Abstract
The market dynamics, and their impact on a future circular economy for lithium-ion batteries (LIB), are presented in this roadmap, with safety as an integral consideration throughout the life cycle. At the point of end-of-life (EOL), there is a range of potential options—remanufacturing, reuse and recycling. Diagnostics play a significant role in evaluating the state-of-health and condition of batteries, and improvements to diagnostic techniques are evaluated. At present, manual disassembly dominates EOL disposal, however, given the volumes of future batteries that are to be anticipated, automated approaches to the dismantling of EOL battery packs will be key. The first stage in recycling after the removal of the cells is the initial cell-breaking or opening step. Approaches to this are reviewed, contrasting shredding and cell disassembly as two alternative approaches. Design for recycling is one approach that could assist in easier disassembly of cells, and new approaches to cell design that could enable the circular economy of LIBs are reviewed. After disassembly, subsequent separation of the black mass is performed before further concentration of components. There are a plethora of alternative approaches for recovering materials; this roadmap sets out the future directions for a range of approaches including pyrometallurgy, hydrometallurgy, short-loop, direct, and the biological recovery of LIB materials. Furthermore, anode, lithium, electrolyte, binder and plastics recovery are considered in order to maximise the proportion of materials recovered, minimise waste and point the way towards zero-waste recycling. The life-cycle implications of a circular economy are discussed considering the overall system of LIB recycling, and also directly investigating the different recycling methods. The legal and regulatory perspectives are also considered. Finally, with a view to the future, approaches for next-generation battery chemistries and recycling are evaluated, identifying gaps for research. This review takes the form of a series of short reviews, with each section written independently by a diverse international authorship of experts on the topic. Collectively, these reviews form a comprehensive picture of the current state of the art in LIB recycling, and how these technologies are expected to develop in the future.
Export citation and abstract BibTeX RIS
1. Foreword: towards a sustainable circular economy in lithium-ion and future battery technologies
Gavin D J Harper1,2, Paul A Anderson2,3 and Emma Kendrick1,2
1 School of Metallurgy and Materials, University of Birmingham, Birmingham B15 2TT, United Kingdom
2 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
3 School of Chemistry, University of Birmingham, Birmingham B15 2TT, United Kingdom
The decarbonisation of society is the most pressing challenge of our age. We are locked into a trajectory where a degree of anthropometric climate change is inevitable; the question now is how much we can decarbonise to mitigate against the worst effects of this man-made change in our world. Key to decarbonisation is our transformation from energy systems reliant on hydrocarbon fuels as a dense store of energy, to a system where emissions-free energy vectors can be used to transport and store energy. Lithium-ion batteries (LIBs) are the best available current technology in mass production for storing electricity and offer high volumetric and gravimetric density relative to other battery storage technologies. We are seeing their adoption in a wide range of applications, and they have enabled electric vehicles (EVs) that are attractive to consumers and are being brought in ever greater numbers.
In front-runner countries like Norway, we have seen the tipping point reached, whereby the number of new EVs sold outnumbers conventionally fuelled vehicles. EVs are beginning to defy the expectations of a long cynical motor industry, and the number of automakers wholeheartedly embracing EV technology in their forward model ranges are now by far in the majority. Yet alongside optimism at the growing number of EVs on the road, there has also been the portents of a potential waste problem that could arise when these vehicles reach the end of their lives. We already see the signs of industry responding to this challenge. Globally, a wide range of firms are engaged in the race to recycle LIBs and recover the valuable and critical materials contained in them.
The adage that one person's trash is another's treasure is true for LIBs, where many see opportunity in the recovery of valuable materials from end-of-life LIBs. Yet a minority of materials contained in current batteries are recycled at present and some are not regarded as recyclable. There is a wider perspective possible than viewing recycling as an end-of-pipe activity. An integrative approach for a circular economy in EV batteries would consider where remanufacture, reuse and repurposing of batteries are appropriate, in order to extract the maximum utility from the materials and energy embedded in their manufacture. This is the happy situation that obtains for the much older—and much simpler—lead–acid battery technology, whose >99% recycling rates are driven by the value of the metals contained.
Applying a degree of foresight to this future circular economy, as industry scales dramatically, we may find that the processes and methods that have proven themselves at low volumes become an encumbrance as volumes dramatically increase. Several converging long-term trends make a circular economy for EV batteries ever more challenging—the price of new batteries is falling, changes in formulation mean that future batteries contain a materials inventory with an ever decreasing value and so the margin for recyclers is under pressure. This leads us to the conclusion that technologies and approaches that may have been appropriate for a low-volume, high material value industry will not necessarily be suitable in a high-volume, low material value industry.
The key goal of our Circular Economy Roadmap for LIBs is to present a range of compelling visions for the future trajectory of the LIB industry from a cross-section of experts. A broad range of knowledge is presented from a range of disciplinary perspectives and international research groups, in the form of 25 topic sections. We open by presenting the market dynamics and their impact on a future circular economy for LIBs. Safety is an integral consideration in the future handling of LIBs throughout their lifecycle and this is considered. At the point of end-of-life there is a range of potential options for LIBs—remanufacturing, reuse and recycling. These potential options are evaluated, as well as improvements in the systems that can be used to make this triage decision. Here diagnostics play an important role in evaluating the state-of-health and condition of batteries and improvements in diagnostic techniques are evaluated. At present, manual disassembly dominates end-of-life disposal, however, given the volumes of future batteries that are to be anticipated, automated approaches to the dismantling of end-of-life LIB packs will be key. Here the digitalisation of the circular economy of batteries, with future designs of batteries providing enhanced, open information from internal diagnostics could aid more efficient processes, where information about the condition of batteries is available prior to disassembly, speeding the processes of triage and reducing or removing the need for gateway testing.
The first stage in recycling after the removal of the cells is the initial cell-breaking or opening step. Approaches to this are reviewed contrasting shredding and cell disassembly as two alternative approaches. Design for recycling is one approach that could aid the easy disassembly of cells, and new approaches to cell design that could enable the circular economy of LIBs are reviewed. For cells that are opened using shredding processes, the subsequent sorting of the black mass liberated from cells is required. Approaches to this are reviewed. For cells that are disassembled different methods are required to delaminate material from the cathodes; this alternative future approach to the recovery of active materials from cells is also considered.
Alongside all of these approaches to materials recovery, there is also a need for measurement and metrology, and evaluation of the recovered materials and a range of approaches to this are presented. Once material is recovered, there is a plethora of alternative approaches for recovering LIB materials. This roadmap sets out the future directions for a range of approaches including pyrometallurgy, hydrometallurgy, short-loop, and direct recycling, and the biological recovery of LIB materials. Furthermore, we also consider anode, lithium, electrolyte, binder, and plastics recovery in a range of approaches that could maximise the proportion of materials recovered, minimise waste and point the way towards zero-waste recycling of LIBs. We also consider some of the life-cycle implications of a circular economy in LIBs, both from a macro-systems point of view, considering the overall system of LIB recycling, but also on a micro-view, comparing and contrasting different LIB recycling technologies. The legal and regulatory perspectives on LIB recycling are also considered. Finally, with a view to the future, we consider how we might recycle some of the next generation technologies that are predicted to come after LIB technologies, looking at how the approaches used to recycle LIBs may find application in the circular economy of new battery types, and identifying gaps for research.
2. Safety in end-of-life lithium-ion batteries
Wojciech Mrozik1,2 and Paul Christensen1,2
1 School of Engineering, Newcastle University, Newcastle upon Tyne NE1 7RU, United Kingdom
2 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
Status
There is growing evidence that lithium-ion batteries (LIBs) are discarded improperly, e.g. mixed either with other recycling fractions (flammable paper, plastic etc) or thrown into general waste, and fires in waste collection vehicles as well as recycling facilities and landfills are a growing phenomenon in recent years [1]. Fires due to LIBs in waste recycling facilities in the UK are costing £158 M p.a [2] and the situation is significantly worse in the USA and Canada, with an estimated 1800 fires in such facilities 2019 [3]. This is a global problem that, as well as the loss of expensive plant machinery, recycling resource, potential closure of the facility and rising insurance premiums [1], has also lead to death or injury of employees [2] and the public [4]. Waste facilities, and the vehicles employed to transport waste materials, often have machines to crush and compact the waste, this triggers thermal runaway in LIBs causing the venting of highly toxic and flammable gases leading to fires [5, 6] or, with larger batteries, vapour cloud explosions as well as the risk of electrocution [7, 8]. As yet these fires have been due to small LIBs, (e.g. mobile phones), hence the hazard has been confined to fire. However, materials recovery may lead to direct contact of battery materials with human operators putting their health in danger. Besides the fire risk, shredding may lead to the release of carcinogenic dust from cathode materials or harmful gases including hydrofluoric acid and hydrogen cyanide [9].
Current and future challenges
Fires due to small LIBs are threating the global waste industry as a sector [10]: the relevant key challenge is to ensure that LIBs and waste electronic and electrical equipment containing these batteries are processed appropriately [11] and not allowed to contaminate municipal waste or dry mixed recycling waste that undergo highly mechanised processing. However the near future will see the processing of significantly larger batteries [10]: for example, there will be an estimated stockpile of ca. 70 000–106 000 end-of-first-life electric vehicle (EV) batteries in the UK by 2025: EV batteries can weigh 500 kg or more and at EoL may still retain 50%–60% of their orginal capacity [12]. EV batteries not suitable for 2nd life applications will have to be recycled (i.e. materials recovery): this may be a major materials flow as the draft standards IEC 63330 & 63338 and the draft EU Batteries Regulation (EUBR) [11] rely solely on the EV battery manufacturers being willing to provide detailed data from first life as means of assessing if the batteries are suitable for 2nd life use. Moreover, it has been suggested that recycling may have to replace 2nd life due to scarcity of the key metals: thus the draft EUBR requires 85% by mass of key metals in LIBs to be recyclates. Thus, increasing demands will be placed upon the nascent LIB recycling industry [2]: this then raises the spectre of informal and inexperienced processing or illegal disposal, as do weak or lax regulations, or reasons such as the absence of incentives [9]. Landfilling LIBs legally or illegally will present major potential safety and environmental problems [11–13] and this leads to a consideration of the access of the public to complete 2nd life EV battery packs, as well as their component cells and modules via online traders: these LIBs are employed by hobbyists to store solar energy and/or exploit time of use billing [14]. Any recycling system must be able to capture EoL LIBs from all sources, and transport them safely [1], to avoid illegal disposal.
Advances in science and technology to meet challenges
Unfortunately, the general perception of the risks and hazards associated with LIBs is either low or confused with other types of batteries, e.g. Ni-Cd. Moreover, it is arguable that high energy density batteries can never be made truly safe: hence the advances to be made must be in the handling, processing and disposal practices of EoL LIBs. A fundamental problem is that LIBs are not designed for 2nd life or ease of recycling [11] and this impacts directly on the purity of any recyclates recovered, however this is down to the battery manufacturers to address. Materials flow analysis should be carried out to ascertain precisely where LIBs enter the waste stream: thus it is generally accepted that it is the public who currently place LIBs for recycling into the wrong waste streams [2] and, if so, a major research theme must be the most effective means to educate and alert the public and to ascertain to what extent the methodology should depend upon culture. One potential approach to this is more effective unambiguous labelling and conveniently located, readily accessible and dedicated collection points. This would be assisted if Life Cycle Analyses included actual data on recycling rates. There have been major advances in detecting and extinguishing fires due to small LIBs in waste facilities, as well as in the design-for-safety of waste sites [1, 2], and these should be promulgated across the global industry. However, EV batteries and e.g. EoL batteries from grid-scale LIB Energy Storage Systems will bring a whole new range of challenges due to scale and volume. Research is needed on collection methodology, safe transport and storage. Again, raising the awareness and education of all stakeholders will also be a key aspect of this research, as will bringing stakeholders together e.g. waste facilities and local Fire and Rescue Services. Effective and efficient fire prevention and mitigation procedures as well as new advances in fire sensing and firefighting are needed, focused solely on large LIBs. A forward look is also urgently required to try and assess the challenges of the next generations of LIBs, including lithium-air [15] and solid state [16].
Concluding remarks
The advantages and challenges associated with LIBs are both due to the very high energy density (energy per unit volume or per unit mass) of these devices. The waste industry is already facing a serious crisis due to fires due to small LIBs from mobile phones, laptops and tablets entering inappropriate waste streams. Further, the throughput and size of LIB waste streams are set to increase very significantly in the near future. Without urgent action, the global waste industry could be under severe threat. Research needs to be undertaken and current best practice disseminated. Finally, over-the-horizon planning for the next generation of even higher energy density batteries needs to commence immediately.
Acknowledgments
This work was supported by the the Faraday Institution as part of its 'SafeBatt—Science of Battery Safety' Project (FIRG028) and 'Recycling of lithium-ion batteries (ReLIB)' Project (FIRG005 & FIRG027).
3. Remanufacture, reuse and repurposing of batteries in second life applications
Simon Lambert1, David Greenwood1, Paul Christensen1, Gavin D J Harper2, Prodip K Das1, Mohamed Ahmeid1, Zoran Milojevic1 and Oliver Heidrich1
1 School of Engineering, Newcastle University, Newcastle upon Tyne NE1 7RU, United Kingdom
2 School of Metallurgy and Materials, University of Birmingham, Birmingham B15 2TT, United Kingdom
Status
In the traditional waste management hierarchy, there are a cascaded series of options for the preferential treatment of waste streams. In the first instance it is preferable to reduce waste overall, but where waste is inevitable, the options in order of preference would conventionally be listed in order of preference as; reuse followed by remanufacture and if these are not possible then recycling with energy recovery and disposal as a last resort. In response to this various waste management hierarchies have been proposed for battery waste which almost universally call for an aspect of reuse [10, 17]. Reuse can be broken down into direct reuse in the primary application or secondary (even tertiary) reuse in a different application. Furthermore, reuse in the primary application can further be broken down into whether the donor battery remains intact (direct reuse) or whether remanufacturing is required (indirect reuse).
Earlier electric vehicles (EVs) and hybrid electric vehicles (HEVs) such as the Nissan Leaf and Toyota Prius had battery designs which were highly maintainable pseudo in-field—i.e. packs consisting of modules containing small numbers of cells were easily replaceable by the dealership. Where battery packs contained faulty cells or modules this meant several unusable packs could be consolidated to incorporate the functioning components of each to be remanufactured into either fully operable packs suitable for the original application or a remodelled pack for repurposing into a secondary application. The potential list of secondary applications for batteries is as numerous as those for new batteries however, given the degradation in energy and power density that will be seen in used batteries, applications with less stringent requirements in these areas will be of most potential (such as gird-connected stationary storage).
More recently design trends by some manufacturers, generally in a drive to improve energy density and cooling performance, have meant that manufacturing techniques and the use of smaller form factors have significantly reduced the interchangeability of battery subcomponents. In some cases, cells are bonded together with glues and adhesives, which whilst efficient to assemble does not lead to easy repair, remanufacture, reuse or recycling [18]. This leads to situations whereby single cell failures in a pack cannot be repaired by the dealership and can result in the need for full pack replacements for small failures, the cost and environmental impacts of which are highly contentious [19] (not least amongst consumers burdened with significant repair costs). Indeed, the so-called 'right to repair' movements are gaining political traction internationally [20].
In terms of general use cases, second-life battery systems are attractive for power-system services (such as operating reserve or frequency regulation) because the typical operating cycle is less demanding than those of electric vehicles (EVs) [21]. The peak power requirements typically require batteries to discharge at 1 C or less, and many services are only fully delivered in contingency situations which occur infrequently. However, power system services are often key to the stable operation of the power system and failure to deliver could have impacts as catastrophic as a full system blackout. Consequently, where second-life batteries are to be used to support power systems then a very high threshold of reliability would be needed. This requires that the grading processes (e.g. [22]) to determine the performance and safety capabilities of batteries destined for second life to be extremely accurate and reliable (see current and future challenges).
There may also be applications for second-life battery systems which are driven entirely by energy price variation. Intermittent renewable energy sources can lead to volatile energy prices (even negative prices during certain conditions) which creates opportunities for arbitrage within energy markets. In this case, second life systems with higher efficiency would be desirable since higher losses would directly impact the profitability of the system. This may also create opportunities for fast returns on systems which can be operated closer to their power and energy limits than newer systems with less consequence for failure due to their lower cost.
Future, net-zero electrical energy systems, which use high volumes of renewable energy to meet the demands of electrified heat and transport, will require balancing and stability services on timescales from milliseconds to months [23]. Energy storage systems—and battery systems in particular—are well positioned to meet many of these requirements. Some of the most likely services at the transmission level include primary and secondary frequency response, operating reserve, and balancing actions [24]. During the August 2019 power outage in Great Britain around half of the frequency response service which stabilised the system was delivered by battery systems [25]. Future transmission networks may also require provision of virtual inertia. Distribution networks will also require services to manage network congestion and restore supplies after network outages.
For second life application reuse scenarios (such as stationary storage), vehicles with low degrees of sub-component interchangeability need to be reused as full packs by technical necessity. This in itself is not necessarily a problem for reuse since particularly for large capacity applications the economies of scale make the lower efficiencies and re-engineering costs more economically viable. There are numerous examples of second life applications utilising large numbers of second life packs [26]. Conversely, whilst the flexibility that the interchangeability of the former design philosophy (i.e. that of the older (H)EV models) gives in reducing unnecessary waste in the primary application reuse scenario it also opens up the possibility of reengineering the underlying batteries, either from cell or small module format, into new battery packs for use in secondary applications. This is perhaps best suited to domestic or small commercial installations where the capacity of a full pack is unnecessary and thus a smaller pack derived from larger vehicle packs would be desirable. There are however few notable examples of this being done at any real scale commercially since the decoupling of the engineering process from the original equipment manufacturer (OEM) combined with a significant lack of regulation in this area has meant concerns over safety have been expressed [27].
Current and future challenges
Different jurisdictions have varying oversight in terms of codifying the use of second life batteries, however, what is generally common across jurisdictions is that there are currently either few or no codes or standards specifically targeted at the regulating the second-life applications of lithium-ion batteries (LIBs). The draft codes IEC 63330 (Requirements for reuse of secondary batteries) and IEC 63338 (The reuse of secondary lithium and nickel metal-hydride cells and batteries after extraction from the application they were first placed on the market with), and the draft EU Batteries Regulation (EUBR) are of direct relevance to the second life LIB market in those jurisdictions and stakeholders in these markets need to be aware of their implication.
Furthermore, a 2021 BSI report [28] identified second-life testing as a gap in standards, and the invalidity of type tests (employed in all international and European standards) on second-life batteries is made explicit in Clause 6.3 of BS EN IEC 63338 (19 January 2021 draft). Perhaps acknowledging the absence of an accepted test to assess the safety of second life LIBs, IEC 63330, IEC 63338 and the EUBR specify only that the safety of these devices is assessed based on the 1st life data in the battery management system (BMS). The absence of reliable testing regimes alongside the OEMs' proprietary data protection prohibiting access to the BMS data may not inspire confidence in the second life market. In addition, the standards and regulations rely on the EV OEM being prepared to pass on potentially valuable intellectual property to third parties which is a significant commercial and security barrier. Finally, there is also a major gap in the regulations governing the safe transport of second life batteries, in that UN38.3 applies only to new batteries placed on the market for the first time, and the requirements of the ADR do not apply to the public. As an example of the limitations of the guidance and regulation in this area, in the UK at present, a member of the public can collect a damaged and potentially unstable EV battery pack from e.g. a breaker's yard and take it home all perfectly legally.
Linking safety [7] and regulatory concerns [29] is perhaps one of the greatest challenges for setting up a second-life economy and is where the responsibility for guaranteeing performance (and safety) lies. A viable business model for second life would have to sit within a framework whereby the technical performance of a second life battery is defined and measurable. In primary applications this is generally linked to capacity (largely analogous to range for an EV) however since the second life application is likely to have very different demands to that of the primary other performance metrics such as power capability may need to be explored. Multiple techniques do exist to define performance and are actively being pursued by researchers these are difficult to achieve at scale or with good commercial viability. Also, since many performance metrics for batteries vary significantly depending on, for example state of charge or temperature, and are essentially interconnected with the application and the recent history of usage a general guarantee of performance of a second life battery for commercial contract satisfaction would be fraught with difficulty.
Advances in science and technology to meet challenges
One of the key factors that will affect the economics of operations at the end of a batteries first life is the time and labour taken to gateway test and sort modules. To that end, investigation has proceeded on the rapid evaluation of battery state-of-health to enable decisions to be made about the onward destination of the battery [26, 29, 30], additionally in the future there may be opportunities to evaluate packs before they leave the vehicle based on enhanced in-vehicle diagnostic data if this can be made available (see the section on the Digitalisation of Battery Recycling).
At present, second use models have been applied to batteries that have not specifically been designed with second life applications in mind. There are diverging trends in automotive pack design—on the one hand, some packs are heading towards a unitary construction where the pack is increasingly treated as a single-unit. On the other hand, others are designing batteries with service, maintenance and repair in mind. The section on design for recycling in this roadmap considers the latter approach.
In order to improve the economics of sorting batteries for reuse or recycle, the removal of manual operations could potentially speed the throughput of battery processing. To this end, robotisation of the testing process could lead to significant efficiency and economic gains [31].
Some of these challenges could be solved through increased standardisation of pack/module designs [32]. Whilst some convergence as a result of manufacturer collaboration and platform sharing is likely, it seems likely that those wishing to reuse batteries will have to contend with variety for the foreseeable future.
Where particular standardisation could aid repurposing decisions is around the sharing, format and transparency of data for batteries [32]. This is discussed in this roadmap around the digitalisation of recycling.
In addition to the technical challenges that need to be solved to enable greater reuse of LIBs, we are also likely to see an evolution of the business models used to consumer energy storage [33]. The capital cost of EVs has the potential to lend itself to energy storage as a service, rather than as a product. Leasing and rental models may in turn give manufacturers greater control over batteries when they reach the end of their lives. Although there is great technical potential, there is a lack of established, mature business models for second use [34]. In the future, conceiving of batteries as part of a product-service-system may aid in overcoming some of the barriers to second life battery adoption.
Concluding remarks
It is doubtless that it is technically feasible to remanufacture and reuse LIBs where their state-of-health permits. There are, however, technical gaps that need to be solved in order to optimise the efficiency of the sorting and grading processes, and design for remanufacture/reuse and recycling. Automated processing will be essential in improving potentially speeding this process and leading to economic gains.
There remain legal, regulatory and safety questions about the desirability of repurposing batteries with packs designed for one application in another application. With research, learning and experience it is anticipated that in time standards and regulatory frameworks will emerge that bring clarity to this new industry.
Finally, whilst reuse is technically possible, some have also drawn attention to the effect that reuse strategies may have on delaying the stocks and flows of critical raw materials back into the supply chain. Some may argue that second use applications are a poor use of older battery chemistries, which may be higher in cobalt content [35]. Whilst reuse exploits the battery to its maximum, improving the energy stored on invested (ESOI)[36] of batteries, it may not be best from a material-efficiency standpoint. Putting a high-cobalt content battery, in a reduced state-of-health in a less demanding second-use application, may make less sense than recycling that battery and sharing the material between a greater number of newer chemistry batteries, operating 'as new'.
Acknowledgments
The authors would like to acknowledge the funding from the UK's Faraday Institution supporting the Recycling of Lithium-ion Batteries (ReLiB: FIRG005, FIRG027 & FIRG057) and Science of Battery Safety (SafeBatt) projects.
4. Gateway testing/triage
Mohamed Ahmeid1,2, Zoran Milojevic1,2, Simon Lambert1,2 and Prodip K Das1,2
1 School of Engineering, Newcastle University, Newcastle upon Tyne NE1 7RU, United Kingdom
2 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
History and status
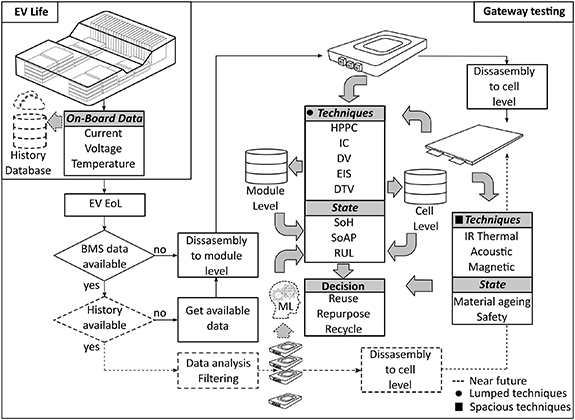
According to automotive standards, the electric vehicle (EV) battery is considered to have reached its end-of-life (EoL) when it loses between 20%–30% of its initial capacity [37, 38]. This figure is typically acquired by means of a battery management system (BMS) that monitors the battery pack status using key operational parameters such as voltage, current, and temperature [39]. In many cases the reported capacity is limited by the weakest cell or module within the pack that in turn dominates the performance of the whole battery system and hence the EoL decision either in the primary or secondary application [40, 41]. The principle of the recycling hierarchy dictates that reuse be prioritised over repair and recycling thus, in addition to the BMS data a reliable gateway testing strategy is paramount in the battery recycling industry as it aims to provide a piece of detailed information about the status of each individual module or cell. This informs decisions on whether each battery cell/module can be (a) reused in the EV, (b) reutilised in less demanding applications [42], or is (c) not economically or functionally viable and must be recycled [43]. Moreover, well-tuned gateway testing programmes are able to determine the appropriate second-life application that a retired battery cell/module can be used in [44] and predicts its remaining-useful-life (RUL) in this application through identifying key parameters such as capacity, impedance, power capability, and internal resistance [45]. As a result, the retired cells/modules can be classified and reassembled for the most suitable purpose. For fully spent batteries, it is feasible that gateway testing at the cell level can be used to comprehend the conditions of constituent parts or materials, this information can be used to inform downstream recycling processes in order to maximise the recovery of the most valuable active materials in shorter loop processes which can be subsequently recycled to produce new batteries [45–47]. This will ensure cycling stability, alleviate fast capacity fade, and increase the safety performance of the new, recycled batteries. However, the uses of gateway testing in sorting retired EV batteries is still somewhat challenging, due to rapid and constant evolution in battery chemistries, different designs and form factors [48], and the limitation of databases that can be utilised gateway testing algorithms must constantly be adapted and improved. In addition, the number of retired batteries has increased significantly in the last decade that must be replaced and recycled [49]. Thus, gateway testing is vital to sort this large number in a rapid and efficient manner. In reality, for high-volume industrial applications, a gateway testing procedure dedicated to EoL assessment is still in relatively early development stages and requires more work, especially in terms of testing time, cost, and reliability. This can be achieved through continuous research effort and investment devoted to developing a practical sorting package that comprehends various battery chemistries and extends the existing lab-based techniques from cell to module and pack level, which can be implemented either in the testing platform of retired batteries or the BMS.
Current and future challenges
The main task of a gateway testing programme is to accurately determine the state-of-health (SoH) of the retired battery that reflects its ability to deliver and store electrical energy. Accurate SoH estimation allows the user to avoid early disposal of the batteries, lowering the ownership cost, and mitigating unexpected failures [50]. Thus, considerable research efforts have been invested in recent years to address the issues related to battery SoH estimation, and several methods from different fields have been applied and reported in the literature [37, 50]. These methods can be classified into three main groups: model-based methods [51], data-driven methods [52], and experimental methods [53]. In the model-based methods, a physical model is adopted to mimic the behaviour of the battery for the estimation of the SOH. This includes the electrochemical model, the equivalent circuit model, or other empirical models. Whereas the data-driven approaches rely on a large set of data to map the relationship between relevant variables such as discharge capacity and SoH [54].
The experimental method is also called direct evaluation and includes capacity, direct current (DC) resistance, impedance, incremental capacity (IC), differential voltage (DV), and differential thermal voltammetry (DTV). Due to their perceived lack of complexity, direct evaluation methods are widely adopted in assessing and sorting EoL EV batteries [37]. Coulomb counting is one of the most common methods used for determining the remaining useful capacity in Ampere hour to predict the SoH of a battery [55]. The measurements obtained from charge/discharge experiments can be further investigated using the IC analysis to capture the ageing signatures and monitor the capacity fade [56]. Alternatively, the DV approach can be employed to analyse the charge/discharge data and estimate the battery SoH by identifying the degradation modes of the battery associated with its electrochemical properties [57]. Similar to previous methods, the DTV method by the ratio of time-varying voltage and temperature differentials curve analysis can be utilised to determine the battery's SoH [58]. For impedance-based SoH estimation, electrochemical impedance spectroscopy (EIS) is a very much exploited non-invasive technique to estimate the actual value of the impedance parameters. The EIS measurements are conducted either in galvanostatic or potentiostatic mode over a broad range of frequencies while the battery is at an equilibrium state, and an equivalent circuit model (ECM) is used to characterise EIS measurements and infer the SoH of the battery [55]. However, the EIS technique is sensitive to temperature and state of charge (SoC) [59]. It could be argued that SoH is a slightly ambiguous term since it generally only refers to a battery's capacity fade. As with the term health in the more familiar organic sense, poor or degenerating battery performance is not just limited to capacity fade. An example of another indicator of battery health is the evolution of the DC resistance as a health indicator for the state of available power which can be quantified, for example, by hybrid pulse power characterization techniques applying current pulses and following Ohm's laws [55] and spectral impedance techniques. Among lumped parameter techniques, spacious IR thermal imaging [60], acoustic [61] and magnetic [62] techniques can be used to detect ageing states of different materials and safety risks on the cell level. A pathway for the current and future gateway testing is schematically shown in figure 1 that illustrates ongoing gateway testing avenues and future directions. Whilst some of the aforementioned methods can result in extremely accurate and reasonably straight forward SoH assessment, a high measurement precision and extensive data processing is required that has an impact on their potential for commercial utilisation in an industrial environment. In retired EV batteries, the lack of history, and variety of configurations in battery modules within the pack add more constraints to this implementation. Therefore, further work is needed in this field to meet the demand of performing SoH assessment at module and pack level of retired EV batteries.
Figure 1. A pathway for the current and future gateway testing.
Download figure:
Standard image High-resolution imageAdvances in science and technology to meet challenges
For the recycling industry to operate at commercially viable scales, to avoid a large and growing volume of EV battery packs waiting for the triage and decision-making for a second life, the priority for the getaway testing/triage process is to be as short as possible testing time yet maintain the accuracy of predicted RUL. It is currently common that EV packs which arrive in recycling facilities are between seven and eight years old and have no data history, even if their BMS is available early EV models are not so sophisticated that the triage process can rely on it.
Typically, batteries are connected in series and/or parallel to form modules, which are then connected in series to form an EV battery pack. Assessment of the SoH on the pack level is a very difficult process because ageing over the pack is influenced by factors such as temperature gradients between modules and cells in the modules, the electrical imbalance between cells and ageing non-uniformity over the cell surface. With expected higher charging rates in the future, ageing inconsistency over the battery pack will only increase. Disassembling modules down to individual cells and then triage of these batteries at the cell level is a time-consuming, expensive and potentially unnecessary process, depending on the module design. It sometimes can even be impossible without irreparable damage to the cells. Triage on the module level presents a good solution from a triage time and SoH accuracy point of view. To date, most of the research and databases for battery SoH diagnostics have been done at the cell level in laboratory conditions. To progress to the greater scales and throughputs required for commercialisation, there is a need for multi-physics databases on the module and pack level which can be utilised in the getaway testing/triage process.
In the future, great assistance in EoL triage should come from OEMs or EV battery manufacturers. Firstly, improved and more optimised cooling systems will lead to more uniform ageing (modules over the pack and cells over the modules). Greater uniformity in ageing over the pack/module will lead to the reduction of gateway testing/triage process time as only a small number of the modules should be tested for battery pack SoH assessment. Secondly, the existence of data history on the module level (e.g. charging/discharging curves and operating temperature) could be of great help for the gateway testing/triage process. Such data can be post-processed and used for the generation of large datasets which can be utilised by data-driven and machine learning approaches [63]. With the increase of cloud computing technologies today, such a scenario is a real possibility as the data need not be permanently stored on or processed by the vehicle's systems. As a result, the gateway testing/triage process will be more focused on data history analysis and processing to assess the battery pack's SoH and RUL.
Concluding remarks
Many techniques are available for Li-ion battery's SoH and EoL assessment on the cell and module level, but they are mostly applicable to laboratory conditions. To enable greater take-up of gateway testing/triage processes for industrial applications, improvements of multiphysics check-up databases on the different levels are needed. Also, research is required into reducing the testing time of these techniques to be reduced and to be as short as possible, which would lead to the potential for further technological improvements and combinations of different techniques & technologies. Lack of battery pack history is one of the main concerns, but with BMS improvements and cloud technologies development, this problem is not unsurmountable.
5. X-ray tomographic imaging in diagnostics for 2nd life batteries
Wenjia Du1,2, Dan J L Brett1,2 and Paul R Shearing1,2
1 Electrochemical Innovation Lab, Department of Chemical Engineering, University College London, London WC1E 7JE, United Kingdom
2 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
Status
Spent batteries from electric vehicles (EVs) may present safety and waste-management issues from their middle to end-of-life [10] and examining the state-of-health (SOH) for secondary application remains challenging. Commonly available diagnostic techniques provide limited and indirect information; for instance, electrochemical impedance spectroscopy (EIS) is widely used to monitor the cell's impedance, indicating its electrochemical performance [64] and, the geometric change of entire cells is typically measured by either the Archimedes' principle or by a gauge metre [65]. Consequently, the internal cell structure and its relation to SOH have remained largely unknown. Non-destructive 3D x-ray computed tomography (CT) can reveal defects, gas evolution, thickness changes, morphology of active materials and alignment of electrodes to complement this understanding.
Current and future challenges
The growing number of EVs present a serious waste-management challenge when batteries reach the end-of-life (EOL). Thus, re-use of these EOL batteries is preferred as they retain sufficient capacity (typically 80% of original value) for less demanding applications (i.e. micro-grids), thus minimising the cumulative burden on the environment and cost. Estimating the SOH of these batteries is essential to ensure safe usage, however diagnostic tools used in this assessment must be non-destructive. It is common to monitor the capacity fade and impedance increase via electrochemical measurements, such as open circuit voltage and EIS. However, SOH cannot be simply determined from a single measurement and complementary understanding of the integrity of the internal architecture of EOL batteries must also be considered. The degradation of EV cells usually involves severe architectural or structural deformation, in particular for pouch cells [66], wherein gas generation may distort the electrode structures and thus lead to internal short-circuits. As the degradation coincides with gas evolution [67] the ageing-induced gas via electrolyte decomposition needs to be considered as a crucial metric of SOH. Invasive methods for SOH assessment are undesirable and make the battery unusable [68], hence, the application of non-destructive x-ray CT provides a significant opportunity to understand the SOH by visualising and quantifying the internal cell structure in 3D. Its non-destructive nature permits investigations without damaging the cell, ensuring those batteries can be re-directed to other non-destructive diagnostics or second-life applications.
It is worth noting that some technical challenges exist that currently prevent the implementation at the scale and speed required for secondary applications. Firstly, there is a balance between the desired resolution and the field-of-view (FOV) as it is difficult to observe small defects in larger format cells [69]. Secondly, the inherent high-aspect-ratio issue for scanning a large flat pouch may limit the x-ray transmission (at a certain angle) during the sample rotation during image collection [70]. Thirdly, it is almost impossible to scan the entire cell module due to the limited x-ray beam energy of conventional x-ray scanners and, the multilayer structure of cell may have similar signal-to-noise (grayscale value) level and suffer from image artefacts which may restrict subsequent analysis, in particular for those features around the tab area. Moreover, large numbers of cells disassembled from millions of EVs require high-throughput characterisation; with current bottlenecks, they are unlikely to be all examined by x-ray CT. Thus, it is vital to deploy x-ray resources wisely and find the most representative cells and fully understand their characteristics to estimate the SOH and predict the lifetime of other batteries. This will also motivate the design and construction of an automated system with fast x-ray acquisition for high-throughput diagnostics.
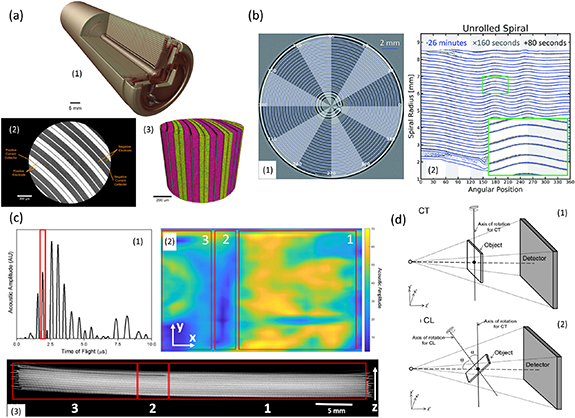
There are numerous x-ray case studies in the literature focusing on both cylindrical and pouch cells, demonstrating how x-ray CT help understand the degradation processes and electrode materials. At cell level, for example, Gelb et al [71] used multi-scale x-ray imaging to investigate the microstructural characteristics and failure mechanisms of an 18 650 Li-ion cell (figure 2(a)). Kok et al [72] developed a novel algorithm to quantify the delamination of the 18 650 jelly-rolls by highlighting the imperfections that arise at different cycle life conditions (figure 2(b)). Robinson et al [73] identified the manufactured defects in a Li-ion pouch with correlative x-ray CT and acoustic spectroscopy, highlighting that SOH can be better understood by multi-modal measurements (figure 2(c)). Based on Faraday's Law, Li and Hou [74] have used x-ray CT to detect the capacity of lithium-ion batteries under various working conditions. They presented a mathematical model by coupling the battery working conditions of the first-life application (i.e. cycle, discharge current, depth-of-discharge, temperature, and actual capacity) with the structural parameters (i.e. gray value of active materials). However, considerable efforts are still required to develop a robust model to enable rapid, accurate determination of the SOH via x-ray imaging.
Figure 2. (a) x-ray CT of 18 650 battery: (a1) 3D image of the entire 18 650 cell reveals the spiral cell architecture, inner mandrel, and cell safety devices; (a2) an enlarged region from (a1) examines finer layer details; (a3) the virtual slices were rendered in a 3D volume. Reprinted from [71], Copyright (2017), with permission from Elsevier. (b) Virtual unrolling of 18 650 Li-ion cells: (b1) slice of a cell after edge enhancement (the blue line shows the contour); (b2) A plot of the radius of the spiral versus the angular position for three different acquisition times. Reproduced from [72]. CC BY 3.0. (c) Diagnostic analysis of a Li-ion pouch cell with a defect using correlative methods: (c1) a sample acoustic signal from the area without the defect highlighting the third layer within the cell as the peak of interest; (c2) a 2D raster of acoustic scans corresponding to the amplitude variations of the peak at ca. 2.0 μs (with a 0.4 μs range applied to account for small ToF shifts); (c3) 2D slice of the reconstructed cell confirming the defective region. Reproduced from [73]. © The Author(s). Published by IOP Publishing Ltd CC BY 4.0. (d) Schematic diagrams showing (d1) a conventional circular scan computed tomography (CT) setup, and (d2) the computed laminography (CL) setup. Reproduced from [70]. © IOP Publishing Ltd CC BY 3.0.
Download figure:
Standard image High-resolution imageAdvances in science and technology to meet the challenges
The growth in maturity of x-ray CT techniques, including hardware (i.e. fast-readout detectors) and software (i.e. reconstruction algorithms), and their flexibility as part of a portfolio of diagnostics techniques, provides an opportunity to understand battery SOH.
Generally, cylindrical cells are much easier to scan by x-ray than pouch cells given their size and rotational symmetry. Although lab-based x-ray CT systems may struggle to cope with large pouch cell form factors (due to the low signal-to-noise ratio issue), the development of x-ray computed laminography (CL) and the application of High-Aspect-Ratio Tomography (HART) may help to overcome this. Using the CL protocol (figure 2(d)), the cell would be able to move closer to the x-ray source by tilting the rotation axis. Thus, this approach minimizes the artefacts and increases the effective FOV of the detector, allowing larger objects to be imaged [70]. Normal x-ray CT applies evenly distributed projections along the rotation angles, whilst the HART protocol [75] provides the capability to scan a thin and wide sample by collecting fewer projections along the wide side and more projections along the thin side, and adaptively altering the exposure time. Furthermore, advanced reconstructions (either via iterative or deep-learning approaches or both) could be used to generate higher quality or even super-resolution x-ray images.
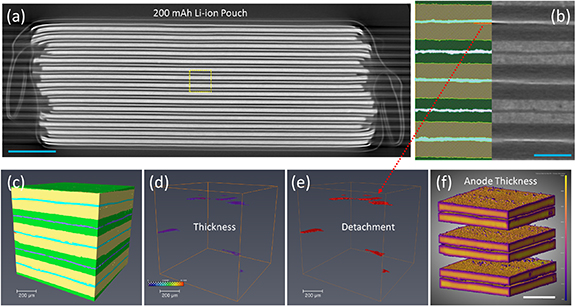
The 'stitching' of tomograms is a practical approach to resolve those small features (hundreds of micrometres) distributed in a large cell (tens of centimetres); here, we present a high-resolution (down to voxel size of 1.6 µm), multiscale advanced x-ray CT study of a small pouch cell in figure 3. The 3D image of the Li-ion cell enables direct visualisation and quantification. It should be noted that time-lapsed x-ray CT can be applied here to capture temporal scale information (i.e. morphological evolution) upon cycling [76]. Thus, 4D (3D plus time) datasets show architectural changes over time and ageing-induced deformation may be further investigated by the digital volume correlation approach. Whilst this study provides fundamental insight into the evolution of cells during cycling, revealing the microscopic changes that influence cell SOH, and therefore their viability for second-life application; the limited FOV associated with high-resolution imaging and the very low throughput means that this technique is unlikely to find a practical application for cell metrology. However, recent advances in high throughput macro-scale imaging indicate the possibility to rapidly evaluate cylindrical cell architectures in minutes (figure 4).
Figure 3. High-resolution x-ray CT (under HART) of a commercial Li-ion pouch cell: (a) 2D cross-section slices from 3D tomographic images of Li-ion cell at pristine state, showing the overall microstructures. The voxel size is 10.4 µm; (b) a high-resolution interior tomography shows 2D microstructures (right) and associated segmentation (left) in the region of interest (ROI), demonstrating the anode (yellow), cathode (green), Cu current collector (CC) (blue), Al current collector (purple) and porosity (red). The separator is difficult to resolve due to low Z. The voxel size is 1.6 µm; (c) 3D volume-rendered image of the ROI in (b); (d) 3D thickness distribution of the pre-existed porosity, the colour bar ranges from 0 to 13 µm; (e) 3D porosity shows the original detachments (manufacture defect) between the Cu CC and anode. We suspect the gas may generate at these areas; (f) 3D thickness distribution of the anode, the colour bar ranges from 0 to 110 µm. The scale bars represent 2 mm for (a) and 200 µm for (b) & (f).
Download figure:
Standard image High-resolution imageFigure 4. X-ray CT of 2nd life 18 650 Cell. The experiments were performed on same cell using same scan parameters (beam energy of 190 kV results in power of 24.7 W, 1 mm Cu filter, bin1 with voxel size of 37 µm, and exposure time of 1 sec) except the projection number: (a) 3001; (b) 1501; (c) 801; (d) 401; (e) 201 projections. The associated acquisition time of tomography: (a) 50 mins; (b) 25 mins; (c) 13.3 mins; (d) 6.5 mins; (e) 3.3 mins.
Download figure:
Standard image High-resolution imageRecent advances in deep learning (DL) using neural networks open up a new segmentation approach that could significantly improve the efficiency of image analysis and provide a pathway to evaluate 'noisy' images, thus allowing even shorter collection times [77]. DL is able to accurately recognise materials with similar contrast, for example, gas and graphite anode, but requires a considerable computing source along with expertise in specialized software packages. The development of open-access battery libraries of x-ray CT/CL scans and other diagnostic datasets related to cell voltages, rate, and impedance will also accelerate battery performance, durability and safety investigations. Electrochemical data can be trained to for predicting cell life by ML [78].
Concluding remarks
It is necessary to perform non-destructive measurements to estimate the SOH of EV batteries at the end of their life, to evaluate their suitability for second-life application. SOH determination must be robust for safe second life operation, which is enabled by hierarchical measurements in combination with other diagnostic tools (i.e. acoustics and EIS). X-ray CT/CL can play an indispensable role in contributing to SOH determination by evaluating specific degradative processes and capacity retention for an EOL battery, non-destructively. Volumetric information (i.e. greyscale, electrode deformation, electrolyte consumption, thickness, volume size and distribution of ageing-induced gas products, etc) can be quantified and correlated with battery operational history. Based on the experimental data acquired, it can be further trained for adaptive models for diagnosing degradation processes that affect battery SOH, thus reducing the cost and decision time for second life evaluation. With the improvements in high throughput imaging and artificial intelligence, we anticipate this will become increasingly valuable for second-life qualification.
Acknowledgment
The authors acknowledge financial support from The Faraday Institution ReLiB2 programme (FIRG027). P R S and D J L B acknowledge the Royal Academy of Engineering for supporting their respective Research Chairs (CiET1718/59 and RCSRF2021/13/53).
6. Battery pack automated dismantling and disassembly
Alireza Rastegarpanah1,2 and Rustam Solkin1,2
1 School of Metallurgy and Materials, University of Birmingham, Birmingham B15 2TT, United Kingdom
2 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
The importance of automating the process of disassembly of electrical vehicle batteries
Following the Paris Agreement [79], governments worldwide are setting targets to achieve environmental performance objectives with the aim of reducing human contributions to climate change. One of the main targets is a shift to electric vehicles (EVs), in order to reduce carbon emissions associated with transport. Recently, the UK government has gone to the extent of pledging to ban the sale of fossil fuel vehicles by 2030 [80]. Also, the EU has recently announced plans to ban the sale of such vehicles by 2035 [81].
These trends suggest that the number of EVs hitting the roads will continue to rise rapidly in the coming years. A direct consequence of this will be an increasing number of EV lithium-ion batteries (LIBs) reaching end-of-life (EOL), once the battery has reached around 70% of its original storage capacity. When the current global >8 million plug-in EV fleet reaches EOL, this will result in a battery waste inventory in the region of 2million tonnes, and 4 million cubic metres [10]. By 2030, a further 125 million of the world's 1.5 billion cars are expected to be electric. A key aspect of manufacturing the LIBs is the 'critical materials' such as cobalt, nickel, manganese and lithium, which represent 65% of the overall cost [82].
The labour costs in Europe, for manual disassembly of LIBs, equate to a majority of the value of extractable materials [10]. Currently labour costs are rising rapidly in Europe and North America with significant labour shortages. Furthermore, human disassembly of LIBs poses significant potential hazards in terms of fire, explosion and toxicity. Lithium-based battery materials can become highly unstable (with abuse, faults or simply with age), burning spontaneously (and inextinguishably) when exposed to air or moisture, while emitting toxic, corrosive and highly carcinogenic gasses, which can also lead to explosion [83].
The most urgent and critical recycling problem, in terms of human and environmental safety, is the safe and efficient recycling of EV LIBs. One of the best ways to encourage high recycling rates, is to make the process economically efficient, to increase the incentives for correct disposal and reduce costs associated with end-of-life treatment. Here robotics and automation can play a key role in improving the economic efficiency, as well as safety, of end-of-life LiB processing.
Current and future challenges
Although fully automating the process of disassembly of LIBs would guarantee cost and time efficiency, it is challenging for a number of reasons. Several studies indicated that the extreme variability of battery pack designs is the main antagonist for full automation. Thompson et al believe that automating this process is hindered by the range of battery pack designs as well as the fixings and glues used to construct them [84].
Gerlitz et al also recognized the use of non-detachable joints, either welding or adhesive, as a challenge to battery disassembly particularly in combination with the inherently hazardous nature of this process [85]. As non-detachable joints require some level of destructive separation, in turn allowing for the possibility of triggering explosions or other accidents. The lack of design standardisation is an obstacle for robotizing the process of disassembly. Additionally, the unavailable specifications as well as the unknown conditions at EOL of LIBs lead to further unpredictability. However, the new EU Battery Regulation proposal includes battery labelling requirements together with the creation of battery passports to assist the circulation of information [86].
Though robots are already used in the manufacturing processes of the automotive industry, they carry out repetitive movements from highly precise positions and operate in controlled environments [10]. The challenge of implementing robots in the dismantling of LIBs lies in the much larger uncertainties tied to this process. The slightest deformation that may occur during the operational life of the battery, possibly due to vehicle collisions or overcharging, can lead to unpredictable uncertainties that have to be dealt with during automated disassembly. Uncertainties requires the ability to adapt to diverse situations with some level of flexibility. While easily provided by a human, it is not as straight-forward for a robot control system. Addressing this challenge requires some form of artificial intelligence coupled with machine vision techniques and other sensing modalities. The hazards inherent to LIB disassembly are a further impediment, as a simple mistake in this context could cause an escalation resulting in the battery exploding or catching fire and possibly harming humans. In conclusion, battery disassembly poses potential risks while also requiring a high degree of precision. However it also involves numerous uncertainties, thus posing a great challenge for automation. Developments at the cutting edge of artificial intelligence and robotics may be the solution to this issue.
Advances in science and technology to meet challenges
In order to ensure a circular economy, all viable processes have to be considered at EOL, namely: reuse, re-manufacturing and recycling. In general, we should try to re-use battery components at the largest scale of assembly, before disassembling further. For example, a pack should be re-used if possible. Only once this has been determined to be no longer useful, should the pack be disassembled to seek some modules which may still be usable. After this, modules might be disassembled to seek re-usable cells. A large part of the value is lost, with each successive scale of disassembly.
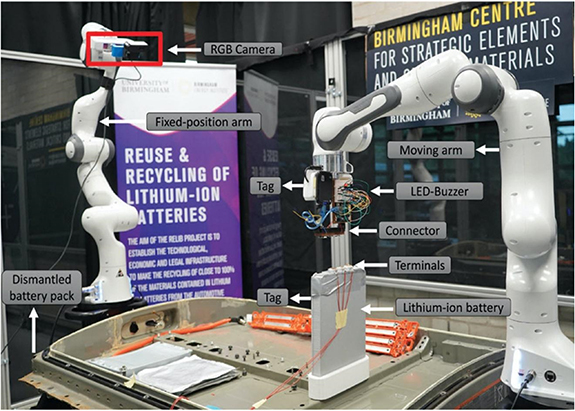
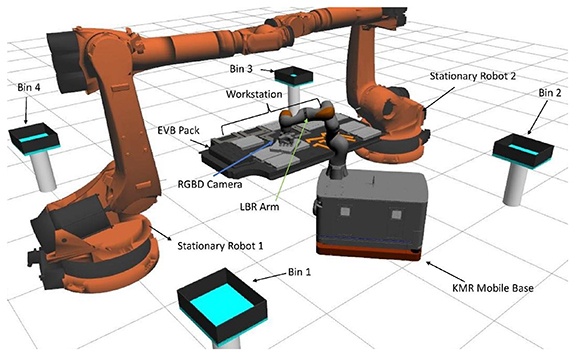
To make these decisions, the state-of-health (SoH) of the batteries has to be determined to establish which of these paths is best suited for a certain battery. Currently, the SoH is analysed through a process of discharge which takes a significant amount of time and highly-trained personnel, and hence is rather costly [19]. For the first time, Rastegarpanah et al proposed a proof of concept technique for robotizing the process of electrochemical impedance spectroscopy testing for estimating the SoH of a Nissan Leaf LIB using a robot (figure 5). In this technique, a custom-designed connector was attached to the end-effector of a collaborative robot (i.e. Franka robot arm) in order to make a firm connection with the battery terminals and to the Potentiostat [31]. The experiment proposed a framework to minimise the human interaction during LIB testing.
Figure 5. Experimental setup used to robotize the EIS testing. Visual Servoing is used to guide the robot towards the terminals of the battery. Then impedance control is used to give compliance to the robot to make a smooth interaction between the end-effector connector and the LIB terminals. Once the connection created, the Potentiostat starts collecting the LIB data. Reproduced from [31]. CC BY 4.0.
Download figure:
Standard image High-resolution imageRastegarpanah et al used extracted parameters from impedance data, and applied various neural network (NN) techniques to estimate the SoH of LIBs [87, 88]. The results suggested the efficacy and high accuracy of NN methods for estimating the SoH of LIBs, in addition such non-destructive methods would save time and costs, and it would allow for better sorting of the batteries at EOL [87].
At the moment there is no study detailing a fully automated disassembly line for EVs LIBs, however there are some studies pioneering human-robot collaborative disassembly systems. A case study for an example of such a hybrid work-station is presented by Wegener et al [89]. However, safety aspects were not considered in this study.
The process of LIB disassembly should be considered from different levels; pack level, module level and cell level. In [90], Choux et al proposed a task planner for automating the process of disassembly of EVBs up to the module level. The proposed framework uses a machine vision technique (i.e. YOL0) to annotate the components of a Audi A3 Sportback e-tron hybrid LIB pack. The proposed method has the capability of recognizing which component to remove first and decide on how to carry out the disassembly without a priori knowledge of the battery CAD models. However, still these methods need improvements in order to be generalized to cope with a variety of battery packs.
In terms of robotic disassembly actions, Rastegarpanah et al proposed an adaptable framework for cutting the battery module, developed by a memory-augmented NN [91]. The developed framework is a proof of concept tested in a simulation environment, and it proposed a trajectory-independent robotic path following for cutting, where the properties of the environment are uncertain. In another work, Rastegarpanah et al proposed a tactile based method for nut unfastening and this method was validated by unfastening the nuts of a Nissan Leaf LIB module [92]. The authors recognized unfastening as a main task in disassembly and showed the generalizability of the proposed method for unfastening the nuts in different sizes and shapes.
In a study carried out by Li et al a method for the automated separation of LIB pouch cells was proposed using a prototype disassembly system. The disassembly system is similar to a Cartesian robot with joints that can slide along rails corresponding to the x-y-z axes, which allows for great precision in controlling the movements. The disassembly system prototype was used to successfully treat inert mock-up cells. However the question of how to handle damaged cells remains a complex challenge.
The stage of post-disassembly or sorting has been investigated by Rastegarpanah et al in [93]. This study proposed a framework for sorting the dismantled LIB components. In this work, a mobile-manipulator robot is used to detect and classify the objects based on a developed Behaviour Tree structure (figure 6).
Figure 6. Sorting the disassembled battery components by a mobile robot based on a developed Behaviour Tree structure. Reproduced from [93]. CC BY 4.0.
Download figure:
Standard image High-resolution imageDespite the above growing body of studies carried out in this field, these challenges remain profoundly difficult, and substantial future developments are still needed towards fully automating the process of dismantling the EV LIBs.
Concluding remarks
The fast-paced growth of the EV market will call for efficient management of used LIBs to prevent ecological consequences of incorrect disposal, and also as an important source to meet the growing demand for battery materials. Therefore, a circular economy for LIBs will be needed to enable a sustainable transition towards electrified transport. A necessary condition for the circularity of the LIB economy is an appropriate infrastructure system for recycling, re-manufacturing, and consequently disassembly of EV LIBs.
Automation will be crucial for efficiently dealing with the large amounts of batteries that will be reaching EOL in the future, as manual disassembly is potentially hazardous and cost and time-intensive. Lack of standards in design, and insufficient data about the manufactured LIBs, are recognized as main barriers for robotizing the process. Towards automating this process, we suggest categorizing the level of autonomy to three classes of fully autonomous, semi-autonomous and manual. The fully autonomous level should be used for safe and repetitive tasks where the robot can independently complete a task successfully. The semi-autonomous tasks are those that require collaboration between human and robot, and some tasks are very complex to be completed by robot and still require the dexterity of human hands. Over time, we should aim to see more fully manual tasks becoming semi-autonomous, and semi-autonomous tasks becoming fully automated.
Acknowledgments
This research was conducted as part of the project called 'Reuse and Recycling of Lithium-Ion Batteries' (ReLiB). This work was supported by the Faraday Institution (Grant Nos. FIRG005 and FIRG027).
7. Cell opening (comminution/shredding)
Roberto Sommerville1,2, Anton Zorin1,2, Jessica L Durham3 and Emma Kendrick1,2
1 School of Metallurgy and Materials, University of Birmingham, Birmingham B15 2TT, United Kingdom
2 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
3 Applied materials Division, Argonne National Laboratory, 9700 S. Cass Avenue, Lemont, IL 60439, United States of America
Status
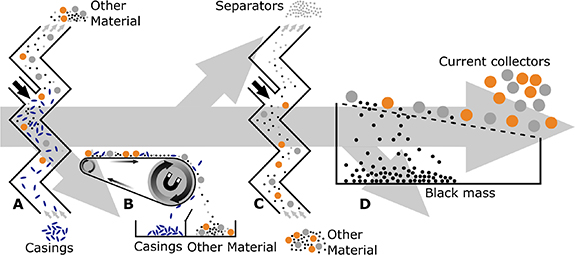
Shredding with subsequent processing is the current state of the art for LIB recycling. The purpose of opening a cell through comminution is to produce a free-flowing material which can be physically processed to isolate the various cell components [94]. For materials to be separable, they must be liberated, i.e: they must be finely divided to such an extent that components are not connected. Comminution is an important step in the recycling process as it allows components to be separated, improving the cost-effectiveness of the downstream purification processes. Comminution is achieved using shredders and mills and commonly takes place in two stages. The first stage opens the cell to produce a coarse intermediate fraction, achieved through a low-speed high torque shredder [95]. For safety, the first comminution process must utilise engineering controls to limit the likelihood of cells undergoing thermal runaway, and to contain the hazardous materials contained within the cells [7]. As the cells are shredded, short circuits will occur, resulting in a rapid discharge of any remaining energy, producing heat. In the presence of flammable electrolyte, these conditions could lead to a fire. In order to limit the likelihood of fire, controls are used such as gas blankets to exclude oxygen, sprays of water to control heat build-up, or a high throughput of air to control the temperature and limit the build-up of flammable gasses [96]. Ideally cells would be discharged prior to comminution to limit the release of thermal energy [95, 97]. After this initial shredding process, physical separation techniques can be used to isolate the separator and casings from the electrodes (see section 8). The second comminution processes the electrodes and separates the active material from the current collector. This is performed with more high-speed processes such as hammer mills [96]. This second comminution process relies on the relative brittleness of the active material coating compared to the much more ductile current collector [98], but will vary between production scrap and end-of-life materials [99]. Further advances in the field will allow for safe, scalable comminution processes which are able to achieve a high degree of liberation. A well liberated product can then be separated downstream, making the entire recycling process more efficient, and more cost effective, due to reduced cross-contamination of materials due to ineffective liberation processes. Examples of waste streams from shredding and size separation is shown in figure 7.
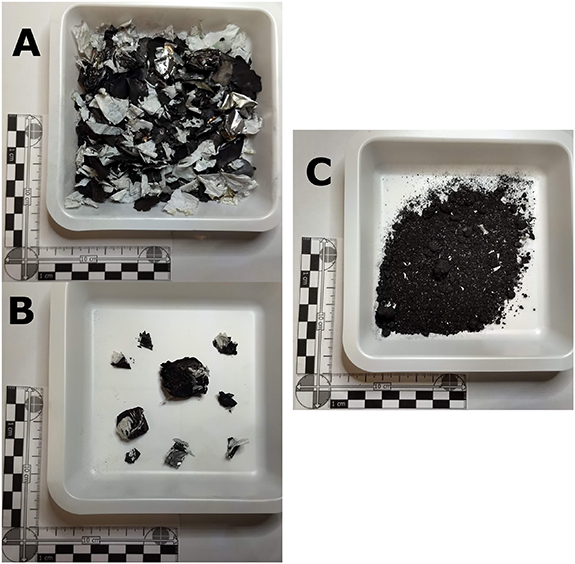
Figure 7. Coarsely shredded material (a). Unliberated material from coarse fraction with inclusions (b). Black mass contaminated with Cu, Al and plastics (c).
Download figure:
Standard image High-resolution imageCurrent and future challenges
The largest challenge for recycling, is the economic viability and environmental impact from the processing. The opening and separation stage being a key consideration particularly with potential harmful gases being produced. The whole of the process must be considered to realise the impact of the different processes. In comminution, the materials are immediately mixed to form a contaminated waste stream. The key challenges are around the safety, energy efficiency and recovery efficiencies. Therefore, the impact of the initial opening step upon the subsequent processes must be considered.
The most pressing challenges include a lack of a global harmonised system for labelling the active chemistries within cells, and the cell formats for cylindrical formats, shredding of the stainless-steel casing does is more energy intense to than laminated aluminium pouches, and the steel recovery is not improved with further comminution as it can be removed through magnetic separation. Consideration is required upon the down stream processes to minimise wasted effort and energy.
Mixed battery feed stocks can lead to contaminated waste streams post-comminution meaning that methods have to be developed that not only separate the components of a cell but also the various chemistries within a cell [100, 101]. This is currently being addressed with coarse shredding such that the current collector and black mass may be separated by sieving [99]. The electrodes are then milled again and undergo subsequent separation processes. These processes have achieved yields over 90% using mixed electrodes, with impurities of 1.9 wt% Cu, 0.8 wt% Al and 0.3 wt% Fe [102]. The current challenge is in achieving a higher yield of black mass whilst also lowering the Al, Cu and Fe contaminants. This would allow for a zero-waste process and permit more efficient and cost-effective downstream processing. Which may lead to direct recycling approaches.
Health and safety, environmental protection, and the viability of the recovered active materials must also be considered [94]. In terms of health and safety: the evolution of VOCs, respirable dusts, and the fire risk must be accounted for and mitigated. This may become easier as battery chemistries evolve, and flammable liquid electrolytes are no longer used. However other risks become more prevalent if finer comminution is required [103]. From an environmental perspective, recycling processes must be energy efficient and waste streams minimised or eliminated. Looking to the future, as the adoption of and age of EVs increases, there will be an inevitable rise in cells that have undergone thermal events coming into the recycling pipeline. These will have to be dealt with in a safe and responsible manner [94].
Advances in science and technology to meet challenges
A better understanding of how state of charge influences build-up of temperature during comminution permits safer comminution of discharged battery packs [95]. Real time monitoring of temperature and volatile organic content allow for improved safety during the initial comminution process [104]. Control measures such as gas blankets can have their purge rates increased in order to cope with sudden gas or temperature build-ups, or scaled back to improve efficiency.
Processing different battery chemistries separately will greatly simplify downstream separation processes and eliminate some forms of contamination. The option to separately process LiFePO4 (LFP), NMC and NCA based chemistries would eliminate Fe contamination in NMC, and Mn contamination in NCA, simplifying downstream processing and making hydrometallurgical recycling more cost effective. This is made significantly easier by proposed 'battery passport' regulations which would require information on battery chemistries be disclosed [11].
Instead of traditional shredding and milling processes, automated lasers or automated high-pressure jets of water (with optional abrasives) have been suggested as methods for opening cells [105]. Water jets would have the advantage of acting as a heat sink during the cutting process and have also been suggested for separating active material from current collectors [106]. Electrohydraulic fragmentation uses shockwaves from a spark discharge under water to break composite materials apart at their interfaces [107]. This method has been demonstrated to preferentially separate anodic active material from current collectors, and with further study could provide an energy efficient method to separate active material from current collectors.
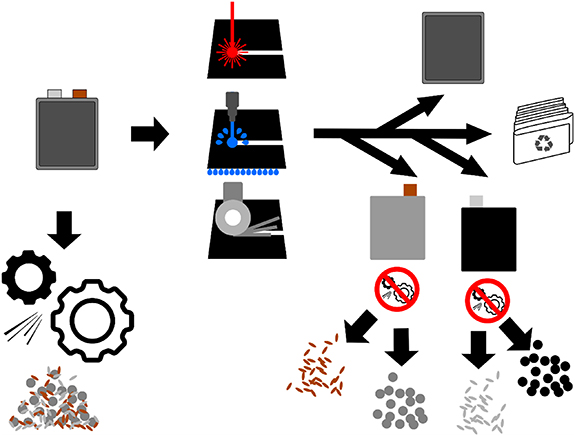
Comminution will inevitably produce fine particles which will contaminate the finest fractions of active materials. To overcome this, cells could be automatically unwound or un-stacked into the sub-cell components of anode, cathode, separator and casing, and each component processed separately in order to limit cross-contamination [101, 108]. (Figure 8) Such a disassembly route would be able to use in-line sensors to separate and identify different electrode chemistries based on their properties, further improving purity. This would be greatly helped if cells were designed to improve recyclability (see section 8). The second comminution phase to produce a black mass could be entirely omitted in favour of more selective methods to separate the active material from the current collector, as shown in section 10.
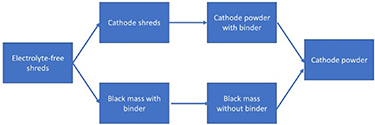
Figure 8. Instead of comminution, opening and disassembly can be utilised to improve material purity.
Download figure:
Standard image High-resolution imageConcluding remarks
Many of the state-of-the-art battery technologies start the process with cell stabilisation and cell opening, this process affects the downstream technologies required for further separation and concentration and requires safety, energy, and environmental impact considerations. Opening safely is key to control personnel and environmental concerns. Challenges arise from the chemical reactions which occur upon opening the cell, and potential for extreme thermal runaway and highly toxic chemicals. In addition, knowledge of the precise chemical content of the cells is lacking, Improvements in cell labelling and chemical inventories are required to pre-sort the battery chemistries and waste streams, as this will improve further extraction efficiencies and reduce energy use on downstream processes.
Technology advancements in automated and intelligent opening methods with advanced monitoring and sensing will enable adaptive control of the processes, reduce any potential the chemical release to atmosphere and reduce the subsequent level material mixing.
8. Cell disassembly and design for recycling
Andrew P Abbott1, Dana Thompson1 and Gavin D J Harper2
1 School of Chemistry, University of Leicester, Leicester LE1 7RH, United Kingdom
2 School of Metallurgy and Materials, University of Birmingham, Birmingham B15 2TT, United Kingdom
Status
Recycling lithium-ion batteries (LIBs) is technically possible and progressing at commercial scale [10, 96]. The economics of the process are however, governed by the purity of the recovered products together with the cost of the process (in hydrometallurgy, the cost of lixiviants and the energy of the mechanical separation steps). Studies on current recycling processes show that recycling spent LIBs leads to a decrease of 7% and 9% in associated CO2 emissions and energy consumption, respectively, compared to LIB production from virgin material [109, 110]. Substantially larger savings in other emissions have also been reported [111, 112]. It is imperative to decrease the amounts of energy and chemicals used to decrease the carbon footprint of the recycling process. Assuming a battery cost of $100 kWh−1, it has recently been shown that the permissible costs for profitable recycling lie in the range $2–6 kg−1 of battery material [108]. The same study also showed that there is a significant difference in the economics of recycling if the cell was dismantled rather than shredded at end-of-life. Disassembly has also been highlighted by Glöser-Chahoud et al as the key enabler to recycling automotive LIBs [113]. The first review on the topic of design for recycle has been published which discusses the aspects of battery design which complicate disassembly and product separation [84].
Many current pack architectures are dominated by small cells with lots of joints to achieve manufacturing economies of scale. Electrical tabs are mostly welded together (although Tesla have developed a tabless cylindrical cell), whilst cells are glued together using adhesives with functional properties to aid thermal management. This makes disassembly for repair or recycle almost impossible. Some designs are putting more active ingredients with less inert components arranged in larger cells. Some cells are combined directly in a 'cell-to-pack' configuration, leading to decreased packaging material and increased energy density. Electrode geometries can also simplify disassembly.
An alternative approach is to use all solid-state batteries where safety and pack opening is less of an issue as there are no flammable electrolytes. The aspects of cell design and process recycling have recently been discussed in an article by Tan et al [114]
Current and future challenges
The main challenges in cell disassembly lie in enabling easy and safe opening at end-of-life while maintaining cell performance and safety during its long and often arduous life. Design for recycle focuses on pack, module and cell architectures that enable subsections to be easily separated, preferably using intelligent robotic manipulation [90]. Current pack disassembly is complex but technoeconomic analysis shows how disassembly could be possible [115]. Hybrid disassembly protocols have also been suggested [116].
In this regard, one of the biggest challenges is the way in which cells are bonded to make modules and modules are assembled to create packs. Structural adhesives are the most common method for bonding and while these ensure the mechanical integrity of the pack, they do it at the detriment to cell disassembly. Clips, adhesives and wires complicate the protocols for autonomous robotic disassembly. This necessitates shredding as the route to material liberation. The challenge being addressed in several ways is how to maintain pack strength to withstand service stresses and accidental impacts without extensive use of structural adhesives. Figure 9 shows where some of these adhesives are used in a typical battery pack. Welding is also commonly used to impart structural strength and causes similar issues particularly with weld failure leading to a decrease in power [117].
Figure 9. Schematic diagram showing some of the uses of structural adhesives in a typical battery pack (Redrawn after). Reproduced from [18]. CC BY 3.0.
Download figure:
Standard image High-resolution imageThe most problematic polymeric components are the binders used in electrode construction. These are heavily fluorinated and have limited solubility in standard solvents, so they are difficult to apply and recycle due to the toxicity of the organic solvents used. They can also become brittle during service leading to in-situ delamination and cell failure. The challenge is to find stable binders which are dispersible using aqueous solutions.
In addition to the chemical and mechanical challenges of recycling there are numerous logistical and legal difficulties including cell formats, labelling and connectors not to mention the varied cathode formulations of which there are at least 16 on the open market [111]. Battery directives in the US, China and EU are beginning to deal with some of these issues.
Advances in science and technology to meet challenges
The main challenge in design for recycle is the development of new pack architectures, which have larger cells containing less junctions, and more material within fewer cells. Some of these are coming to the market [84]. These have fewer wires and connectors, which should simplify automated disassembly.
Using larger cells where the buzz bars can act as a structural component should reduce the need for structural adhesives such a polyurethanes and epoxy resins which are a major barrier to disassembly. A recent review has highlighted the new area of de-bondable adhesives where external stimuli can be used to cause intended failure of an adhesive bond [18].
To efficiently recycle cell materials in a pure form it is important to be able to separate anode from cathode foils. A recent study has shown how skimmers could be used to separate electrodes from a jellyroll cell [118]. This uses the cell membrane to aid separation although there are some signs that this becomes brittle through extended cell use. Early separator designs used to use simple polyolefins but recent designs have used composites, often with inorganic components which make recycling very difficult. Some pack materials are also layered with metallic foils and these also pose recycling challenges.
More ductile, water-dispersible polymers are becoming more common in electrodes, particularly in anode formulations and these are easier to recycle at end-of-life. Adhesiveless electrodes would also aid separation and recovery. Ultrasonic methods have been shown to significantly increase the rate of recycling [101, 119].
Development of legislation to incentivise manufacturers to recycle their spent LIBs could result in LIBs being designed for recycling. The 2019/2020 EU Battery Regulation addresses this with minimum recycling efficiencies increasing from 50% to 65% by 2025 and 70% by 2030. Declarations of levels of recycled content in new LIBs is another factor included [11, 120]. Simplifying design for disassembly would enable more material to be recovered in a purer form at a lower cost compared to using virgin material, which will further improve the economics of cell manufacture.
Concluding remarks
Recycling LIBs is proven to aid in the reduction of greenhouse gases, however to optimise the life-cycle impacts from cradle-to-cradle the energy and chemicals used must be kept to a minimum. Disassembly is the key to greater value retention during recycling, by keeping waste streams separate and enabling for a purer final product. Some present pack and cell architectures hinder this particularly through the extensive use of thermosetting adhesives, so simplified designs are required with larger cells and fewer fixings to allow for efficient robotic separation and increased recovery rates. Ultimately, coordination within the LIB supply chain is needed whereby manufacturers recognise the issues that waste processors face with spent batteries. Circularity in automotive steel and Lead Acid Batteries have already been achieved with over 99% being currently recycled. The challenge going forward is to design the power storage unit so that its components can be recovered and repurposed with a similar efficiency.
Acknowledgments
The authors would like to thank the Faraday Institution ReLiB project (Grant codes FIRG005, FIRG027 and FIRG057) and the UKRI Interdisciplinary Circular Economy Centre for Technology Metals (TechMet) Grant No. EP/V011855/1 for funding.
9. Physical processing & sorting of mixed waste—black mass separation
Roberto Sommerville1,2, Anton Zorin1,2, Jessica L Durham3 and Emma Kendrick1,2
1 School of Metallurgy and Materials, University of Birmingham, Birmingham B15 2TT, United Kingdom
2 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
3 Applied materials Division, Argonne National Laboratory, 9700 S. Cass Avenue, Lemont, IL 60439, United States of America
Status
Physical sorting of shredded lithium-ion batteries (LIBs) is used to reduce the quantity of material being fed into subsequent processes, reducing the energy demand and cost of these processes. Casing materials and separators are isolated from the current collectors and active material [121]. This can take place after all comminution has finished, or between the initial cell opening comminution process, and the active material/ current collector comminution process [96]. Removing the casings prior to the second comminution step reduces the volume of material being processed downstream, and the energy needed to remove the active material from the current collector. Separating the current collector from the active material allows metallic Al and Cu to be recycled, preserving the significant embedded energy costs of Al production and also Cu production, whilst reducing the volume of material being presented for hydrometallurgical recycling, which is not a cost-effective recycling route for the comparatively low-cost Al or Cu [96].
Casings are currently removed through two approaches, density and magnetic separation as shown in figure 10. Density separation can be performed using air in a zig-zag separator, where more dense materials such as steel and Al casings from cells and modules fall to the bottom, and lower density materials such as electrodes and plastics leave at the top [102]. A second zig-zag separator can also be used to isolate the low-density separator materials (which report to the light fraction) from the more dense electrodes (which report to the heavy fraction), and yet again to separate Cu from Al foils after removal of the active material [102]. Magnetic separation can be used in lieu of density separation to remove steel casings prior to the second comminution phase, but will not be effective at removing Al or plastic casings [94].
Figure 10. Current applications of physical processing: Removal of separators via density (a) and magnetic (b) separation. Removal of separators via density (c) separation, and separation of active material from current collectors via screening (d).
Download figure:
Standard image High-resolution imageSize separation is commonly used to separate the fine active material powder from the more coarse current collectors after the second comminution phase, and can be achieved using a vibrating screen [94, 99]. Electrostatic separation exploits differences in surface conductivity in order to separate materials. This technique has been shown to be effective at removing the non-conductive separator from the conductive electrode materials, achieving both a high grade and recovery [122]. Further advances in physical separation processes will improve the cost effectiveness of downstream separation processes, and may pave the way towards direct recycling of active materials.
Current and future challenges
A mixture of LIB cell chemistries are entering the recycling system. One way to simplify processing techniques would be to sort batteries by chemistry before they are opened, as is currently the case for portable batteries. Currently the majority of LIB cells fall into one of 3 formats: prismatic, cylindrical, and pouch. However this does not prevent new formats that better suit yet undeveloped chemistries emerging. This means that sorting processes must be designed to be resilient and able to deal with the variety of cell and hence electrode stack formats.
After comminution the subsequent mixture of separators, foils, casing and black mass that is present within the cell must be separated. As separators start to incorporate ceramic coatings for a higher temperature of failure, this presents a further recycling challenge in separating the polymer and ceramic subcomponents of the separator [7]. In situations where the batteries have not been sorted by chemistry, the black mass requires characterisation to determine which chemistries are present, and separation of anode from cathode and ideally cathode from cathode. Anodic and cathodic active materials can be separated using froth flotation and magnetic separation [123, 124]. Froth flotation is heavily affected by the presence of hydrophobic binders such as polyvinylidene fluoride (PVDF) [125], and flotation processes could be greatly improved by separation of valuable active components from less valuable inactive components such as conductive additives, binders, and other nano-material [126].
Using physical processing techniques to concentrate the valuable cathodic critical materials is essential for an efficient hydrometallurgical recycling process, as the lower value Cu and Al will not be recovered hydrometallurgically. The pinnacle of physical processing techniques might be able to provide a high enough purity of active material for direct recycling, but currently faces challenges, as the purity required is very high (section 19). This approach could reduce energy and resource demands as well as costs but requires an intact crystal structure from the active material [127].
Advances in science and technology to meet challenges
Advances in techniques are necessary to improve concentration of critical materials and components which contain the active materials. Through improvements in processes such as electrostatic separation, materials can be separated based on differences in conductivity, allowing a high proportion and purity of separators to be removed from current collectors, and allowing a mixed feed of Al and Cu current collectors to be separated as shown in figure 11 [101]. Improvements in these physical processing techniques will make downstream processes such as hydrometallurgy more cost effective, due to decreased contamination with materials such as Cu and Al which are not currently recovered through hydrometallurgy.
Figure 11. Various cell components for separation, and options for separating and recovering each component.
Download figure:
Standard image High-resolution imageImprovements in fine particle separation techniques allows separation of anodic and cathodic active material at high purities. It is possible to use froth flotation to separate graphite at recoveries of over 96%, but entrainment of cathodic materials is problematic, and some cathode particles have been shown to report to the froth phase, limiting the recovery of the cathode. The presence of a hydrophobic binder on the cathode further hampers separation efforts [123]. Froth flotation is also relevant for separation of different pristine cathodic active materials with only a small impact on their electrochemical performance [128], though again the presence of binder would alter the separability of the components. Wet High Intensity Magnetic Separation has shown promise in separation of different active materials from each other [9]. Advances in the understanding of the magnetic susceptibility of the cell components will allow for more selective separation of active materials. Separation of conductive carbon from the active material will allow further flexibility in the recycling of active materials, separating the conductive additives and binders from the potentially outdated active material which may have lost the necessary structure to perform adequately [129, 130].
Improvements in binder systems which allow active materials to be more easily separated from the current collector will facilitate the use of more benign solvents in the recycling process. PVDF is commonly used though is challenging to separate and recycle [131]. Designing cells with recyclability in mind will allow for the binder system to be separated and recycled without having to mitigate the effects of thermal binder removal or resorting to expensive or hazardous solvents [132].
Concluding remarks
Separation of the component parts to concentrate the materials streams is key to enabling high recovery efficiency of the critical materials contained within the battery. This is currently performed through size separation and a stream of subsequent physical sorting processes. Challenges arise in the efficiencies of the processes, particularly when the precise chemistry of the technologies going through the processes are not known. This can lead to many contaminated waste streams which require different downstream processes for extraction and affect the efficiencies for recovery of the different components. Improvements in component properties and designing the components of the cell for ease in separation would enable significant improvements. All the mechanical separation processes exploit the specifical material properties, for example, density, magnetic susceptibility, wettability, solubility, conductivity. If the material components can be separated more easily in the comminution process, then the highly specific and tailored techniques can be developed specific to the materials in the mix.
10. Delamination processes—black mass production
Andrew P Abbott1 and Dana Thompson1
1 School of Chemistry, University of Leicester, Leicester LE1 7RH, United Kingdom
Status
Most current hydrometallurgical processes for lithium-ion battery (LIB) recycling utilise shredding as a pre-treatment, allowing complex materials to become more accessible in a safe manner. The shredded materials are then further processed to concentrate the required material component, such as the cathode material into a fraction known as black mass [94]. This is advantageous for small consumer electronics as this allows for them to be treated in bulk irrespective of the various battery designs. It is also used for automotive packs that are constructed from small cells. However, scaling this technique up to large electric vehicle (EV) battery packs tends to produce waste streams of lower purity and lower value compromising the economics of recycling and leading to material being down-cycled rather than recycled. Since the adoption of EVs will see at least 16 kt of battery waste p.a. by 2028 in the UK alone, it is vital that the quality of the materials is maintained to create a closed loop in automotive battery material [133]. By developing more sophisticated approaches to LIB pre-treatment, such as disassembly and separation, purer waste streams and therefore greater recovery rates and product value can be achieved. This becomes important to enable economically feasible (toll-free) recycling processes and allow battery prices to decrease [10, 108]. To maximise the cathode and anode active material components and reduce contamination from the current collectors or other battery components, delamination from the current collector is required. This is obtained through delamination of the active materials components from the current collectors, and binder negation to separate out the conductive additives and polymers.
Current and future challenges
Industrial pretreatment methods involve mechanical crushing in an inert atmosphere with subsequent size separation [134]. Manual disassembly and separation is currently not feasible due mostly to the small cell size and the extensive use of structural adhesives. This results in loss of the active material in coarser fractions mixed with metals from the battery casing and polymeric components. The resulting fine fraction, or black mass, contains impurities from the binder, carbon, Al and Cu foils, requiring further treatment and resulting in low-purity products [135]. The electrode delamination process during the physical separation stages is reliant on processes which shear the electrode mass off the current collector substrate. This of course leads to significant current collector contamination. Binder negation can be used to facilitate the black mass separation, and this is achieved at a larger scale through heat treatments at high enough temperatures to decompose the polymer binder, helping to remove the components from the metallic substrates.
Laboratory-scale pre-treatments involve separation of the active material and current collectors via various methods such as Al etching with NaOH, binder dissolution with organic solvents such as N-methyl-2-pyrrolidone (NMP) or dimethylformamide, or ultrasonic washing with NMP [136, 137]. However, these processes involve the use of expensive and toxic organic solvents and are too slow for implementation on an industrial scale. Shredding and sorting produces a mixed black mass which contains multiple components; future challenges are to produce a single product black mass, such as a graphite or cathode material. This will facilitate direct-loop recycling processes where the components can be directly re-used in the battery manufacturing processes, with little further processing. Currently, no recycler in the EU produces single active component black mass of sufficiently high quality to be directly recycled into batteries of similar quality from which they were removed. Currently to reclaim the transition metals for short loop recycling, the black mass is treated with an acid and the metals are leached into solution for further salt precipitation [138].
Clearly, separating anode and cathode materials before delamination would result in delaminated active material of much higher purity and value and should decrease some down-stream processes [101]. Separation requires battery packs which are easy and safe to disassemble prior to hydrometallurgical processing. However, several disassembly challenges remain due to the lack of standardisation and the way in which cells and modules are glued together [139]. A universal recycling process for cathode active material would be ideal for simplicity, however cell chemistry can vary from fleet to fleet and is constantly changing with the development of next generation batteries. Therefore, a simple but essential requirement for efficient delamination processes is pack labelling so that recyclers can aggregate batteries of similar chemistry and minimise cross contamination. Furthermore, the use of an organic binder causes difficulties in separating the black mass from the current collector. The use of water dispersible binders would help significantly in not only increasing delamination rates but also producing higher purity products and decreasing fluorinated polymer residues. A global effort is required to phase out the use of polyfluorinated alkyl compounds.
Zero waste challenges: using water or solvent based delamination methods produces high levels of contaminated waste which needs disposal or further processing. High temperatures produce gaseous emissions, CO2 and possibly hydrogen fluoride from the fluorinated polymers which also needs to be taken into consideration, with release to atmosphere minimised wherever possible. Both energy and environmental impacts need to be considered for the whole process [135, 140].
Advances in science and technology to meet challenges
Binder negation: Research into non-fluorinated binders has increased significantly with a variety of carbohydrate and protein-based molecules which can be processed in water, which are popular. In particular, a combination of carboxymethyl cellulose and styrene butadiene rubber is often used for anode construction and similar materials have also been used for cathode construction [141]. The largest contributions to recycling costs are solvent and energy costs and so binders which can be dispersed in aqueous solutions are essential. New aqueous bio-based binder systems are being extensively investigated, with the benefit being that they can be more easily removed after end-of-life is reached.
Green solvents. Alternative lixiviants for cathode active material processing include organic acids such as ascorbic acid, which can also act as a reducing agent [142]. Deep eutectic solvents such as a mixture of ethylene glycol and choline chloride have been used as they are cheap and non-toxic compounds that can be used without the need for additional reducing agents or solvent extractants [143]. Solvents, solvent mixtures and binder removal agents with lower environmental impact are required.
The cost of recycling must be lower than the cost of mining virgin materials [144]. A recent review demonstrated that significant improvements in the economics of recycling could be obtained by segregation followed by delamination as opposed to shredding [108]. Lower temperature regeneration methods are also essential.
Delamination methods. Another aspect of the recycling process is the processing time. With such a large volume of material that will need processing it is essential to delaminate electrode foils rapidly. Simply stirred reactors are relatively slow with delamination in the order of 10 s of minutes to hours. This can be accelerated using low power ultrasound to get delamination on the order of 10 s of minutes. To meet the challenges of electrode separation during pre-treatment, the use of high-power ultrasound has been shown to effectively delaminate electrodes containing a polyvinylidene fluoride (PVDF) binder in a matter of seconds. By positioning the electrodes 2.5 mm away from the sonotrode face and feeding the individual electrode streets through the unit at a rate of 2 cm s−1, the NMC/PVDF/Al cathode and graphite/CMC/SBR/Cu anode were delaminated in 0.5 M NaOH and 0.5 M citric acid, respectively. It was found that delamination started 0.5 s after entering the high-power ultrasound region [119].
Further aspects of design for recycle are discussed in this roadmap and elsewhere [84].
Concluding remarks
In the short term, hydrometallurgical processing using mineral acids and bases working on shredded material will predominate as the main method of safe battery disposal and recovery of some of the technology critical metals. A focus upon reclaiming the active material components for direct recycling will reduce the circular cost and result in lower emissions, working towards a zero waste and zero emission process. This will only be possible with high purity material streams, likely only with disassembly processes rather than shredding. The critical barrier to economically favourable delamination and recycling is the design of the pack, module and cell to enable separation and the development of water dispersible binders which will simplify the deposition and recycling of the active material. Battery design will be slower to introduce due to the capital invested in cell manufacture, but appropriate binder improvements could be a drop-in technology with shorter lead-in times. Delamination and binder negation methods which use low energy and have low environmental impact are required. Ultrasonic delamination techniques, particularly for production scrap recycling, could also be easily scaled to cope with a growing stream of material. Green solvents and low level of lixiviant additives to water processes will help to speed up these processes.
Acknowledgments
The authors would like to thank the Faraday Institution ReLiB project (Grant Codes FIRG005, FIRG027 and FIRG057) and the UKRI Interdisciplinary Circular Economy Centre for Technology Metals (TechMet) Grant No. EP/V011855/1 for funding.
11. High resolution & in-situ microscopy for lithium ion battery recycling research
Nigel D Browning1,2,3,4, B Layla Mehdi1,2, Mounib Bahri1, Felipe Schanider-Tontini1 and D Nicholls1
1 Mechanical, Materials & Aerospace Engineering, University of Liverpool, Liverpool, L69 3GH, United Kingdom
2 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
3 Physical Science Division, Pacific Northwest National Laboratory, Richland, WA 99352, United States of America
4 Sivananthan Laboratories, 590 Territorial Drive, Bolingbrook, IL 60440. United States of America
Status
Atomic resolution imaging and spectroscopic methods in the scanning transmission electron microscope (STEM) have been used extensively to study the structure and composition of state-of-the-art Li-ion and beyond Li battery electrodes [145–148]. These methods allow the intercalation of Li (and other ions) to be tracked with single atom sensitivity, and the formation of secondary phases in the electrodes/solid electrolyte interphase (SEI) to be uniquely identified on the sub-nanometre length scale. In all of these experiments, care has to be taken in the preparation, handling and storage of the samples as the battery electrodes and any SEI layers are extremely reactive to the atmosphere and can change substantially with only a few minutes exposure—the use of glove boxes to prepare samples and some form of vacuum transfer (stage, bag etc) into the microscope is essential for the most representative results to be obtained. Another key issue in performing high resolution imaging and analysis is the effect of the electron beam, which if the lowest dose methods are not used, can damage/change the structure and chemistry of the sample significantly during the experiment [149, 150]. As these advanced high-resolution methods are only sensitive to the solid part of the battery, i.e. not the electrolyte, it is often hard to interpret how and why the structure of the electrode changed during the degradation process. To overcome this limitation, operando liquid cells have been developed for STEM [151–153] and also for SEM [154] that permit structure/chemistry changes in the electrodes to be observed during electrochemical cycling in all forms of liquid electrolytes. For these liquid cells, the low mass/density of Li metal causes a contrast reversal in the experimental images that makes the identification and tracking of Li metal easily quantifiable [151]. However, even though the liquid is typically only ∼100 nm thick, coupled with the Silicon Nitride windows, this degrades the spatial resolution possible with the in-situ methods and it is not possible to track dynamics on the atomic scale. More recently, open cell stages have been used [155] to overcome this spatial resolution issue and permit the dynamics of ion diffusion to be observed with atomic resolution. While not having the ideal operando geometry, when the open cell results are correlated with both the static and the operando methods, a complete atomic scale picture of the evolution of structure under dynamic electrochemical conditions is achieved.
Current and future challenges
Although there have been many uses of high resolution and in-situ/operando methods to study battery systems, the overwhelming majority of them focus on pristine materials and the first few (up to a few hundred) charge/discharge cycles. Understanding how the structure, composition and chemistry of the system changes as the batteries reach end-of-life and need to be recycled is going to be the major challenge going forward. Methodologies that are developed for recycling the starting compositions, for example NMC 111 or NMC 811, may not be as effective when the batteries are composed of a heterogeneously mixed array of loosely connected inorganic/organic nanostructures where the composition of neighbouring grains can vary from NMC 111 to NMC 811 or include even more varied and distinct phases and surface layers. It is also not clear that the safety requirements will be the same for end-of-life batteries. For example, how will these multiphase nanocomposites react to air exposure, stress, and temperature fluctuations during the initial dismantling and processing steps? An example of the type of complexity involved in the final battery microstructure is shown in figure 12. Here the end-of-life battery has smaller grains with much wider varying compositions, distinct grain boundaries with secondary phases and an abundant distribution of Al in every defect, when Al was only present in trace amounts in the initial battery/cathode powders [148]. In addition to being more complex in the understanding of the recycling processes, these types of samples are also harder to characterise by STEM (and other advanced methods) than the pristine materials, as the ability to quantify fluctuations in composition on the nanoscale is at the very limit of the sensitivity and precision of the methods. These discussions so far have focused on the ability to observe the materials, rather than the recycling process itself. The chemical leaching methods that are standard are clearly going to be able to be studied in the in-situ stages that are currently available. However, there are other approaches to recycling, such as the use of genetically modified bacteria [156], where the ability to observe the process is going to be more challenging—there are all the issues of complex, corrosive chemical interactions and the biological processes that are linked to them (which are very sensitive to the electron beam conditions).
Figure 12. STEM-EDX maps showing the presence and distribution of Al in (a) a pristine and (b) an end-of-life cathode from a first generation Nissan leaf battery. Over the extended cycling of the battery, Al containing phases appear to increase and form primarily at grain boundary triple pockets. Large Al phases has an effect on degradation, recycling and second life applications of LIBs. Reproduced from [148]. CC BY 4.0.
Download figure:
Standard image High-resolution imageAdvances in science and technology to meet challenges
There are already developments that are underway that aim to address the challenge of observing dynamics in the chemical/biological interactions underpinning recycling strategies. One method that avoids the main issues with beam damage caused by the beam is to use a sub-sampling and inpainting approach that is broadly termed as compressive sensing [157, 158]. Figure 13 shows the post acquisition demonstration of compressive sensing to a transition electron microscopy (TEM) image of microtomed resin embedded bacteria used to create transition metal nanoparticles from battery recycling leachates [159]. The image uses less beam current than conventional approaches and shows the potential to be able to observe the dynamics of such processes at high spatial resolution using the existing in-situ stage technologies. To address the issue of the reactivity of the end-of-life materials, work is also underway to use gas stages to examine the effects of air exposure, mechanical deformation and temperature on the reactivity (and hence safety) of the final system. Coupled with the atomic resolution capabilities to examine these phenomena on the atomic scale, provides a comprehensive set of characterisation tools for understanding the fundamental processes involved.
Figure 13. (a) Post acquisition sub-sampled image of a bacteria, that is reconstructed using inpainting and denoising algorithms [157–159].
Download figure:
Standard image High-resolution imageConcluding remarks
High resolution and in-situ/operando scanning transmission electron microscopy offers numerous opportunities to characterise the dynamics of the degradation and recycling processes for advanced battery systems. As there are no limitations in the materials, chemicals and/or biological systems that can be examined by these methods, the development of these characterisation methods in the future has the potential to provide insights into the process of recycling irrespective of the battery technologies that are being used.
Acknowledgments
This work was performed in the Albert Crewe Centre (ACC) for Electron Microscopy, a shared research facility (SRF) fully supported by the University of Liverpool. Aspects of this work were supported by the UK Faraday Institution (EP/S003053/1) through the FIRG005 (ReLiB), FIRG013 (characterization) and FIRG001 (Degradation) projects.
12. Thermal pre-treatment
Christin Stallmeister and Bernd Friedrich
IME Process Metallurgy and Metal Recycling, Institute of RWTH Aachen University, Intzestraße 3, 52056 Aachen, Germany
Status
Thermal pre-treatment is an already industrial implemented process step in the recycling chain for whole end-of-life battery cells/modules, shredded batteries and also an option for production scrap [138]. The process is at the beginning of the recycling chain either after sorting and discharging or shredding [138]. In the most common case, it is followed by further mechanical treatment and sorting before the black mass enters hydro- or pyrometallurgical treatment, as shown in figure 14 [138, 160].
Figure 14. Integration of thermal treatment in battery recycling routes.
Download figure:
Standard image High-resolution imageAs thermal treatment options incineration or pyrolysis are possible [160]. Pyrolysis means to heat up the material under the absence of oxygen [161, 162], which leads to the decomposition of organic binders, separator and electrolyte by cracking reactions [160]. Oxygen-free atmospheres can be achieved by using inert atmosphere like nitrogen or under vacuum conditions [163].
Additional to organics, parts of volatile halogens such as fluorine are removed via the off-gas [161]. The temperature range is limited by the melting point of aluminum (660 °C) [138], because this would hinder following mechanic processing and separation steps.
Thermal pre-treatment offers crucial advantages for the recycling of LIBs:
- It provides safe and controlled deactivation of battery cells without the risk of thermal runaway as it could occur during shredding [160].
- The removal of organics, especially the binders, improve the delamination of the current collector foils in following mechanical shredding and sorting steps. This is important for recovering single grade intermediate products like copper and aluminum foils, as well as reaching high yields of the valuable black mass [163, 164].
- If the black mass is subsequently treated hydrometallurgical, removal of organics showed to be beneficial in context of the leaching behaviour as well. Higher leaching efficiencies and better kinetics are reached when thermal treatment between 500 °C–600 °C is included in the process chain [163, 165].
- There is also the opportunity for thermal induced phase transformations of lithium, nickel- manganese- and cobalt oxides as observed by Lombardo et al [162, 166]. This enables the implementation of further, new recycling approaches and process steps, like early-stage lithium recovery [167] or magnetic separation [168].
Deeper understanding and optimization of reactions and mechanisms during the heat-treatment of batteries and battery shredder are crucial to increase recycling rates. Therefore, the thermal treatment is under intensive research. Already known are some basic information on off-gas composition [161] and reductive reactions of metal oxides from batteries depending on the process temperature and atmosphere [162, 166].
Current and future challenges
Although some studies on thermal treatment of batteries have already been carried out, there are still research issues and challenges left. During the process unavoidable by-products such as oils, organic and fluorine containing hazardous off-gas are produced, resulting in complex gas treatment systems [163]. In some pyrolysis plants the off-gas is already thermally used [169] but purification and recyclability of produced oils are not investigated yet.
Especially fluorine is problematic in the following recycling steps [138], so a complete and controlled mobilization during thermal treatment via the off-gas would be advantageous.
Furthermore, the furnace technology must be able to cope with the challenging feed material: To circumvent dismantling to cell level, treatment of whole modules is aspired, in case that preliminary shredding is not considered. Here, monitoring of temperature during the heat treatment process at different points of the module is necessary to ensure homogenous heating of all cells and early detection of exothermal reactions like thermal runaways, as carried out by Kaindl in lab scale [170]. This is of special importance if damaged cells or modules are processed. Expected upcoming challenges in this context are continuous material feeding and handling of sudden changes in off-gas volume.
So far, some information about chemical reactions and mechanisms during pyrolysis are known. However, the impact of thermal treatment conditions on following process steps like mechanical processing and hydrometallurgy is not fully investigated. Because of the organic and especially binder removal, pyrolysis enables improvement of new process options like recovery of graphite by flotation [123, 171]. But formation of pyrolysis coke could be challenging in this context. Negative influence on the separation of graphite from the metal oxides during flotation is assumed [171] and the effect of coke on the performance of graphite as a secondary product should be investigated.
Moreover, embrittlement of aluminum foil has been observed in some tests [172], which would hinder the mechanical separation and affect hydrometallurgical treatment.
Another common challenge over the whole recycling chains of LIBs is the lithium recovery. Due to cross contamination of the different products like slag and flue dust in pyrometallurgy or the filter cakes and precipitation products in hydrometallurgy with lithium, as well as chemical consumption, optimization of lithium recovery is still necessary [160, 167]. Thermal treatment could play an important role in this context as discussed in the following paragraph.
This shows that optimization of process parameters will mainly influence the following process steps but plays an important role regarding the full recycling chain.
Advances in science and technology to meet challenges
To overcome the presented challenges, different advances in science and technology are needed. Regarding the off-gas treatment, special burner designs, e.g. connected with heat exchangers could be used. Energy analysis will show, if an autothermal process operation is possible. In this context, the fluorine and phosphorus distribution must be considered. Removal of the halogens via the off-gas would be advantageous for further processing [138] but poses special requirements regarding gas cleaning. Separation of halogens in a lime absorber or scrubber are possible solutions [173]. Furthermore, detailed analysis of produced oils needs to be carried out. Afterwards, utilization or recycling concepts could be worked out [174], like usage in petrochemical applications.
Pyrolysis can be carried out in different furnace types, divided in batch and continuous processes [175]. Important are efficient and safe furnace solutions, which could work with lock systems, oxygen shielding and explosion protection. Productivity is also an important criterion for industrial recyclers and could be improved with continuous processes. Digitalization will play an important role for process monitoring and is under current research for the whole battery recycling process [176]. Especially in the case of critical, damaged battery modules digitalization and machine learning bear the potential for enhanced safety and process control.
The influence of the formation of pyrolysis coke on the recovery and usability of graphite from batteries is not completely investigated yet. But first investigations on measurement of coke content in pyrolyzed black mass have been carried out [171]. This is the first approach to further research like parameter studies on influence of pyrolysis conditions on coke formation and its impact on following process steps like flotation. The reuse of separated and coke containing graphite in new batteries could be tested in lab scale to investigate the influence on performance and cycle stability. Avoidance of coke formation may be possible under defined oxygen contend during pyrolysis.
Another approach under current research is the early-stage lithium recovery [160, 167]. Thermal treatment conditions like temperature, holding time and atmosphere are investigated to produce water soluble lithium compounds [160, 167, 177]. Afterwards the mechanically separated black mass can be water leached to recover lithium salts like lithium carbonate, without chemical consumption [160, 167, 177]. Additional (supercritical) CO2 treatment to optimize lithium yields during leaching is also possible [160, 167]. Afterwards the black mass can be treated either hydro- or pyrometallurgical [160, 167].
Concluding remarks
Thermal treatment of battery-scrap is already implemented in some recycling routes due to several advantages. It mainly influences the following process steps and herby contributes to the overall recycling efficiency. Therefore, parameter adjustment for the production of an optimal black mass product for further processing is necessary and under current research. Moreover, digitalization could contribute to enhanced furnace technology and process control.
13. Pyrometallurgy
Marcus Sommerfeld, Christin Stallmeister and Bernd Friedrich
IME Process Metallurgy and Metal Recycling, Institute of RWTH Aachen University, Intzestraße 3, 52056 Aachen, Germany
Status
End-of-life batteries and production scrap containing cobalt, or nickel and cobalt are currently either recycled in dedicated smelters or added into pyrometallurgical primary and secondary smelters treating nickel-, cobalt- and copper-containing materials [138]. However, the treatment in a dedicated smelter bears the option to enrich lithium in either the slag phase or in the flue dust, which is difficult if batteries are not the main raw material due to the dilution of lithium [178]. Before smelting, various pre-treatment steps are optional to remove several fractions for dedicated processing. Thermal treatment is optionally used to remove the organic components like binders and electrolytes, and to eliminate the risk of explosions during downstream processing. Dismantling, comminution and separation are carried out to obtain metal fractions containing copper, aluminum or steel, which can be used in dedicated recycling facilities [179].
The smelting operation itself works as a fast and efficient splitting operation:
- Elements with a low oxygen affinity present in the feed material like cobalt, nickel and copper are accumulated in an alloy or matte phase, while a major share of iron will be enriched in the metal as well, manganese will be distributed within the slag and metal phase [178, 180]
- Elements with a high oxygen affinity like aluminum and lithium are enriched in the slag phase, while lithium is partially transferred into the flue dust as well, due to the low vapour pressure of lithium-containing compounds like Li, LiF and Li2CO3 [178, 180]
- Carbon and residual organic components are oxidized and leave the furnace as off-gas [178, 180]
- Volatile elements, especially fluorine are mainly enriched in the flue dust [178, 180]
Depending on the pre-treatment, the intermediates, metal, slag and flue dust can be processed by various routes. Cobalt, nickel and copper will be recovered from the metal or matte phase, if iron was not removed by mechanical pre-treatment, partial oxidation could be an option to remove iron and manganese. Lithium can either be enriched in the slag or flue dust [178, 180] and recovered by hydrometallurgy [121, 181–183], depending on the process temperature, fluxing and pre-treatment. However, if aluminum is not removed, lithium will be slagged yielding a slag with a low lithium content [178, 180].
Current and future challenges
The biggest challenges during the process are caused by lithium, fluorine, graphite, and aluminum.
If lithium and fluorine enter the smelting process, they could either be enriched in the slag or flue dust. However previous investigations have shown cross-contamination in both mass flows [178, 180]. The recirculation of flue dust to the melt would prevent lithium losses, but is problematic because of simultaneous fluorine enrichment [178]. It will accumulate in the slag and may influence further processing and usability as a product. Furthermore, fluorine causes corrosion of the refractory lining and hazardous off-gas components that require complex gas cleaning [138, 184].
Selective recovery of lithium from slag or flue dust also needs to be optimized. Problems due to silica gel formation in hydrometallurgical treatment can be caused by silicate slags which are commonly used [138]. In addition, the processing of large quantities of slag is necessary for lithium recovery. This also requires high energy and chemical consumption, therefore it is not carried out industrially, yet [138, 184]. Moreover, leaching residues and precipitated impurities lead to environmental issues [138].
In industrial practice graphite is not separated from the black mass before the smelting step. Previous investigations have shown that graphite contained in Li-ion-batteries exceeds the needs of reductive agents for reduction of battery oxides. This can cause problems due to dispersed graphite in the slag and therefore non-processable melt. Blending with other residual streams leads to a useful utilization of graphite as a reducing agent [180, 185, 186]. However, this means downcycling a valuable and critical (EU) [187] raw material.
Aluminum is another challenging component in the case of pyrometallurgical battery recycling. During the melting process the aluminum is oxidized and accumulated in the slag, so it does not contribute to the recycling efficiency [184]. Another option is the mechanical separation of most of the aluminum foils and casings before the melting process [138]. This keeps the slag volume low and opens an opportunity for recovery. Nevertheless, the separation from the copper foils and sufficient delamination takes great effort [188] and it is not clear yet if the generation of proper feed material for the aluminum recycling industry is possible.
This shows that most of the challenges in pyrometallurgical battery recycling deal with possible pre- and post-processing steps and their impact on the products as well as recycling efficiencies. In particular, the combination of suitable process steps still needs to be determined.
Advances in science and technology to meet challenges
To overcome the previously mentioned challenges, optimization and research are necessary to ideally couple unit operations in the pre-treatment and downstream processing line. Furthermore, new unit operations in the pre-treatment or downstream processing are regularly investigated and might be beneficial to overcome challenges in the pyrometallurgical process.
One approach currently investigated to overcome several challenges includes early-stage lithium recovery. Thermal pre-treatment is carried out, to convert lithium compounds into water-soluble lithium carbonate. Lithium is then extracted by water washing and can be recovered early in the process chain [167, 176, 189]. Another option is the usage of (supercritical) CO2 in aqueous media to recover lithium [160]. Simultaneous fluorine removal also needs to be investigated in this process, as fluorinated gases are currently an issue in every thermal process step. The delithiated residue is then treated via pyrometallurgy to recover remaining valuable metals in an alloy, while a heavy-metal and lithium-free slag is produced, which might be usable in construction materials. Via removing the lithium before smelting, the formation of hazardous leach residues generated during slag treatment is avoided [190]. To recover graphite and avoid downcycling, flotation of black mass could be an option [123, 171].
Another approach currently investigated is a lithium pre-concentration by mineral processing of low-grade slags. In a first step, the lithium-containing slag is comminuted and treated by flotation to obtain LiAlO2-crystals and lithium-free tailings. LiAlO2 is further treated by hydrometallurgical methods, while the flotation rejects are evaluated as substitutes in building materials [176]. Combining mineral processing and hydrometallurgy can significantly decrease the amount of leach residues and could be a viable option for slags with a lower lithium content.
Even though the recovery of lithium is currently more challenging than metal treatment, research regarding downstream processing is still carried out. The majority of processes to recover pure products from the metal alloy are considering hydrometallurgical process steps, which are still continuously improved to increase metal yields, purities and efficiency, but can be already considered common industrial practice. Besides hydrometallurgical recovery steps, a novel liquid alloying step followed by vacuum distillation could be applicable for battery alloys. Zhang et al [191] were able to separate copper from a cobalt alloy made from primary resources by alloying copper with liquid magnesium. The cobalt-containing fraction was removed mechanically from the MgCu-alloy, while magnesium can be separated using vacuum distillation, yielding a copper fraction and magnesium distillate [191].
Concluding remarks
While smelting end-of-life lithium-ion batteries and intermediates is relatively straightforward, the combination of upstream and downstream processing bears several options to recover components, not recoverable by smelting. Figure 15 shows a flowchart containing several options and pathways considerable for battery recycling. While thermal treatment, mechanical processing, smelting and the final recovery of metals from the alloy or matte are already industrial practice, early-stage lithium recovery [160, 167, 176, 189], flotation [123, 171] or mineral processing [176] are currently under investigation and bear potential benefits:
- Flotation could separate graphite usable for further applications [123], while during smelting it could only be used for energy generation or as a reducing agent [178, 180]
- Early-stage lithium recovery could make slag or flue dust treatment for lithium recovery obsolete and bear the option to process batteries in primary nickel and cobalt smelters [192]
- Mineral processing could be an option to enrich lithium from low-grade slags, which might not be suitable for hydrometallurgical processing otherwise [176]
Figure 15. Industrial and potential process routes for pyrometallurgical battery recycling.
Download figure:
Standard image High-resolution imageBased on our experience and literature [178, 180, 183, 192], yields for aluminum, copper, graphite, iron, manganese, nickel, cobalt and lithium for direct smelting (Route 1, figure 15) are displayed in figure 16(a). However, combining all potential processing steps (Route 5, figure 15) will yield a significantly higher recycling efficiency as shown in figure 16(b).
Figure 16. Recovery and losses of valuable elements during direct smelting of batteries (a) and combined pre-treatment and smelting (b).
Download figure:
Standard image High-resolution image14. Upcycling of cathode materials using hydrometallurgical processes
Laura L Driscoll1,2, Abbey Jarvis1,2, Emily C Giles1,2, Paul A Anderson1,2 and Peter R Slater1,2
1 School of Chemistry, University of Birmingham, Birmingham B15 2TT, United Kingdom
2 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
Status
Hydrometallurgical processes can be summarised as attempting to draw the target metals such as Li, Co, Mn and Ni (typically from the fine fraction of black mass) into solution for further processing. Two key requirements are needed to conduct efficient leaching on separated cathode materials: a leaching medium and a reducing agent. The most prevalent combination of reagents in the literature are mixtures of sulphuric acid and hydrogen peroxide, which aim to draw insoluble M3+ into solution as soluble M2+. Once in solution, the leachate can be treated with a number of elegantly designed processes to precipitate out the insoluble target through, for example, variation of the pH of the solution [10]. Early examples (such as Li et al) include the treatment of LiCoO2 (LCO), in which the sulphuric/peroxide combination of reagents were used to leach the spent material, leading to recovery of Co as the oxalate and the remaining Li recovered as the carbonate via the saturated sodium carbonate method [193]. The recovered constituents (recovery rate of 99.5% for Co and 94.5% for Li) can then be recombined to generate LCO through a solid-state procedure with performance comparable to the pristine material (this is defined as a closed-loop process). However, in order to achieve greater electrochemical performance and lower cost, cathode chemistries have developed over the years, including the structurally similar but modified LiNix Mny Coz O2 (NMC) phases, the Mn-rich spinels (often including an NMC/NCA component to aid shelf life, NCA = LiNi1-x-yCoxAlyO2) and the relatively inexpensive LiFePO4 (LFP). This wide variety of materials and components can pose a significant challenge for recycling processes, especially when differing cathode chemistries are mixed together. Examples where a mixed leachate has been upcycled through a short-loop recycling process include the synthesis of NMC from a mixed phase feedstock (Gratz et al [194]), in which the oxide components were dissolved and drawn into the solution while the resistant LFP remained in the insoluble fraction [194]. Once in solution, the targeted mixed metal hydroxide can be precipitated, combined with a Li source, and heated to form the equivalent NMC phase [190]. However, particularly for lower value cathodes, such as LFP, lengthy acidic dissolution processes and the subsequent recovery can add to the cost of recycling of these materials. Therefore, direct recycling strategies, in which the functional cathode is recovered and then repaired with as little reprocessing as possible, are favoured (albeit not yet implemented at industrial scale) for lower value systems [195].
Current and future challenges
Unless using a feedstock of production scrap, the cathode materials entering the recycling process are likely to originate from cycled batteries that have undergone a certain degree of capacity fade, and hence been subject to degradation [196]. However, many publications which claim to recycle spent lithium ion batteries are actually using pristine or artificially aged cells when evaluating the effectiveness of their recycling method, and are untested on real end-of-life materials. They are also tailored to one type of cathode material and rely on the presumption that there is a pre-sorting of cathode chemistries into separate waste streams. At present commercial batteries, or the waste stream in general, can contain a blend of cathode chemistries, and so processes that can effectively separate cathode mixtures are required. Scaling up lab-based processes to an economically viable industrial scale is also a challenge, if high energy and waste disposal costs are incurred (hydrometallurgical processes can produce large volumes of hazardous waste), particularly for batch-based processes.
Another challenge is separating the binder, which is most commonly polyvinylidene fluoride (PVDF), from the cathode material. While PVDF is soluble in N-methyl-2-pyrrolidone (NMP), NMP is too toxic and expensive to form part of an industrial scale recycling process [197]. Given the low decomposition temperature of PVDF, another commonly used strategy is to remove the binder thermally. However, the decomposition of PVDF can produce hydrogen fluoride which, as well as being a pollutant, can fluorinate the cathode material or remove lithium from the structure as LiF [198]. While these detrimental effects can be mitigated by adding excess LiOH.H2O with additional prolonged high temperature annealing [132], this would be an energy-intensive and expensive step in a direct recycling process and be likely to cause particle sintering.
Moreover, the chemistries used in commercial lithium-ion batteries are constantly evolving. For example, layered-oxide materials are moving to more Ni-rich compositions that are stabilised using surface coatings and more complex particle morphologies such as larger 'single crystal' primary particles or core–shell structures [199]. Next-generation cathode materials such as Li-rich layered oxides, anion redox cathode materials, as well as solid-state batteries are also being developed [200]. This presents a challenge for recyclers as by the time a battery reaches its end-of-life and enters the recycling chain, the cathode chemistry will be obsolete. This is why it is important to develop flexible recycling processes that can convert or 'upcycle' older cathode materials to modern chemistries.
Advances in science and technology to meet challenges
There are a number of challenges associated with recycling of Li-ion battery materials as discussed above. A particular area of focus is the importance of hydrometallurgical recycling processes to evolve with next generation cathode chemistries. Here, upcycling of recycled cathode materials to next generation materials will be essential. Although hydrometallurgical processes for single phase cathode materials have received growing research interest, development of processes for blended and mixed cathode materials also need to be considered including the ability to upcycle to next generation materials.
Zou et al [190] have demonstrated that mixed-cathode chemistries (LiCoO2, LiMn2O4, LiNi0.33Mn0.33Co0.33O2, and LiFePO4) can be separated and recycled to form LiNi0.33Mn0.33Co0.33O2 using H2SO4 and H2O2. By controlling the pH to precipitate impurities, the remaining Co/Mn/Ni ions in solution can be subsequently used to synthesise the desired final phase, LiNi0.33Mn0.33Co0.33O2. In addition to the use of inorganic acids, Li et al [201] have used citric acid combined with H2O2 to leach mixed-cathode chemistries (LiCoO2, LiCo1/3Ni1/3Mn1/3O2, and LiMn2O4), and then resynthesised (with extra metals added to achieve the correct ratio) LiNi0.33Mn0.33Co0.33O2 using a sol–gel method.
Less commonly investigated is the selective leaching of blended cathode materials. Here selective leaching can be used to separate lower and higher value materials (figure 17). Work carried out in the Faraday Institution ReLiB project has developed a methodology whereby lower value Mn-rich phases can be selectively leached from higher value Ni-rich layered materials [202]. This allows for cleaner waste streams which can then be utilised in the synthesis of current and next generation cathode materials.
Figure 17. Flow chart showing the potential processes involved in dealing with a mixed waste stream.
Download figure:
Standard image High-resolution imageTo lower the cost of the recycling process, direct recycling of cathode materials is a preferred solution, for example hydrothermal relithiation. In this process the cathode material is typically heated in an autoclave to about 200 °C in a saturated, high ionic strength, aqueous lithium solution such as 4 M LiOH followed by a final higher temperature heating step in air/O2 [203]. This will, however, only regenerate the original cathode, and so may not be viable if this cathode chemistry is now obsolete.
Further developments in hydrometallurgical leaching, combined with upcycling of cathode materials, need to be considered to ensure that end-of-life battery materials can be fully utilised to prepare the current and next generation of battery materials. These methods will be particularly important for not only recycling of single-phase materials, but also mixed-cathode waste streams.
Concluding remarks
While methods exist for the hydrometallurgical processing of cathode black mass from used batteries, many of these were developed for single cathode waste streams and high value LCO cathodes. The change to high Ni content NMC/NCA and LFP cathodes means that new lower cost methods are needed. Moreover, the cathode from a used battery may be 10‒20 years old, and so the cathode chemistry may now be obsolete, such that a direct recycling process is no longer viable. Therefore there is a pressing need to develop efficient processes for the upcycling of obsolete cathode materials into current and next generation cathode chemistries. Furthermore, as the number of end-of-life electric vehicle batteries increases, the prevalence of blended cathode chemistries and mixed cathode waste streams is likely also to increase as, in the absence of greater standardisation, bespoke processes for every single variation will prove impractical. In these cases, selective leaching processes can provide one avenue to make the upcycling process more cost effective, by creating cleaner waste streams that can then be employed to manufacture the required cathode chemistry. Overall, the challenge is to develop a suite of effective and flexible low cost and high throughput processes that can be tailored both to the waste input and the remanufactured cathode chemistry required.
Acknowledgments
We would like to thank the following funding bodies who have supported this work: the Faraday Institution's ReLiB (FIRG005, FIRG027 and FIRG057), and CATMAT (FIRG016) grants.
15. Biological methods for recycling lithium ion batteries
Virginia Echavarri-Bravo1,2, Giovanni Maddalena1,2 and Louise E Horsfall1,2
1 School of Biological Sciences, University of Edinburgh, Edinburgh EH9 3BF, United Kingdom
2 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
Status
The use of biology for recycling metals contained in spent lithium-ion batteries (LIBs) is still in its infancy, but can feasibly utilised to enable a circular economy for LIB (figure 18). Most of the advances to date have been developed in the area of biohydrometallurgy (bioleaching and bio-oxidation) [204], which is based on the same principles as hydrometallurgy but with lixiviants being produced biologically, generally by microorganisms of the fungal and bacterial kingdoms. The use of natural deep eutectic solvents has also been successfully demonstrated for the selective delamination of LCO battery material [143]. Selective biohydrometallurgy together with other biological methods such as bioflotation, biosorption and bioreduction [205] are potentially useful for the separation and purification of metals from LIB leachates. These processes facilitate metal purification steps by either concentrating ions onto biomolecules that are easier to purify or by directly precipitating ions via metabolic processes. However, to date, they have been somewhat overlooked or tested only at bench-scale despite their success in recovering gold at a commercial scale from electronic waste [206].
Figure 18. Biological approaches for enabling a circular economy for LIBs.
Download figure:
Standard image High-resolution imageBiological systems have the potential to support greener recycling methods for LIBs as acids for selective delamination/leaching processes can be produced from sustainable feedstocks at relatively low temperatures (<40 °C) and are biodegradable. In contrast, conventional chemical synthesis of acids is more hazardous as they are produced at higher concentrations, involve fossil fuel derived compounds and require energy intensive processes. Furthermore, the biological lixiviant contains other undefined metabolites which can make the leaching process more efficient and more selective than using equal concentrations of chemically synthesised acids [207] and therefore resource-intensive downstream processing is not necessarily of further benefit. Unfortunately, the lack of data associated to sustainability metrics and standards for biological processes, especially those at a large scale, hinders the use of common decision tools, such as life cycle assessment, for enabling accurate comparison with conventional methods.
On the whole, biological approaches do not intend to replace chemical processes but to enhance their sustainability. Current advances in the fields of engineering biology and bioinformatics are currently being used to harness the mechanisms responsible for biological-metal interactions, which will rapidly advance the development of metal bio-recycling. Molecular and metabolic engineering have already shown the practicality of biology for the selective adsorption of rare earth elements [208] and for the controlled precipitation of heavy metals [209]. Moreover, biological methods can up-cycle metals into nanoparticles rather than the devaluation that is a widely accepted consequence of recycling.
Current and future challenges
Presently LIB recyclability is very low and the incorporation of biological solutions into the recycling process is crucial to ensure this technology meets the zero-carbon emissions targets and that there is a sustainable and more equitable management of resources [210]. One of the main challenges in the field of spent LIB recycling is the wide range of battery chemistries that complicates the standardisation of the process, however this should not impede the development of biorefineries for the production of bio-based reagents (i.e. lixiviants) required in hydrometallurgy. This type of indirect bioleaching, when microorganisms are not in contact with the spent battery material, would overcome some of the issues associated with bioleaching (e.g. metal toxicity, long cultivation time) [211]. Even though there is abundant renewable organic material and waste that can be valorised for the production of relevant acids or lixiviants [206], the supply of feedstock can be unreliable due to losses during primary production and industrial processing [212]. The complexity of biological processes including the composition of lixiviants are often regarded as a 'black box' which is translated into higher variability of the processes or end-products, thus requiring prolonged testing for ensuring standardisation and homogeneity.
Bio-based solutions require long-term consideration since, under the current linear economy context, they cannot compete with fossil fuel dependent technologies and products marketed at prices that do not incorporate actual environmental costs.
Scaling-up bioprocesses requires a high initial investment; the processes and products synthesised by engineered microorganisms involve considerable optimisation due to the difficulty in replicating laboratory conditions in an industrial/commercial context. The infrastructure required for the scale-up is usually larger than for chemical methods, and needs to be adapted to accommodate slower processes compared to more conventional recycling methods that take place at higher temperatures. Larger infrastructure and higher volumes of water are needed [213] which requires consideration when siting biological recycling plants.
Other challenges of biological approaches are to enhance metal selectivity and to improve removal/recycling yields. The most relevant metals contained in vehicular LIBs are the critical metals Co and Li followed by the transition metals Mn and Ni which are essential in all vehicular LIBs, and Al and Cu present in the current collector. Unselective recovery of these metals with the exception of dissolved Li was demonstrated by Calvert et al [214] using hydrogen sulfide produced by sulfate reducing bacteria. The separation of the recovered metals from biomass may also be regarded as a purification obstacle discouraging further developments in the field.
Advances in science and technology to meet challenges
Some of the challenges associated to the scale-up process could be overcome by the development of industrial strains using metabolic engineering supported by advances in synthetic biology, systems biology and evolutionary engineering. The design and construction of more efficient and resistant microorganisms in order for them to be more productive, whilst consuming less resources and better tolerating extreme conditions [213, 215] will support the viability of biological-based projects. Moreover, faster growing microorganisms could be used as new hosts for the production of lixiviants in biohydrometallurgy through the use of synthetic/engineering biology [216]. Automation and machine learning with the increasing genetic information available will reduce the time and effort needed for the development of industrial strains, while the costs of DNA sequencing and synthesis are no longer a serious impediment [217]. The synthesis and screening of potential industrial strains does require significant initial investment but once achieved they self-replicate and regenerate [218].
Alternatively, inhibition due to metal toxicity might be addressed by employing microorganism-free systems, concomitantly easing downstream processing requirements. Selective delamination alongside metal bio-separation developed under microorganism-free conditions would facilitate the purification and separation process of the recycled metals when in nanoparticulate form [215]. Alternatively, microbial mass might not be a handicap if carbonisation is incorporated into the synthesis of metal-based catalysts [219]. Selective biorecovery of metals is still in its early stages but advances using synthetic biology show it has a promising future [215].
There is also scope for reducing freshwater consumption by promoting the use of sea water in feedstock preparation. This will require the development of halophilic organisms and the use of sea water in cooling systems would further reduce dependence on freshwater. Biological cycles are renewable and inspired the concept of circular economy. Re-using the fermentation water and progressing to the reuse of microbial biomass [220] will enhance the circularity of processes using microorganisms for LIB recycling.
Perhaps one of the key solutions to support recyclability and to reduce metal use is the development of biodegradable batteries as demonstrated by Delaporte et al [221] who employed cellulose in Li-ion electrodes. This plant polymer, the most abundant biological compound on earth, served as both a support and a binder without the need for incorporation of a current collector (usually made of Cu and Al). The properties of other abundant biological polymers such as alginate and chitosan are gaining relevance as these also exhibit properties suitable for use as aqueous binders. Further developments in this field will provide much needed alternatives to in-use fluorinated binders, which are toxic and cannot be recycled [222].
Concluding remarks
The biggest challenges to employing biological methods for recycling of LIBs have yet to be solved, as they require inter- and trans-disciplinary solutions. More active collaboration between academic subjects is needed to overcome the barriers imposed by the lack of common meaning within the language used by disciplines. Efficient communication and academic collaboration is perhaps also the key to interest industry in bio-based solutions as, quite understandably, companies do not consult biologists when looking at battery-related issues. However, it is time to follow biotechnologists' principles who, a long time ago, incorporated the concepts of circularity and sustainability into their processes, principles that are still being overlooked by many material scientists today. The continuous development of faster and higher resolution analytical techniques confirms the precision of biology to create, evolve, build and grow materials to perform successfully. With the recent advances in DNA technologies it is time to reach beyond bioinspired and biomimetic materials and actually integrate biology into technology platforms [223]. Microorganisms exhibit an extraordinary range of abilities insufficiently investigated and underutilised; if once they changed the atmospheric composition to enable the diversity of life on our planet, surely they could be part of the solution to maintain it.
Acknowledgments
This research was supported by the Faraday Institution ReLiB project (Grant Codes FIRG005, FIRG027 and FIRG057) and EPSRC Fellowship (EP/N026519/1).
16. Direct cathode recycling
Linda Gaines and Qiang Dai
Argonne National Laboratory, 9500S. Cass Ave., Lemont, IL 60439 United States of America
Status
The cathode contains numerous critical materials and can represent as much as 50% of a cell's cost—it is therefore the first target for recovery when the material is recycled. A desirable strategy would be to recover this material in a nearly usable condition and minimize the treatment needed before putting it back into a cell. One such promising strategy is Direct Cathode Recycling, which is defined as the recovery, regeneration, and reuse of the cells' positive active material directly without breaking down its chemical structure. Direct recycling has been demonstrated at the bench scale for both end-of-life cell material and manufacturing scrap.
For pyrometallurgical (smelting) and hydrometallurgical (leaching) recycling processes, both of which are already commercialized, most of the value that can be obtained from either spent cells or scrap is from the cobalt in the cathode, recovered in an alloy or as a salt. However, batteries for electric vehicles are rapidly evolving toward low- or no-cobalt cathodes, thus reducing the potential revenues from pyro and hydro processes. But the properties of the cathode material's crystal structure impart value to it over and above that of the elements contained. Therefore, by retaining the crystal morphology, direct recycling offers to yield reusable cathode material that will retain high value, even as the prevailing chemical composition changes [224]. Other cell components can be recovered as well, providing additional revenues and avoiding disposal costs. In addition to being the most valuable component of the cells, the cathode's production processes account for a large fraction of the cell's energy use and environmental impacts, including greenhouse gas emissions [225]. Recovery intact minimizes required process steps for recovery and therefore offers the potential for reuse with minimum impacts, as well as minimum costs.
Current and future challenges
Due to the continuous evolution of battery chemistries and long product life, end-of-life battery streams are likely to contain multiple cathode chemistries, and so the cathode materials available for recovery in spent cells may be obsolete. Conventional recycling technologies essentially treat batteries as rich ores and use processes that are similar if not identical to virgin production to recover battery materials and therefore can better adapt to changing chemistries technologically, albeit possibly at a higher cost and/or a lower revenue.
In contrast, the success of direct cathode recycling hinges on whether we can separate cathode materials into different streams based on chemistry with sufficient purity and yield and subsequently convert the recovered cathode materials—which are likely degraded as a result of lithium loss over the numerous charge/discharge cycles the battery has undergone—into materials that can be used for new battery manufacturing. Many of the processes being developed involve separation of the materials in the different layers of the cell structure from each other (see figure 19). Materials within a layer may require separation as well (i.e. removal of binder from cathode).
Figure 19. Layers within a Li-ion cell.
Download figure:
Standard image High-resolution imageThese challenges are associated with end-of-life battery recycling, but in the near future, battery manufacturing scrap is going to be a significant feedstock for recycling plants [224]. Unlike end-of-life batteries, manufacturing scrap usually contains a single, known chemistry and therefore would not require cathode/cathode separation. Similarly, manufacturing scrap contains pristine or slightly degraded cathode materials and would only need minimal processing after separation before it can be returned to the battery supply chain. Therefore, direct cathode recycling can be particularly promising for manufacturing scrap.
The battery industry is moving toward nickel-rich chemistries, which help reduce costs and improve power density but have stability and capacity issues [226]. Strategies such as cathode doping and coating have proven effective in overcoming these issues, but they may pose challenges to direct recycling. Future research is needed to understand if cathode modification strategies would complicate cathode separation and/or regeneration. Over the next few decades, the battery industry is expected to move toward a new generation of batteries. Conventional recycling technologies—and the direct cathode recycling technology we discuss here—focus on recovering materials from lithium-ion batteries (LIBs) for new LIB manufacturing. Future research is needed to develop technologies that can upcycle LIBs into materials for next-generation batteries, such as Li-S and Li-Air.
Advances in science and technology to meet challenges
To facilitate separation of cathode materials based on chemistry, technologies are being developed that can achieve pre-sorting (before batteries are shredded) [38] and cathode/cathode separation after shredding based on inherent properties of the cathode materials, such as hydrophobicity and magnetism [128]. Research is also under way to understand what impurities may be present in the recovered cathode material and what effects they may have on the electrochemical performance, stability, and lifetime of the cathode material [227–229]. In the future, robotic disassembly could obviate the need for shredding and reduce the number of material separation steps required.
Figure 20. Two possible routes from shreds to cathode.
Download figure:
Standard image High-resolution imageSeveral relithiation technologies (to replenish lost lithium) are also being developed to regenerate the recovered cathode material so that it can have electrochemical performance comparable to pristine material, while minimizing the cost and the environmental impacts of the process [230–233]. In addition, some research groups are exploring the possibility of changing the transition metal stoichiometry of the cathode material, potentially enabling the upcycling of obsolete chemistries into more current formulations [234].
Successful commercialization of direct recycling requires collaboration of research efforts on each unit operation of the recycling process, as well as an in-depth understanding of the dynamics, economics, and environmental impacts of the battery supply chain. At the U.S. Department of Energy's ReCell Centre, each team working on a unit operation is developing the technology with scale-up in mind, informed by technoeconomic analysis and Life-cycle Assessment (LCA) to ensure the technology is cost-effective and environmentally friendly. Since there could be various recycling process designs to achieve the same goal, depending on what unit operations are used and in what order, as shown in figure 20, the teams are working together, again informed by analysis, to determine the optimal process design for direct recycling, for both end-of-life batteries and manufacturing scrap.
Research on battery material supply and demand [235], as well as costs and environmental impacts for the entire supply chain beyond recycling, including logistics (e.g. packaging, transportation, and storage, among others) and virgin material production, will also inform the development of a direct recycling infrastructure.
Concluding remarks
Direct cathode recycling may offer profitable recycling, even for cathodes that contain little or no cobalt. For a country like the United States, which as of 2021 had no installed cathode capacity, directly recovered cathode material from end-of-life batteries could represent the first source of domestically produced cathode material, helping to complete the battery supply chain and moderate price fluctuations. Simple direct processes could also be used very quickly for manufacturing scrap, enabling efficient return of the material to the cell production line without it having to be exported and sent thousands of miles for processing. Direct recycling offers the opportunity to recover cathode and other materials in their highest-value form, thus making LIB recycling profitable and environmentally beneficial, possibly without the need for government regulations or incentives.
Acknowledgments
This work was performed through the ReCell Center, which gratefully acknowledges support from the U.S. Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office. The submitted section 16 has been created by UChicago Argonne, LLC, Operator of Argonne National Laboratory ('Argonne'). Argonne, a U.S. Department of Energy Office of Science laboratory, is operated under Contract No. DE-AC02-06CH11357. The U.S. Government retains for itself, and others acting on its behalf, a paid-up nonexclusive, irrevocable worldwide license in said section 16 to reproduce, prepare derivative works, distribute copies to the public, and perform publicly and display publicly, by or on behalf of the Government. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan. http://energy.gov/downloads/doe-public-accessplan.
17. Electrolyte recovery and recycling
Shiva J Jethwa1,3, Albert L Lipson2 and Gary A Leeke1,3
1 School of Chemical Engineering, University of Birmingham, Birmingham B15 2TT, United Kingdom
2 Applied Materials Division and ReCell Center, Argonne National Laboratory, 9700S. Cass Ave., Lemont, IL 60439, United States of America
3 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
Status
Often overlooked in terms of operation and function, the electrolyte is an indispensable conducting medium responsible for the transfer of charge between both electrodes through the movement of lithium-ions [236]. There are several types of electrolyte chemistries, though the most common within the majority of applications is an aprotic non-aqueous solution. Standard electrolyte composition is broken down into three integral components, the solvent, conducting salt and a large array of additives. The solvent comprises the largest share on a volumetric basis and dissolves the conducting salt and the additives within a combination of carbonates. The bulk of solvents can be categorised as aliphatic and aromatic ethers or esters, the most prevalent include dimethyl carbonate, ethyl-methyl carbonate, diethyl carbonate, ethylene carbonate and propylene carbonate [237]. Lithium hexafluorophosphate (LiPF6) is the most common conducting salt in electrolytes, which is due to its well-balanced properties as opposed to other salts. Alternative salts are beginning to be employed to try to improve the performance of the battery further, including lithium bis(fluorosulfonyl)imide and lithium bis(trifluoromethanesulfonyl)imide [238].
When recovering the LIBs most commercial processes implement pyrometallurgical and hydrometallurgical routes to focus upon the recovery of high-value components, whilst often neglecting the electrolyte. Commonly within these processes the electrolyte would be incinerated or be treated with chemicals to break it down. Currently, there are very few commercial recycling processes that reclaim and process the end-of-life (EOL) electrolyte, due to the low economic value of the material and the toxic flammable nature of the electrolyte and its decomposition products [239]. However, from an environmental and safety point of view, the recovery and recycling of the electrolyte is a crucial and necessary step to mitigate against rising greenhouse gas emissions and reduce the dangers associated with processing battery waste material. There are currently three promising techniques for electrolyte recovery. Solvent extraction, vacuum extraction and supercritical CO2 extraction.
Here we will address the challenges and needed advancements to overcome the challenges to recover and recycle the electrolyte.
Current and future challenges
The challenges of recovery and recycling electrolytes can be broken down into three areas: the dangers of EOL material, cost of processing and standardisation. As LIBs reach EOL their capacity and discharge ability is reduced as a result of numerous ageing phenomena. Though the ageing within LIBs does not exclusively impact the degradation of LIBs operational abilities, it results in the formation of hazardous and corrosive compounds within the cell.
LiPF6 independently is thermally stable at temperatures under ∼107 °C, but once dissolved within the solvent and in contact with electrodes that can span in voltage from 0 V to over 4.3 V vs. Li/Li+ this stability is significantly reduced. Furthermore, any exposure to moisture will cause the hydrolysis of LiPF6 and results in the formation of hydrogen fluoride (HF), which imposes significant health risks if it does not react with other components of the cell [240]. In a 2015 paper published by Grìtzke et al [241], shredded NMC/graphite LIB material was transferred into storage containers and analysed over a period of 20 months using headspace gas chromatography-mass-spectrometry. The standard compounds within the electrolyte were identified, but in addition, organo(fluoro)phosphate compounds were identified with structures that closely resembled chemical nerve agents. Dimethyl fluorophosphate and diethyl fluorophosphate were characterised in concentrations of 1.12 ± 0.09 and 1.00 ± 0.20 g kg−1. Although these concentrations are exceptionally small, the concentration would be exponentially amplified under increased temperature, proving to be hazardous and more so when applied to the large volumes predicted to pass through recycling plants within the near future.
Reclamation of the solvent component has proven successful, although the large variation in polarity amongst aliphatic and aromatic carbonates create an imbalance of organic carbonates recovered. In comparison, the conducting salt, LiPF6 is more challenging to recycle due to its volatile nature that can compromise process safety. In addition, the practice of not removing the LiPF6 results in downstream contamination of the recycling process with fluoride that can cause corrosion, poorer yields, and safety concerns. Currently, the electrolyte is not standardised and the composition of solvent and salt fluctuates significantly amongst manufacturers, as a consequence it is difficult for recyclers to produce a consistent product.
From an economical perspective the electrolyte accounts for roughly 13% of the total cost of a new cell and after EOL this value is far less when compared to the value of the electrodes and their respective current collectors [242]. However, from an environmental perspective if conventional processing methods persist the generation of greenhouse gases will continue to rise. Based upon the number of LIBs expected to reach EOL by 2030 and the carbon dioxide (CO2) released when the LIB electrolyte is incinerated, CO2 emissions are forecasted to reach upwards of 90 000 tonnes by 2030, as presented in figure 21. Assuming, incineration is the sole practice for the disposal of the electrolyte and LIBs continue to be recycled at a rate of 5% per year. Although if current recycling rates were to rise, emissions would increase too, unless alternative practices were adopted [243, 244].
Figure 21. Projected CO2 emissions by 2030 as a result of LIB electrolyte incineration. aCarbon dioxide equivalencies were obtained from the United States Environmental Protection Agency [245].
Download figure:
Standard image High-resolution imageAdvances in science and technology to meet challenges
For practical recycling of electrolyte to become standard practice, many challenges need to be addressed as shown in figure 22. Once the electrolyte is extracted by either a solvent based process or one incorporating supercritical CO2 it then needs to be processed into a salable product. Ideally, a process would be developed that could separate out each component of the electrolyte. However, such a process would require a large number of steps that makes it economically unfeasible. One of the main challenges to these processes is the strong coordination between the salt and the solvent coupled with the low thermal stability of the salt [238, 240]. This makes simple evaporation processes to recover the pure salt impractical. New processes that can perform this separation at lower costs than conventional processing, such as chromatography, could enable the salt to be extracted and resold.
Figure 22. A general electrolyte recycling process with the challenges that are needed to be overcome during each step to make recycling a reality.
Download figure:
Standard image High-resolution imageAn additional challenge is the introduction of new lithium salts into the electrolyte to improve performance [246]. Separation of these salts will be very difficult to achieve in an economically feasible manner. If these salts cannot be separated, there may be a very limited market for the mixed salts. Alternatively, this may require manufacturer and possibly product specific processing to enable the reuse of the salts by those manufacturers. Although during the lifespan of batteries, the choice of electrolytes in new batteries may change making the recycled electrolyte obsolete. This would relegate any recovered salts to chemical feedstocks.
Beyond the issues with separations, the lack of stability of the salts in contact with moisture adds an additional challenge to recycling [240]. Performing the entire recovery process in a dry room would add significant cost and energy usage to the process. An understanding of the breakdown kinetics is needed to design these processes at scale and thereby recover the electrolyte with the needed low acid content. In addition, the practical effect of the contact of the electrolyte with other components of the battery, which can effectively trap HF that is formed during the process, should be explored. Several alternative options can be considered to a full dry room including inerting the process environments or simply limiting the exposure time. Understanding the practical air stability of electrolytes can be difficult experimentally since they will be highly scale and equipment dependent.
If the ideal electrolyte cannot be directly recycled into new battery electrolyte new strategies are needed to gain maximum value while mitigating any environmental impacts. Processes could be developed that enable the spent electrolyte to enter the manufacturing stream for new salts at an earlier stage. Likely this spent electrolyte would be concentrated to reduce volume and recover the volatile carbonate solvents before being transferred to a chemical manufacturer.
Concluding remarks
Reclamation and recycling the electrolyte is clearly an area that requires further development. EOL electrolyte material can introduce toxic compounds via a range of degradation pathways, combined with the poor thermal stability of the conducting salt makes the implementation of a commercial recycling process substantially more complicated.
The lack of standardisation of the composition of the electrolyte, and difficulties associated when separating the salt from the solvents is the key challenge behind reprocessing EOL electrolyte directly into a new LIB. The electrolyte is typically unrecoverable within the majority of large scale processes, though few companies have managed to extract and reprocess it, achieving a standard that is fit for resale in the chemical industry. Although, for substantial advancements it would prove beneficial if future electrolyte blends are developed by both LIB manufacturers and recyclers, establishing a product that could be implemented into a circular economic model aimed to reduce greenhouse emissions and hazardous LIB waste.
Acknowledgments
This work was supported by the Faraday Institution, ReLiB Project [FIRG005, FIRG027 and FIRG057] and the ReCell Center, which gratefully acknowledges support from the U. S. Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, and the Vehicle Technologies Office.
18. Lithium recovery from lithium-ion batteries
Thomas Cowell1, Joseph Gresle Farthing1, Greta Mariani1, Amy Smith1, Zubera Iqbal1,2, Rabeeh Golmohammadzadeh3,4 and Emma Kendrick1,2
1 School of Metallurgy and Materials, University of Birmingham, Birmingham B15 2TT, United Kingdom
2 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
3 Department of Chemical and Biological Engineering, Monash University, Clayton, Victoria 3800, Australia
4 Institute for Frontier Materials, Deakin University, Geelong, Victoria 3200, Australia
Status
The reported global mine production of lithium in 2021 is 100 K metric tons [247]. About 55 K MT of lithium comes from Australia, extracted from Spodumene rock, and 26 K MT from Chile and the Salar brines [248]. This extraction levels are reported to be 82.5 K metric tons in 2020 and expected to grow further. Lithium chloride is extracted from evaporating water from high lithium content brines, whereas lithium from spodumene is extracted using a hydrometallurgical leaching process, first the spodumene is heated to change the phase, and then heated with sulfuric acid to generate lithium sulfate. The concentration of other impurity metals in the ore is key as they affect the solubility of the lithium. The impurities are removed using calcium oxide and sodium carbonate, lithium carbonate is then precipitated using sodium carbonate after further concentration of the lithium through evaporation. Producing 1 tonne of lithium carbonate by this method is excessively resource-intensive, from 1 tonne of lithium carbonate, 1.34 tonnes of spodumene is used, 1.66 GJ of energy from natural gas and 1.1 GJ (0.3056 MWh) of electricity, with a total energy expense of 2.76GJ, and half a tonne each of sulfuric acid and soda ash, also consuming 24 tonnes of water [248].
Currently lithium recovery from batteries is not widely performed, even though the concentration in spent LIB's is higher than the primary sources. In a lithium-ion battery (LIBs), lithium is contained in the electrolyte (∼1 Mol.) and the cathode. During use of the battery, lithium is transferred into the negative electrode and forms a thin interface upon the surface which is called solid electrolyte interphase (SEI). At end-of-life, when the materials are recycled, the content of lithium in the different fractions can vary depending upon the state of charge and state-of-health. If the cells are discharged first then the lithium is contained mainly within the cathode fraction, with lower concentrations in the electrolyte and the negative electrode interface. However, if there is a lower state-of-health with large SEI growth then greater levels contained in the anode is possible. Typically, most of the lithium is reclaimed from the transition metal containing cathode fraction, even though all the waste streams will contain some level of lithium, the concentrations of lithium in the other fractions are low and difficult to extract. The main sources for lithium recovery in the battery waste streams which are from the shredded waste, which contains electrolyte and cathode, waste water from any washing processes which may include the organic electrolyte, and the upgraded transition metal and cathode or anode and negative electrode waste streams.
Current and future challenges
The major challenge is the many different recycling processes that need to be considered when reclaiming lithium. When using the widely accepted pyrometallurgical process [134, 249], a primary metallic alloy product which containing the valuable metals, nickel, cobalt, and copper is produced. A slag-former or reducing agent is added to form the alloy, and the lithium, aluminium and manganese are contained within this slag or light fraction and typically not extracted further due to the high cost and low yields [250]. In addition, lithium is lost at the high temperatures involved in pyrometallurgical processes, >1200 °C, and is often lost in the toxic flue gas.
Hydrometallurgy encompasses the range of techniques which rely on the use of aqueous solutions to extract valuable metals from the spent LIBs which are being recycled [121, 251]. Reductive leaching where the cathode 'black mass' concentrate is mixed with a reducing agent and leaching with a mineral acid such as H2SO4. This technique is advantageous over pyrometallurgy due to its lower energy cost, lower emissions, and potential for lithium recovery. However significant pre-treatment such as density or magnetic separation, to concentrate the transition metal containing black mass. Using this method undissolved residues, such as the plastics and graphite can be removed, prior to further selective precipitation of the metal salts [252]. The metal salts are then selectively precipitated at different pH's [253]. Similar to extraction from ores, lithium is recovered at pH14, by concentrating the lithium and adding Na2CO3. The other metals are selectively removed earlier with less alkaline solutions, (∼pH 7.5 for MnCO3, 9 for NiCO3, 14 for Li2CO3) [251].
A combination of methods can allow for better extraction of valuable and critical elements. Many pyrometallurgical hybrid techniques use a high degree of mechanical separation, to concentrate the important components before pyrometallurgical or hydrometallurgical processes are used. This can reduce the contaminants in the waste streams, such as aluminium or copper, which cause differences in solubility for salt precipitation [134]. One method which uses the aluminium current collector as the reducing agent in the pyrolysis process, causing a thermite reaction, has been investigated [254]. The advantage here is the minimised need for mechanical separation while still recovering lithium, although the aluminium is oxidised in this process.
Alternative methods for lithium recovery from battery waste are bio-hydrometallurgy, this is a specialist branch of hydrometallurgy, which utilises weaker acids obtained from naturally occurring microorganisms, which are Iess energy intensive [255–258]. This process can selectively leach different metals, as well as lithium, from the material and provide possible higher concentrations of lithium from the waste processing. The main challenges LIBs recycling using bio-hydrometallurgy is the reaction time, which can take up to weeks before we obtained high enough recovery rates.
Advances in science and technology to meet challenges
Rapid development of combined processes of the pyro-hydro, mech-hydro, and hybrid types, where efficiency of recovery of material is the primary goal is being performed. However, looking at the future of industrial recycling, it is important to not only consider efficacy, but also the complexity/length of the process, the cost of the equipment/chemicals, the environmental impact of the process in terms of hazardous chemicals, and its energy consumption. Some of the advances to meet these challenges are discussed below and compared.
Lithium from pyrometallurgical processes is difficult to process due to its high levels of impurities and relatively low levels of desirable elements, therefore it is usually discarded. Methods to extract lithium from this slag are now being developed [259]. One method is to concentrate the lithium into components which can be more easily extracted. Engineered artificial minerals (EnAM) where the lithium is concentrated into specific minerals is one way to further extract lithium. Through the slag forming agent and the furnace profile the formation of high lithium containing compounds such as spinel is possible, this can be further separated through mineral processing methods such as froth flotation or density separation [260, 261]. Reaction coupled separation such as chlorination [262] and sulfation [263] roasting and produces soluble lithium containing salts which can be more easily removed with water or hydrometallurgical processes. For example, 99.5% selective lithium recovery from washing with water was achieved after heating in a sulfur dioxide atmosphere.
Reductive leaching requires improved efficiencies, and higher purity streams for sequential extraction of the different components, and in particular lithium [264]. Vieceli et al investigated the effect of reducing agent on leaching efficiency It was found that sodium metabisulphite was a promising reducing agent that could increase the leaching efficiency of elements such as Co from ∼48% to ∼58% [265].
Biological leaching methods can be used to extract or leach out lithium and have lower costs and environmental impact. Lithium is easily dissolved in the acidic media produced by the bacteria, although a reducing agent is required to reduce the transition metals such as cobalt, nickel, and manganese to a lower oxidation state to aid dissolution [266]. Bacteria can be used directly or indirectly in the bioleaching of spent LIBs. In the direct method, bacteria are metabolised in the presence of spent LIBs and the acids are consumed by spent LIBs as they are produced. However, in the indirect bioleaching method, the bacteria produce acid in the absence of spent LIBs and the lixiviants are used for leaching of spent LIBs [257, 267]. Fungi is another type of microorganism that can be employed for the recovery of metals from spent LIBs. Aspergillus Niger (A. niger), which is the most common fungi that have been used for recovery processes, consumes sucrose and glucose and produces a mixture of organic acids including malic acid, gluconic acid, citric acid, oxalic acid [268–270]. The concentration of each organic acid varies by change in nutrients and growth condition [271]. Unlike bacterial bioleaching, in fungal bioleaching, no reducing agent is required. This is attributed to the presence of a mixture of organic acids in which some of them are also reducing agents (e.g. oxalic acid) [269]. The synergetic interaction of the organic acids also allows for more selectivity and higher efficiencies [268]. The contacting methods in fungal bioleaching are classified as one step, two step and spent medium [270, 272]. In the one step method, the spent LIBs are in contact with the fungi during incubation, with the bioleaching and fermentation of nutrients simultaneously occurs [269]. In the two-step approach, the fungi are cultured and the spent LIBs are added to the culture medium when a sudden drop in pH is observed [271]. And in the spent medium approach, a cell-free-acidic medium is used in bioleaching of spent LIBs from the growth of fungi, where waste materials are absent. This allows for fungi growth in the absence of toxins, and at the same time allowing the use of higher pulp densities in the bioleaching process [269, 271].
Complete lithium recovery efficiencies depend upon the lithium concentration and the impurity levels in the solution. The lithium product can be also precipitated out using a solvent extraction processes however these require large quantities of organic solvents. Membrane based separation technologies can be utilised to improve selectivity of lithium separation. There are several types of membrane separation including membrane distillation crystallisation, selective electrodialysis and electrochemical ion pumping, which can be used in combination with these improved efficiencies of extraction to remove lithium. Reverse Osmosis [273]. This process works by using a reverse-osmosis, or membrane osmosis system to concentrate the lithium-containing salts in the leachate. By increasing the salt concentration, the precipitation of lithium is maximised, and the precipitation of impurities minimised, leading to more efficient, higher purity Li2CO3 product.
Alternative methods for lithium reclamation include ionic sieves such as ion exchange and absorption methods. Adsorption methods work by absorbing the lithium onto the surface or into the of high surface area materials, whereas ion exchange method swop the cations from the solid to the water. Receiving increased attention is the electrochemical lithium ion pumping process which uses both faradaic and surface absorption materials.
Concluding remarks
Current lithium extraction processes are from the hydrometallurgical leaching of lithium into solution and subsequent precipitation of lithium salts. High lithium containing spent battery waste could be considered as a secondary ore. Similar processes for lithium extraction are used for primary and secondary ores, and typically consist of a heating step followed by leaching into solution and subsequent concentration and precipitation.
The main challenges in extraction are from the impurities found in the primary and secondary sources and the concentration levels in the waste streams, and future research into concentration and purification of the lithium species is required. There are a wide variety of process steps for battery recycling and hence many waste streams to consider and the precise methods of concentration and purification depend upon the recycling route being used.
The key technical challenges are:
- (a)Low lithium selectivity in the recycling processes
- (b)Low concentration of lithium in the different waste streams
- (c)Lithium is soluble and can be difficult to precipitate out separately from other transition metals
- (d)Lithium is volatile and easily lost through high temperature processes
- (e)Binder negation can cause further segregation of lithium from the transition metal black mass
There are many future challenges with new chemistries adoptions, these may have different components which cause differences in the method for recycling, selectivity, and recovery efficiency of lithium. Solid state batteries, for example contain lithium in the polymer or solid state separator.
Technology advancements can improve the efficiency and yields for lithium recovery at each step of separation, concentration, leaching and precipitation. However, to achieve 100% recovery of lithium other waste streams which occur after washing, physical separation and electrolyte removal need to be considered.
Acknowledgments
This work was supported by the UK EPSRC/Faraday Institution through the research project 'Recycling of Lithium-ion Batteries (ReLIB)' (Grant Nos. FIRG005, FIRG027 and FIRG058).
19. Anode recycling and reuse
Luke Sweeney1,2 and Paul A Anderson1,2
1 School of Chemistry, University of Birmingham, B15 2TT, United Kingdom
2 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
Since wide-scale mass production began in the 1990s, the components of lithium ion (Li-ion) batteries have been subject to significant change. The cathode, for example, originally a LiCoO2 (LCO) layered structure, has seen the development of [LiNix Mny Coz ]O2 and [LiNi0.8Al0.05Co0.15]O2 layered oxide phases, LiFePO4 olivine and LiMnO2 spinel cathode chemistries. The electrolyte has also changed, with a plethora of additives to increase ionic conductivity and aid solid electrolyte interface layer (SEI) formation. However, the use of graphite as an anode material has remained almost unchallenged [274]. Listing the properties of graphite is perhaps the best way to explain the difficulty in replacing it; low volume expansion upon lithiation, good conductivity and high capacity (compared to cathode materials) coupled with its natural abundance and low cost, all make it a difficult material to replace. Higher capacity materials such as silicon, silicon oxide, tin and iron oxide [275–278] have motivated many groups to study their application as Li-ion anodes but, despite considerable progress in some areas, poor capacity retention in these materials means graphite is still the dominant commercial anode material with additives such as SiOx only being added in small volumes (approx. 2%–5%) to commercial lithium-ion batteries.
Graphite has been largely overlooked in battery recycling. Within pyrometallurgical techniques, it is often used as a reductant for the more valuable transition metals found in the cathode (nickel and cobalt). Alternatively, hydrometallurgical techniques which extract nickel and cobalt using acids can recover graphite, however, graphite recovered in industrial hydrometallurgical processes is (in the best case scenario) processed as a raw material or (the most likely scenario) sold as scrap to the highest bidder. The graphite used in lithium-ion anodes is either natural or synthetic and, owing to China having large natural abundance of flake graphite and low energy costs, the production of both is dominated by China, with western countries having limited natural graphite resources, both in terms of mining and refining. Currently, 100% of uncoated spherical graphite, a refined graphite product essential for automotive electrification, comes from China [96], and this makes it vulnerable to geopolitical supply chain disruption, evidenced by natural graphite being listed as a critical raw material by the EU [279]. Despite the lack of natural graphite, the West will accumulate large amounts of already processed graphite in end-of-life (EoL) lithium-ion batteries, which could in principle be recycled.
Current and future challenges
Once lithium-ion batteries (LIBs) are shredded, a common process within recycling, graphite typically makes up around 20 wt% of recovered shredded material [180]; however, some of the main advantages for using it—its abundance and low cost—do not provide a strong incentive for recycling. In addition to economic factors acting as a deterrent to recycling there is the lack of a process which can rejuvenate EoL graphite into electrochemical grade graphite. With this in mind, some papers have reported novel recycling processes for recovering graphite which show good electrochemical performance [281, 282]. However, comparing electrochemical results from published recycling techniques remains challenging as often the graphite comes from an EoL Li-ion battery with unknown provenance. Two main processes account for graphite aging: changes in morphology and the introduction of impurities. The latter are commonly introduced to the graphite structure from the SEI or, if exposed to air, SEI decomposition products, manganese dissolution from the cathode and, if over-discharged to 0 V for safe transport, copper dissolution from the anode current collector.
Morphological changes, i.e. damage to the spherical structure of the graphite particles, are caused by excessive cycling, through repeated expansion and contraction upon lithiation and delithiation. Such volume changes cause microstructure cracking to occur [280] revealing new graphite for electrolyte reduction, and subsequently increasing the internal resistance of the battery upon electrolyte removal (figure 23). Removing contaminants from the graphite can be done via acid washing with 5 M sulphuric acid 35 w w−1 % H2O2, as reported by Ma et al [282]. Other variations of acid washing have also been reported to help purify EoL graphite [283]. Restoring particle morphology, by reforming the spherical shape and removing microstructure cracking poses a greater challenge, particularly if we wish to avoid high temperature annealing. A low temperature annealing step (40 mins at 500 °C with NaOH powder) has also been tried [282]. However, despite this treatment, high first cycle losses were reported indicating excess exposure of the graphite to the electrolyte. Within this research on recycling graphite little mention is made of restoring the amorphous pitch coatings used to reduce first cycle loss, typically found on battery grade graphite. An exception is work by Zhang et al, who dispersed heat-treated EoL graphite in an ethanol/phenolic resin solution followed by a 950°C 1 h N2 heat step, forming a thin amorphous carbon coating which was shown to reduce the first cycle loss [284]. Ultimately, the degree of damage to the graphite particle morphology will depend on cycling history, and the variability in battery usage makes the development of a universally applicable, low temperature, reprocessing route a desirable but extremely challenging goal.
Figure 23. A transmission electron micrograph illustrating crack formation from the electrochemical cycling of graphite. Reprinted from [280], Copyright (2011), with permission from Elsevier.
Download figure:
Standard image High-resolution imageThe lack of a rapid characterization technique to determine the condition of graphite and, from that, possible future applications, makes it impossible to separate graphite that might be reused in an LIB from that too damaged for electrochemical applications. Any such technique would need to be quick and highly automated to be used within recycling plants. In terms of electrochemical performance, a prominent feature of EoL graphite is that, despite having been in a battery, the crystal structure is still able to intercalate lithium reversibly and reach close to the theoretical capacity of graphite of 372 mAh g−1 [285]; however, high first cycle losses have been reported, which jeopardizes reuse as an anode material, as excess electrolyte reduction leads to higher internal cell resistances through electrolyte depletion and formation of a thicker SEI layer.
Demand for high energy densities has led manufacturers to look towards additives such as silicon or SiOx , both of which display high capacities, and therefore adding small amounts can result in noticeable increases in capacity. However, as both these materials crack and fragment to a greater degree than graphite [286, 287] direct reuse is likely to be challenging without high temperature annealing.
Advances in science and technology to meet challenges
Different states of health (SoH) of batteries often found within EoL feedstocks for recyclers mean that ideally batteries should be evaluated in order to determine if they can be used for second life or need to be recycled. Some of the most commonly used characterization techniques for graphite, as well as their advantages/limitations, can be found in table 1. Measuring open circuit voltage (OCV) and internal resistance to determine SoH [288] of the battery is the most widely used method to distinguish between those suitable for second life and those needing recycling but, unfortunately, such measurements are a composite metric of the performance of the full cell from which the condition of the graphite is difficult to infer. OCV measurements can also present a problem as the complete discharge of batteries to 0 V—often a requirement for safe transport—renders OCV measurements useless and second life applications impossible. Once the battery has been dismantled and separated, determining the condition of the graphite powder presents a unique challenge with characterization techniques either yielding little information or being too time intensive or costly for commercial scalability. Some spectroscopic techniques, however, such as raman spectroscopy, could be suitable for industrial application as they are quick, non-destructive and require little or no sample preparation. In the context of raman the ratio of the intensity of the formally forbidden D and allowed G peaks (Id/Ig) can be used as a quantitative indicator of disorder within graphite systems [289]. Yet, the presence of defects from other sources within the battery, such as carbon black additives, SEI layer/decomposition products and carbon coating makes the spectra difficult to deconvolute and, despite raman being a promising technique, a clear cutoff point within the spectra that indicates the graphite needs to be remanufactured is yet to be identified.
Table 1. Summary of characterization techniques used to evaluate the condition of EoL graphite.
| No cell disassembly required | |||
|---|---|---|---|
| Technique | Information extracted | Limitations | References |
| Open circuit voltage (OCV) | Yields information on state of charge (SoC) and, with an internal resistance measurement, SoH Industrially scalable | The SoH measurement may not indicate graphite condition | [288] |
| Electrochemical impedance spectroscopy (EIS) | Real part of EIS spectra can be used to infer battery SoH Testing can be incorporated within a BMS (battery management system) | Like the OCV limitations, it only measures the whole cell, rather than the graphite condition | [290] |
| Cell disassembly required | |||
| Technique | Information extracted | Limitations | References |
| X-ray diffraction | Excellent for detecting crystalline impurities (e.g. Cu metal, Li2CO3) Provides information on bulk crystallite structure. Can detect graphite exfoliation | Time consuming Little change in bulk structure from cycling i.e. graphite is still hexagonal | [285] |
| Raman spectroscopy | Quick scans are possible Small penetration depth means amorphous organic contaminants can be detected Id/Ig ratio can be used to infer crystallinity and crystallite size Has potential for industry application | Highly localized technique meaning multiple scans needed to give a representative picture of the sample Peak fitting is required, but could be automated | [291] |
| FT-IR | Very cheap setup Very easy to detect surface organic impurities in graphite, as electrochemical graphite has no peaks Industrially scalable | Gives no information on the morphology and structural changes in graphite | [292] |
| SEM | Excellent at revealing topographic information undetectable by raman and XRD | Not industrially scalable Expensive set up | [293] |
| Surface area measurements | Important parameter for SEI formation Can indicate degree of surface damage within the graphite structure | Long measurement time Can only be done on small amounts of sample Unlikely to be industrially scalable | [294] |
| XPS | Excellent tool for graphite surface characterizationVery powerful when used in conjunction with SEM | Not scalable | [295] |
In addition to research trying to replace graphite as an anode material, there has been significant activity to improve graphite itself as an anode material with techniques using doping [296], amorphous carbon [297] and polymer coatings [298]. Graphite 'upcycling' (a recycling process in which the end product has a higher value than the virgin material) to graphene has also been reported [299], but the current limited supply of EoL graphite coupled with the high demand for electric vehicle (EV) grade graphite places uncertainty in the current scalability of such upcycling techniques, especially if similar graphene production processes could be applied to lower grades of graphite. As yet, no significant effort has been made to develop previously researched methods for graphite rejuvenation, with the aim of improving the electrochemical performance of aged graphitic anodes, for low cost application in an industrial context, nor indeed for the more energy intensive and CO2-emitting processes of repurification and annealing they would seek to delay or avoid.
Concluding remarks
Although natural graphite is classed as a critical material it is widely dismissed in large-scale recycling processes. Given the volumes that will soon begin to flow there is little sign that high value markets will emerge quickly to be mass consumers of EoL graphite. Reusing EoL graphite is an essential component in creating a closed loop recycling system for EV lithium-ion batteries. Recycled graphite has shown promising electrochemical properties in terms of specific capacities, but has been shown to suffer from lower first cycle coulombic efficiencies when compared to its manufactured form. Little research has hitherto gone into investigating low impact surface modifications which could play a key role in extending the usable life of anode materials before the need for complete remanufacture or disposal.
Acknowledgments
The authors (PAA) would like to acknowledge the ReLiB Projects (FIRG005, FIRG027 & FIRG057), UKRI Interdisciplinary Centre for a Circular Economy in Technology Metals (Met4Tech—EP/V011855/1) EPSRC Grant: Thermal Recovery of Functional Coatings (TRefCo—EP/W019167/1), and the University of Birmingham (LDS) for financial support, and the ReLiB network for the support and guidance relating to battery recycling. We would also like to thank all of the members of floor 5 and 6 of the Haworth building, at the University of Birmingham, for all their support whilst working in science.
20. Plastics recovery and reuse markets
V Goodship
WMG, International Manufacturing Centre, University of Warwick, Coventry CV4 7AL, United Kingdom
Status
For the purposes of this review, the term plastics should be considered to refer to any polymeric based formulations and components which may be reused, recycled or recovered as part of lithium ion battery disposal systems. The shear versatility, ease of manufacture and low cost of plastics over the past 70 years has led to a global challenge for environmental management. With catastrophic leakage of plastic waste into the world's ecosystems, oceans and food chains, accumulated microplastics have even more recently been found in human placenta [300]. In 2017 it was reported that over 8 billion tonnes of plastics had been globally produced [301], and they continue to provide irreplaceable materials for a wide variety of sectors and applications forming a large material subset for the wider global e-waste strategy [302]. Perhaps due to the toxicity, value or critical nature of many lithium ion battery materials, versus the relatively low densities of individual polymeric components, plastic recycling has for the most part been overlooked by the battery community beyond easy to recover high volume outer battery packaging plastics, which can be dismantled, sorted and mechanically recycled as part of larger collections and disposal of e-waste [303], but this ubiquitous use of plastics also extends inside battery technology. Therefore, in lithium ion batteries alone these materials can be found in everything from a stand-alone material (such as a module spacer section), a multi-layer material (i.e. copper wire coating or pouch material), part of a protective outer casing composite material as well as connector blocks, cell holders, packaging components, separators and electrode binders. They also form a key material component for future research, environmental emission analysis and increased energy density technology in developments for polymer solid state batteries. In a circular and zero emission marketplace for battery manufacture and disposal relative to other processes, their versatile nature can potentially offer both new materials and key paths to incorporating carbon offsetting additives currently entering the market i.e. [304]. The nature and fate of plastic materials used in batteries such as those in figure 24, should therefore also be considered alongside other design and waste management issues for end-of-life batteries, to address the totality of battery waste. Generic components, as parts of our larger e-waste problem will not be discussed further here, see for example [302, 303], instead the focus will be on issues with interior battery specific components: namely anode, separator and pouch.
Figure 24. Recovered flaked (light fraction) plastic from a lithium ion battery following an initial shredding and physical separation process. Contaminants can be seen.
Download figure:
Standard image High-resolution imageCurrent and future challenges
One of the long-term challenges is to predict and prepare for which technology may develop in terms of plastic material usage and therefore potential recyclate markets. Holistically, plastic recycling rates globally continue to climb, but are still relatively low at 18%[305] and barriers and recommendations are well documented and include a range of technical, economic, legislation and technical know how barriers [305]. A review of polymer research in active materials, membranes and binders [306] provides the industry with a glimpse of what future waste streams may contain.
Polyvinylidene fluoride (PVDF) provides an interesting case study in this respect, since it is an established binder material but not the only material being used for this application. It has traditionally undergone pyrolysis during electrode recovery operations with evolution of greenhouse gases as well as hazardous fluorocarbons. Despite this material being a very well known inert, stable, versatile polymer used across many sectors, very little work has up to now been published in the recovery and subsequent reuse of the PVDF polymer. In fact, relatively little is even still known about its long-term stability in battery service. It was recently reported by [307] that PVDF with properties of virgin PVDF material could be recovered from laboratory based cathode materials using a combination of THF: N-methyl-2-pyrrolidone. However, a colour change was noted in extracting these materials from end-of-life commercial batteries. A similar change, attributed to gelation is also noted and vividly illustrated in a recent comprehensive review paper [131]. There is also still very little known of the fate of the polymers during long-term exposure to highly corrosive battery environments or even if their deterioration in service contributes to any subsequent battery failure mechanisms [308, 309]. Further, impurities or the use of additional materials can also cause hitherto unforeseen consequences, as reported in a study where silicon presence effected PVDF stability [308]. This is not confined just to the well-used PVDF material and could equally be applied to a material as commonplace as the polypropylene inner pouch material, parts of a separator component or alternative binders such as identified in [306]. So while PVDF recovery and reuse potentially offers a stable and reliable material supply with a wide range of potential applications more research is clearly needed in both quantifying the recovered properties and in the green and economic recovery routes to get there.
Advances in science and technology to meet challenges
By studying PVDF, some broader challenges, such as uncertainties in binder identity, low volumes and mixed materials prevent internal polymeric component recovery developing. One challenge is to increase the accuracy of sorting techniques [310] beyond just the generic and deep diving into understanding the recovered (figure 25) state-of-health of plastic wastes. Utilizing enhanced digital techniques such as coupled chemometric techniques along with AI and machine learning (ML) will enable key properties of polymeric materials to be rapidly determined and their fate automatically directed accordingly towards mechanical, chemical recovery or conversion and/or energy recovery options. The low volumes of mixed and potentially contaminated materials remain long-term challenges for the industry. A further missing component lacking in the field is reliable training datasets to enable sorting and recovery, based on materials in a known range of state-of-health to accurately model an actual waste stream. These are needed to test and validate these developing advanced systems.
Figure 25. Flaked (light fraction) plastic (from figure 24) following enhanced laboratory scale chemical cleaning processes.
Download figure:
Standard image High-resolution imageGiven the problems looking at a single common polymer such as PVDF, it becomes even more complex when looking at multi-material components. These components have a technical and economic value higher than their individual material costs; examples are multi-layer separators and pouch materials. These materials de-value once they are melted and mixed, so the most viable reuse or remanufacture often remains one of reuse and repair. Technically complex battery separator materials can be recovered by dismantling but retain high levels of cell contamination of hazardous and potentially valuable materials. This not only currently restricts further melt processing, but these porous structures still contain high value, low quantity, contaminant materials which could potentially be part of further secondary recovery processes. This is further complicated by a number of different separator formats in commercial use. Examples of more complex waste streams are mixed material packaging materials such as pouches which may contain thin layers of multiple polymers (PET, PP, adhesives) sandwiching a core metallic based materials (such as aluminum or similar). There is both an opportunity and a challenge. As more efficient methods of recovery, separation, reuse and recycling are developed recovered plastics will provide opportunities not just to feed into new battery technologies but also for other market sectors in a circular economy. In attempting to provide more generalized solutions, it is clear dismantling and sorting technology enablers will play a key role in further incentivizing the recovery of these plastic components.
Concluding remarks
Inside batteries in particular, there is still a need to fundamentally understand the effects of internal battery environments, temperature, contamination, and volume changes shrinkage on the longer term performance of the recovered plastic. With regard to zero emission, recovered and reused components have the potential to be modified with carbon negative additives to reduce the overall environmental burden on recovery. There is no doubt going forward those plastic materials provide tremendous opportunity to the battery community for a variety of environmentally acceptable and emission offsetting materials for the next generation of batteries whatever their ultimate chemistry. However, the limited attention to polymeric components has so far produced a legacy of poor and varied design choices, providing a complex and contaminated waste stream for plastic recyclers. Transfer of knowledge from polymer design, manufacturing and recycling into the battery scale-up processes are a key component to more holistic and successful recovery strategies. Reducing the complexity of polymer material usage by clear standardisation of materials, enabling more advanced AI assisted sorting technologies and giving confidence to recovery markets will ensure the past legacy of plastic waste mismanagement is not repeated in future energy markets.
Acknowledgments
This work was financially supported by Innovate UK (Faraday Battery Challenge Programme) R2LIB (Reclamation and Re-manufacture of lithium ion batteries) Project (Number 104425). Figures 24 and 25 were produced from this work with material originally sourced from Mr Sam Haig at RS Bruce Battery Recycling, UK.
21. Recycling of small and consumer lithium ion batteries
Zheng Li1 and Gavin D J Harper2
1 Virginia Tech/Li Industries, Blacksburg, VA 24060, United States of America
2 Li Industries, Inc., School of Metallurgy and Materials, University of Birmingham, Birmingham B15 2TT, United Kingdom
Status
The Recycling of small and consumer lithium ion batteries (LIBs) presents a range of different challenges from recycling large-scale batteries used for automotive and stationary energy storage applications. However, whilst at a different scale, and serving different sectors, they are linked by competing for the same critical material resources [311].
Between 2014 and 2019, the use of LIBs in small devices, including mobile phones, laptop computers, digital cameras, toys, e-cigarettes and garden and power tools doubled [312]. There are currently over 7 billion mobile phones, a billion laptop computers and a billion more tablets—largely reliant on LIBs as a power source [144].
From a waste management perspective, LIBs used in consumer electronics are particularly challenging as their small scale creates challenges with ensuring that they are segregated from other household and other types of battery wastes. It is essential to ensure that they do not contaminate other mixed waste streams, as being highly energetic, they have been identified as the cause of a number of large fires, igniting adjacent combustible materials in municipal materials recovery and recycling facilities [7] and leading to extensive property damage and potential risk to life.
It is also harder to ensure high collection rates for these batteries. For vehicles and large-scale batteries, many legislatures have prescribed disposal routes for vehicles, for example, which ensure that these larger LIBs find their way back to approved treatment facilities for further onward processing. For small LIBs, however, it is much harder to ensure that consumers dispose of these batteries responsibly. Being small and of relatively low value, there is little financial incentive towards correct disposal, there is also a lack of resource to educate end users to increase the awareness of correct disposal, and it is difficult to compel consumers and others in the reverse logistics supply chain to dispose of products correctly at end-of-life [313].
Current and future challenges
The stake holders in the recycling supply chain of small and consumer LIBs (figure 26) are categorized into three major roles: (a) sources, which include LIB producers, end product producers, end users, electronic waste collectors, retail channels and specialized channels (e.g. cell phone or automobile repair shops); (b) intermediaries, which are the companies that process and move EOL LIBs to recyclers, including sorters and collectors; and (c) recyclers, including those who repair and repurpose batteries for other uses (e.g. LIB re-conditioners) and those who extract the materials from EOL LIBs.
Figure 26. The small and consumer lithium-ion battery recycling ecosystem.
Download figure:
Standard image High-resolution imageFrom a design perspective, the small size of consumer LIBs makes materials handling for sorting batteries more straightforward than with larger scale batteries, however, the variety inherent in batteries used for consumer electronics makes it even harder to design standardised processes for disassembly due to the lack of uniformity. Furthermore, a product design challenge which frustrates the recycling of end-of-life LIBs from consumer electronic is the trend towards 'sealed in' batteries that are not user replaceable in mobile phones and portable electronics.
Among various intermediaries, it is generally agreed across the battery recycling industry that sorting is one of the least efficient and most costly processes. There are also concerns about the quality of sorting (i.e. the accuracy of the sorting process). First, in the main, current sorting is done by high-cost, low-accuracy manual processes, and very often suffers from labour shortage, delays in schedule and unsafe working environment. Second, sorting is currently limited to general batteries types rather than more specific battery chemistries, which is a critical consideration in the pricing model of battery collectors. Third, low sorting quality and the lack of more precise sorting (e.g. cathode chemistry-based sorting) hinders recyclers from adopting more promising and potentially lucrative recycling technologies, such as direct recycling, which requires more refined inputs of LIBs waste streams.
For recyclers of small and consumer LIBs, the decrease in high value raw materials and transition from LCO to less cobalt rich chemistries presents a challenge for recyclers trying to valorise scrap LIBs from consumer electronics, as the margins on recovered materials are squeezed. The trend for decrease in cathode material component values will necessitate more efficient recycling methods for recycling at scale to be profitable (Gaines, 2019). Furthermore, the trend towards embedded LIBs, necessitates additional, costly disassembly steps at the end of product life, which further constrains the economic margins for value recovery.
Advances in science and technology to meet challenges
To meet these aforementioned challenges, innovative technology solutions have been proposed and developed in the LIB recycling ecosystem. In the area of battery sorting, Refind Technologies has developed a vision-based automated battery sorting process that can identify all cylindrical batteries up to D size and sort all 9 V batteries into four classes: alkaline, NiMH, NiCd, and lithium batteries [313]. Li Industries further develops the Smart Battery Sorting System that integrates multiple sensors and utilizes machine learning to allow unique and more refined battery sorting (e.g. based on electrode chemistries of the LIBs) [314] An effective LIB sorting to obtain uniform waste stream would enable and facilitate the adoption of direct recycling as a primary approach for LIB recycling. The prevalence of direct LIB recycling would significantly reduce the production cost of cathode materials and mitigate the negative environmental effect of current LIB recycling methods [10, 111, 135, 195, 315, 316].
The trend for decrease in cathode material component values will necessitate more efficient recycling methods for recycling at scale to be profitable [317]. Furthermore, the trend towards embedded LIBs, necessitates additional, costly disassembly steps at the end of product life, which further constrains the economic margins for value recovery. The complex nature of technology products with integrated batteries frustrates simple battery removal [318]. Automated consumer electronics product disassembly has been demonstrated, e.g. Apple Daisy [319]. These automation processes need to be tailored for specific consumer product.
Automated disassembly at the cell level is challenging due to various types of cell design and internal structure. For lithium-ion pouch cells with z-fold internal structure, an automatic disassembly system has been designed and prototyped for dismantling and separating cathode sheets, anode sheets, separators, and Al laminated film housing from lithium-ion pouch cells [118]. Compared to the destructive pre-treatment widely adopted in the metallurgical process, automated disassembly and separation has a great potential to achieve a higher material recovery rate of all cell components. To further improve the accuracy, safety and effectiveness of the disassembly and separation, the automation system can be advanced to cyber physical system which integrates computing, communication and control to achieve collaborative and real-time interaction through feedback loops of interaction between computational processes and physical processes [320].
Concluding remarks
Effective recycling of small and consumer LIBs can alleviate negative environmental impact and shortage of critical materials, and thereby improve the sustainability of LIBs. The challenges of recycling these LIBs include difficult materials handling, low end-of-life LIBs collection rate, high-cost and inefficient sorting, and ineffective recycling operation. These challenges can be addressed by developing smart and automated collection and sorting system, effective disassembling process, and scalable direct recycling technology specifically designed for small and consumer LIBs.
Acknowledgments
The authors are grateful to the following funders for support—the Faraday Institution's ReLiB project (Grant Numbers FIRG005, and FIRG027) and the UKRI Interdisciplinary Circular Economy Centre for Technology Metals (TechMet) EP/V011855/1
22. Life cycle assessment of recycling processes
Jacqueline Edge1 and Laura Lander2
1 Dept. Mechanical Engineering, Imperial College London, London SW7 2AZ, United Kingdom
2Department Engineering, King's College London, London WC2R 2LS
Status
Quantitative data from life-cycle Assessment (LCA) informs holistic strategies to reduce pressure on raw materials, minimise environmental impacts and achieve a circular battery economy, providing guidelines to industry and policy. LCA studies have already demonstrated that lifecycle costs and greenhouse gas (GHG) emissions can be reduced by up to 50% through the incorporation of recycled materials into new batteries [321]. Figure 27 summarises the energy consumption reductions possible by different recycling methods. The importance and benefits of recycling lithium and other low value materials has been highlighted by several studies, particularly as regulations, such as the EU's proposed procedure 2020/0353/COD, mandating a minimum recycled content, come into effect and evolve [322]. Recycling is key to displacing high impacts incurred during the raw material extraction and refinement stages [315, 321, 323, 324]. Different strategies may be necessary for chemistries incurring lower extraction impacts, such as LiFePO4 (LFP), where recycling benefits are weaker [324]. Wider use of green electricity and low impact, low cost or high yield recycling processes are two ways in which the benefits of recycling could be enhanced.
Figure 27. Life Cycle Assessment studies on recycling of lithium cobalt oxide (LCO)-graphite cells shows how cell production energy can be reduced by increasing the use of recycled material, considering each component of the cell individually and comparing different recycling processes. Reprinted from [325], Copyright (2018), with permission from Elsevier.
Download figure:
Standard image High-resolution imageIn order for battery materials recovery to be widely adopted, recycling processes need to yield sufficient quantities of high quality materials, at low cost and low impact, especially as revenues from materials recovered from future batteries cannot be relied upon [325]. LCA studies on lithium-ion battery recycling processes are still few, mostly due to the paucity of data available in this new and still evolving field. Recycling strategies for cathode materials are well documented [321], but are lacking for other battery components. While methods, system boundaries, assessed impact categories and assumptions can vary significantly across the literature, making comparisons difficult [323], a number of effective tools have been developed by Argonne National Laboratory: (a) the GHGs, Regulated Emissions, and Energy use in Transportation (GREET) model [326] provides environmental impacts and energy modelling, (b) BatPaC [327] enables cost modelling of EV packs and (c) EverBatt [328] uses GREET and BatPaC to calculate the cost and emissions of recycling processes for various chemistries, recycling processes and locations, modelling closed- (recovered materials used to make new batteries) or open-loop (materials used in other products or industries) battery recycling. Another notable tool is SWAVE (Strategic materials Weighting And Value Evaluation), developed to qualitatively compare sustainability and materials recoverability of over 44 commercial recycling processes [96].
Of the recycling methods (see sections 13, 14 and 16), pyrometallurgical (smelting) recycling has the highest environmental impact and can even result in recycling causing more harm than benefit [17, 315, 329], due to high energy consumption, toxic emissions [10, 17, 330] and production and treatment of waste slag [331]. In addition, the materials are recovered in their pure form, requiring repetition of materials processing steps to produce battery precursor materials [317]. Hydrometallurgical (leaching) processes incur burdens through water use and chemical outputs [330, 331], but both hydrometallurgical and direct recycling lead to improved lifecycle environmental impact [315, 321, 323]. Generally, direct recycling is considered to incur the lowest impacts and achieve highest revenues [317, 325, 332] and is therefore particularly indicated for low value chemistries [111, 325].
Current and future challenges
There is a need for a consistent, quantitative framework for LCA of batteries and their recycling processes [333] that addresses all impact factors, defines unambiguous measures and units and clearly defines detailed, cell chemistry-specific process flows for battery end-of-life treatment [324]. This will enable studies to consider and declare their system boundaries and ensure that important elements, such as disassembly processes and transport of materials between steps, are not overlooked. Figure 28 shows the possible end-of-life pathways for automotive batteries, all of which need to be considered within such an LCA framework.
Figure 28. Stages of the battery lifecycle, showing the possible processes and routes that materials may follow. It is hoped that extensive recycling will eventually eliminate the final, landfill stage and its associated risks.
Download figure:
Standard image High-resolution imageThe benefits of recycling vary, depending on which impact category is addressed [324], therefore a standard framework should include quantitative measures for tracking wider impact categories, such as habitat destruction, aquatic ecotoxicity [331] and production of persistent chemicals. LCA studies need to keep up with an increasingly diverse LIB waste stream (variety of chemistries, form factors and dimensions) and processes still under development, to suit each. Direct recycling methods, in particular, present new logistical flows and are still emerging. Emerging biohydrometallurgical processes (see section 15) have not yet been assessed from an LCA perspective. Dealing with the inherent uncertainty around emerging battery chemistries, such as sodium ion batteries, is challenging and only a few studies have attempted to assess their recycling impacts [324], but they are hampered by a lack of transparency and reliable data at industrial scales [321]. Rinne et al included process simulation to scale up life cycle inventory data from laboratory-scale analysis [334].
Recovered materials re-entering the battery manufacturing stream may need to be reprocessed or reconditioned before use and these steps need to be included in LCA, as well as assessments of their effectiveness during the use phase of the lifecycle [325, 335], so that process flows which produce high quality materials, capable of truly displacing virgin materials, can be identified. This is especially difficult, because of degradation during both use and subsequent recycling, as well as the long lifetimes of EVs, such that recovered materials may be about a decade behind current technologies.
Holistic assessments which include the fate of the full range of battery components, including plastics, electrolyte, binders and housing, as well as lower revenue materials [96, 321, 335], would highlight the need for more materials to be recovered, leading to better processes and process flows. LCA studies have tended to focus on cathode materials, but more attention is needed for graphite recovery [96].
The commercial and technical feasibility and environmental benefit of battery recycling processes is highly variable [324, 325], presenting a complex problem requiring thorough, consistent LCA to be performed on all battery chemistries, form factors and emerging recycling processes, so as to formulate the best recycling strategies for each battery chemistry [336]. This is particularly relevant to the decreasing value of component materials, as batteries move away from cobalt [317].
Advances in science and technology to meet challenges
Recent reviews [10, 325, 335, 336] provide clear definitions for end-of-life strategies and summarise the latest recycling processes, which are continuously being advanced through various initiatives, including Argonne National Laboratory's ReCell Center [337], the Faraday Institution's ReLib project [338] and the LithoRec project [339], among others. In addition, new materials, cell form factors and pack designs are being developed and scaled up for commercial production, which may require bespoke dismantling and recycling processes. All of these rapid advances require LCA and techno-economic studies to keep up with impact, cost and benefit assessments, identifying the most promising strategies for each battery chemistry. Tailoring whole lifecycle, particularly end-of-life, strategies to each chemistry to identify the key trade-offs and achieve a truly circular battery economy, requires more attention [96]. All of this highlights the need for LCA tools which are flexible and easy to implement and extend. The qualitative methodology of SWAVE [96] could be extended to provide quantitative data and cover more impact factors. To achieve this and match the breadth of SWAVE will require stronger collaboration between academia and industry, sharing vital data for analysis [96]. In order to assess closed-loop recycling, LCA tools need to account for the post-recovery performance of recycled products, particularly as components undergo repeat recycling, so that the effectiveness of additional steps for restoring materials to a usable quality can be compared. There is a need to establish accurate, quantitative measures for other impacts across the value chain, particularly biodiversity, social and safety impacts.
Concluding remarks
Life-cycle Assessment is a powerful tool for designing tailored, holistic strategies for recycling batteries, to make them more cost effective and sustainable, however a consistent, quantitative framework for benchmarking processes against each other has not yet been defined. The focus, at present, is on recovering cathode active materials and is largely limited to retrieving the high value minerals, but the aim should be to recover all components within the battery. LCA studies have provided a strong case for more extensive recycling, but more data and analyses are needed to design the most effective strategies for each different battery type and application. These strategies provide a vital feedback loop to inform the design of future batteries, so that they include features which make their eventual recycling lower impact and financially viable.
Acknowledgments
This work was carried out with funding from the Faraday Institution (faraday.acuk; EP/S003053/1), Grant Number FIRG025.
23. Life cycle assessment of recycling systems
Viet Tien Nguyen1, Robert J R Elliott2, Oliver Heidrich3, Margaret Slattery4, Qiang Dai5 and Gavin D J Harper2
1 London School of Economics and Political Science, United Kingdom
2 Business School, University of Birmingham, Birmingham, B15 2TT, United Kingdom
3 Water Group, Environmental Engineering Group, School of Engineering
4 Energy Systems, Energy and Efficiency Institute, University of California Davis,
5 Argonne National Labs
Status
In general Life Cycle Assessments (LCAs) rely on well-defined system boundary, which involves choices in multiple dimensions such as technology, geographical location and time horizon [340]. In this roadmap, we draw the distinction between a 'micro' level LCA analysis, focusing on the differences between different processes within the recycling plant, and the broader system-level 'macro' analysis which considers the circular economy system as a whole, including all the steps of transportation and logistics from the end user through to finished materials.
For the case of Lithium Ion Battery Recycling, whilst there is a significant technical focus on the improvement of processes within the system boundary of the recycler, the wider system context and design is under-considered. To optimise the global battery recycling system, the reverse logistics from end-user back to the point of resource recovery need careful consideration. End-of-life logistics and the location of these activities has been identified as having a significant impact on the impacts of such reverse supply chains [330]. Some analyses will focus on, for example, the chemical processes taking place within a recycling plant, but neglect the broader aspects of whether disassembly vs. comminution (covered elsewhere in the roadmap) is required to realise these subsequent processes. LCA analysis of the broader system can lead to a more intelligent configuration of resources to minimise the environmental burden of the transportation of wastes and their valorisation.
The location of the global supply of technology critical metals and the processing capacity for lithium ion batteries does not align with the location of consumption [341]. The refining of materials and LIB manufacturing is also highly geographically concentrated [341]. Whilst this may be of concern to policy makers from a critical materials security standpoint, it also results in enhanced environmental impacts through shipping long distances.
Notwithstanding the dynamics of the supply of primary materials, nations still have an opportunity to shape the industries that may produce a secondary supply of critical raw materials through the remanufacture, reuse and recycling of batteries as these industries are still in their relative infancy given the anticipated volumes of end-of-life LIBs anticipated. To this end the time dimension matter as LCA can inform policies and recycling should be considered together with other contemporary technologies. More specifically, careful life-cycle assessment of a range of recycling system-level scenarios, that include the wider boundary of transport and logistics operations could in turn lead to the optimisation of this future industry identifying preferable facility locations in relation to anticipated waste arisings and onward consumers of recycled products from those facilities [342].
Finding the optimal locations for the aggregation and collection of waste, the final destination for the processing and categorisation of waste and the onward logistics to remanufacture, reuse and recycling operations is not a straightforward problem [343]. To this end macro-level life-cycle assessment of the whole-system could potentially aid in better decision making. Technological developments may influence the future configuration and topology of the closed-loop circular supply chains for battery materials [344]. A holistic appraisal of the lifecycle impacts of any given recycling technology should not just consider the LCA of the technology within the plant, but also the wider implications for transport and energy use that arise from the broader system implied by this waste treatment pathway [345]. The scale at which future technologies may operate economically may dictate system topologies that tend towards either centralised or distributed infrastructure [346].
Indeed, overcentralisation of recycling infrastructure has the potential to result in 'diseconomies of scale' [347], that are associated with excessive transportation burdens. This also means excessive pollutions and fire risks related to transportation and less opportunities for technology diversification.
Current and future challenges
It has been highlighted that whilst there has been a focus on end-of-life processes, transportation is under-investigated. In a literature evaluation that considered 60 studies about electric vehicle (EV) battery end-of-life, 70% considered collection and transportation a challenge to LIB reuse and recycling [342], and 63% identified a policy or research gap in this area [342]. However, less than a third of the studies considered included transportation in their analysis when evaluating the economic or environmental impact of recycling, despite the fact that reducing transportation costs is seen as crucial for profitable recycling [332]. Indeed it has been found that profitable recycling can only be achieved within a tight alignment of waste feedstock chemistries, processes and locations [332].
The impacts of transportation on the techno-economics of recycling are significant and in-country vertically integrated recycling performs better on both economic and environmental grounds compared with overseas recycling [332]. This also has positive impacts for risk reduction and critical materials security [332].
One model that has been well adopted in the life cycle analysis of recycling solutions, is Argonne National Labs Everbatt model [328]. Within Everbatt there is a 'Transportation and Collection' model which considers the movement of batteries from their last user to a collection site—then onwards transportation to a recycler. The recovered materials are then transported to a cathode producer, then finally to the battery manufacturer. Additionally, consideration is optionally given to the transportation of manufacturing scrap, which is presently an important feedstock for recycling facilities in the absence of large-scale EV retirement. The Everbatt model then uses the GREET (The Greenhouse gases, regulated emissions and energy use in transportation) model [348] in order to model the emissions from transportation modes employed in. Whilst freestanding LCA models could and have been developed, the Everbatt model, is well refined for a static model, and has been applied in many different context and scenarios. To some degree, this provides a helpful baseline model as it allows comparisons to be drawn between different analyses using the same baseline model.
Depending on the distances and routes travelled, different modes of transport may be appropriate, and these will have varying impacts. For example, rail has lower emissions compared with heavy-duty trucks; however, it offers less flexibility regarding timing and location. The Everbatt model allows for the modelling of a wide range of transport modes including medium-duty truck, heavy-duty truck, rail, barge, and ocean tanker. Furthermore, the model also allows the user to specify whether cargo is subject to from hazardous material transportation requirements, which may have significant implications for the total shipping cost [342].
However, future LCA analyses should not just extend to the material flows through the recycling system, but also encompass a broader circular economy conception that includes process routes through remanufacture and reuse [349]. Finally, the location of refining and production infrastructure must be considered, as the environmental and economic benefits of distributed recycling facilities are greatly reduced if recovered material must be exported for further processing before they can be reused in the battery value chain. Building and optimising advanced models that can produce meaningful insights remains a significant challenge given the many uncertainties around the future circular economy of Lithium Ion Batteries.
Advances in science and technology to meet challenges
Several studies have attempted to add spatial and temporal dimensions to LCA to aid the planning of recycling infrastructure [330], whilst the Argonne National Labs model provides a sound LCA model for recycling systems, such process-based static modelling can be dramatically enhanced if combined with an additional layer of reverse logistic demand projection and geo-spatial optimization [350]. This has been attempted, but clearly there is scope to further evolve the modelling of the broader recycling system. Finer grained, dynamic models may even find future application in 'real time decision making' if they can be updated with live data, allowing optimum decisions to be made about the best options given live data on energy costs, carbon intensity, the prices of materials and reagents and other live data.
In combination with other packages, reverse logistics supply chains can be modelled using Geographical Information Systems approaches to build Geospatial Supply Chain models [350] which can take into account a fuller mapping of the nature of the networks used to convey materials flows through the recycling system. Furthermore, given that the anticipated materials flows through recycling systems are likely to vary over time with changing waste flows, it is also necessary to map the temporal dimension of these changes over time.
It is possible to conduct life cycle analyses at different scales [351]—the product level, the organisation level, the consumer level and the regional level and the global level. The choices of technology selection in the processes for recycling and reuse of lithium ion batteries will in turn influence the shape, form and geographical distribution of the future lithium ion battery end-of-life industry, and modelling of the geospatial form of this future industry will be key to good decision making and planning. Of course, the growth of the industry that surrounds the circular economy of Lithium Ion Batteries is the product of the decisions of many different actors, but to a degree, legal an regulatory perspectives, as well as co-ordination of any targeted investment by state actors informed by the best models can aid in steering the direction of the industry towards an optimal configuration.
Approaches to a comprehensive whole-systems life-cycle assessment of a future circular economy of Lithium Ion Batteries, are to a degree limited by present tools and available data, there is significant scope for advancement on both of these fronts to improve our understanding of planning future efficient circular economy systems for lithium ion batteries.
Concluding remarks
Whilst there is an understandable focus on improving the technical potential of recycling processes and using LCA as a tool for benchmarking these against each other, consideration should be given to the wider system boundary of the recycling system, including transport, logistics and movement of materials and the concomitant impact of all of these steps which add embodied energy and impact to the final product.
Many LCA studies of LIB recycling draw the system boundary at the process for materials recovery and neglect the wider context around collection and transportation [341]. Understanding these pathways and the impacts of these material flows will be key to optimising the environmental impacts of a circular economy of Lithium Ion Batteries [342].
Given the complex nature of the evolving market in end-of-life Lithium Ion Batteries, and the lack of transparency in this market, there is a need for a greater data-driven understanding of the waste flows that arise at end-of-life. To this end, enhanced waste statistics in this area would be an invaluable aid to improved decision making.
Acknowledgments
The authors are grateful to the following funders for support—the Faraday Institution's ReLiB fast-start project (Grant Numbers FIRG005 and FIRG006) and the UKRI Interdisciplinary Circular Economy Centre for Technology Metals (TechMet) EP/V011855/1
24. The digitalisation of lithium ion battery recycling
Dan Reed1, Iain Styles1 and Gavin D J Harper1
1 School of Metallurgy and Materials, University of Birmingham, Birmingham B15 2TT, United Kingdom
Status
The future landscape for the recycling of lithium-ion batteries (LIBs) will be different from the present scale of recyclers operations. Recycling and repurposing operations will need to be undertaken at an ever increasing scale against the backdrop of increasing variability in cell design and chemistry combined with squeezed margins through the use of decreased value raw materials through transitioning to low/no cobalt formulations and the decreasing cost of batteries.
A step-change in a circular economy for batteries will be achieved through embedding the concept of design for recycling, using next-generation recycling technologies and retaining and/or upcycling the structures engineered into the active materials. To achieve this mechanically disassembling and pre-sorting enables the use of slicker hydrometallurgical techniques that are most effective when specifically targeted or direct recycling.
One of the keys to enabling this enhanced approach to recycling LIBs is the digitalisation of battery data, and a more integrated approach to the use of digital data in recycling.
A combined suite of digital technologies (figure 29) would enable a more efficient circular economy by providing critical up-front data. The onboard computer, battery management system (BMS) can contain primary knowledge about the formulation, make and assembly of batteries. This would greatly aid disassembly and inform the recycling path. Secondary information regarding use-phase, state-of-health (SoH) and any failure and causes of failure that could be collected in real-time during operation could significantly reduce the time taken to triage a battery pack, enabling faster, smarter, more informed decisions to be made. This could dramatically aid in the reduction of some of the transactional costs that are associated with battery waste management [352].
Figure 29. Technologies contributing.
Download figure:
Standard image High-resolution imageDigitalisation could also be a key enabler for new types of business model, including 'Product Service Systems'[353], that could lead to new patterns of consumption, and 'consuming' batteries in radically new ways that deliver greater value from finite materials.
Currently digitalisation tools are only being employed to a limited extend in waste management [354]. Combining the onboard digitalisation, with an external primary survey (triage) involving robotic visual inspection [93], retrieval and processing of onboard information enables critical decisions about reuse (complete or partial) or guides the disassembly by autonomous or semiautonomous robots.
A greater emphasis on digital data in battery systems could be used real time to yield significant benefits in the use of the battery pack within electric vehicles (EVs), due to greater insights about current SoH, estimation of remaining life and diagnosis of faults.
Increasing the accessibility and transparency of on-board digital data to the future recycling industry would cut the time taken to triage each pack upon arrival, enable autonomous disassembly whilst increasing safety by self-identifying abused or damaged cells hidden within a pack.
Current and future challenges
The challenges facing the digitisation for battery recycling are related to firstly the active onboard monitoring and digital information regarding manufacture, and secondly the acquiring, processing and using of information at the end-of-life to guide the most effective or profitable recycling route.
These challenges are technically solvable with the implementation of greater sensing, making information available and use of advanced data processing. The technologies exist, however, many are undergoing the translational phase of being deployed in real-world use cases.
Some of the biggest challenges to ensuring the greater degree of penetration of digital technologies into vehicle batteries are organisational and cultural. With pack and vehicle manufacturers, focusing on the sales/market share and technical properties which give their product competitive advantage, many are presently unwilling to divulge any potential information which could aid competitors about their battery cells, pack or management system.
The regulatory push towards the 'right to repair' and need to recycle could push for access to the current digital information locked within the BMS, with marginal additional cost to manufacture. Without a compelling need to share information this is likely to be restricted to the minimum information possible.
The limited choice and cost to repair (given limited competition in the EV service, maintenance and repair space) is likely to translate into consumer disapproval, who in time may feel the burden of operating, maintaining and potentially disposing of their vehicle. The inability to access information and diagnose the SoH of a battery pack will be reflected in the costs to insure vehicles, as insurers reflect the risk of repairing expensive crash-damaged vehicles. If digital technologies can aid in quick diagnosis and repair, this will translate into improved perception and confidence in models which implement such technologies.
The challenges around the future use of digital information and digitisation in the battery recycling industry will centre around the triage of EoL batteries and the subsequent choices. One of the first decisions that needs to be made with any EoL vehicle battery pack is 'can this pack be reused in another application?'. Through rapid diagnostics to determine the performance of any pack, cell or module that can be reemployed in another application, extending its useful life, retaining the valuable materials and embedded value in service for longer.
Once cells are beyond the point of being useful batteries they must be dismantled, their components recovered and recycled for use in new batteries. Knowledge of the manufactured housing and cells is a useful starting point, however, after service and potential damage it is necessary to collect and use real time information regarding the disassembly and recovery of components. This requires large amount of real-time data to be collected and used to rapidly guide autonomous disassembly. Potentially requiring computational power and algorithms far beyond what would be seen as standard within the current industry.
Advances in science and technology to meet challenges
Verifying the provenance of the materials and ensuring custody of ethical supply chains is considered of paramount importance to automotive brands with reputations to defend and protect. Given concern over the potential for unethical sourcing of some materials in the EV battery supply chain, manufacturers are keen to ensure robust verification and traceability of materials. Blockchain has been advanced as one technology that could potentially be used in order to keep an inventory of materials in the battery [355, 356]- in addition to leading to better verification of contents for ESG, there is also the potential to use this information to better aid the sorting process at the end-of-life for batteries enabling more efficient recycling at end-of-life.
Digital twins [357, 358] of battery systems compare measured physical values with digital norms to enable evaluation of batteries and prediction of future performance. Embedded digital twin models could aid in providing onboard diagnostics whilst batteries are in service, and the ability to access this information with open-standards could greatly aid the process of triaging batteries at the end-of-life, potentially reducing or eliminating the need for gateway testing.
Artificial Intelligence and machine learning have the potential to help with many problems at the end-of-life [359]). Where information is incomplete or not known, AI based processes could help infer information that is unknown about batteries from previously learned situations. This could be useful in packs which are not designed for disassembly.
To make batteries truly smart, they could be networked (e.g. via 5G) to enabling reporting back to the manufacturer, software updates to be issued, and potentially allowing machine learning approaches to spot the early signs of failure, or patterns of usage that might preface or accelerate a failure. This could be used to issue an early warning to the user that includes a prediction of the consequences. The advantages of this approach, improving on the 'digital twin', is that it can also draw on aggregate data from other batteries, a 5G-connected battery could enable this to be done in the cloud by the manufacturer, who would in turn gather better data to improve their design.
Given future volumes of batteries reaching end-of-life, manual dismantling would seem essential to deal with many dismantling functions cost effectively [113]. Cheap, commercially available robots enabled by clever AI approaches that enable them to deal with the variety inherent in end-of-life scrap [87]
Concluding remarks
With Industry 4.0 a contemporary and persistent theme in industrial transformation, it seems highly likely that further scope for digitalisation of various stages of the LIB's lifecycle is inevitable. The full realisation of the integration of these techniques has already been coined 'Recycling 4.0' [360]. Whilst digital techniques for process control and monitoring in manufacturing are nowadays a given, there remain unrealised opportunities for the further integration of digital technologies at different stages of the LIB's lifecycle.
At the industry's current stage of development, manual processes are pervasive in the end-of-life management of LIBs. This presents numerous potential safety concerns [7] and hazards, not to mention the lack of economy in labour-intensive manual operations.
Automation for the removal, sorting and disassembly of batteries holds much promise to speed operations at the end-of-life. It has been observed that waste management is 'inexorably developing towards digital industrialisation' [361]. However, the full potential of these technologies in the circular economy of lithium ion batteries has not been fully realised. If information on the batteries state-of-health can be garnered from connected technologies before battery removal, post removal testing could be eliminated.
These technologies could aid greatly in the processing of existing batteries, however, batteries that were designed for end-of-life with digitalisation borne in mind at the design stage could lead to an enormous improvement in the efficiency of end-of-life operations.
Whilst many of these technologies have been demonstrated in the lab in isolation, the opportunity remains to integrate them and demonstrate them at scale. Furthermore, the even greater opportunity is to leverage these models to enable new product service systems and business models that could lead to greater resource efficiency in the circular economy of lithium ion batteries [362].
Acknowledgments
The authors are grateful to the following funders for support—the Faraday Institution's ReLiB project (Grant Numbers FIRG005, FIRG027 and FIRG057) and the UKRI Interdisciplinary Circular Economy Centre for Technology Metals (TechMet) EP/V011855/1
25. Battery recycling: legal & regulatory
Jyoti Ahuja, Aleksandra Cavoski and Robert Lee
Birmingham Law School, University of Birmingham, Birmingham B15 2TT, United Kingdom
Status
The move to sustainable and safer use of batteries throughout their life cycle is primarily driven by two main objectives: the need to reduce negative environmental impacts in sectors such as transportation to meet climate neutrality targets; and the wider promotion of a circular economy [11]. This paper explores the lessons learnt from the European Union as the EU framework provides the most recent attempt at reform of battery regulation. The EU has a long lineage of addressing the disposal and recycling of batteries, starting with the Council Directive 91/157/EEC on batteries and accumulators containing certain dangerous substances [363]. This 1991 Directive was repealed in 2006 and replaced by Directive 2006/66/EC on batteries and accumulators, which for years provided a broad framework for treating batteries (hereinafter: the 2006 Batteries Directive) [364]. However, the 2019 evaluation of the Directive, [366] recognised that, despite fulfilment of its intended benefits, the Batteries Directive 'is too general on the nature and extent of the objectives to be achieved and on important measures that the Member States have to implement'. The EU was also led by a third objective which is a need to strengthen the functioning of the internal market. Moreover, the evaluation found that the Batteries Directive did not sufficiently incorporate technical advances, as illustrated by growing importance of chemistries such as lithium-ion batteries (LIBs) not specifically addressed by the Directive [365].
The vehicle for legal change came with the launch of the European Green Deal and one of its underpinning strategies, the Circular Economy Action Plan. The European Green Deal recognised the need for 'a safe, circular and sustainable value chain for all batteries, including to supply the growing market of electric vehicles (EVs)' [366]. The newly proposed Batteries Regulation [11] envisions the modernisation of the EU's legislative framework underpinned by several objectives including:
- improvement of collection and recycling of batteries;
- better recovery of valuable material; and
- the improvement of sustainability and transparency requirements for batteries [367].
A regulation, rather than a Directive, allows for a more centralised and uniform set of rules.
Current and future challenges
The 2006 Batteries Directive, operating in conjunction with the end-of-life vehicles Directive (2000/53/EC), lacked the mechanisms to facilitate a circular economy in EV batteries as they flood onto the market. Issues include:
- a lack of definitional clarity;
- inadequate safety and design requirements and
- the absence of a robust extended producer responsibility (EPR) framework for EV batteries [368].
Drafted before the electric mobility transition, the Directive contains three broad categories of batteries: portable, industrial and automotive. End-of-life management of (largely consumer) portable batteries, are subject to the most rigorous provisions within the Directive. Automotive batteries are confined to starting, lighting or ignition batteries. Industrial batteries are those designed solely for industrial or professional use, but currently include EV batteries. These two latter categories are subject to less stringent EPR obligations, with industrial (including EV) battery producers facing no proactive collection obligations or targets, but are simply required to take-back on request. Article 2(12) of the Regulation proposes a distinct category for EV batteries: 'any battery specifically designed to provide traction to hybrid and EVs for road transport' [11].
With more stringent recycling/reporting requirements, greater clarity will now surround the repurposing and remanufacturing of battery packs. Article 12 and Annex V of the Regulation addresses some safety gaps for stationary battery energy storage systems (BESS) by stipulating that these must be safe during operation and use [11]. Producers must provide evidence of safety testing, using methodologies set out in Annex V. Article 14 and Annex VII of the Regulation requires that rechargeable industrial batteries and EV batteries must contain a battery management system (BMS) that stores data to evaluate the state-of-health and expected lifetime of batteries [11], which must be provided to purchasers. Better evaluation of residual value aims to facilitate reuse, repurposing or remanufacturing of the battery.
The proposal could usefully extend further by requiring adequate safeguards for installation, location and maintenance of domestic BESS. It could be more explicit, also, on producer responsibility obligations and liabilities upon transfer of an EV battery from vehicle manufacturer to battery repurposer [27]. The Regulation states that repurposing constitutes a waste treatment operation, so that repurposed batteries are classified as 'new products' which must comply with product requirements and standards set for new batteries [11], but does not explicitly clarify repurposing responsibilities and liabilities.
Advances in science and technology to meet challenges
There are currently no agreed standards for lithium-ion battery (LIB) design/manufacture for first use, although these are in development. Economic feasibility of recycling is hampered by the complexity and lack of standardisation of EV LIBs, which are manufactured by different companies with varying design configurations, cathode chemistries and physical shape [10, 369]. The specific chemistry of an EV battery is usually not labelled, thus neither third-party battery refurbishers nor recyclers can know which kind of battery they are receiving [369]. While the proposed Regulation states that more stringent battery labelling will be required, we contend that this must also include labelling of battery chemistries (an aspect not mentioned in the proposal) to facilitate more efficient sorting and recycling.
To allow repurposing operators to comply with specified quality control criteria via access to BMS, advanced diagnostic functionality must be embedded within BMSs to facilitate a circular economy approach to EV batteries [10]. To date, battery manufacturers have designed batteries to ensure that they perform as intended in the vehicle rather than with a view to second use [369]. However, the liability principles that would apply if a repurposed battery causes harm remain unclear, especially if causal evidence links battery failure to original manufacturer.
One can anticipate greater emphasis on eco-design, but this needs to facilitate design for reuse or for disassembly and materials recovery. Despite benefits of putting EV batteries to second use, widespread repurposing could potentially disadvantage recycling and circular economy goals, especially where access to critical materials is needed to serve the demand of ongoing EV battery manufacture. The Regulation seeks to promote recycling by mandating that industrial and EV batteries should contain minimum levels of recycled content, which will rise over time. There is a tension, however, between this promotion of recycling and the facilitation of EV battery repurposing. This might be resolved by better planning based on economic and lifecycle data to clarify the relative circular economy merits of different end-of-first-life pathways [27].
Concluding remarks
As a part of the wider policy objective to achieve climate change neutrality by 2050, the electric mobility transition is one of the key policies at both the EU and national levels. However, this transition also brings numerous legal and regulatory challenges. The European Commission has recognised the imperative for a new legal framework that addresses inadequacies in current battery regulation, which is clearly unsuited to govern the EV battery transition. To that extent, the new 2020 proposal for a Regulation to reform the 2006 Batteries Directive is welcome. The proposal contains some novel and forward-thinking strategies to improve sustainability of the EV battery value chain; as well safety of use, second-use and disposal.
We have outlined some proposed measures that will address regulatory gaps to meet current and future challenges in EV battery management. However, while positive, we contend that some aspects of the new proposal might go further to advance effective end-of-life management for complex and rapidly advancing EV LIB technologies. These include the need for mandatory labelling of cathode chemistries to support recycling/remanufacturing and greater clarity about battery ownership and liabilities in the battery repurposing chain. We highlight concerns about the potential of widespread repurposing to disrupt the potential of recycling to secure the supply for critical minerals needed for battery manufacture. Policies must be guided by consideration of the relative long-term merits of recycling versus repurposing at the end of first life, based on robust economic and lifecycle data.
Acknowledgments
This work was supported by the Faraday Institution (Grant Numbers FIRG005 and FIRG006) and UKRI Interdisciplinary Circular Economy Centre for Technology Metals (Met4Tech) Grant No. EP/V011855/1.
26. Next generation chemistries and recycling considerations
Emma Kendrick1,2, Elizabeth Driscoll1,2, Paul A Anderson2,3, Gavin D J Harper1,2, Jen Baker4 and Peter Littlewood2,5
1 School of Metallurgy and Materials, University of Birmingham, Birmingham B15 2TT, United Kingdom
2 The Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot OX11 0RA, United Kingdom
3 School of Chemistry, University of Birmingham B15 2TT, United Kingdom
4 Swansea University, Swansea University Bay Campus, Fabian Way, Swansea, Wales, United Kingdom
5 University of Chicago, 5801S Ellis Ave, Chicago, IL 60637, United States of Americ
Status
Lithium-ion batteries (LIBs) are well established and will remain a key technology for many years, however, due to the extensive use of critical materials and the constant requirements for improvements in energy, power, life-time, cost and sustainability, a greater diversification in battery chemistry is required [370]. The term 'battery' refers to the whole assembly and not just the cell; the battery packs are disassembled to liberate the modules and cells. In this section we discuss the range of future cell chemistries specifically, assuming that the cells can be easily separated from packs, as these in turn will require end-of-life treatment.
When considering sustainability, the full materials life cycle needs to be considered, from materials sourcing and extraction, to manufacturing of the materials, components, cells and batteries, use in a device (1st life) and potentially use in a different application after the first (2nd life), through to recovery of the materials for re-use at end-of-life. Sustainability requires energy and environmental impact assessment at each stage of the battery materials' lifecycle. Therefore, when considering other battery chemistries, cradle to cradle Life Cycle Analysis (LCA), supply chain, and environmental impacts need consideration, in addition to the performance metrics. For example, a 1 kWh battery may require from 30 to 50 kWh of energy to produce. Some of these effects can be mitigated by improvements in supply chain management from mining to refining, but in the long term we must strive for a closed cycle in terms of CO2 emissions and the minimum possible energy input.
One of the shortcomings of the present suite of technologies, and a driver for change, is the use of Critical Raw Materials in the manufacture of the active cathode materials for these batteries. Whilst some Li-Ion cathode chemistries like LiFePO4 use less critical materials, lithium availability is also a common challenge and, despite the fact that it is not rare in the earth's crust, given the increasing demand for battery packs, while production lags and greener extraction methods are developed [371] there is a push to develop energy storage devices that can employ less critical and more earth-abundant materials. This development follows two main routes: first using a similar architecture to LIBs but with different chemistry such as sodium ion batteries [372]; and flow cell batteries which contain the electrolyte in a separate tank and utilise a range of chemistries from vanadium [373] to zinc bromide to lead-based systems [374]. The flow cell architecture is much more suited to materials recovery at EOL but, owing to the lower energy density and bulky tanks, cannot replace lithium ion in mobile applications.
Peters et al [375] demonstrated that the basis for recycling at the end-of-life is less compelling for sodium-ion in comparison to LIBs. That said, in accordance with the waste management hierarchy, there is an imperative to seek to recycle all waste products in preference to less preferred options like energy recovery and disposal, and in other spheres, less valuable materials are recycled, however, for complex technology products like future batteries the economic case for doing so is as yet unproven.
Alternative battery chemistries (figure 30) can be loosely classified into three fields: (a) Lithium metal anode batteries [376] (b) Alkali-ion alternatives to lithium [377] and (c) Multivalent batteries [378].
Figure 30. Next generation battery technologies being investigated, and their estimated power and energy densities.
Download figure:
Standard image High-resolution imageLithium metal anodes can increase energy densities significantly and are employed in solid-state configurations, which have either polymer or ceramic lithium-containing solid membranes [105]. Here the lithium metal is partnered with a composite cathode which often contains high voltage cathode materials in conjunction with the solid electrolyte system. Other lithium metal systems include lithium-sulfur and lithium-air, where the electrolyte can be liquid, polymer, or ceramics [379].
Owing to the increasing criticality of lithium supply in particular regions [370], alternatives are being investigated. It is possible to substitute the lithium-containing materials for those of other cations. In this respect sodium and potassium are receiving significant attention. Both sodium and potassium materials can be utilised as drop-in replacements for lithium-based chemistries where alkali metal containing transition metal oxides, polyanion or sulfide materials can be used as the positive electrode with carbon-based negative electrodes [377]. Graphite can be utilised for both Li and K, whereas hard carbons are utilised for Na. Owing to the higher abundance of these materials, the supply chain is less restricted, with a focus upon nickel- and cobalt-free positive electrodes, and synthetic carbons.
Pushing the boundaries of energy density further, multi-valent materials such as Zn2+, Mg2+, Al3+ are being investigated [378]. These are found in similar configurations to the alkali metal ion batteries, with metallic or carbon negative electrodes. The combined configuration of higher valence and metallic anodes significantly increases the theoretical capacities and energy densities of these battery systems; however, they are currently not mature, and to date the power and cycle life is still limited.
Current and future challenges
In all cases not only the materials choices but performance, safety, manufacturing, recycling and the ultimate life-cycle sustainability require consideration. The use of critical materials in LIBs is high, with cobalt, lithium, graphite and phosphates all on the critical materials list for Europe [187], meaning that there is a supply risk, either due to their lack of abundance, geographical location, or supply chain. The move to other battery chemistries could introduce new materials which have lower supply risks, and there is an opportunity to look only towards low risk and low-cost materials, such as sodium-ion or potassium-ion batteries (NIB, KIB). One consideration, however, with low value materials, is the economic viability for reclamation at end-of-life. High value materials combined with lower efficiency methods of reclamation or recycling can still make economic sense, but for lower value battery materials, greater effort in high efficiency recovery of all the materials for reuse will be required. In every battery system there is a power, energy and lifetime trade-off. When optimising high power, energy will decrease and vice versa. Decoupling power and energy is a challenge. The increase in power and energy also typically reduces the lifetime of the cell. Life-time or time 'in-use' also needs to be considered as part of the materials lifecycle, therefore decoupling the performance properties and maximising lifetime is a critical challenge for all these technologies. Safety considerations in manufacturing and recycling are also a critical factors.
To increase energy density, metallic anodes can be used, as in lithium metal batteries, for alternative alkali metals, potassium and sodium are much more reactive in oxygen and air than lithium and may cause safety issues during manufacturing and disassembly. In terms of manufacturing, metallic lithium can react with nitrogen, oxygen, and water in the atmosphere to produce an insulating layer, and highly reactive sodium and potassium cannot be used even in a dry atmosphere only an inert atmosphere. Metals such as magnesium and aluminium, have strong passivating layers which require removal, and often highly corrosive electrolytes are utilised, also posing safety issues from the electrolytes [380]. For high-capacity cathode materials such as the high nickel content lithium and sodium layered oxides, residual compounds on the surfaces such as hydroxides and carbonates are formed which cause instabilities in the inks and slurries for coatings, ultimately hindering the potential lifetime of the cells [381]. The move to metallic anodes and solid-state cells means that new manufacturing processes are required, and it is no longer a 'drop-in' technology to current manufacturing processes [379]. This adversely impacts the cost and sustainability assessment.
All electrochemical cells are multi-component systems, and current recycling technologies do not recover the full range of material components, whether through pyrometallurgical, hydrometallurgical, short-loop or direct recycling from mechanical processing [94]. When the complexity of the cell increases, the difficulty in sorting the components of the cell also increases. Metallic anodes are reactive with water and potentially provide safety issues. In solid state systems where the materials incorporate polymer or ceramic electrolytes, difficulty in separation because of the binding between the components occurs, and these are significantly more complex to separate than with low polymer content binders and liquid electrolytes [105]. If the cell or battery is easy to disassemble, or designed in a manner in which all the components are easily separated, copper, aluminium and steel would be in principal easy to recycle, as effective recycling routes already exist.
Advances in science and technology to meet challenges
Materials: The extension of current cell chemistries to metallic anodes, solid state, potassium and sodium, and multi-valent materials offer potential improvements in energy density, power, lifetime and cost. All of these performance parameters need confirmation in larger proof of concept cells to provide assurance of the technology before they can be incorporated into new devices. A key consideration in designing new batteries is sustainability and a materials life-cycle Assessment. However, often and to-date the LCA takes places retrospectively, rather than at the beginning of understanding a material's viability towards sustainability. To some extent this is unavoidable as the pace of the electric vehicle transition is leading to products reaching the marketplace in large volume well before large volume, high-throughput, efficient recycling processes are available to assess.
A whole life approach considers materials, manufacturing, lifetime, re-use and recycling, all of which impact sustainability. Incorporating principals of sustainability at the design stage is crucial as this will reduce critical materials use, waste, cost and environmental impact, creating a more circular engineering approach to battery design (figure 31).
Figure 31. Schematic to show the different considerations in sustainability for next generation battery technologies.
Download figure:
Standard image High-resolution imageManufacturing and recycling: Pyrometallurgical approaches are elegant in their simplicity but may yield less valuable returns from future batteries. In common with the pattern of reducing the quantity of valuable critical materials from LIB, if critical materials are removed from future battery chemistries, value recovery from pyrometallurgy becomes more challenging. For future multivalent batteries, the manganese would end up in the slag phase, but copper and iron may be recovered. Whether this would lead to significant value recovery, or some metals would just act as a useful reductant for other products in the smelter as aluminium is at present remains to be seen [97]. For hydrometallurgical processes, there may be concerns over selectivity with the presence of new elements that may poison the process, e.g. iron control in recycling Na0.76Mn0.5Ni0.3Fe0.1Mg0.1O2 batteries [382]. Low energy and environmental impact manufacturing processes which reduce the use of heat and energy could be used in these new cell designs [383], for example, polymer-containing composite electrodes or solvent-free processing. The move to lithium metal requires dry or inert atmospheres for processing, and often, even if processing occurs in inert or dry atmospheres, a passivation layer requires removal before assembly. For the case of metallic negative electrodes, it should be noted that these cells are manufactured in the charged state rather than the discharged state, such as for LIB and NIB. This impacts the safety of the manufacturing processes, transport and storage and they may need careful control and monitoring [383]. An alternative is to use metallic alloying metals such as tin, silicon or germanium, and polymer and ceramic membranes, which could offer improved safety solutions.
Design for disassembly and recycling processes can be utilised to maximise the potential for reclamation of the critical materials and limit additional processing of 'black-mass' for short-loop and direct recycling processes. Easily removed binders for separation of the material components are key [84]. However, if polymer and ceramics are used as the electrolyte, and are contained within the positive electrode, this will make separation significantly more difficult [105]. Key areas for research are delamination, binder negation, and mixed material separations. With the complexity of the cells and chemistries, a greater degree of highly mixed waste streams are produced. To separate these materials, the specific properties of the material components, conductivity, density, magnetic susceptibility, and surface properties, can be exploited in the separation process, and this can also be a consideration when designing a new battery.
Performance and parameterisation: Sustainability considerations for 'in-use' are also required; longer 1st life and evaluations for 2nd life applications extend the lifetime before recycling is required. Lifetime can be maximised through operation and control of the charge and discharge cycles of the cell, through to design of the materials and components. Electrolyte additives and formation processes are often used to form more stable solid electrolyte interphase layers and hence improve performance [384]. Much of this is done by empirical design and is difficult to predict. To improve the predictability of this, multi-scale models can be utilised for design and performance purposes, and accelerate a new technology to market, whilst also designing best charging, discharging regimes for maximum lifetime [385]. For sustainability care should be taken from the outset to ensure that any additives used are fully compatible with proposed end-of-life recycling processes.
Table 2 provides a summary of some future battery technologies, their characteristics and the potential circular economy issues that may arise should they become successful in the market.
Table 2. Future battery technologies and their potential circular economy issues.
| Type: | Battery characteristics: | Circular economy issues: |
|---|---|---|
| Sodium, Potassium Ion Batteries [377] | Hugely decreased environmental impacts and reduced concerns around materials criticality. | How can we make recycling relevant with low-cost materials? |
| Multivalent ion batteries(Mg2+, Ca2+, Zn2+) [378] | Potentially greater capacity due to greater ion valency. Use of more earth-abundant materials. | Similar challenges with cathode recycling for LIBs. Challenge with meaningful value recovery if using more earth-abundant materials. |
| Li-Sulfur or Li-Oxygen batteries [386] | Potentially cheaper than Li-ion | The value of the materials used in Lithium-Sulfur batteries is lower than in Lithium Ion Batteries. |
| Higher energy in Li–S/Li–O bonds. | ||
| Active materials are earth-abundant. | ||
| An experimental scheme for recycling Li-S batteries has been suggested employing NaOH and HNO3 leaching. | ||
| Less concern over supply chains associated with these batteries. | ||
| Organic Flow Batteries. [387] | Electrode materials are liquid and flow through the battery. Reactions take place in solution. Scalable solution suitable for large scale grid storage. Organic polymers and molecules. Self-healing. | Refurbishment of electrodes and electrolytes could make this the ultimate circular economy battery. |
| All Solid State Batteries. [105] | Differences in construction with ASBs mean that present recycling processes for conventional LIBs are not straightforward to transfer to this technology despite commonalities in cathodes. | An experimental plan for LLZ + NMC recycling has been proposed, involving a combination of thermal treatment and hydrometallurgy. It has been suggested that the requirement for strong acids to dissolve the garnet structure would lead to undesirable environmental consequences and thus should be avoided. Selective leaching processes being developed for blended and mixed cathode streams may be well suited for this. |
| Lithium Metal ASSBs. | Advantages over current LIBs; mechanical integrity of SSE inhibits dendrite growth. Superior thermal/electrochemical stability leading to higher energy density. [105] | A proposed sustainable design and recycling strategy for Lithium Metal ASSBs has been proposed and evaluated using Everbatt. This employs direct recycling and has the potential for electrolyte recovery. [388] |
Concluding remarks
When developing and considering the next generation of battery technologies and chemistries there is an opportunity to embed sustainability from the design stage. This ensures a circular lifecycle approach to materials, previously only considered retrospectively at end-of-life. It may be that the case for recycling becomes less compelling if future battery technologies employ more, lower-cost, earth-abundant materials that reduce our reliance on critical materials. Indeed, if uneconomical, an alternative approach may be to design such batteries with benign materials for 'graceful degradation' to enable disposal, however, it is most likely that recycling will be required. Research and techniques from the recycling of LIBs may be applicable to these new technologies for example automated testing, removal and disassembly approaches. In addition, for functional materials recovery, it may be possible to use similar methods, as many exploit the specific material properties such as magnetic, conductivity and surface chemistry. Regeneration of materials for re-use may also be possible, using heat, near infrared, hydrothermal or ultrasonic methods to recover materials properties [386].
Design for disassembly using highly soluble binders, and well separated components enables greater materials recovery at end-of-life, however, the move to mixed materials components and composites, such as in solid state batteries and even hybrid or multivalent battery technologies, introduce a greater degree of component mixing, thus creating more difficulty in separation at end-of-life. Whilst materials recovery is important, other aspects of a circular materials life cycle also need consideration, such as lifetime, maximising first and second life of the battery.
In summary, advances in power, energy and cycle life, are required, whilst consideration with respect to the sustainability in materials supply, manufacturing, lifetime and recycling are also needed. Metrics and monitoring of the materials lifecycle in these new technologies will enable a greater circular economy input to battery design.
Acknowledgments
Funding TREFCOP/W019167/1 (GH, PAA, JB) ReLiB EK, ED, PAA FIRG005, FIRG027 & FIRG057, EK acknowledges SIMBA, which has received funding from the European Union's Horizon 2020 research and innovation program under Grant Agreement No. 883753.
27. Integrating responsible innovation in lithium-ion battery recycling
Sampriti Mahanty and Frank Boons
Sustainable Consumption Institute, Alliance Manchester Business School, University of Manchester, Manchester, United Kingdom
Status
The search for alternative energy sources has been extensive in the last two decades to replace the massive exploitation of fossil fuels and the consequent emissions and pollutants. However, energy from most renewable sources is intermittent in nature, making storage systems essential for the continuous supply of energy from such sources [389]. Lithium-ion batteries (LIB's) are emerging as one of the most popular and common energy storage systems due to the increasing demand of electric vehicles, as well as residential and consumer utility scale applications. This increasing demand of LIB's has also led to a significant demand on mineral resources thus challenging its long-term sustainability [390]. Resource scarcity and supply of materials for LIB's is particularly of concern due to short lifetime of a device, which can result from design obsolescence, 'upgrades' to newer smartphone models, or even the Limb nearing its own end-of-life [391]. Thus, an inevitable downstream consequence from increasing LIB demand and production has been spent LIB's. Scarcity of resources and the increasing volume of spent LIB's have led to the increased focus on the reuse and repurposing of second-life batteries (this has been explored in section 4 of the roadmap). Moreover, if improperly handled, there is potential for discarded LIB's to be eventually landfilled or stockpiled, leading to contamination and wastage of natural resources. Due to inflammable substances (electrolyte and separator), challenges with Lithium as a reductant and highly reactive and flammable metal and toxic substances (cobalt) in the spent LIB's, simple landfill of the spent batteries potentially poses significant threat to the environment and human health [111, 336, 391]. Such issues around safety have been addressed in section 2 of the roadmap.
Recovering stocks of metal from spent LIBs can not only reduce pollutants, but also supplement the metal sources, thus mitigating resource constraints. It is generally acknowledged that recycling of spent LIBs (covered in depth in this roadmap) is critical for the sustainable development of the LIB industry. Thus, there is immense attention being paid to recycling technologies by researchers and practitioners. To bring this into perspective: there are over 500 academic review papers on LIB recycling technologies 26 . However, as argued by Yang and colleagues [392], developing such technologies in silos from economic and environmental considerations will limit their practical applicability. Moreover, there is a need to integrate the societal aspect into the recycling technologies which is key to achieving scalability, practicality, and long-term sustainability in LIB's [393]. This roadmap has considered some of the broader systemic issues in recycling systems and their impacts in section 23, as well as providing a comprehensive view on improving recycling technologies with a view to the future scalability of these technologies as volumes increase.
Current and future challenges
While there are several recycling technologies that are being developed for LIB recycling, the key challenges are to take into consideration the environmental, social, and economic impact of these recycling technologies (this has been evaluated in section 22, which focuses on the LCA of recycling processes). It is important to make sure that the recycling technologies that are being developed do not result in 'unintended consequences' in the long run. For instance- a recycling technology which results in more environmental damage. A number of grand challenges that we are facing today are unintended results of solutions to past problems [394]. In the case of LIB's, they were developed as a sustainable alternative to fossil fuels. However, currently we are grappling with sustainability and safety related issues pertaining to the LIB's itself (discussed in detail in section 2 of the roadmap). Moreover, another challenge is how to integrate the social aspect in LIB recycling. LIB recycling will require a shift in everyday practices of consumers in the sense that new modes of production and consumption will develop. It is therefore important to bring societal values and consumer perspectives into the debate on LIB recycling. This work has been labelled as 'consumption work' [395]. For instance- the disposal of a spent battery or bringing in a used smartphone for recycling of the battery are activities that are to be carried out by the consumer. Thus, the argument is to integrate the societal aspects that must be considered along with development of recycling technologies.
Advances in science and technology to meet challenges
Responsible innovation (RI) emerged as an academic discourse and field of praxis to transform innovation practices to become more anticipatory, reflexive, inclusive, and responsive [396]. RI principles are summarized in the AREA framework which denotes Anticipate, Reflect, Engage, and Act [397]. As a process RI seeks to promote creativity and opportunities for science and innovation that are socially desirable and undertaken in the public interest. Activities following RI principles serve aligned purposes of avoiding unintended consequences and of proactively aligning with societal needs [398]. Societal needs are often identified 'responsively' through the engagement of public and private stakeholders through inclusive or participatory approaches as they then influence development efforts [399]. And yet, most innovation activities within RI are identified ex-ante to public or stakeholder engagement and often follow the continuing development of the technologies such as bio- or nanotechnology [394]. RI seeks to avoid unintended consequences as these pose risks that can be anticipated and managed before innovation is fully implemented [400]. Further to an early involvement of stakeholders, the avoidance of unintended consequences also enables evolving or anticipatory governance arrangements. These arrangements allow to manage risks responsively and ensure social acceptability and desirability [401]. Integrating RI principles in the case of LIB recycling means identifying and mitigating the unintended consequences that could arise due to the technology. It also ensures engagement with a diverse range of stakeholders such that the process is inclusive and takes into account the social aspect of the technologies. By now, the tools to bring the AREA principles into practice are publicly available and an evidence base is growing on their effectiveness [402].
Concluding remarks
In this short piece, the argument is based on the widespread assumption that LIB recycling is of utmost importance to the long-term sustainability of the LIB industry. However, when it comes to the recycling of LIB industry it is important to take into consideration the environmental, social, economic or any unintended consequence of the technology. This can be achieved by integrating RI principles throughout in the innovation process. This ensures an anticipatory, reflexive, inclusive, and responsible approach to the recycling technology thereby avoiding any unintended consequences.
This roadmap presents a comprehensive view of the current state of the art technologies for a circular economy of LIB's, as well as a comprehensive view on how that technology might evolve in the future. Carefully considering the principles of RI alongside developments in the science and technologies that will enable a circular economy of lithium ion batteries, will ensure best practices and outcomes as that industry evolves.
Acknowledgments
The authors would like to acknowledge the funding from the UKRI Interdisciplinary Circular Economy Centre for Technology Metals (Met4Tech) Grant No. EP/V011855/1.
Data availability statement
No new data were created or analysed in this study.
Footnotes
- 26
Google scholar search dated 22 October.