Abstract
Lignocellulosic biomass is an agricultural waste material abundantly produced in large quantities on earth. Rice husk (RH) is a type of lignocellulosic biomass and a huge byproduct of rice milling. Notably, the rice plant collects silica from the soil and stores the collected silica in the form of silicic acid inside the cellulose micro-compartments of the plant. Therefore, RH obtained from rice milling contains a significant quantity of amorphous silica, which can further be used for several other purposes. Furthermore, silica-rich RH can be employed as a raw material for the production of biofuels and biochars instantaneously via thermochemical processes such as pyrolysis and gasification. This article thoroughly explores a prospective method use to produce biosilica and energy from RH at the same time, which is currently under investigation. Moreover, this study also discusses current improvements in the synthesis of RH silica materials and their long-term use, particularly in energy and environmental functional materials. In terms of the environment, RH silica materials can remove heavy metals and organic pollutants in soil amendment, wastewater treatment, and gas purification via adsorption, catalysis, and integrative methods. In essence, there are numerous research and development obstacles to overcome in the production of biosilica and biofuels, respectively, from RH, and this review article highlights all of them.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nowadays, nanoscience is becoming one of the most promising technologies for multidisciplinary studies. In particular, nanoparticles are among the most potent materials and are widely used in different fields, such as catalysis, drug delivery, ceramics manufacturing, chromatography, anticorrosion treatment, etc. In addition, biomass is a renewable energy resource obtained from plantation, agricultural, forestry, and animal waste [1]. Notably, it was discovered in 1938 that rice husk (RH) contains silica [2] (table 1). In particular, RH contains cellulose and hemicellulose in various proportions (56%–75%), and 13.6%–34.8% of lignin [3, 4]. All the rice in the world is grown over 140 million ha of land throughout Asia [5]. Generally, waste materials, such as RH, peanut shells, bamboo leaves, and sugarcane bagasse, are burned after harvest, and hence we lose a large number of nutrients contained in these waste materials. In particular, these waste materials are enriched with silica and carbon (table 2). The utilization of biomass has proven to be a viable alternative to fossil fuels. Advanced biofuels and bioproducts generated from lignocellulosic biomass hold the potential for significant depletion in greenhouse gas emissions and enhanced domestic energy generation in nations with sufficient renewable sources and/or areas appropriate for energy crop cultivation. In light of the present situation, this study outlines some of the most recent breakthroughs in lignocellulosic pretreatment and transformation into solid, liquid, and gaseous biofuels. Meanwhile, RH impacts the environment negatively if it is left on the farmland. On the other hand, burning RH into the air converts elements like carbon, oxygen, and hydrogen from the RH into combustible gases, such as methane, monoxides, hydrogen, and ash [6]. Moreover, the increased rice field size causes several environmental issues (e.g. greenhouse gas emissions, soil depletion, pesticide usage), one of the most serious of which is dealing with post-harvest rice straw treatment [7–9]. Over the past few decades, scientists have discovered various methods for limiting As absorption in rice grains. These methods include the use of chemical immobilizing agents, electrokinetics, phytoextraction, and soil cleansing, etc [10, 11]. Therefore, in situ immobilization is now widely used because of its efficiency, cost-effectiveness, and accessibility[12]. Rice straw may be harvested from the field, heaped or distributed, integrated into the soil, or employed as mulch for the indicate [13, 14]. For instance, rice straw can be used as a natural fertilizer in fields [15–17]. Yang et al also revealed that rice straw as well as other crop waste can be utilized as additional feedstock for ecological clean-up and soil cleansing [18].
Table 1. The composition of RH ash. Content, % wt.
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | P2O5 |
|---|---|---|---|---|---|---|---|
| 93.4 | 0.05 | 0.06 | 0.31 | 0.35 | 1.4 | 0.1 | 0.8 |
Table 2. A comparison table of the amount of silica in various raw materials.
| Serial no | Raw material | Production route | Biosilica yield (%) | References |
|---|---|---|---|---|
| 1 | Rice hull ash | Alkaline precipitation | 60% | [30] |
| 2 | Bagasse fly | Alkali extraction and acid precipitation | 80% and 73% | [31] |
| 3 | Chrysotile | Acid leaching | Above 90% | [32] |
| 4 | Sand | Thermal treatment and acid leaching | 99.7%–99.9% | [33] |
| 5 | Banana peel ash | Sol–gel method | 0.36% | [34] |
| 6 | Bamboo leaves | Sulfuric acid treatment | 52% | [35] |
| 7 | Coconut husk | Chemical treatment | 90% | [36] |
| 8 | Corncob | Sol–gel method | 27% | [37] |
| 9 | Cigarette butt | Sol–gel method | 0.1% | [37] |
| 10 | Wheat straw | Thermal and chemical methods | 50%–55% | [38] |
RH is the most beneficial agricultural waste in the world since it includes a lot of organic substances, such as cellulose, hemicelluloses, and lignin, and generates a significant amount of RH ash [19]. So, thermochemical processing of RH utilizes the highly organic compounds of RH to generate adsorbents (biochar and activated carbon) for environmental protection through pollutant removal, building materials (natural fibers), and biofuels [20]. In addition, RH ash has been identified as a suitable raw material for the production of biosilica because RH ash contains trace levels of alkali metals (e.g. Na and K), alkaline metals (e.g. Ca and Mg), transition metals (e.g. Al, Fe, Cu, and Zn), and non-metals (e.g. Cl, S, and P) [21]. Notably, biosilica is a precursor for the development of silica derivatives, used as pollutant removal adsorbents, epoxy resin additives, composite material constituents, refractory ceramics, concrete material constituents, and catalysts [22]. As a result, most researchers have started to focus their efforts on developing environmentally acceptable pretreatment processes for RH used before biosilica production from the RH. As stated before, the rice plant absorbs silica from the soil and stores the absorbed silica inside the cellulose micro-compartments of the plant in the form of silicic acid [23]. Moreover, RH may be a natural reservoir and a suitable precursor of amorphous nano-biosilica because the silica in RH is present naturally in the form of nanoparticles. Many studies have followed various biosilica production methods, including calcination [24], the sol–gel method [25], and chemical precipitation [25, 26]. Notably, the sol–gel method of producing biosilica takes a long time but produces exceptionallly pure and homogeneous nano-biosilica [27]. Operationally, the sol–gel method of biosilica production involves the condensing of the originated silica molecule of the production and low-temperature hydrolysis [28]. Meanwhile, sodium silicate is treated with an inorganic acid during the precipitation of sodium silicate in the chemical precipitation method of silica production to produce precipitated nano-biosilica. In particular, the sol–gel method of biosilica production is simple, easy, and quick. However, this biosilica production method has the disadvantage that the biosilica nanoparticles produced in this method can be easily altered by different causes [29]. On the other hand, the calcination method of biosilica production produces nano-biosilica from RH in a more commercial and straightforward manner. Notably, the goal of this study is to identify the most effective technique and examine the optimal circumstances for synthesizing highly pure biosilica from RH through acid leaching and chemical extraction, which is a potential catalyst for the manufacturing of biofuels.
2. Biosilica production from RH
Bakar et al experimentally produced biosilica from RH. First, they used sodium dodecyl sulfate solution to wash the RH. After washing, it was then rinsed with distilled water to remove detergent residues in the RH [39]. Thereafter, the rinsed RH was dried at ambient temperature for 24 h before being pulverized, and the pulverized RH was dried at 100 °C for more than 24 h in an air oven. This drying produces unleached RH, which is considered to be cleansed RH. The cleansed RH was then treated with a 0.5 N sulfuric acid (H2SO4) solution at 60 °C for 12 h with steady stirring. Further, the acid-leached RH from the previous step was vacuum-filtered and rinsed with distilled water to remove acid residues, and this vacuum-filtering and rinsing process was repeated until the water pH was neutral. Finally, unleached and acid-leached RH ashes were produced by heating the unleached and acid-leached RHs for 2 h at 100, 150, 200, and 250 °C, respectively, in a muffle furnace. The functional groups of the unleached and acid-leached RH ashes, respectively, were identified using a Fourier transform infrared spectrometer with attenuated total reflectance adapter. Further purification and drying of the ashes each produced biosilica. Figure 1 shows the schematic production of biosilica from RH.
Figure 1. Schematic production of biosilica from RH.
Download figure:
Standard image High-resolution image2.1. Routes for the production of biosilica from RH
RH can be used to produce biosilica in a number of ways. Generally, there are four routes, namely the hydrothermal, biological, chemical, and thermochemical routes. In particular, the hydrothermal, biological, and thermochemical routes can be used to convert RH into energy as well. Figure 2 illustrates the four production routes of biosilica from RH.
Figure 2. Various routes for the production of biosilica from RH.
Download figure:
Standard image High-resolution image2.1.1. Hydrothermal route for the production of biosilica from RH
Hydrothermal synthesis is typically defined as the direct crystallization of ceramic materials from their respective solutions into an aqueous medium at high temperatures (higher than 25 °C) and pressures (greater than 100 kPa) [40]. Since RH contains organic compounds and metal oxides, the production of biosilica from RH using the hydrothermal route involves high temperatures and pressures, an acidic or alkaline (basic) medium having strong RH-oxidizing properties, RH organic compound decomposition, and transforming the trace metals of RH into soluble ions (figure 3). In addition, the purification of the biosilica produced via this method can use only water as the purifying agent. However, the decomposition of RH organic compounds involves a complicated decomposition procedure. Notably, the hydrothermal route for the production of biosilica from RH still requires the incineration of the untreated or pretreated RH even though the soaking time is less than the incineration time. In addition, the amorphicity of the silica in RH is unaffected using this route. Also, the typical oxidative medium used in this production is an acid with strong oxidative activity, such as H2SO4 and HNO3 [41], and Wu previously used this route to produce biosilica from RH [42].
Figure 3. Flow chart of the hydrothermal route for the production of biosilica from RH.
Download figure:
Standard image High-resolution image2.1.2. Chemical route for the production of biosilica from RH
There are several chemical routes that can be used to produce biosilica from RH (table 3). Notably, the alkaline and alkaline earth metal contaminants of RH are eliminated, and the amount and purity of the biosilica produced are increased by acid leaching the RH at the start of the RH pretreatment under this route [43–46]. The acid-leached RH is then treated with NaOH solvent at 6–8 bar for their reaction, and the solution is agitated for 1–2 h at 180 °C–200 °C [30, 44, 47–49]. The slurry resulting from the reaction is then vacuum-filtered to provide a thick, viscous Na2SiO3 solution that can be titrated with H2SO4, HCl, or H3PO4 [44, 47, 48, 50]. The titrated Na2SiO3 solution is then kept at atmospheric pressure without stirring, and precipitation occurs within the solution when the solution pH drops below 10 [30, 46, 51]. The frothy-textured white precipitate of the solution is then aged for 1–3 h before being centrifuged [52] or dried [47]. Generally, the precipitate is dried at 70 °C for a day [46, 52] or at 105 °C for 4 h [43]. Zarib et al examined the effect of RH acid leaching on the biosilica purity (biosilica content) in RH ash of the biosilica production from RH under this route [53]. In particular, first, RH powder was made by crushing the RH thoroughly, and the RH powder was leached with sulfuric and citric acids at concentrations of 0.1, 0.5, and 1 mol l−1 for 30, 60, and 90 min, respectively, in this examination. Finally, the biosilica content of the acid-leached RH was shown to increase as the acid concentration and the leaching time were increased in the experiment. Notably, the citric acid leaching of RH produced a slightly higher amount of biosilica (99.3%) than that produced under the HCl leaching of RH (98.6%) in the analysis.
Table 3. Recent studies on the production of biosilica from RH.
| Year of study | Raw material | Reaction temp (°C) | Reaction time (h) | Production route/method | Biosilica yield (%) | References |
|---|---|---|---|---|---|---|
| 2017 | Rice husk | 550–880 | — | Thermochemical processes | 10.6 | [79] |
| 2015 | Rice husk | 700 | — | Chemical activation | [80] | |
| 2019 | Rice husk | 24 | Extraction process | 62.83 | [76] | |
| 2016 | Rice husk | 500–900 | 2 | Acid leaching | 99 | [39] |
| 2020 | Rice husk ash | 550 | 24 | Thermochemical | 93.08 | [81] |
| 2021 | Rice husk | 700 | 2 | Extraction | 99.7 | [82] |
| 2019 | Rice husk and straw | Up to 1000 | — | Combustion process | 98 | [3] |
| 2021 | Rice husk | 800 | 2 | Carboxylic acid leaching | 89.91 | [83] |
| 2018 | Rice husk | Sonication process | [84] | |||
| 2017 | Rice husk | 110 | 48 | Hydrothermal process | [85] | |
| 2015 | Rice husk | 900 | 7 | Acid treatment | 55–80 | [35] |
| 2015 | Rice husk | 300 | — | Thermal method | 95.85–99.62 | [86] |
| 2019 | Rice husk | 600 | — | Thermochemical | 60 | [87] |
| 2020 | Rice husk | 400 | 10 min−1 | Thermochemical | 37.71 | [88] |
| 2016 | Rice husk | 699.85 | — | Acid pretreatment | 99.761 | [89] |
| 2017 | Rice/wheat husk | 700 | 6 | Thermal and chemical | 95.55 | [38] |
| 2020 | Rice husk | 650 | — | Combustion process | [90] | |
| 2017 | Rice husk | 550–800 | — | Thermochemical processes | [57] | |
| 2019 | Rice/wheat husk | Sol–gel method | 80–97/50–55 | [91] | ||
| 2019 | Rice husk | 100 | 1 | Sol–gel synthesis | [92] | |
| 2020 | Rice husk and wheat straw | 80 and 550 | 4 | Sol–gel method | [93] | |
| 2017 | Rice husk | 600 | 2 | Pyrolysis | 99.33 | [94] |
| 2020 | Rice husk | 550 | — | Bottom-up process | [95] |
2.1.3. Thermochemical route for the production of biosilica from RH
The thermochemical route for the production of biosilica from RH involves calcining or burning the RH to produce RH ash, containing biosilica. The most common thermochemical route is the calcination and fixed-bed combustion of RH [54, 55]; however, pyrolysis of RH [56, 57] and fluidized-bed combustion of RH [58, 59] are other popular approaches in this route. Notably, the calcination of RH in the absence of air turns the RH into RH ash, but a high calcination temperature can create crystalline biosilica in the RH ash [60]. Moreover, acid leaching of RH reduces the impurities in the RH and increases the porosity of the biosilica produced from the RH (by preventing the crystalline biosilica creation in the RH ash) as compared to the porosity of the biosilica produced from the unleached RH under the thermochemical route [61, 62]. Generally, alkaline earth and iron oxides, as well as water- and acid-soluble impurities (of the alkaline group) are categorized as impurities of RH [3]. In addition, RH can be leached at temperatures above 700 °C [48, 49], and the reduction of alkali and alkaline earth metals in the RH by leaching inhibits the eutectic reaction of amorphous biosilica from creating crystalline biosilica during the production of biosilica from RH under the thermochemical route. Pa and Kein employed oxalic acid to leach RH at various acid concentrations, leaching times, and leaching temperatures (70, 80, and 90 °C, respectively) [63]. Subsequent calcination of the acid-leached RH at 800 °C for 3 h was performed, and the biosilica content in the calcined RH (RH ash) increased from 92.4% to 99.3% when the acid concentration, leaching time, and leaching temperature were increased.
Bakar et al showed that acid leaching of RH at a temperature of 500 °C–900 °C could alter the biosilica content in the RH ash and increase the biosilica surface area [39]. Consequently, 99.08% and 99.58% pure biosilica were produced from the RH via the thermochemical route after leaching the RH with high HCl and H2SO4 concentrations, respectively. In addition, Chakraverty and Kaleemullah showed the calcination of RH at 500 °C–700 °C in a muffle furnace [64]. In particular, RH treatment at lower temperatures led to amorphous biosilica in the RH ash produced by the treatment, and an increasing treatment temperature resulted in a drop of the unconfined compressive strength (UCS) of the RH ash to 2.2% of that produced by the lower-temperature RH treatment. Lee et al also burned 100 g RH in a tiny furnace. They observed that when the burning temperature increased from 950 to 1050 °C, the corresponding UCC of the RH ash produced by the burning decreased from 0.76 to 0.17 (also resulting in white RH ash) [65].
Bakar et al also acid-leached RH with 10% HCl at 100 °C for 2 h and 5% citric acid at 80 °C for 20 min, respectively [39]. Subsequently, amorphous biosilica was found in the RH ash produced by calcination of the acid-leached RH at 750 °C for 5 h. As the biosilica surface area increased from 56.6 to 316 m2 g−1 due to the acid leaching of RH, the corresponding purity of the biosilica increased from 91.3% to 99.0%. In particular, citric acid was selected for acid-leaching the RH because of the non-corrosiveness of citric acid. Likewise, Hincapie-Rojas et al produced biosilica from RH by leaching the RH for 1 h with 1 0 M sulfuric acid (H2SO4), nitric acid (HNO3), or hydrochloric acid (HCl), calcining the acid-leached RH at 700 °C, and crushing the calcined, acid-leached RH for 18 min [66]. Umeda and Kondoh calcined RH at 650 °C–1150 °C and then leached the calcined RH with 5% citric acid at 50 °C for 15 min [67]. This acid leaching increased the biosilica content of the RH ash produced from the calcination of the acid-leached RH from 94.58% to 99.14%. Umeda et al performed RH pretreatment with H2SO4 at 1%–5% H2SO4 concentrations [68]. However, impurities in the RH were effectively eliminated by leaching the RH at the 1% H2SO4 concentration; nevertheless, leachingat the 3% H2SO4 concentration resulted in a 99.3% purity of the biosilica produced from the acid-leached RH. Mahmud et al also examined the effect of acid leaching of RH on the purity and surface area of the biosilica produced from the acid-leached RH [69]. They calcined the acid-leached RH at 700 °C in a muffle furnace, and produced 99.77% pure biosilica.
2.1.4. Combined thermochemical and chemical routes for the production of biosilica from RH
RH is commonly burned in air to create reactive RH ash, and biosilica is easily recovered from the RH ash with a hot NaOH solution using a combined thermochemical and chemical route [47, 70]. In particular, a solvent-to-feed ratio (RS/F) of 4 [49], 3 [71], 6 [30], 7 [72], and up to 12 for NaOH treatment temperatures of up to 60 °C [71], respectively, resulted in a chemical reaction between RH ash and NaOH in the production of biosilica from RH using the combined thermochemical and chemical route. Carmona et al produced nano-biosilica from RH using the combined thermochemical and chemical route. In particular, they used a mixture of 10% citric, 10% phosphoric, and 0.2 mol l−1 phosphoric acids to leach the RH at 100 °C for over 2 h [73]. Then, they calcined the acid-leached RH at 650 °C for 1 h and produced amorphous biosilica by treating with NaOH.
Azat et al studied the production of amorphous biosilica having a purity of 84.81%–99.66% from RH by acid-leaching the RH, calcining the acid-leached RH at 600 °C for 4 h, and then treating the RH with NaOH [43]. Likewise, Azat et al used 2 mol l−1 HCl to study the HCl leaching of RH at 90 °C for 2 h. RH ash was produced by treating the calcined (at 600 °C for 4 h), HCl-leached RH with 2 mol l−1 NaOH at 90 °C. In particular, the HCl leaching increased the purity of the biosilica produced from the RH from 83.8% to 99.7% with a biosilica surface area of 980 m2 g−1 corresponding to a 99.7% biosilica purity. This large surface area of the biosilica was due to the HCl leaching and variability of the RH [74]. Likewise, Todkar et al produced biosilica from RH by burning the RH at 800 °C–850 °C in a muffle furnace and treating the burnt RH (RH ash) with 12% NaOH, resulting in a 90% biosilica recovery from the RH ash [72]. Costa and Paranhos produced biosilica from RH by calcining the RH at 500 °C–800 °C for 1–4 h and then treating the calcined RH (RH ash) with NaOH [46]. The amorphous biosilica from this production had an average purity of 98.3% and a recovery of 80.88%–99.37% from the RH ash. However, the biosilica purity increased to 95.9% with acid leaching of the RH before the calcination, and the corresponding biosilica surface area increased from 60.3 to 169.7 m2 g−1. Also, the size distribution of biosilica nanoparticles from this production was 51–198 nm. Notably, Phoohinkong and Kitthawee reported a rapid and low-cost method of producing nano-biosilica from RH under the combined thermochemical and chemical route [75]. Their experiment involved the calcination of the RH at 900 °C for 2 h and treating the calcined RH with 2 mol l−1 NaOH at 80 °C for 1 h. The amorphous biosilica nanoparticles from this production had a size range of 10–100 nm.
Fernandes et al produced biosilica having a purity of 99.61% and surface area of 290.03 m2 g−1 from RH ash [49]. Mittal also used NaOH and HCl in his procedure for extracting biosilica from RH ash [47]. He discovered that 65% of the biosilica had been recovered from the RH ash and the recovered biosilica was 98% pure. Kalapathy et al presented a straightforward procedure for extracting biosilica from RH ash. In particular, this procedure used 1 mol l−1 HCl for the acid leaching of the RH ash and 0.1, 0.25, 0.5, and 1 mol l−1 NaOH for the treatment of the acid-leached RH ash to extract biosilica from the acid-leached RH ash, respectively, in an acid-leaching experiment at 100 °C for 1 h [30]. The biosilica recovered from the acid-leached RH ash by this procedure was amorphous and had a purity of 93%. However, when the NaOH concentration was increased in the treatment of the HCl-leached RH ash, the corresponding biosilica recovery increased proportionally to 91%. However, treatment of the RH ash (that was not HCl-leached) with 1 mol l−1 NaOH resulted in a 2% drop in the corresponding biosilica recovery compared to that of the HCl-leached RH ash with 1 mol l−1 NaOH. Likewise, Mehta and Ugwekar used 0.5, 1, and 2 mol l−1 NaOH, respectively, to treat RH ash having different weights in the range of 10–80 g at 60 °C for 2 h to extract biosilica from the RH ash [71]. Notably, the biosilica recovery from the RH ash treated with 0.5 mol l−1 NaOH dropped to 45.3% as the RH ash loading increased steadily from 10 to 80 g. When the NaOH concentration was increased to 1 mol l−1, the corresponding drop was from 64% to 51.6%. However, when the NaOH concentration was 2 mol l−1, the biosilica recovery increased from 66.11% to 71.15%, corresponding to an increasing RH loading from 10 to 80 g. Setyawan and Wulanawati were also able to extract biosilica from RH ash without acid leaching the RH ash [76]. The concentration of NaOH used in this extraction was 0.5, 0.75, and 1 mol l−1 and the RS/F was 4, 5, and 6, respectively. In addition, the NaOH treatment of the RH ash was carried out at 80 °C for 1 h. Notably, the silica recovery from the RH ash increased to 62.83% when the RS/F and NaOH concentration increased to their respective maximum values. In addition, the biosilica from this extraction had a purity of 86.17%.
Selvakumar et al used 1 mol l−1 of HCl, H2SO4, and HNO3, respectively, to leach RH ash for 2 h in the extraction of biosilica from the RH ash [77]. Subsequently, NaOH with a concentration of 0.1, 0.25, 0.5, and 1 mol l−1, respectively, was used to treat the acid-leached RH ash to extract biosilica. Notably, the maximum silica recovery from the RH ash of this extraction was 85% when RH ash was leached with HCl. Likewise, Ghosh and Bhattacherjee used H2SO4 for the acid leaching of RH ash and 0.5, 1, and 1.5 mol l−1 NaOH, respectively, to treat the H2SO4-leached RH ash for 1 h to extract biosilica [78]. The biosilica recovery from the RH ash was 95%–98% and the surface area was 150–200 m2 g−1 in this extraction. In addition, Haq et al studied how the variation of NaOH concentration, extraction time, and RS/F, respectively, affected the extraction of biosilica from RH ash [96]. Notably, the silica recovery from RH ash of this extraction increased from 35% to 40% when the extraction time was increased from 30 to 60 min, and reached 82% when the extraction time was increased from 90 to 120 min. In general, raising the NaOH concentration to more than 1 mol l−1 did not increase the silica recovery in this extraction. Also, an RS/F of 5 was found to be ideal for this extraction. In addition, other solvents that can be used to treat RH ash in the extraction of biosilica from RH ash include hydrogen fluoride and ammonium hydroxide [72, 97]. In particular, Ma et al extracted biosilica from RH ash leached with 100 g HCl. The HCl-leached RH ash was treated with a 2–5 mol l−1 solvent at 100 °C–150 °C for 1–5 h, respectively, to extract biosilica from the RH ash. Notably, the amount of biosilica recovered from RH ash in this extraction increased as the extraction time increased from 1 to 2 h, but an extraction time of longer than 2 h showed little effect on the amount of biosilica recovered. In addition, a treatment temperature of 100 °C–120 °C increased the biosilica recovery, while a temperature of more than 120 °C had little effect on the amount of biosilica recovered in this extraction. Similarly, increasing the solvent concentration from 2 to 4 mol l−1 increased the amount of biosilica recovered proportionally, while increasing the solvent concentration to 5 mol l−1 did not increase the amount of biosilica recovered in this extraction. Further, the highest amount of biosilica recovered in this extraction was 94.6%, with a 50–60 nm pore size distribution in the corresponding biosilica extracted. Unfortunately, this extraction involves a very corrosive chemical [72].
2.2. Biological route for the production of biosilica from RH
Fungi, bacteria, cyanobacteria, diatoms, Porifera, and Californian red worms, respectively, have been used as fermentation agents in the biological route for the production of biosilica from RH [57, 98, 99] (table 4). Silica-based material is the principal food source for these organisms, and the silica in the food consumed by the organisms accumulates in their respective cells [57] via a bio-silicification process. So, the in vivo silica can be extracted from these organisms, and a homogenizer is typically used to kill the organisms' cells. The extracted biosilica is then precipitated, purified, and dried, which, in particular, produced 90% pure biosilica under an acid-leaching and baking time of 7 h for the RH [98]. Notably, microbial fermentation of RH with white rot fungi increased the silica concentration in the RH from 23.7% to 31.3%–49.0% [100]. Likewise, the fermentation of RH with Californian red worms produced 88% biosilica nanoparticles from the fermented RH in 5 min [98, 99]. However, the fundamental disadvantage of the biological route for the production of biosilica from RH is the long production time [99, 100].
Table 4. Various biological routes for the production of biofuels.
| Serial no | Source | Method | Biofuels | References |
|---|---|---|---|---|
| 1 | Rice husk | Enzymatic hydrolysis | Bioethanol | [109] |
| 2 | Rice husk | Anaerobic digestion | Bio hydrogen | [110] |
| 3 | Rice husk | Anaerobic digestion | Biogas | [111] |
| 4 | Rice husk | Fast pyrolysis | Bio-oil | [112] |
| 5 | Rice husk | Hydrothermal liquefaction | Biochar | [112] |
| 6 | Maize, wheat, rice and sugarcane | Biomethanation | Methane | [113] |
| 7 | Rice bran | Screening process | Bioethanol | [114] |
3. Production of biofuel from RH
Biodiesel (a substitute for diesel) and bioethanol (a substitute for gasoline) are two of the most popular and first-ever produced biofuels. These biofuels are derived from vegetable oils, seeds, and lignocellulosic biomass. In particular, lignocellulosic biomass (acquired from non-edible plant wastes, such as RH, wheat straw, and rice hull) and agricultural waste are the two most popular raw materials for producing biofuels [101]. In addition, algae [102, 103], maize and sweet sorghum [104], physic nut [105], palm [106], sugarcane [106], and others are the most frequently used raw materials for the production of biodiesel. However, post-harvest wastes of rice (such as RH) are considered one of the most promising energy resources because they are renewable and are available in large quantities due to the multiple crops of rice planted [107].
It is necessary to take preventative measures to address complex situations, such as a worldwide increase in energy demand, an increase in the environmental pollution from different waste streams, etc, must encourage humanity to take preventative measures to address such a complex situation. Hence, biofuels are being viewed as a promising candidate to fulfill energy requirements and for improved food security, a more stable pattern of land use, and a reduction in the use of conventional energy resources that are non-renewable and that have put pressure on the environment worldwide. Currently, significant attempts are being made to generate bioenergy from biomass, such as agricultural or wood residues (cellulose and lignin biomass), which have a small or no market, and can help to mitigate a wide range of environmental problems and global issues, such as climate change, greenhouse gas emissions, increasing worldwide energy demand, and so on [107]. The different routes for the production of biofuels from RH are shown below. In addition, figure 4 shows a schematic production of biofuels from the post-harvest residue of rice.
Figure 4. Schematic production of biofuels from the post-harvest residue of rice (rice straw waste, in this case) [107].
Download figure:
Standard image High-resolution image3.1. Biological route for the production of biofuels from RH
Generally, various biochemical processes and technologies have been used to produce ethanol, hydrogen, and methane through the conversion of biomass. Saxena et al described a number of biochemical processes and technological routes to produce ethanol and hydrogen from biomass [108]. The following are some of the cases of this biological route.
3.1.1. Biofuel production from anaerobic digestion of RH
Micro-organisms in the absence of oxygen can convert biomass into biogas, which is a combination of methane and carbon dioxide. Generally, heat and energy are generated by using the biogas as fuel. Related to this, a review of various anaerobic digestion techniques used in the production of biofuels was conducted by [115].
RH and other organic waste mixtures have been proven to be suitable as raw materials for the production of biofuels using anaerobic digestion [111]. In addition, investigations have been conducted to increase biomass digestibility by using different pretreatment methods for biomass, such as acid pretreatment, heat pretreatment, biomass size reduction, and planting [116]. Notably, the pretreatment of lignin biomass with alkali is among the most effective pretreatment methods for biomass in the context of anaerobic digestion of biomass. Furthermore, according to He et al, RH treated with NaOH produced more biogas (64.5%) than untreated RH (27.3%) [117]. Notably, many developing countries, including China [118], India [119], Honduras [120], Colombia, Ethiopia, Tanzania, Vietnam, Cambodia, and Bangladesh [121], have used small-scale gas digesters.
3.1.2. Bioethanol production from fermentation of RH
Enzymatic hydrolysis, fermentation, and a pretreatment step are the basic steps involved in the production of bioethanol from lignocellulosic biomass. The enzymatic hydrolysis of lignocellulosic biomass converts cellulose into glucose and then hemicelluloses into glucose in the lignocellulosic biomass, and the glucose is further converted into various pentoses and hexoses [109]. Selected micro-organisms also ferment the glucose into bioethanol. Generally, simultaneous saccharification and fermentation or separate enzymatic hydrolysis and fermentation can be used to convert cellulose and hemicellulose fractions in rice straw and RH into bioethanol. Binod et al described some possible technologies for the production of bioethanol from RH [122]. Likewise, Chen and Qiu reported on the latest research advancements of their research group in fractional conversion-based technology for the production of bioethanol from RH [123].
Meanwhile, a mathematical programming model was developed by Kaylen et al to assess the economic feasibility of converting lignocellulosic biomass into bioethanol [124]. According to them, the co-production of higher-value compounds with bioethanol from lignocellulosic biomass [124] could make bioethanol comparable to gasoline. Alternatively, Gnansounou and Dauriat used the value engineering and target costing technique as their approach for the above-mentioned economic feasibility study [125]. As a result, they pointed out the importance of reducing the cost of bioethanol produced from lignocellulosic biomass by utilizing all resources efficiently.
Other extensive research has also been conducted over the past few decades on lignocellulosic biomass as a raw material for bioethanol production. However, commercialization of this production is yet to be completely realized. According to Sukumaran et al, the production of bioethanol from lignocellulosic biomass goes a long way to fulfilling the energy demands of India; however, the relevant processes are still in the early stages of development [126]. Hence, they stressed that the cycle of this bioethanol production must be improved, and integrated production systems must be developed economically to realize the full potential of bioethanol production on a large scale [126]. There are also similar limitations on the production of bioethanol from lignocellulosic biomass in China, where the lignocellulosic biomass-based bioethanol production is limited to pilot plants [127].
3.1.3. Biohydrogen production from fermentation of RH
The production of biohydrogen from the fermentation of agricultural waste, such as RH, is a relatively new research area compared to the well-known anaerobic digestion method for the same production involving agricultural waste. In particular, anaerobic bacteria in this fermentation causes the production of biohydrogen, volatile fatty acids, and carbon dioxide during the fermentation. Generally, there are two types of fermentation, namely the photo and dark fermentations, involving various species of bacteria that perform differently under various circumstances. The fundamentals of the production of biohydrogen from agricultural waste and the limiting processes of the production were discussed by [110]. Furthermore, Argun and Kargi found the operating mode of the production of biohydrogen from agricultural waste that provided the highest hydrogen production rate and yield under dark fermentation, light fermentation, and a combination of the two [128]. In addition, the fermentation of carbohydrates, such as that used with rice or agricultural wastes, with other biomass is also a potential way to produce biohydrogen. However, additional industrial-scale operations must continue to be developed for the production of biohydrogen from RH to be economically viable.
3.2. Thermochemical route for the production of biofuels from RH
There are two main thermochemical routes for the production of biofuels from RH. In the first type, the RH is used directly as fuel for combustion and generating heat and power. In the second type, the RH is converted into other valuable forms of energy sources before being used to generate heat or power. Goyal et al discussed several thermochemical methods, such as direct combustion, gasification, liquefaction, hydrogenation, and pyrolysis of renewable energy resources, such as RH [129].
3.2.1. Direct combustion of RH (as a biofuel)
Generally, the direct combustion of biomass (as a fuel) in the presence of enough air in the combustion chamber is used to produce steam. Subsequent use of the steam in steam turbines generates electricity. Fixed- and fluidized-bed combustion of biomass, such as RH, are the two main types of biomass combustion technology. For example, Natarajan et al [130] summarized earlier research on the burning of RH in fluidized-bed combustion systems. In addition, Wibulswas et al determined the economic sustainability of building steam power stations in a rice mill by comparing the gasifier internal combustion engine and boiler-turbine systems [131]. The determination showed that both systems seemed to be financially viable for meeting the energy requirements.
Sookkumnerd et al used an economic model to determine the financial return of RH-based steam engines for rice mills in Thailand [132]. The determination demonstrated that installing steam engines in rice mills of 45–120 tons of rice/day capacity was cost-effective. Subsequently, Sookkumnerd et al included the earnings from the sale of additional electricity generated by the steam engines to a power grid in their study. Consequently, it was found that adding grid-connected generators to the RH-based stream engines improved the financial performance of rice mills with a daily capacity of 120 tons of rice [133].
The use of RH to generate electricity for rice-milling industries was also evaluated by [134]. In particular, the evaluation was based on analyzing three different types of power stations with varying capabilities to consider the various energy requirements of rice-milling industries. Bergqvist et al also investigated the lifetime cost, energy savings from the electricity generator, sale of the RH ash, and the feasibility of implementing the clean development mechanism (CDM) in the electricity generation. The investigation concluded that the electricity generation at large units would be cost-effective if the earnings from RH ash sales or CDM were included in the evaluation. However, smaller electricity generation units needed the inclusion of the earnings from the RH ash sales and CDM in the evaluation to deem that these units were financially sustainable.
Currently, producing heat and power from RH is a well-known technology. For example, according to Carlos and Khang, 44 RH-based electricity-generating plants exist in Southeast Asia alone. Notably, the high silica content of RH ash leads to many industrial power producers using RH as a single fuel to generate extra revenue [135]. However, approximately 80% of the RH-based electricity-generating plants have a maximum electricity generation capacity of 10 MW [135] because of the limited availability of RH in the respective region of these electricity-generating plants.
3.2.2. Parameters affecting the RH combustion performance
3.2.2.1. Effect of combustion temperature
When RH is burned, RH ash with a high proportion of crystalline biosilica is produced. On the other hand, RH produces powerful reactive amorphous biosilica when it is burned under regulated settings. In particular, the RH ash produced at lower temperatures (773–873 K) of this production is found to contain mostly amorphous biosilica. Related to this, rubber, paper, paint, pesticides, and fertilizers are produced from amorphous silica, which is one of the white industrial minerals. In addition, fine-chemical synthesis uses amorphous silica as an adsorbent or catalyst in the synthesis because of its high purity, tiny particle size, and large surface area. Notably, high-purity amorphous silica can be manufactured by heating the RH at a temperature of 773–1673 K before and after RH combustion/burning for various heating times [136–138]. In addition, chemicals including HCl, H2SO4, HNO3, NaOH, and NH4OH, have been used as leaching acid and extracting solvents in this production, along with the RH heating before and after RH combustion/burning.
According to Patil et al, longer RH combustion times may change the biosilica in the mineral phase of the RH ash from amorphous (with shorter RH combustion times) to crystalline [139]. Carbon residue removal from RH ash through re-incineration of the ash is also frequent, although it necessitates greater temperatures and more time for the re-incineration. Notably, a temperature of 700 °C or lower is advised for this re-incineration to minimize the detrimental consequences.
3.2.2.2. Combustion of acid-leached RH
Numerous researchers [140] have performed initial leaching of RH with the solutions of HCl, HNO3, H2SO4, NaOH, or NH4OH at a temperature of 500 °C–1400 °C for various times [141, 142] before the RH combustion to remove most of the metallic impurities in RH and produce biosilica with a large specific surface area. In particular, complete, acid-leached RH combustion produced white RH ash, but complete, unleached RH combustion produced light brown RH ash under the same circumstances [45]. Moreover, the amorphousness of the produced biosilica was not affected by the acid leaching of the RH. Real et al discovered that leaching RH with an HCl solution before burning the RH at 600 °C might produce reasonably pure biosilica (about 99.5%) with a large specific surface area (roughly about 260 m2 g−1) [140]. In addition, when the white RH ashes from the combustion of RH at 600 °C were leached with HCl, amorphous biosilica with the same purity as that produced from the unleached RH combustion was produced. However, the corresponding surface area of the produced biosilica reduced to 1 m2 g−1 with the additional acid leaching of the RH. Meanwhile, the production of black biosilica particles in this biosilica production was greater when unleached RH was used in the production compared to black biosilica particle production when an acid-leached RH was used in the production. This difference in black biosilica particle production is caused by potassium in the RH, which is eliminated greatly by acid-leaching the RH [141]. RH is also known to produce white, amorphous, and chemically pure biosilica when processed with HNO3 and subsequently calcined at 873 K (the produced biosilica purity is 98.5%). Other acids, such as sulfuric, oxalic, and citric acids, may also be used to leach RH; however, experimental data reveal that HCl leaching of RH results in the production of biosilica having a greater surface area from the RH combustion than that of the biosilica produced from the combustion of RH leached with sulfuric, oxalic, and citric acids, respectively [143].
3.2.2.3. Combustion of HCl-leached RH
Acid solutions, such as HCl, HNO3, H2SO4, and others, may act as leaching agents to acid-leach RH before the RH combustion. However, Liou and Yang found that HCl is more effective than other acids as an RH leaching agent [143]. Many researchers have found HCl to be the most efficient leaching agent for RH to produce significant amounts of amorphous biosilica with better pozzolanic characteristics from the acid-leached RH combustion. Furthermore, Vayghan et al [144] showed that HCl leaching of RH can effectively remove impurities, such as aluminum oxide (Al2O3), calcium oxide (CaO), iron (III) oxide (Fe2O3), magnesium oxide (MgO), and most significantly, destructive alkalis like potassium oxide (K2O), from the RH [145]. It has also been reported that a potassium oxide (K2O) component was identified in the RH ash even when the RH was acid-leached before RH combustion. In particular, the presence of potassium in RH causes surface melting and catalyzes the crystallization of the biosilica produced from RH combustion [146]. Basically, carbonization of degraded organic matter in RH happens when the RH is burnt, and subsequently, carbon is produced. However, when the RH combustion temperature is constantly raised and adequate oxygen is provided to the combustion, the carbon will change into carbon dioxide. Additionally, when the RH combustion temperature exceeds the dissociation temperature of potassium oxide (K2O), non-oxidized carbon is produced from the combustion. Hence, the molten glass component blocks the area of interaction between oxygen and CO2. As a result, the melted surface favors biosilica crystallization, making the RH ash less reactive in pozzolanic processes [144]. Since acid-leaching of RH eliminates the primary biosilica crystallization catalyst from the RH, acid-leaching of RH is responsible for the increase in the pozzolanic activity index.
3.2.2.4. Effect of direct incineration of RH without pretreatments
The direct incineration of RH produces biosilica with various degrees of purity, irrespective of whether pretreatments of RH were performed before the production [147–153]. In addition, the surface area and brightness (whiteness) of the biosilica produced from direct incineration of RH are affected by the temperature of incineration, incineration/holding time, and pretreatment processes used for the RH. For example, RH can be converted to white, gray, or light gray RH ash, respectively, depending on the incineration temperature [148]. In particular, an incineration temperature of 300 °C–450 °C merely turns RH into carbonized RH, whereas an incineration temperature of 500 °C–650 °C turns RH into white or gray RH ash, depending on the incineration/holding time.
An increasing incineration temperature of RH appears to cause phase shifts in the RH after a certain temperature. For example, an incineration/holding (soaking) temperature of 500 °C–650 °C and time of 2.5–6 h are regarded as excellent for the manufacturing of white amorphous biosilica from the direct incineration of RH, although crystallinity sets in the produced biosilica when the incineration temperature exceeds 700 °C. Also, impurities in the RH affect the amount of operational phase in the biosilica produced from this incineration, cristobalite or tridymite (tridymite and cristobalite are high-temperature, low-pressure polymorphs of silica, forming stably above 870 °C (tridymite) and 1470 °C (cristobalite)). Furthermore, direct incineration of RH at too high incineration temperatures produces biosilica with a much lower surface area and hence lower reactivity compared to those of biosilica produced from RH using other production methods/routes [148].
Hamdan et al [154] described how RH may be directly incinerated in open air or in a muffle furnace [137]. The direct incineration of RH has been employed by certain researchers [151–153] to produce biosilica from the RH, despite the purity of the produced biosilica being less than 95%. It is thus possible to accomplish total incineration of RH in either static or moving air, resulting in varying effects on the biosilica produced from the incineration. It has recently been discovered in India that a TORBED-like reactor may be used to incinerate RH [155].
3.2.2.5. Effect of metallic impurities in RH
The metallic contaminants in RH make it hard to produce biosilica with a purity of more than 97% by direct incineration. For example, Chandrasekhar et al observed that oxides, particularly K2O, in RH result in a black color of the biosilica particles produced from the direct incineration of RH [156]. Notably, the biosilica produced from RH and oxides, particularlysodium and potassium oxides, may have good compatibility. This good compatibility causes surface melting of the biosilica particles and accelerates the crystallization of amorphous biosilica into cristobalite, as shown by the study findings, and giving one explanation for the black biosilica particles produced [140, 147, 157, 158]. Also, Kalapathy et al were unable to produce biosilica with a purity of 98% from their sol–gel treatment of RH ash with alkaline (bases) and acids even after 14 h of sol–gel treatment [159]. This inability could be due to the impurities in RH ash. In particular, the biosilica particle reactivity is reduced because the oxide impurities of RH on the biosilica particles reduce the particle size due to the melting of the particle surface by the oxide impurities. Hence, the odds of producing biosilica with a high purity and surface area from the direct incineration of RH are typically maximized with various RH pretreatment procedures, which may either be done via an acidic or alkaline (basic) medium.
3.2.2.6. Parameters affecting RH ash chemical extraction
Several aspects, such as the incinerating conditions (temperature and time), the rate of heating, the burning apparatus used for RH incineration, pretreatment of the RH (acid leaching or not), geographic region/origin, rice crop type, and usage of fertilizers to grow the RH crop, affect the RH ash characteristics. In particular, an increasing RH incineration temperature changes the color of the RH ash from gray (with a temperature of 500 °C) to whitish gray with a pinkish tone (with a temperature of 800 °C). In addition, RH ash produced at higher RH incineration temperatures is thinner and more brittle, with a more pronounced pinkish color, than the RH ash produced at lower RH incineration temperatures. On the other hand, according to Bakar et al, an RH incineration temperature of 500 °C–900 °C has no significant impact on the amount of biosilica produced from the incineration [39].
3.3. Production of biofuels from gasification of RH
The gasification of biomass is a process in which biomass is transformed into synthesis gas (syngas) instantly within a gasifier under controlled air conditions. Syngas can generate heat in an internal combustion engine or generate heat and electricity in a cogeneration plant.
The gasification reactions are given below [160, 161]:
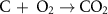
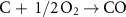
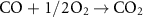
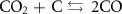
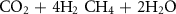
Notably, Kapur et al identified the unit cost of electricity produced by RH gasifiers and compared it to that of electricity generated by utility companies and diesel-powered generators [162]. Also, Abe et al examined the possibility of biomass gasification generating power in rural areas [163]. Their examination found that although agricultural leftovers, such as RH, may have a high energy density, providing a biomass gasification power generation system in the long term may need planting to ensure an adequate supply of energy resources for power generation. Hence, the sustainability of large-scale RH gasification for power generation depends largely on the power generation plant location, as the location impacts the accessibility of RH energy resources and the related logistical costs. In general, biomass gasification and related power generation technologies are well-established on an industrial level and have respective power generation capacities ranging from 200 to 10 000 kW.
3.4. Pyrolysis
Pyrolysis is an endothermic route in which organic waste is degraded at a high temperature without oxygen. Reactor temperatures generally range from 250 to 600 °C depending on the nature of the biomass feedstock and the distributions of the desired outputs (i.e. proportion of solid, liquid, and gaseous products). Neves et al claimed that when heat is added to the furnace during the pyrolysis, the polymer structure and constituents of biomass constituents (such as cellulose, hemicellulose, and lignin) begin to degrade [164].
When compared to other methods like gasification and combustion, pyrolysis is in the initial phase; these methods are accompanied by secondary gas-phase breaking and/or oxidation reactions to produce gaseous products. Overall, pyrolysis may be divided into two major categories: traditional (slow) and fast, depending only on the heating rate. Accordingly, the following formulas are used to describe slow and fast pyrolysis: theating ≫ tr for slow pyrolysis and theating ≪ tr for fast pyrolysis [165], where theating is the duration of time needed to heat the fuel to the pyrolysis temperature. The distribution of the product in the pyrolysis method, which is frequently carried out at atmospheric or slightly higher pressure, may be changed by changing these variables. Due to the variations in temperature, heating rate, retention time, and end products, pyrolysis is classified as slow, intermediate, fast, ultra-fast, or flash pyrolysis. Torrefaction is a kind of moderate pyrolysis that is applied to transform biomass into carbon-rich solid fuels [165, 166].
The process typically takes place in an inert gas environment, although rarely in vacuum [167] or in hydropyrolysis, which produces a pressurized hydrogen atmosphere [168]. Both a primary and a secondary reaction may occur during pyrolysis. Combustible and non-combustible gases are produced during primary pyrolysis (usually between 250 and 500 °C) as a consequence of the thermally induced breakdown of the chemical bonds in the molecules of the organic raw materials. Processes including cracking, polymerization, dehydration, condensation, and charring are examples of secondary pyrolysis. Studies from the recent literature also provide an interesting overview of the chemistry involved in the reaction of pyrolysis [169, 170]. The size of the feedstock is a significant factor that may affect the pyrolysis process in addition to the temperature and heating rate. Fast pyrolysis requires smaller particles to maximize liquid products. As a result, a wider variety of biomass feedstocks may be used in intermediate pyrolysis as there is no maximum limit on the particle size requirement [171]. Additionally, the liquid byproducts of the intermediate pyrolysis process have shown a notable improvement in terms of their qualities, which are comparable to those of traditional diesel and biodiesel [172, 173].
Slow pyrolysis, a traditional process for synthesizing charcoal that has been around for centuries [174], is characterized by modest heating rates and a lengthy solid and vapor retention period. The biomass feedstock is burnt at high temperatures in multiples of 100 °C min−1 in intermediate pyrolysis, and the vapor's retention period ranges from 10 to 30 min [175, 176]. Fast pyrolysis, in comparison, often demands high heating rates between 10 and 1000 °C s−1 along with a brief retention time of around 0.5–2 s. Depending on the dry biomass content, fast pyrolysis yields mainly bio-oil, which accounts for 65%–80% of the final product [177, 178]. Fast pyrolysis produces liquid end products with a moisture content of 20–30 wt% [179] and a variety of organic molecules, such as acids, alcohols, aldehydes, phenols, sugars, furans, nitrogen, and oxygenated compounds [180]. Likewise, flash pyrolysis, also known as very fast or ultra-fast pyrolysis, occurs at high heating rates of between 1000 and 10 000 °C min−1. An ablative process is analogous to the process that takes place during flash pyrolysis [181]. The secondary breakdown processes, which result in the generation of lighter gaseous products, should occur before the cooling and condensation of volatile compounds created during the main reactions in order to optimize the yield of liquid products in the final product.
Mostly, biomass is transformed into bio-oil or syngas for energy generation depending on the type of pyrolysis used, whereas only 10%–20% is turned into a solid form (biochar). Biochar, often referred to as biomass-generated charcoal, is a carbonaceous solid waste and ash formed from the organic and inorganic constituents of biomass [182]. Biochar may be employed as a catalytic support and a source of carbon nanotube precursors in addition to its primary usage as activated carbon for filtering and purification [183, 184]. Non-combustible gases, including CO, CO2, and other light hydrocarbon gases, are also emitted during pyrolysis on top of the charcoal and combustible vapors. The final yield dispersion and physio-chemical characteristics of the end products are significantly influenced by the pyrolysis process parameters, including the temperature, heating rate, pressure, and retention time, as well as the quality and content of biomass feedstock [185].
4. Common findings and emerging prospects
The use of RH as a biosilica and energy source is particularly appealing, due to its low cost and wide accessibility. The amorphous biosilica that is synthesized is a promising raw material for a wide range of sectors. Furthermore, several scientists have investigated RH based on the exclusive use of a single criterion for burning or extraction. This showed that RH, which has a high silica content and heating value, was mostly used to produce energy or biosilica. There are many methods of using RH. Calcination and chemical extraction are both effective methods for producing biosilica. The biological method is undesirable since it takes more time to process. The hydrothermal method has also been investigated; however, it has drawbacks due to its poor energy output and high carbon concentration in the ash.
Burning is a frequent method of power generation. Due to the insufficient combustion air supply, pyrolysis and gasification methods suffer from poor energy generation and a relatively high UCC in ash. High-temperature combustion is necessary to optimize the energy output, but this increases the chances of crystalline silica in the ash and also produces a significant number of NOx emissions. Pretreatment must be performed in order to maximize biosilica generation, and the burning temperature must be kept below 700 °C. However, this will result in an increase in CO discharge and UCC in ash. In order to compete with the synthetic approach, it is also predicted that the manufacturing costs of RH to energy and biosilica might be low.
Additionally, one of the future possibilities to be explored is an industrial-scale exploitation of RH that concentrates on both energy and biosilica generation. This possibility clearly aligns with the sustainable development idea that stresses zero-waste discharge.
Several studies have described laboratory-scale research using rice straw and husks burned in a muffle furnace to generate RH ash with a high silica concentration. A current and comprehensive analysis of the industrial-scale burning of RH in a suspended compressor, accompanied by RH ash chemical extraction with NaOH and RH ash acid leaching with various acids, as well as methods for biosilica extraction from RH, is still lacking. Thus, this review paper serves to compile, highlight, and discuss the vast RH prospects and limitations of its high heating value, high silica concentrations in ash, and low bulk density and different routes for biosilica production. Additionally, this study addressed the generation of biosilica from RH via chemical extraction and acid leaching, concluding that chemical extraction increases the silica concentration in ash.
5. Challenges and future prospects
This review examines studies on the use of RH for energy and the manufacturing of biosilica, which has produced useful outcomes but has revealed some enormous challenges. The difficulties, shortcomings, and results of several RH incineration and extraction techniques are also thoroughly outlined.
The ash content within different varieties of dried RH may be up to 25 wt%. A maximum silica content of 99.77 d b wt% has been reported in RH ash. Moreover, RH has a significant combustion temperature as well, ranging from 12 to 26 MJ kg−1. However, most experts agree that the weak physical characteristics of rice husk make it difficult to address combustion difficulties.Additionally, the chemical extraction of RH ash combined with an acid-leaching treatment produced biosilica with recovery rates of up to 98%, purity levels of up to 99.7%, and surface areas of up to 400.69 m2 g−1. Existing studies on converting RH to energy and biosilica have mostly been conducted on the lab scale, and the technology for commercially viable manufacturing has not yet been designed. The RH burning method to produce energy and amorphous biosilica in an industrial-scale synthesis contributed to a significant prospect. However, a conflicting criterion on the use of RH for both the development of biosilica and energy arises, and therefore operational parameter optimization must be developed. Consequently, a future viewpoint on realizing and growing green chemical industries and independent renewable green power plants via this issue needs to be acknowledged and is capable of promoting national prosperity. Additionally, pure nano-biosilica and large quantities of biosilica will be developed from RH in the future. The amorphous silica produced by this approach may be used in a variety of items, such as filters for leather and paper products and anti-sticking chemicals. It is a crucial catalyst in the chemical sector and a raw ingredient for the synthesis of silicon.
6. Conclusion
The significance of converting waste to energy or material is growing. Waste RH biomass is regarded as a sustainable and renewable energy source in this context, and offers great promise as an inexpensive precursor for the development of value-added products. Extracting silica from RH not only maximizes its potential value but also reduces the environmental problems associated with the recycling of RH. Thermochemical processes such as pyrolysis and gasification are considered to be the most effective methods of converting RH to biofuels (e.g. bio-oil, vapors) and biochars concurrently. The current study looked at the production of an amorphous silica made from RH using hydrochloric or sulfuric acid leaching, accompanied by controlled burning at 200 °C–600 °C for 3 h. As a result, there is a growing interest in the synthesis of valuable products from renewable resources. In this respect, RH is used to manufacture pure amorphous and crystalline biosilica using thermal and chemical methods, which can be used for other purposes. This review reveals that RH-based biosilica decreases the dependency on expensive silica, which is commercially used as a catalyst for fine chemical synthesis. In conclusion, RH, which is rich in amorphous silica, has the potential to become a source of inexpensive precursor for the synthesis of high-value silica/silicon compounds for practical use.
Acknowledgments
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government (MOTIE) (20210310100020, Production of advanced biofuel from lignocellulosic biomass by a combination of fast pyrolysis and supercritical ethanol upgrading). This work was also supported by the Ministry of Environment's waste resource energy recycling professional training project (YL-WE-22-001).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).





