silica-rich MBS addition may lead to an improper SiO2 to Al2O3 ratio, impacting geopolymer formation and increasing ion transfer in the GPC. The MBS pozzolanic reaction can enhance chloride resistance by improving pore refinement and filler effects [20].
Table 4. RCPT value of different geopolymer mix.
| Mix. ID | M1 | M2 | M3 | M4 | M5 | M6 | M7 |
|---|---|---|---|---|---|---|---|
| Average charge passed in (Coulombs) | 1762 | 1989 | 2339 | 2787 | 2565 | 1713 | 2919 |
| Chloride ion penetrability | Low | Low | Medium | Medium | Medium | Low | Medium |
The enhanced ability to withstand chloride is linked to the enhancement of the pores and the impact of the MBS pozzolanic reaction. The combined influence of fly ash and bagasse ash with MBS boosts the compaction of solid materials by occupying the small pores in geopolymer concrete [30].
7.4. Carbonation
The control mix M1 with only GGBS exhibited more coloured area which is the noncarbonated zone this may be attributed to the fact that calcium rich precursor had improved the pore structure this is in line with the result observed by [39]. The mix M4 with 15% SBA showed increase in the carbonation depth attributed to the fact the blending of SBA will increase the porosity which will favours the condition for carbonation as shown in figure 7. The physical alterations in the mortar caused by the addition of SBA content included a steady increase in the number of pores and voids in the matrix along with unreacted particles, which raised porosity and lowered compressive strength similar results were observed.
Figure 7. Carbonation results for geopolymer different mixes.
Download figure:
Standard image High-resolution imageThe incorporation of MBS along with SBA in the mix M6 showed the results in which the coloured area is more compared to other mix as discussed already the colored area is non-carbonated region. This can be due to the fact that the optimal addition of the MBS will reduce the voids and increase the compactness of the structure there by reducing the carbonation. Increase in both SBA and MBS beyond 15 and 8% (M7) respectively into the GGBS mix increased the carbonation due to the increase in the voids which is supported by reduced the compressive strength.
The carbonation usually depends on the factors such as pH, porosity and humidity [40]. The conventional concrete forms a dense CaCO3 layer as a result of the carbonation reaction, which causes CO2 infiltration to the concrete surface to decrease with the length of the exposed period. Here, the present study utilised GGBS, SBA, and MBS, which is being activated by the alkaline solution makes up the majority of the ingredients in most of the mixes varieties of concrete, and sodium carbonate (Na2CO3) are the main by-products of the carbonation reaction similar results were observed by [41].
7.5. Resistance to acid attack
7.5.1. Mass loss percentage
When geopolymer concrete is exposed to an acid erosion medium for an extended period, the mortar will separate from the matrix, exposing the aggregates, which can result in concrete mass loss and structural damage. Figure 8 depicts the mass loss rate of all GPC when exposed to a sulfuric acid solution for 28 and 90 days. As illustrated in the figure, the mass loss rates of every GPC specimen rapidly increased over time in immersion. The geopolymer concrete with MBS replacement experienced a lower mass loss than the control geopolymer concrete with GGBS. The experiment's results indicate that the acid resistance of GPC based on MBS and GGBS was significantly lower than that of GPC based on GGBS. As the duration of exposure lengthened, the weight of all GPC samples exhibited a significant drop after 28 days.
Figure 8. Mass loss percentage due to acid curing.
Download figure:
Standard image High-resolution imageThe mass loss rate of GGBS-MBS-based GPC was more than 15% after a 90-day exposure period, which is consistent with the observed phenomenon depicted in the figure. The specimens' mass loss rate for the GGBS-based GPC stayed below 8% after 90 days of exposure. However, the M6 sample experienced a mass loss rate of only 4.44%, the lowest value observed after exposure to the acid solution for 90 days. According to the findings regarding mass loss rate, it was observed that the GPC specimen labelled as M6 exhibited the most effective resistance to sulfuric acid corrosion. The weight reduction is linked to the extent of neutralising various GPCs when exposed to the sulfuric acid solution.
7.5.2. Compressive strength after acid exposure
Figure 9 illustrates the reduction in GPC's compressive strength following exposure to the sulfuric acid solution. GPC that included GGBS, SBA and MBS in the alkaline mixture had initially exhibited higher compressive strengths than those that didn't include MBS, since the formation of geopolymer gel was enhanced due to the reactive silica present in MBS. After being exposed for 90 days, the GPC specimens with GGBS failed but maintained some of their compressive strength. The specimens containing high-silica MBS and activated with high concentrations of alkali activators exhibit higher compressive strength than the others. This is due to MBS acting as a filler, blocking the pores of specimens, and thus reducing the infiltration of the harsh chemical through the pore network. Visual analysis of the GGBS-based GPC specimens from optical microscopic images revealed in figure 10.
Figure 9. Decrease in compressive strength due to acid curing M1-M7.
Download figure:
Standard image High-resolution imageFigure 10. Optical microscopic image of (a) M1 (b) M3 (c) M6.
Download figure:
Standard image High-resolution imageThe deterioration starts at the Interfacial Transition Zone, which enhances the neutralization depth and mass loss and leads to reduced compressive strength. M6 exhibited the minimum deterioration of all the specimens, where the MBS acts as a filler, and the neutralization depths are reduced. Here, the bond between mortar and aggregate is not affected much. Therefore, the strength reduction is not significant.
8. Characterization study and microstructural analysis
8.1. X-ray diffraction analysis
The x-ray diffraction of different geopolymer concrete is given in figure 11. It is clear from the figure that various minerals are formed as a result of chemical reactions.
Figure 11. XRD of M1, M3, and M6- A-Albite, C-C-A-S, S-C-S-H, Q-Quartz, M-Mullite, Z-Zeolite.
Download figure:
Standard image High-resolution imageThe mix M1 showed the mineral Q quartz at 26.22° which is crystalline in nature the zeolite and albite minerals are also formed at 27.64°, and 54.48° respectively. The Calcium silicate hydrate C-A-S-H is responsible for the strength development formed at 29.06° 2θ [33, 34]. The mix M3 also showed a similar trend but an additional mineral calcium silicate hydrate CSH mineral is formed as a secondary strength forming mineral due to the incorporation of MBS at 50.06° 2θ apart from C-A-S-H at 29.36°. The mix M4 Showed minerals such as Quartz and Mullite at 26.52°, 20.67° 2θ respectively which is due to the incorporation of SBA into the matrix. this mix depicted enhancement in the peak of C-A-S-H and C-S-H gel due to the hybrid reaction of geopolymer and conventional reaction. Mix M6 showed a diminished in the intensity of zeolite and mullite minerals but the strength forming C-A-S-H and C-S-H peaks are well established in this mix at 29.59 and 50.36 respectively which is supported by the compressive strength results [42].
8.2. Morphological characteristics
The SEM images of different Concretes as shown in figure 12. The concrete M1 shows the formation of the dense matrix, portlandite, void, and cracks. It also exhibits thermal cracks due to GGBS in the matrix [34], as already discussed in the water absorption test results. The formation of portlandite reduced drastically in rest of the mix due to the formation of secondary strength forming minerals such as C-S-H which consumes portlandite and silica in presence of moisture [43], which was observed in the XRD test results for the same. The Mix M3 exhibits partially reacted and no reacted SBA the incorporation of SBA into the matrix reduces the fissures or cracks due to reduction in GGBS which is responsible of heat inception, it also witnesses the formation dense glassy matrix which contributes to the strength. The Mix M6 who's strength resembles the control samples compact topography and traces of reacted and inert SBA and MBS, SBA sometimes led to the formation of voids [44], in the mix which is countered by the MBS due to its micronized silica particles. Hence the combination of SBA and MBS proved to be vital in enhancing the strength parameter of geopolymer concrete.
Figure 12. SEM Images of geopolymer concrete (a) M1 (b) M3 (c) M6.
Download figure:
Standard image High-resolution imageThe EDS of M1, M3, and M6 are summarised in the table 5. The Ca/Si ratio for M1 is 0.60 and the same ratio forM3 and M6 was 0.68 and 0.59 respectively this can be due to incorporation of silica rich SBA in M3 and blending of SBA, MBS in M6 which contributes to the strength forming minerals and also reduces thermal cracksas shwon in figure 13.The Ca/Al ratio for M1 M3 and M6 are 1.82, 2.26 and 1.78 respectively which indicate towrds reduction in the calcium content due to replacement of GGBS with SBA in M3 and blending of SBA, MBS in M6 and also some tracesesof alumina present in SBA.
Figure 13. EDS for geopolymer concrete M1 (a) M3 (b) M6 (c).
Download figure:
Standard image High-resolution imageTable 5. Atomic ratio (%) of geopolymer concrete.
| Ca/Si | Ca/Al | |
| M1 | 0.60 | 1.82 |
| M3 | 0.68 | 2.27 |
| M6 | 0.59 | 1.78 |
9. Conclusion
The effects of substituting SBA and MBS with GGBS on the compressive and flexural strength of geopolymer concrete were examined in this study at room temperature. The following conclusions have been made possible by appropriate experiments and tests:
- The compressive strength of GGBS and SBA incorporated mortar cannot produce the required performance as its reactivity is moderate beyond 10% blending, which showed a reduction in strength and similar to SBA and addition of MBS beyond 8% significantly reduced the compressive strength.
- Blended geopolymer-hybrid Concrete (combination of SBA 10% and MBS 8% with GGBS 82%) showed more than 6% improved strength compared to the control Geopolymer concrete.
- The lowest water absorption was observed for mix M6 consist of 10% of SBA and 8% of MBS hybrid mortar, similar behaviour was exhibited in sorptivity test showing the role of SBA and MBS in filling the voids and reducing the thermal cracks offered great resistance against the moisture absorption and capillary suction.
- In RCPT analysis the hybrid mortar consisting of SBA, MBS mix showed the lowest charge passed, and highest chloride ion penetration resistance. In carbonation test the area affected by CO2 is lesser for M6 compared to other mixes. This shows the lowest penetration of CO2 due to lower porosity of the mix.
- Decrease in compressive strength and weight loss due to the sulfuric acid exposure is minimum for M6. Therefore, high stable geopolymer concrete mix in the acidic environment is obtained for M6.
- Mix M1's XRD revealed the development of C-A-S-H as well as the minerals Q quartz, zeolite, and albite. Similar trends were also seen in Mix M6, albeit an extra C-S-H mineral developed.
- SEM results initially reveal the presence of C-A-S-H in the M1 mix; however, the incorporation of SBA witnessed the formation of C-S-H and MBS, making a dense and compact structure, resulting in fewer cracks for M6.
- EDS results showed changes in atomic ratios of Ca/Si and Ca/Al for M1 and M6, which followed the XRD and SEM results.
- The incorporation of 10% of SBA and 8% of MBS in GGBS based geopolymer mortar proved to be a vital to have good mix which stood firmly against compressive flexure, durability and acid exposure properties.
Data availability statement
The data cannot be made publicly available upon publication because they are not available in a format that is sufficiently accessible or reusable by other researchers. The data that support the findings of this study are available upon reasonable request from the authors.