Abstract
Glioma is one of the most malignant types of cancer and most gliomas remain incurable. One of the hallmarks of glioma is its invasiveness. Furthermore, glioma cells tend to readily detach from the primary tumor and travel through the brain tissue, making complete tumor resection impossible in many cases. To expand the knowledge regarding the invasive behavior of glioma, evaluate drug resistance, and recapitulate the tumor microenvironment, various modeling strategies were proposed in the last decade, including three-dimensional (3D) biomimetic scaffold-free cultures, organ-on-chip microfluidics chips, and 3D bioprinting platforms, which allow for the investigation on patient-specific treatments. The emerging method of 3D bioprinting technology has introduced a time- and cost-efficient approach to create in vitro models that possess the structural and functional characteristics of human organs and tissues by spatially positioning cells and bioink. Here, we review emerging 3D bioprinted models developed for recapitulating the brain environment and glioma tumors, with the purpose of probing glioma cell invasion and gliomagenesis and discuss the potential use of 4D printing and machine learning applications in glioma modelling.
Export citation and abstract BibTeX RIS
1. Introduction: glioma as a clinical and engineering challenge
Gliomas are tumors of the central nervous system (CNS) that originate from glial or progenitor cells. They represent 28% of all primary brain and CNS tumors and 80% of all malignant tumors [1]. Categorized by their histological and morphological properties [2], types of gliomas include astrocytomas (high grade astrocytoma is termed glioblastoma (GBM)), oligodendrogliomas, ependymomas, and mixed oligoastrocytic gliomas. While the cell of origin of GBMs is still not fully understood, possible cell types that give rise to GBMs include astrocytes, neural stem cells (NSCs), NSC-derived astrocytes, and oligodendrocyte precursor cells [3, 4]. On the other hand, oligodendrogliomas are known to originate from oligodendrocytes [5]; and ependymomas from ependymal cells [6]. In addition to histological classification, most gliomas are assigned malignancy grades I through IV, using the World Health Organization (WHO) classification system, with WHO grade IV representing the highest malignancy [2, 7, 8]. GBMs, which are grade IV gliomas, tend to be very aggressive, invasive, and neurologically destructive, leading to a high mortality rate and a poor prognosis. The median survival for GBM is 15 months, with only 5% of cases reaching 5 year survival, despite available treatment options including neurosurgery, radiation, and therapeutic drugs, such as Temozolomide (TMZ) [9].
There are two types of GBMs: primary GBM, also termed de novo GBM, usually manifests as an invasive tumor with no prior evidence of a lower grade lesion, and secondary GBM, which is generally observed in younger patients and arises from a low-grade astrocytoma within a 5–10 year time frame [9, 10]. Despite these distinctions, both types are characterized clinically by rapid proliferation and diffusive invasion, making it difficult to perform standard treatment options [11, 12]. It is thought that secondary GBM occurs when malignant astrocytes in a low-grade glioma de-differentiate, which may be due to the accumulation of genetic mutations in the pathways controlling glial differentiation. However, the pathogenesis of primary GBM is less established. While it may arise due to a mechanism similar to that of a secondary glioma, it is also possible that it is caused by malignant transformation of glial precursor cells. There remains a lack of understanding of molecular pathways leading to GBM and the main mechanisms behind the differences in genetic mutations between its two types. Hence, several questions with respect to gliomagenesis remain unanswered: Which genotypes give rise to a glioma and which pathways are involved in that process? What is the timeline for the progression of glioma? Furthermore, what are the factors that influence gliomagenesis?
While much remains undetermined regarding gliomagenesis, a unique characteristic of glioma is that its cells migrate and invade healthy brain tissue [13]. It is hypothesized that the mechanisms of invasion are reminiscent of the mechanisms by which early glial cells migrate during embryogenesis [9]. Glioma cell invasion is responsible for the high mortality rate associated with the tumors: it has been observed that after surgical removal, most recurring tumors arise within 1 cm of the resection area because malignant glioma cells had already invaded beyond the bulk tumor into healthy tissues [14, 15]. To our knowledge, there is no approved approach targeting this aggressive hallmark of gliomas. Therefore, further studies are required to understand the biology behind the invasiveness of glioma cells. By gaining more insight into the processes associated with glioma cell invasion, it may be possible to therapeutically target invading tumor cells, to reduce the spreading of such cells into normal brain tissues, and to contain the tumor within manageable limits.
Angiogenesis, which refers to the formation of microvasculature, is an important hallmark of cancer [16]. It supports malignant growth of tumors by delivering oxygen and nutrients to them, while removing their waste products [13]. The occurrence of angiogenesis in GBM is striking and it may be a promising point of intervention in the development of agents inhibiting tumor growth [9]. One major well-known mechanism for angiogenesis is that the rapid cell proliferation in GBMs leads to ischemia and hypoxia, leading to the release of vascular endothelial growth factor (VEGF), which in turn induces blood vessel formation [13]. According to an alternative theory, tumor cells may have a direct effect on existing blood vessels. Glioma cells implanted on the brains of mice were observed to migrate toward existing vasculature and induce expression of angiopoietin-2 (Ang2), which causes destabilization and death of blood vessels. Vascular apoptosis, in turn brings about necrosis and hypoxia in the tumor mass, triggering VEGF-mediated angiogenesis [17]. Angiogenesis serves as an important therapeutic intervention point, as inhibitors of VEGF (Avastin) has been widely used in the clinic, along with standard drugs like TMZ. However, despite its promise in preclinical studies, Avastin did not increase overall survival of patients [18]. Furthermore, anti-angiogenic treatments led the tumors to become even more invasive [19], suggesting the unmet need to better understand the cellular interactions between tumor cells and their microenvironment.
Although there has been progress in elucidating molecular pathways governing malignant gliomas using conventional two dimensional (2D) in vitro studies and in vivo animal models, the heterogeneity and the complex immunosuppressive microenvironment of gliomas have proved challenging to recapitulate [20, 21]. Furthermore, conventional 2D in vitro models have been insufficient in mimicking the dynamic microenvironment of tumors [22]. Similarly, in vivo studies of animal models have remained limited in their ability to replicate the human condition and to allow modifications of experimental variables with precise control and reproducibility [23]. As such, translation potential of preclinical studies into effective clinical solutions has been extremely low with only 5% of anticancer agents proving efficient in Phase III trials [24]. It is therefore essential to engineer three-dimensional (3D) glioma tissue models and to study each process closely in a controllable and tunable environment in a highly reproducible and high-throughput manner. Hence, here we review reported 3D bioprinted glioma models, which are proposed as ideal platforms to conduct biomimetic studies on glioma microenvironments, anti-cancer drug sensitivity, and mechanisms of angiogenesis and vascularization.
2. 3D modeling in tissue engineering
In pursuit of an alternative to organ transplants, tissue engineering has emerged as a promising field of research [25]. 3D tissue models have been very critical for mimicking the exact microcellular environment and cell–cell interactions. The pioneering works in this domain have identified and characterized 3D matrices, which are vital for cell anchorage and precise replication of the cellular microenvironment, and therefore, have led to the ability to create living tissues from a source of cells [26, 27]. However, existing cell patterning techniques, such as soft lithography, have largely been incompetent in patterning neural cells in 3D. They involve a fixed-form mold along a transverse or axial plane, thus lack any flexibility in positioning cells as per the neural pathways [28, 29].
Three-dimensional fabrication is particularly promising for neural tissue engineering as a potential alternative to autograft-based approaches used in peripheral nerve repair [30, 31]. This strategy avoids common shortcomings of grafting, such as dual surgery, donor site morbidity, and aberrant regeneration [30]. These novel tissue-engineered channels were also found to enhance neural regeneration. The peripheral nerves in the CNS do not regenerate as readily as they do in the peripheral nervous system (PNS) [32, 33]. Thus, the scope of autologous grafting for CNS injuries remains limited. This inadequate regeneration capability of CNS axons stems from various physical and chemical obstacles, such as glial scar and myelin, respectively [34]. However supplementation of supportive extracellular matrix (ECM) components, molecules guiding cell adhesion, and neurotrophic factors were reported to be successful in counteracting these issues [35, 36].
In recent years, tissue engineering has been applied to more complex constituents of the nervous system (brain and spinal cord), where nerve grafts were not a viable option [35]. The growing need to address these factors while devising a therapeutic strategy against CNS injuries truly highlights the need for tissue engineering in this field. Microfluidic models, in particular, have provided remarkable spatial control over co-cultured cells, and generated controllable signaling gradients, demonstrating significant advantages over conventional methods to engineer 3D models [37]. Several studies have also established bottom-up tissue fabrication techniques, which are essential to replicate the complex microenvironment and cellular interactions in a neural circuit [38]. Whether recreating the stratified layers of the cerebral cortex, or the intricate neural pathways connecting it to the lower spinal cord, a rapid and precise user-controlled technique has been deemed essential [38, 39]. Consequently, there has been tremendous growth in user-defined manufacturing, primarily 3D bioprinting, for neural tissue engineering. Among the fabrication techniques used for production of 3D models, 3D bioprinting as an emerging method is reviewed in detail here, providing a glimpse into this paradigm changing fabrication method [40–43].
2.1. 3D bioprinting
Three-dimensional bioprinting has emerged as an effective strategy for regenerative medicine [44–46], as well as many other fields including organ-on-a-chips [47, 48], cancer research [49, 50], and pharmaceutical engineering [51–54], owing to its exceptional versatility in cell positioning. 3D bioprinting offers rapid freeform prototyping capabilities to the field of tissue engineering, facilitating instant fabrication of living tissue constructs, unlike other existing technologies [55]. In addition to having high-throughput and reproducible layer-by-layer construction of 3D scaffolds, bioprinting has also been recognized as one of the ideal techniques for controlling the pore size and introducing interconnected channels [56]. The large surface area offered by 3D printed fiber meshes promotes cell attachment, growth, and diffusion of nutrients [57], giving them a definite advantage over traditional techniques, such as solvent casting [58], phase separation [59], and melt molding [60].
Due to the readily achievable ordered 3D structure with controlled and aligned topology, 3D-bioprinted constructs have been reported to induce desirable cell attachment and growth as well as intracellular communication, which is often regarded as one of the most distinctive features of the nervous system [61]. In addition to providing the ability to construct tissue models with a homogeneous cell distribution and uniform spacing [62], 3D bioprinting also provides exceptional control over the 3D architecture and spatial positioning of neural cells [63]. Control over spatial positioning of neural cells using microfluidics has enabled 3D models to mimic unique microenvironments, microvasculature, and cell-cell signaling patterns of gliomas [64]. 3D-bioprinted models of cancer have contributed to our understanding of the disease significantly and have served as excellent models to test new drugs and therapies in vitro [49]. Furthermore, 3D printing not only saves expenses and time during manufacturing but also enables the usage of clinical research products by constructing intricate models and designing free-form geometric biomedical devices [41, 42, 65, 66].
2.2. Biomaterials for 3D bioprinting
According to the working principle of 3D printing, the main components of bioink (including natural or synthetic biocompatible polymers) allow 3D construction that mimics in vivo conditions [67]. However, the development of ideal bioinks that possess proper strength and viscosity as well as the formation of 3D tissue structures has remained challenging. Therefore, studies focusing on novel bioink formulation and enhancement of 3D bioprinting techniques to resolve the problems associated with structural resolution, preserving cells, and production time are emerging [68].
Various types of biomaterials have been used as the main component of 'bioink' by encapsulating cells and providing them with a suitable microenvironment during the 3D bioprinting process [69]. Furthermore, bioinks have to possess several rheological properties to ensure high viability and resolution of the 3D model, including viscosity, shear thinning and thixotropy [70]. Viscosity of the bioink is critical during printing, as insufficient viscosity directly affects the print quality or printability [71]. A printable bioink requires a relatively high viscosity, which in return can cause clogging of the nozzle tip. Shear thinning materials and thixotropic biomaterials facilitate the deposition of bioink by altering viscosity under shear stress [70], since shear stress can cause deformation of cells processed by 3D bioprinting application. Biomaterials such as hydrogels, alginate or gelatin have the ability to prevent cells experiencing stress during bioprinting [72, 73]. Natural polymers, such as collagen, fibrin, gelatin, and alginate are preferred in different bioprinting techniques, as their thermo- and stimuli-responsiveness enhances their printability. In addition, they are abundant in bioactive groups, which provide relevant cues for cellular processes, such as migration, proliferation, and differentiation [69]. Yet, each of these natural materials has its own advantages and limitations.
Collagen provides remarkable cell adhesion and proliferation in the microenvironment, however, it lacks mechanical stability and an adequate level of viscosity required for 3D printing [74]. Gelatin, one of the derivatives of collagen, demonstrates temperature-dependent reversible gelation behavior, making it a suitable candidate for 3D bioprinting. Furthermore, its properties, such as an abundance of cell-binding domains and the presence of matrix metalloproteinase recognition sequences, favor optimal cell functioning. However, similar to collagen, gelatin is also deficient in mechanical strength and viscosity [69, 74]. Alginate, another natural polymer obtained from brown seaweed, although being cost-efficient and showing high biocompatibility and low toxicity, has an insufficient number of cell-binding domains. Another naturally occurring polymer, fibrin, provides cells with a large attachment area and thus promotes their adherence and proliferation. However, it has a fast degradation rate, making it unsuitable for long-term cell culture [74]. Gelatin methacryloyl (GelMA) is a commonly used bioink due to its tunability and biocompatibility. However, it undergoes a rapid sol–gel transition at room temperature, which creates difficulties in its stereolithography (SLA) bioprinting [75]. Nevertheless, GelMA can be altered and allow for rapid printing while maintaining mechanical stability and resolution. This provides high biocompatibility and resolution to GelMA and contributes to the versatility of bioink availability in further bio fabrication applications.
Alternatively, synthetic polymers which have great mechanical characteristics, are advantageous for 3D printing and tissue engineering applications owing to their convenient cost-efficient synthesis procedures [76]. However, they are relatively inert and lack bioactive groups compared to natural polymers. One example of synthetic polymers is polyethylene glycol (PEG). PEG is a low-cost biocompatible polymer with easily tunable chemical structure and physical properties. However, it has a shortage of cell-adhesive domains and is unresponsive to shifts in temperature or light exposure [69, 74]. Polycaprolactone (PCL), another synthetic polymer widely used as a bioink, possesses great biocompatibility, degradability and stiffness [68]. Although PCL is known to have a low melting point of 63 °C, making it quite attractive for 3D bioprinting, it cannot be used for cell encapsulation in its liquid form due to its harmful effect on cells [76]. Furthermore, in its pure form, it is rather hydrophobic, which diminishes cell adhesion and growth rate. Similar to PEG derivatives, other synthetic polymers such as polyvinyl alcohol (PVA) have easily tunable properties. PVA is a biodegradable, biocompatible, thermostable and water soluble synthetic material and has poor cell affinity [74]. While PVA enables control over mechanical and chemical properties, it requires further functionalization to ensure bioactive properties in cell culture platforms [77]. Polyurethane (PU) is another synthetic polymer that is appropriate for bioprinting. PU is biodegradable, waterborne, and possesses low toxicity [78]. PU-based hydrogels can be used to produce photosensitive, thermosensitive, and pH-sensitive hydrogels. Together, biomaterials for 3D printing come in various properties with specific characteristics suitable for the target application.
2.3. 3D bioprinting methods
Technologies predominantly used for 3D bioprinting are Nozzle based (e.g. extrusion-based bioprinting and inkjet- or droplet-based bioprinting) and light-induced bioprinting (e.g. two- or multi-photon polymerization based bioprinting (TPP or MPP), SLA, digital light processing (DLP), laser-assisted, and computed axial lithography (CAL) bioprinting (figure 1). They offer relatively time-efficient, accurate, and effective ways over conventional methods and create favorable microenvironments for cellular development and activity [76, 79].
Figure 1. Schematics of 3D bioprinting methods. In nozzle based bioprinting, (A) extrusion-based, and (B) inkjet- or droplet-based platforms are used. In light-induced bioprinting, the utilized methods include (C) two- or multi-photon polymerization based bioprinting (TPP or MPP), (D) stereolithography (SLA), (E) digital light processing (DLP), (F) laser-assisted, and (G) computed axial lithography (CAL). (A), (B), (D), and (F) [92] John Wiley & Sons [© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim]. (C), (E), and (G) [130] John Wiley & Sons [© 2020 Wiley-VCH GmbH].
Download figure:
Standard image High-resolution image2.3.1. Nozzle-based bioprinting
One of the well-established nozzle-based methods is extrusion-based bioprinting, where 3D tissue scaffolds are formed with the continuous extrusion of biomaterials (figure 1(A)). Extrusion-based bioprinting is a cost-efficient method, allows the use various biomaterials and cell types such as stem cells, and it operates with less cell damage during printing, resulting in rapid modeling of controllable structures [80]. Although extrusion-based bioprinting offers speed in 3D bio fabrication, some of its restraints are low resolution (∼100 µm) [81], shear-stress, and limited biomaterial availability due to the need for rapid gelation. These limitations can be resolved to some extent when printing parameters such as nozzle diameter and pressure are adjusted accordingly [82]. Advanced approaches in extrusion-based bioprinting include multi-nozzle [83], coaxial [84, 85] and hybrid bioprinting. The multi-nozzle technique allows for parallel printing of a combination of bioinks, possibly composed of different biomaterials and cells, while minimizing their cross-contamination. However, it is a highly complex and expensive method. Coaxial bioprinting involves multiple nozzles positioned coaxially, resulting in a core and shell alignment. This property appears to be suitable to produce vascularized structures, which is crucial for the functionality of a range of tissue constructs. In hybrid methods, a combination of techniques is adopted to improve biomimicry of tissue complexity [86]. For instance, multi-nozzles systems can be integrated with coaxial nozzles or electrospinning, another technique used to fabricate tissue engineering scaffolds [87, 88]. Since 3D bioprinting systems usually pose challenges in mimicking surface nano topographies, using such hybrid techniques can improve the functionality of scaffolds, help constrain cell migration, and particularly aid in constructing bone and cartilage tissues [80, 89]. On the other hand, inkjet 3D bioprinting (also known as droplet-based), in which the structure is formed by the produced droplets either using a heating system or a piezoelectric actuator (figure 1(B)), has lower costs and allows for multiple printheads to work simultaneously, enabling fast and high-resolution printing. Despite its ability to sustain high cell viability, it exhibits low cell density [90]. Piezoelectric actuation, however, carries a higher risk of cell death with possible rupture of cell membranes due to high shear stress, making them less attractive in the field.
2.3.2. Light induced bioprinting
MPP is an absorption process carried out via femtosecond lasers (figure 1(C)). The method requires a high laser intensity, and photopolymerization can be achieved at sub-micrometer degrees with pulses from the laser [91]. Upon exposure to light, a photoinitiator catalyzes the photopolymerization by releasing photo-radicals. However, there is a limited number of biocompatible photoinitiators due to their toxicity profiles. Another limitation of MPP can occur as a result of photopolymerization; the resin should be transparent to infrared light. Since the available materials are only transparent near-IR, their use in MPP is limited. Additionally, this method has a lower processing rate than many other currently used scaffolds. This slow rate makes it difficult to obtain high resolution on larger scales, although progress has been made in increasing printing rate. Despite limitations in biocompatibility, scanning rate, and transparency of the materials, this method remains promising for its use in tissue engineering.
In SLA, a 2D bioink layer is selectively photopolymerized layer-by-layer along the z-axis (figure 1(D)). Since the cells are not exposed to any shear stress, the method provides higher cell viability than nozzle-based printing. Yet, because the method utilizes light for polymerization, it imposes a transparency limitation to the bioink for uniform crosslinking. This requirement also limits the bioink cell density to be around 108 cells ml−1. Nonetheless, it offers fast and high resolution (∼1 µm). The laser-assisted bioprinters consist of a thin bioink layer, a donor layer, and an energy-absorbing layer. Situating below the energy-absorbing layer, the donor layer releases a high-pressure bubble as a laser beam is applied to the desired location on the absorbing layer. This bubble releases a droplet of bioink to a platform below. The 3D structure is constructed with the movement of this platform along the z-axis. Due to cells not experiencing shear stress directly, the technique allows for high cell viability (>95%) [92]. Similar to SLA, bioprinting using DLP technology is another technique, where polymerization is achieved through the use of projection light (figure 1(E)) [93]. In addition to its ability to produce complex products, DLP has a high resolution, efficiency, accuracy, printing speed and mild working conditions [94]. However, photosensitive polymers limit the materials that can be used in this application.
Laser assisted bioprinting is another promising technique to engineer tissues with physiological functionality [95, 96]. A pulsed laser is used as an energy source and allows biomaterials on the ribbon to be deposited onto a receiving substrate (figure 1(F)). The nozzle-free and non-contact process in this method allows for high cell viability and high resolution outcomes, producing complex bioengineering scaffolds that mimic physiological conditions at high accuracy [97]. Other advantages of laser assisted bioprinting over its native counterparts include high-throughput printing and high reproducibility [98]. However, the high costs associated with the technique along with the relatively low bioprinting speed pose some limitations. Additionally, the use of metallic surfaces induces the risk of metallic particle contamination. Computed axial lithography (CAL) is a method that uses the superposition of 2D images, particularly computerized tomography scans, to generate 3D models using photopolymerization of photosensitive materials (figure 1(G)) [99]. Unlike traditional methods. Where layer-by-layer approaches are utilized, CAL simultaneously fabricates all points within a 3D geometry. Thus, volumetric synthesis allows for faster and highly scalable 3D printing compared to layer-by-layer or point-by-point additive manufacturing (AM) methods. In addition, while the use of biomaterials is limited by viscosity restraints in other AM methods, CAL allows for the use of higher viscosity materials, thereby widening potential applications.
3. Emerging applications of 3D bioprinted glioma models
Microfluidic organ-on-chip technologies have offered comprehensive models for investigating the treatment of CNS and PNS diseases [100]. In addition, analysis of complex biological systems such as blood–brain barrier (BBB), brain tumors, neurotransmission and axon propagation using microfluidic chips has proven more convenient than conventional models [100]. 3D bioprinting holds promises for addressing a variety of research challenges in drug delivery, regenerative medicine, and functional organ replacement. Furthermore, 3D printing technology can be used to create in vitro models that possess the structural and functional characteristics of human organs and tissues by spatially positioning cells and bioinks [69]. In this section, emerging applications of 3D bioprinted glioma models, which are utilized to gain more insight into the biology of gliomas, are reviewed briefly.
3.1. Examining native signaling patterns of glioma microenvironments
Two-dimensional culture models and in vivo animal models have proven insufficient in recapitulating the tumor microenvironment. Therefore, 3D models have attracted great attention to study glioma biology recently. In one seminal study, a 3D bioprinting approach to study the interactions between GBM cells and macrophages was established by forming a mini-brain model [101]. This mini-brain model was engineered using a two-step bioprinting process. Two mouse-derived cell lines, RAW264.7 macrophages and GL261 GBM cells, were utilized to fabricate a larger brain model with an empty cavity and a tumor model to fill the empty cavity (figures 2(A)–(C)). The model was completed using photo-crosslinking of the construct. It was demonstrated that the model could maintain its 3D structure as the bioink remained stable over time and that paracrine and juxtacrine signaling interactions could be elucidated in this model. Similar to findings in in vivo models and contrary to those in 2D models, increased expression of ECM-remodeling enzymes by macrophages and an upregulation of GBM-specific markers were observed. Additionally, exchange of cytokines and growth factors were observed in the GBM and macrophage laden mini-brains. These findings suggested that paracrine signaling interactions and crosstalk were established, mimicking physiological conditions (figure 2(D)). Further, the effects of novel targeted therapeutic agents, such as STAT signaling inhibitor or CSF-R inhibitor, on tumor growth were investigated by observing relative gene expression profiles upon treatment, demonstrating the model's suitability to study GBM progression (figures 2(E) and (F)). This proof-of-concept study suggested that more complex bioprinted models can be developed and utilized in tracking immunomodulatory, chemotherapeutic, microcellular interactions in cancer models and rapid drug screening.
Figure 2. Three-dimensional bioprinted mini-brain model for glioblastoma (GBM) therapeutic research. (A) Cell-laden GelMA-gelatin mixture was prepared as the bioink for the 3D bioprinting process of the mini-brain concept, which included two steps: first, the larger brain model with a cavity was printed with encapsulation of mouse macrophage cell line (RAW264.7). Second, this cavity was supplied with mouse glioblastoma cells (GL261) embedded into bioink and the whole construct was undergone photo-crosslinking. (B) Dimensions and schematic side view of the cell-laden 3D bioprinted mini-brains. (C) Cross-section in the frontal plain of the fabricated mini-brains. The GBM region is featured in red (scale bar: 5 mm). (D) Schematics of the procedure for paracrine signaling between individually bioprinted RAW264.7 and GL261 cell-laden mini-brains in coculture, in order to study the interactions of cancer cells with macrophages. Gene expression results after culturing for 4 d and after treatment with either AS1517499 or BLZ945 on day 1 and 3 post-bioprinting (E) for selected genes in RAW264.7, and (F) for selected genes in GL261. *p < 0.05, **p < 0.01. [101] John Wiley & Sons [© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim].
Download figure:
Standard image High-resolution imageIn another study, biomimetic tri-regional GBM models were 3D printed via DLP-based bioprinting using patient-derived GBM cells, human endothelial cells, and hyaluronic acid derivatives, to mimic native tumor heterogeneity [102]. Stiffness patterns of the three regions, namely the tumor region, the acellular ECM region, and the endothelial region, were correlated to the GBM stroma, normal and pathological brain parenchyma, and brain capillaries. Two models with different stiffness conditions of the ECM region were used to capture the tumor microenvironment, as well as biophysical and biochemical cues in different states of GBM progression, mimicking GBM-remodeled stroma and healthy brain parenchyma. Furthermore, it was demonstrated that the stiff condition enabled a single-cell diffusion pattern, hypoxia, stemness, and angiogenic potential, while the soft condition facilitated an expansive growth pattern as well as rapid proliferation of cells. Additionally, primary GBM tissue signatures and enrichment of gene sets associated with mesenchymal and proneural subtypes were observed in the stiff condition, whereas gene sets associated with classical subtypes were enriched in the soft condition. Distinct angiogenic patterns and endothelial growth patterns were also explored for both conditions through the incorporation of human umbilical vein endothelial cells (HUVECs) into these models. Endothelial proliferation and sprouting were observed in the stiff models, while only proliferation was observed in the soft models. Furthermore, the stiff model showed higher drug resistance to anti-cancer drugs. Through the observed differences between these conditions, it was demonstrated that the tri-regional stiffness patterned GBM models recapitulate tumor microenvironments and biomimetic cell–ECM interactions. Therefore, this model or similar ones can be applied to assess other tumor-stromal interactions in GBM.
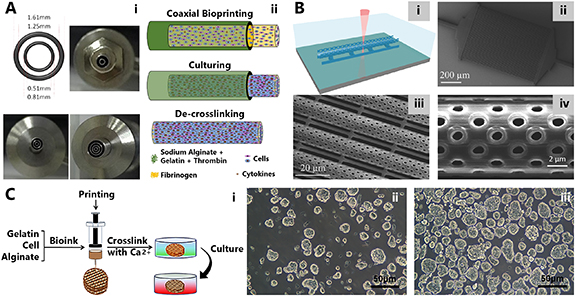
Another 3D model to capture the complex microenvironment of gliomas was fabricated using coaxial 3D bioprinting (figure 3(A)) [103]. The bioprinter utilized in this study contained a coaxial nozzle, constructed by an outer nozzle and an inner nozzle. While the outer nozzle was used for the hydrogel, the inner nozzle was used for cell suspension in the fibrinogen. Various modifications of these nested nozzles were achieved by changing their diameters. To capture cell–cell interactions, a high-density core surrounded by a biofabricated shell scaffold was engineered using a layer-by-layer approach. While biomaterials generally confine the connections between the cells during the bioprinting process, a solution was proposed for this challenge using coaxial bioprinting and biomimetic tumor microenvironment was formed. Furthermore, this ex vivo tumor model promoted interactions between various cell types by creating tissue-like fibers constructed by tumor and stromal cells. The alginate shell was proven to exhibit high permeability, integrity, and continuity. Although the resolution was not higher than other techniques, it was suggested that it could be increased using various modifications to nozzle sizes. High cell viability and cell–cell interactions were the main advantages of cell-laden core–shell tumor fibers, yet cell proliferation rate was significantly lower than that of 2D models. Additionally, other major drawbacks including the lack of control over shell strength, spatial precision, and the potential impacts of the unremovable apoptotic debris on cellular activity, remained unresolved for this model.
Figure 3. Three-dimensional bioprinted models for brain cancer studies. (A) One more advanced approach in extrusion-based bioprinting is coaxial 3D bioprinting. This method was used for fabrication of self-assembled multicellular heterogeneous tumor fibers. (i) Various types of nozzles were utilized for 3D bioprinting. (ii) Schematic representation fabrication process of the tumor fibers which included coaxial bioprinting, in vitro culturing and de-crosslinking. Reproduced from [103]. CC BY 4.0. (B) A 3D microfluidic chip for biomimicry of blood–brain barrier in real (1:1) scale. (i) TPP process was utilized for constructing the porous microtubes. Scanning electron microscopy (SEM) images of (ii) the fabricated microfluidic chip with 50 tubes in parallel (scale bar: 200 µm), (iii) the tubes (scale bar: 20 µm), and (iv) pores with diameters: 1.05 ± 0.08 μm (scale bar: 2 µm). [109] John Wiley & Sons [© 2017 The Authors. Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim]. (C) Glioma microenvironment for glioma vascularization research fabricated with (i) 3D bioprinting approach using human glioma cell lines U118 and GSC23. Cell spheroids of (ii) U118 and (iii) GSC23, isolated from 3D hydrogel scaffolds (scale bar: 50 μm). [111] John Wiley & Sons [© 2020 Wiley Periodicals LLC].
Download figure:
Standard image High-resolution imageIn another study on immune interactions in glioma, DLP based 3D bioprinting was utilized for preparation of tumor microenvironment in a reproducible and scalable platform [104]. To assess the impact of cellular interactions in tumors, 3D models were fabricated using glioblastoma stem cells (GSCs), GSCs with astrocytes and neural precursor cells, in the presence and absence of macrophages. Particularly, the 3D tetra-culture models, where M2 macrophages were mixed with GSCs, achieved high imitation of patient-derived GBM tissues as demonstrated by the upregulation of GBM tissue specific genes, hypoxia, cancer stemness, signature cellular interactions, immune activation, and increased invasiveness, and M2 polarization. Through this model, the human tumor microenvironment was fully recapitulated using brain, immune, stromal components, allowing for the mimicking of essential biochemical and electrical cues. This model also allowed for the application of functional genetic screens using whole-genome clustered regularly interspaced short palindromic repeats based approach. Accordingly, specific molecular dependencies of GSCs in bioprinted structures relative to sphere cultures were identified. It was suggested that this highly reproducible model could be used to investigate cell–cell interactions and signaling, immunological interactions, drug sensitivity, invasion patterns, as well as apply novel functional screens in GBMs.
To optimize printability versus mechanical properties required for soft tissue modeling, a new filler-free bioink for micro extrusion bioprinting was proposed [105]. Hyaluronic acid, which is naturally found in neural ECM, along with gelatin were used, and both were conjugated with dopamine to enhance the elasticity of the hydrogel for cell culture. Subsequently, these natural polymers were crosslinked to Pluronic F-127 for its low elastic (storage) modulus with a thiol-catechol mechanism, allowing the bioink to display Herschel–Bulkley fluid behavior. Furthermore, two curing reactions were compared: photocuring under 365 nm UV exposure and chelation with Fe3+. Cured bioinks had elastic modulus between 6.7 and 11.7 kPa, with chelation showing a lower modulus profile. This modulus range was in alliance with the native neural tissue modulus. Alginate, a standard bioink that requires fillers for the rheological appropriation, was shown to have about 10-fold higher modulus value, which increases even more without the added fillers. In this platform, Schwann, neuronal, and glioma cell viability showed no significant decrease with respect to alginate counterparts. Through the fabrication of soft, free standing neural tissues, high ECM biomimicry was achieved, and the use of this model in neuroscience research, disease modelling, drug discovery, and regenerative medicine was suggested.
3.2. Searching for mechanisms of anti-cancer drug resistance and sensitivity in gliomas
Encapsulating tumor microenvironments can prove tremendously useful in elucidating molecular mechanisms and translating laboratory-based drug discoveries to high efficacy outcomes in clinical settings. In fact, the lack of adequate models is thought to be one of reasons preventing the success of drug candidates in clinical trials. To engineer a proof-of-concept model that can mimic the complex structure and biology of GBMs and serve as a rapid drug screening platform, a 3D GBM model was fabricated using a nozzle-based 3D-bioplotter with five ink cartridges [106]. The effect of hydrostatic forces, sheer stress, tension, and compression forces, as well as swelling equilibrium on ECM–cell and cell–cell interactions were formulated and the fibrin 3D-bioink gelation time was optimized according to rheological and mechanical characterization of GBM tissue. A desirable degree of swelling in addition to long-term culture capability was established. Furthermore, a stroma bioink comprised of patient-derived GBM cells, astrocytes, and microglia and a vascular bioink comprised of thermo-reversible Pluronic F127, endothelial cells, and pericytes were used, generating a 3D bioprinted penta-culture. Additionally, a perfusion chip, delivered through the hollow channels of the model, was used to evaluate long-term flow, imaging, and drug-response. The incorporation of peripheral blood mononuclear cells, circulating tumor cells (CTCs), and various agents into the model was demonstrated, suggesting that the platform can serve as a rapid, robust, customizable drug screening array using patient-derived tumor, stromal, and immune cells. Moreover, while no difference was observed between three different patient-derived GBM cells when treated with TMZ using a 2D model, different sensitivities to TMZ was observed when the same cells were grown in the aforementioned 3D model. The success of recapitulating the tumor microenvironment using the 3D model was also validated through gene expression profiling, suggesting that the model could be used as a personalized drug-screening platform.
Anti-cancer drug resistance is a major cause of GBM recurrence in patients and glioma stem cells (GSCs) are thought to be one of the main causes of drug resistance in glioma patients. To overcome obstacles associated with studying GSC related mechanisms of drug resistance by ensuring GSC enrichment, an in vitro model was fabricated using a 3D multi-nozzle bioprinter [107]. Using gelatin, alginate, and fibrinogen as bioink, a highly viable glioma model was constructed. Structural stability of the 3D printed scaffolds was maintained for 15 d, indicating higher viability and proliferation capacity of cells compared to its 2D counterparts. Furthermore, mRNA expression of GSC markers, CD133 and Nestin, were not only significantly higher than that in 2D models but also increased in a time-dependent manner, suggesting that the 3D model provided a better structure for GSCs to expand and enrich. Additionally, epithelial–mesenchymal transition (EMT) through which some epithelial cells gain stem like properties were explored. mRNA expression of EMT-associated transcription factors, Snail and Twist1, were upregulated in the 3D bioprinted scaffold. It was also suggested that the observed increase in the expression of hypoxia related factors VEGF and HIF1-α could be related to enrichment of GSCs and enhanced tumorigenicity. Lastly, as higher drug resistance was observed, the use of this model in drug efficacy trials can be plausible.
Similarly, to investigate drug resistance in GBMs, a microenvironment with GSCs (GSC23) and glioma cells (U118) was constructed using coaxial extrusion-based bioprinting [108]. High viability (∼93%) with increased cell–cell and cell–ECM interactions after printing was displayed using hydrogel microfibers with shell-GSC23/core-U118 (G/U) organization. Moreover, fiber-like cell aggregates were formed from the proliferation of core-U118 cells into multicellular spheroids. The gene expressions of biomarkers related to drug resistance and tumor invasion were observed to be higher in G/U hydrogel microfibers than shell/core-U118 (U) microfibers. Drug resistance was further studied using the most common chemotherapy drug in GBM treatment, TMZ, and G/U microfibers were observed to display higher resistance than U microfibers. Although drug resistance and sensitivity can be evaluated in an accurate and precise manner using this technique, challenges associated with obtaining a pure population of core cells were not eliminated; removal of apoptotic cells and cell debris remained unfeasible before shell structure was dissolved and cytokines secreted by shell-cells were non-functional.
To achieve a controllable model for CNS drug trials and neurovascular disorder research, a biohybrid BBB model was developed with a 1:1 scale to native neurovasculature (figure 3(B)) [109]. A microfluidic chip containing porous tubules was fabricated with TPP method. To mimic native tissue dimensions, the tube diameter was adjusted as 9.5 µm with an average of 1 µm for pore diameters. The tubules were surrounded by a monolayer of bEnd.3 endothelial cells and were tested for dextran permeability as well as trans-endothelial electrical resistance (TEER). With significant reduction of dextran concentration in the extra tubular environment of bEnd.3 barring scaffolds when compared to microtubules without the cells, barrier functionality was confirmed. Furthermore, the maturation of tight junctions and satisfactory dextran diffusion through membrane and TEER was observed, suggesting that permeability of compounds or anti-cancer drugs could be studied using this model. The TEER was elevated in the case of bEnd.3 carrying tubules. Additionally, the model's use for cancer drug screening was also considered and U87MG human GBM cells were shown to grow on the tubule scaffolds surrounded with bEnd.3 cells. The inflow of dextran through capillaries was higher in this biohybrid system. Given the resolution and adjustability of the material with TPL, this BBB model exhibited suitability for modeling various pathologies, including brain cancers.
To capture pathology specific features of GBM and complex tumor microenvironments, a GBM-on-a-chip model was engineered using patient-derived tumor cells, vascular endothelial cells, and silicon bioink in an extrusion-based bioprinting procedure [110]. Mimicry of biochemical, physiological, and structural features of native GBM as well as the intrinsically complex brain structure were achieved through the development of cancer-stroma compartmentalization in a concentric-ring structure, establishment of an oxygen gradient (leading to central hypoxia) using selectively gas permeable biomaterials, and fabrication of a brain derived decellularized ECM without compromising strong heterogeneity of ECM. It was demonstrated that assessment of anti-cancer drug combinations on patient-derived GBM-on-a-chip models remained superior to that of conventional methods in predicting drug resistance and responsiveness to treatment options. Furthermore, the potential applications of the proposed model in identifying appropriate and effective personalized cancer treatment were indicated. It was also demonstrated that the GBM-on-a-chip platform could be employed to identify most potent combinations of chemotherapy and chemoradiotherapy candidates, which would enable physicians to make effective and personalized data driven medical decisions. Given the robust mimicry of complex GBM ecology and pathological features as well as the relatively short one- to two-week development time, the practicality and suitability of GBM-on-a-chip models for point-of-care testing in clinical settings, was proposed.
3.3. Studying vascularization and angiogenesis in gliomas
The development of an extensive and abnormal vascular network has remained one of the defining features of GBMs. To elucidate the mechanisms of tumor vascularization and angiogenesis, glioma microenvironments were fabricated from human glioma cell lines, U118 and GSC23, using an extrusion-based 3D bioprinting approach (figure 3(C)) [111]. Both models formed spheroid shaped tumors in 3D hydrogels, mimicking in vivo tumor growth patterns. Additionally, the upregulation of VEGF, a potent marker of angiogenesis and vascular permeability, demonstrated the potential of these models in angiogenesis studies. Furthermore, tubule-like structures were formed in both 3D-GSC23 and 3D-U118 models. A higher level of VEGF and greater number of tubule-like structure formation was observed in 3D-GSC23 along with abundant mitochondria and well-developed Golgi apparatus. Thus, it was suggested that 3D-GSC23 had higher tumor vascularization potential. Similarly, to investigate the cell–cell interactions between vascular cells and GBM cells, a 3D glioma-vascular niche model was engineered [112]. Using patient derived GSCs, HUVECs and collagen type I with matrices with variable laminin concentrations, an endothelial-like fluid vascular channel was formed and embedded into a collagen-based structure with a GBM cluster located closely. GSC invasion of matrices was demonstrated, and more aggressive invasion patterns were observed at higher laminin concentrations. Furthermore, it was suggested that the model could be tailored to promote or inhibit vascularization patterns for studies investigating glioma and endothelial cell interactions. The model's potential use in recapitulating tumor cell behavior in patients, namely vascular cuffing and angiogenesis mechanisms, was also predicted.
To explore mechanisms involving tumor growth and metastasis, a vascularized tumor model was generated using 3D bioprinting techniques [113]. Using 3D printed, nanomaterial-functionalized, stimuli-responsive capsules containing growth factors, easily manipulated dynamic chemical environments were established. Furthermore, tumor cells, stromal cells, and infused vascular cells were 3D printed and the dynamic environment was manipulated externally upon laser irradiation of the capsules resulting in the release of growth factors and directing cell migration. In addition to the use of microcapsules, perfusable vessels were also introduced, leading to the generation of CTCs. To accurately mimic tumor metastasis mechanisms, chemotactic pathways were programmed. Thus, temporal, and spatial control was achieved through the use of 3D printing and programmed release capsules. It was demonstrated that the model provides the ability to induce post fabricated modifications via cell–cell interactions induced by the capsules, suggesting potential advantages over traditional methods in studying molecular mechanisms regulating vascularization. Furthermore, migration of cells into fibroblast-laden fibrin and tumor cell invasion into vessels was observed, indicating that intravasation could occur. While the complexity of tumor microenvironments was not captured fully, the model proved effective in establishing vascular invasion and angiogenesis pathways in tumors.
Similarly, to advance the ongoing research in tumorigenesis and anti-cancer drug susceptibility in neural tissues, the use of a GSC model to mimic the exact microenvironment of a brain tumor was suggested [114]. A combination of gelatin, alginate, and fibrinogen was chosen as the bioink to encapsulate the properties of GSCs (SU3 cells) for 3D bioprinting of the tumor matrix. The cells maintained their intrinsic characteristics and were found to grow into spheroids within three weeks, pushing aside the surrounding hydrogels and mirroring in vivo tumor growth, unlike 2D models that were observed to become apoptotic in two weeks. Thus, 3D models were suggested as better long-term alternatives for tumorigenesis studies. Compared to conventional 2D approaches used for studying tumor cell response to TMZ, the 3D bioprinted model showed more resistance than its 2D counterpart, thus mimicking in vivo tumor microenvironment more closely. Additionally, high expression of tumor angiogenesis markers was observed in the 3D model, suggesting that the model could serve as a functional platform for angiogenesis mechanism studies. Furthermore, due to the high potential for vascularization of GBM cells, the GBM tumor model has been regarded as an ideal microenvironment to examine in vitro tumor angiogenesis leading to migration. In this regard, GSCs were modeled using micro extrusion-based 3D bioprinting technology and a higher stability and proliferation rate was observed (approximately 86%) for the bioprinted tumor cells compared to conventional culture application [72]. Bioink, which surrounded GSCs with biomaterials was comprised of alginate, gelatin, and fibrinogen. In addition to exhibiting the features of GSCs and behaviors associated with vascularization, such as stemness, an increase in the characteristics and the expression of related genes were observed when this approach was employed.
4. Future perspectives
4.1. Advancement into 4D printing
Three-dimensional printing technology is evolving into a novel approach called 4D printing, which is based on the use of smart materials (substances with inherent properties or functionalities that can reshape or transform in response to external stimuli such as shape memory polymers (SMP) or alloys, and smart nanocomposites), the functionality of AM machines, and innovative design processes [115–117]. It has been suggested that 4D printing could provide a high throughput rapid processing platform for drug evaluation in GBM organoid models [118]. 4D cell-culture arrays were fabricated using thermo-responsive SMP, which formed 3D arrays and self-transformed into histological cassettes upon heating. The use of these arrays in single cell-derived GBM patient derived organoid-like models allowed for the scaling of screening capacity and simultaneous screening of organoid models. Utilizing this programmable self-transformation ability of the platform, the need for manual transfers were eliminated, demonstrating advantages over traditional 3D printing applications for cancer drug testing. Additionally, it was revealed that the 4D models were successful in recapitulating heterogenous cell populations and capturing expression profiles and tumor cell phenotypes in patients with GBM through a 2 week drug testing period. Although it is evident that further studies in reproducing neural architecture and preserving tumor microenvironment components would be beneficial, 4D platforms remains promising in providing an alternative, effective, and high throughput platform for personalized cancer drug testing.
Four-dimensional bioprinting has also provided significant advancements in the medical industry, allowing for more precise production of custom smart implants, instruments, and devices. It is designed to replace traditional scaffold fabrication methods, with the primary goal of fabricating implants with specific geometric parameters and greater versatility in changing the shape of the model [119]. Organ printing, tissue engineering, and self-assembling human scale biomaterials are all examples of how 4D printing is transforming the healthcare industry. Furthermore, 4D printing can help with advancement of biomedical splints, stents, orthodontics devices, and biomimetic setups in accordance with human development [120–122]. In the field of bioprinting organs and tissues, 4D bioprinting can introduce bioink-printed microtissues (e.g. cell-laden microgels) that can undergo maturation through cellular coating, self-organization, and/or matrix deposition to shape functional tissue constructs over time [123, 124]. 4D-printed structures' self-remodeling and functional maturation properties could help in the fabrication of biologically complex hierarchical constructions similar to native bone tissue. Multiple 4D-printed techniques could also be used to create microvascular systems and nervous networks [125]. The structural and functional transformation features of 4D bioprinting could be used to develop and monitor printed models with specific shapes, sizes, functional times, and working sites over time, meeting tissue engineering and clinical application requirements. By using stimulus-induced structural deformation or cell-assisted tissue maturation, 4D bioprinting holds great promise for creating engineered functional tissue structures and regulated drug delivery. However, there are several challenges in this area that need to be addressed in future studies in order to advance the science in the mentioned perspective: The number of responsive materials that can be printed is limited, and the majority of them are only responsive to one type of stimulus [126]. Additionally, deformation of 4D bioprinted constructs is currently restricted to basic deformations including folding/unfolding and self-organization.
4.2. Integration with artificial intelligence
Machine learning (ML), one of the subdivisions of artificial intelligence, which includes approaches such as supervised-, unsupervised-, reinforcement-, and deep-learning, is a set of algorithms that aim to discover estimated mathematical expressions of the real world by using historical data. Complexities that occur with the increasing values of input and output, specifically in bioprinting, demonstrate the need for using ML technology in related fields [127]. ML has already been emerging to solve challenges that are encountered at the stages of pre-processing, processing, and post-processing of 3D bioprinting [127]. A predictive ML model was developed to predict cell viability depending on four crucial parameters used in SLA-based bioprinting: UV intensity, UV exposure time, gelatin methacrylate concentration, and layer thickness [128]. Although projection SLA has proven to have several advantages in providing high cell viability owing to its printing mechanism, using the 3D bioprinting method has remained a challenge. Moreover, apoptosis of the cells was observed as a result of exposure to UV irradiation in SLA-based bioprinting. In addition, the prediction of cell viability of existing physics-based models has remained limited because of the complexity of cell biology and cell recovery mechanisms during the bioprinting process. These challenges were overcome as a result of the use of ensemble learning that covers ridge regression, k-nearest neighbors, random forest, and neural network. Results have revealed that this data-driven modeling approach has shown high accuracy in predicting cell viability.
Similarly, another ML method, Bayesian Optimization (BO), was used to estimate the filament formation of GelMA and hyaluronic acid methacrylate bioinks and the layer stacking of the 3D scaffold in extrusion-based 3D printing [129]. The use of BO in bioprinting has provided rapid and less tedious results compared to the traditional method of trial-and-error optimization. Big Data, which can be sourced from diagnostic images stored in health institutions, experimental data, and other databases, has been crucial for ML and thus, in building the infrastructure for bioprinting. Furthermore, in the nanomaterial field, the open-access database has provided a wide range of opportunities for modeling and applying ML in said web-based constructions. Contrary to the nanomaterial field, despite the abundance of research data in bioprinting, the restrictions on database access has continued to limit the use of ML in bioprinting, hindering improvements [127]. The use of ML in bioprinting is not limited to ensuring the optimization of research time and efficient bioprinting processes. The challenges associated with mimicking and maintaining structural and biological functionality has been considered one of the greatest challenges in generating artificial tissues and organs. However, cell-based mathematical models and software may provide some solutions for stimulating the biological functions and modeling the tissue, namely generate digital twins of organs. Additionally, by utilizing magnetic resonance imaging (MRI) and patient-specific live tissue models might increase the accuracy of the digital twin model. Thus, the importance of ML-based programmable design for 4D bioprinting is evidently promising in improving 3D bioprinting technology and translating research into medical applications [66, 127].
5. Conclusion
Treating glioma, one of the most malignant types of cancer, still remains challenging using current treatment procedures. To expand the knowledge regarding the invasive and aggressive behavior of glioma, various modeling strategies were proposed in the last decade, ranging from 2D environments to 3D structures and very recently 3D- and 4D-bioprinted models. Some of these emerging bioprinted models were reviewed in this article. Developed for recapitulating the brain environment and glioma tumors, these models offer a convenient means to investigate the treatment of CNS and PNS diseases and to develop patient-specific treatment options. In addition, the role of smart materials and artificial intelligence techniques were discussed to show their importance in opening new doors of studies, improving available technologies, and translating research into medical applications.
Acknowledgements
S T acknowledges Tubitak 2232 International Fellowship for Outstanding Researchers Award (118C391), Alexander von Humboldt Research Fellowship for Experienced Researchers, Marie Skłodowska-Curie Individual Fellowship (101003361), and Royal Academy Newton-Katip Çelebi Transforming Systems Through Partnership award for financial support of this research. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the TÜBİTAK. This work was partially supported by Science Academy's Young Scientist Awards Program (BAGEP), Outstanding Young Scientists Awards (GEBİP), and Bilim Kahramanlari Dernegi The Young Scientist Award. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. We kindly appreciate contribution of Irem Sultan Ilci, Melis Oktayoglu, and Sevval Doga Kul.
Data availability statement
No new data were created or analysed in this study.