Abstract
There has been growing and recent interest in using non-edible feedstocks, such as waste animal fats, as an alternative to vegetable oils in biodiesel production to address the food versus fuel debate. Waste animal fats are cost effective and yield good quality biodiesel. Therefore, waste animal fats are appealing and excellent feedstocks to produce biodiesel. Commercially, the biodiesel is obtained by transesterification reaction of triglycerides present in oil/fat with alcohol in the presence of homogeneous base catalysts. However, free fatty acids found in low-quality oil feedstocks are particularly sensitive to homogeneous base catalysts, necessitating extra acid pretreatment and neutralization procedures that not only raise the overall expense of producing biodiesel but also create environmental contamination. Optimistically, the use of solid catalysts can offer an environmentally friendly, cost-effective and practical route for the manufacture of biodiesel from inexpensive oil feedstocks, including waste animal fat. The present review article covers catalyzed transesterification/esterification using various catalysts with particular focus on the use of heterogeneous catalysts when using waste animal fat as feedstock for biodiesel production. In particular, the properties of biodiesel obtained from waste animal fats are also compared to the biodiesel properties of standard organizations, such as the European Committee for Standardization (ISO) and the American Society for Testing and Materials (ASTM). Moreover, this paper also offers future research directions that can direct researchers to fill in knowledge gaps impeding the creation of efficient heterogeneous catalysts for long-term biodiesel generation. To the best of our knowledge, the valorization of waste animal fats from slaughterhouses is not feasible and has some techno-economic concerns. However, this technology is more desirable considering the environmental point of view to address the pollution problems caused by these wastes.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
With the increase in population, fossil fuels are rapidly running out due to the increasing global consumption of these fuels [1]. The primary resources of fossil fuels, such as petroleum, coal and natural gas, are non-renewable and diminishing [2]. Notably, with the rising population, the energy consumption is approximately 12 billion tons of fuel oil equivalents worldwide annually [3]. Recently, fossil fuels have been used to generate power that accounts for 80% of power consumption, with the transportation sector accounting for 58% of the power consumption [4]. Figure 1 shows the worldwide consumption of various fuels in 2021 [5].
Figure 1. Worldwide consumption of various fuels in 2021 [5].
Download figure:
Standard image High-resolution imageThe most important environmental problem caused by the use of fossil fuels and different wastes is global warming due to the emission of CO2 during their use [6, 7]. Hence, the elevated energy consumption for human pursuits, such as the consumption for transportation and powering the industrial zones, and the health issues related to the environmental pollution from fossil fuel use necessitated the search for renewable energy resource-based fuels that can replace fossil fuels [8]. Consequently, renewable energy resource based and sustainable fuels/energy sources (such as biofuels, solar energy, hydro energy, wind and tidal energy sources) have been considered candidates to minimize the use of non-renewable energy resources [9]. Renewable resources are an intriguing option for overcoming the energy crisis because waste feedstocks are readily available for the production of bioenergy resource-based fuels (biodiesel, bioethanol, biogas, bio hydrogen, etc) using a variety of alteration/conversion technologies for the feedstocks [10]. Notably, biofuels are alternative energy sources and environment friendly, as they are biodegradable, non-toxic and renewable, and have low carbon monoxide (CO) emissions and low sulfur content [11–13].
Biodiesel is one of the most important biofuels and an alternative energy source because it possesses similar properties to conventional fossil fuels, such as high flash point, high cetane number, good lubricity, non-toxicity, is a low sulfur contaminant, etc [14–16]. Biodiesel is a mono alkyl ester of long-chain fatty acids produced from a range of feedstocks that includes vegetable oils and waste animal fat [17, 18]. Generally, the production cost of biodiesel is determined by the feedstock used. The edible oils used as feedstock for biodiesel production are palm kernel, castor, soybean, hazelnut, Jatropha, mustard and sunflower oils [19, 20]. Some edible oils, such as soybean oil, rapeseed oil and palm oil, are commonly used for biodiesel production in the USA, European countries and Malaysia, respectively [21]. However, when biodiesel is produced from edible oil, the feedstock cost rises by more than 70% compared to any other feedstock that could be used in the biodiesel production [22]. Although using vegetable oils to produce biodiesel has some advantages, which include liquid nature portability, ready availability, renewability, high heating value, low sulfur and aromatic content, and biodegradability, some drawbacks are that they are highly viscous and have lower volatile properties [23]. In addition, its use also causes food shortages and seems unsuitable and expensive due to the high production cost [24]. This limitation has been overcome by using non-edible oils, such as Jatropha, rubber seed, jojoba, tobacco seed, sea mango, neem, candlenut, mahua, karanja, etc [25]. Even India is producing biodiesel by using Jatropha oil on a commercial scale [21]. Moreover, biodiesel can be produced from various waste animal fat feedstocks, such as chicken fat, beef tallow, fish oil, mutton fat, pork fat and waste cooking oil (WCO) [26]. Table 1 shows the yield in tons per hectare of land of different biodiesel feedstocks with their oil content. It can be concluded that micro algae are the most promising feedstocks among all others as it yields 50 tons per hectare and oil content of 70%. In addition, there is no requirement of huge land area as it is easy to grow micro algae throughout the world.
Table 1. Yield comparison in tons per hectare of land of different biodiesel feedstocks and their oil content [27].
| Biodiesel feedstocks | Yield (tons per hectare) | Oil content (%) |
|---|---|---|
| Micro algae | 50 | 70 |
| Eucalyptus (leaves) | 15 | 1 |
| Pongamia | 10 | 50 |
| Palm | 5 | 43 |
| Jatropha | 5 | 40 |
| Neem | 4 | 45 |
| Mahua | 3 | 35 |
| Kusum | 3 | 11 |
| Paradise | 2.5 | 35 |
| Coconut | 2.26 | 45 |
| Moringa | 2 | 55 |
| Pomace | 2 | 40 |
| Okra | 1.9 | 19 |
| Jojoba | 1.8 | 54 |
| Peanut | 1.2 | 60 |
| Rapeseed | 1 | 55 |
| Castor | 0.81 | 42 |
| Sunflower | 0.80 | 57 |
| Orange | 0.60 | 01 |
| Sesame seed | 0.59 | 48 |
| Coffee bean | 0.39 | 15 |
| Rubber seed | 0.15 | 43 |
In a broader sense, biodiesel production costs depend on the feedstock, catalyst and alcohol used in the production. Thus, feedstocks, such as WCO and waste animal fat can be used to reduce the cost of biodiesel production [28]. Waste animal fat is abundantly available from slaughterhouses, farming industries and restaurants. However, valorization of waste animal fats from these sectors is not a feasible approach and there are some techno-economic concerns. This approach is more favored from an environmental standpoint to handle the pollution issues brought on by these wastes. Waste animal fat is obviously unsafe for consumption as food but can be used to produce biodiesel. Waste fats have been demonstrated to be the most widely used renewable and sustainably promising sources for biodiesel fuel with the least expensive production costs globally compared to the sources of edible and non-edible oil. Furthermore, the high fatty acid content and high fatty acid methyl ester (FAME) conversion efficiency of these fat and oil sources make them desirable when suitable homogeneous or heterogeneous catalysts are used. In addition, these waste fats provide high-quality fuel that meets international standards and does not compromise food security. Notably, triglycerides and unwanted impurities are found in waste animal fat that must be eliminated or modified before being sent for further processing and transesterification reaction. These impurities can be removed by washing, drying, filtration, centrifugation, evaporation, decantation, etc. Otherwise, the final product will be affected by these impurities. Eventually, inadequate biodiesel separation and purification lead to a higher content of contaminants, which could have an impact on engine performance.
Waste animal fat can easily be found in slaughterhouse waste and the food sector and contains free fatty acid (FFA) content with other constituents. When the amount of FFA in the waste animal fat is less than 15% by quantity, the fat is called yellow grease; otherwise, it is called brown grease [29, 30]. Moreover, yellow grease is superior to brown grease for biodiesel production due to low FFA content as excess FFA content results in soap formation (saponification reaction) [31]. Furthermore, when waste animal fat is used to produce biodiesel, fossil CO2 deficiency is higher. In particular, the use of waste animal fat-based biodiesel reduces CO2 emissions by about 80%, whereas using vegetable oil-based biodiesel only reduces CO2 emissions by 30% compared to the use of fossil fuels [32]. Meanwhile, the need for bioenergy resource-based fuels continues to rise, and these fuels are expected to account for 30% of the transportation fuel of the world by 2050 [33]. Consequently, biodiesel production has been increasing gradually every year worldwide and reached 45 million tons in 2019. Notably, 91 plants in the United States produced more than 5.6 million tons of biodiesel in 2019 [34]. In addition, approximately 80% of modern diesel vehicles are designated for using B20, which combines petroleum fuel with 20% biodiesel [35].
Transesterification reaction is the most popular and successful way to make biodiesel from non-edible oil because it is straightforward and affordable. Alcohol and triglycerides combine in this process to create biodiesel (FAMEs) [36]. By using both homogeneous and heterogeneous catalysts, this biodiesel manufacturing process can be expedited. The use of homogeneous catalysts for the synthesis of biodiesel raises several questions. These include the creation of sludge and challenging product purification, rendering them unsuitable for use in the synthesis of biodiesel [37]. The development of solid heterogeneous catalysts that exhibit extraordinary catalytic efficiency under operating reaction circumstances for the generation of biodiesel is therefore a continuing trend [38]. It is important to note that there are many issues with using heterogeneous catalysts, including difficult experimental procedures and reaction setups for catalyst synthesis, lengthy reaction times, swelling characteristics, leaching of active sites, pricey pretreatments, and low oil to biodiesel conversion rates [39]. These concerns drove scientists to create solid heterogeneous catalysts (biomass derived and green nanocatalysts) that are more effective, inexpensive and ecologically beneficial [40]. There are a number of reviews in the literature focusing on different kinds of catalysts used in biodiesel production from different feedstocks with respect to different aspects [11, 13, 15, 17, 19, 24, 41]. However, there are no up-to-date and in-depth reviews of various catalysts used in biodiesel production from waste animal fats. Hence, the theme of this paper is to present a quick review of different biodiesel production methods and a detailed review of the homogeneous and heterogeneous catalysts employed in biodiesel production from waste animal fats as feedstocks due to continuous trends in the use of waste animal fats. In addition, the advantages and disadvantages of different homogeneous and heterogeneous catalysts employed for biodiesel production are comprehensively discussed. Importantly, the properties of biodiesel obtained from different feedstocks including waste animal fats with the European Committee for Standardization (ISO) and the American Society for Testing and Materials (ASTM) are compared in detail. Finally, the challenges and prospects in this research field are presented.
2. Production methods of biodiesel
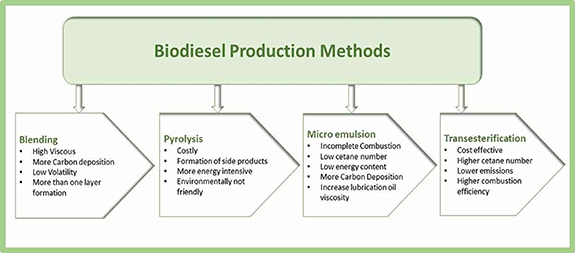
Pyrolysis (thermal cracking), dilution, microemulsion and transesterification/esterification are methods used to produce biodiesel. These methods are explained below [42].
2.1. Pyrolysis (thermal cracking)
Pyrolysis uses heat or catalysts to change one substance into another when there is no oxygen or air present [43]. When compared to other ways of cracking, the procedure is fast, less polluting, and effective [44]. New technologies are currently being employed to produce biodiesel because the pyrolysis method is no longer effective. The thermal degradation reaction is depicted in figure 2.
Download figure:
Standard image High-resolution image2.2. Dilution/blending
Diesel is mixed with vegetable oil to operate engines. To maintain the entire power without variation or engine adjustment in pre-combustion chamber engines, Caterpillar Brazil employed 10 wt% vegetable oil in 1980. Diesel was not introduced at that time to replace vegetable oil in its entirety, but a blend of 20% vegetable oil and 80% diesel fuel was successful. About 75 wt% diesel and 25 wt% sunflower oil were used to combine the fuel. Because of the low viscosity, the proportion of fuel can be reduced while still improving engine efficiency [17].
2.3. Microemulsion
Microemulsion is a term used to describe the dispersion of an optically isotropic fluid microstructure, which is created spontaneously by two normally immiscible liquids and many ionic amphiphiles [45]. Spray properties can be improved by explosively vaporizing all micelle low-boiling components. The micro-emulsion of 25 wt% and 53 wt% sunflower oil in diesel produced the same engine performance.
2.4. Transesterification
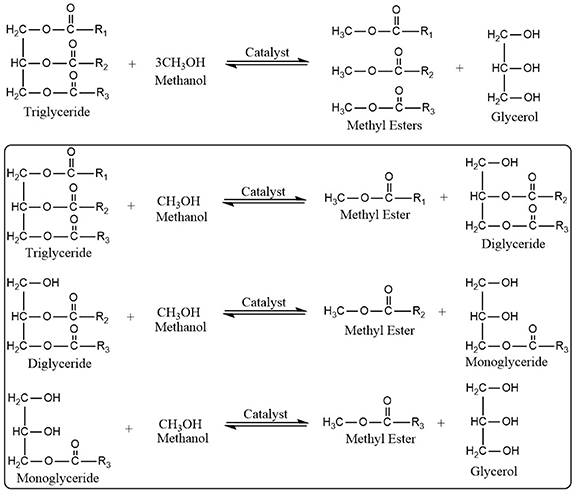
This is by far the preferred method for producing biodiesel. Esters and glycerol are produced as a result of the interaction between fatty acid or oil and alcohol. High temperature and pressure are necessary for a non-catalytic reaction, and the non-catalytic method has a low yield. The reaction rate and yield are often improved by the catalyst. In the process, alcohol and FFA combine to create FAME and water [46]. Excess alcohol alters the equilibrium in the forward direction since it is reversible. Transesterification involves a series of subsequent reversible processes. Over time, triglycerides change into diglycerides, monoglycerides, and finally glycerol, as depicted in figure 3.
Figure 3. Schematic illustrations of the conversion of triglycerides into methyl esters with the steps involved (enclosed in box) in the reaction.
Download figure:
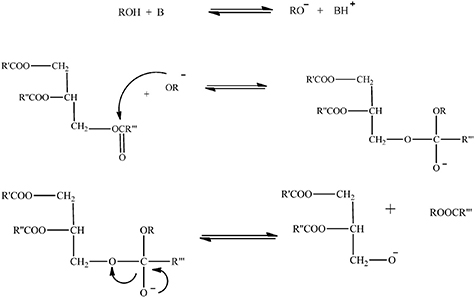
Standard image High-resolution imageCatalyzed transesterification involves the use of alkali/basic and acidic catalysts. Figure 4 shows the three steps involved in alkali catalyzed transesterification. The methoxide ion (the alcohol anion) strikes the carbonyl carbon of triglycerides in the first phase of the process, forming a tetrahedral intermediate. In the final stage, the tetrahedral intermediate produces a diglyceride and fatty acid ester after reacting with alcohol in the second step to create a methoxide ion [47]. Alcohol is combined with catalysts including NaOH, KOH, K2CO3 and others to generate alkoxides.
Figure 4. Reaction mechanism of alkali/basic catalyzed transesterification. Adapted from [17]. CC BY 4.0.
Download figure:
Standard image High-resolution imageAccording to some researchers, alternative use of an acid catalyst is more tolerant of FFAs. The mechanism of catalyzed acid transesterification is depicted in figure 5. After a nucleophilic assault on alcohol, protonation of the ester carbonyl group causes carbocation, which produces an intermediate tetrahedral [48]. The catalyst is renewed by this intermediate, which removes glycerol and creates a new ester. Triglycerides contain more water and FFAs, necessitating the employment of an acid catalyst. However, when an alkaline catalyst is present, transesterification proceeds approximately 4000 times more quickly than when only an acidic catalyst is present.
Download figure:
Standard image High-resolution imageFigure 6 depicts the advantages and disadvantages of the four different biodiesel manufacturing techniques.
Figure 6. Advantages and disadvantages of four different biodiesel production methods. Adapted from [39] (2022), reprinted with permission of the publisher (Taylor & Francis Ltd, www.tandfonline.com).
Download figure:
Standard image High-resolution imageTransesterification, the least complex technologically among these four techniques, is the most recommended way for producing biodiesel. In addition, transesterification has the enormous benefit of producing biodiesel within a very short time. Because it does not produce any byproducts, which are difficult to separate, it has the highest biodiesel output of any biodiesel manufacturing technique [39]. In addition to the biodiesel production method, the layout of the equipment, the process parameters, the reaction kinetics of the process, and the catalyst all have an impact on productivity and biodiesel yield. Figure 7 illustrates a few example variables influencing the biodiesel yield during biodiesel manufacture.
Figure 7. Different factors affecting the yield of biodiesel during the production.
Download figure:
Standard image High-resolution image3. Catalysts used in biodiesel production by transesterification/esterification
Different catalysts are used in the transesterification for biodiesel production. In particular, the catalysts are used to speed up the transesterification reaction and increase the biodiesel production yield. In addition, the catalysts for this transesterification are classified as homogeneous, heterogeneous and enzyme catalysts.
3.1. Homogeneous catalysts
The most widely used catalysts in transesterification for biodiesel production are homogeneous catalysts, which may be acidic or basic. In general, homogeneous catalysts are easy to use and time-efficient.
3.1.1. Homogeneous basic catalysts
Transesterification of vegetable oils or waste animal fat into biodiesel is usually achieved using a homogeneous basic catalyst, such as KOH, NaOH, CH3ONa and CH3OK [49]. These catalysts have many advantages, such as their requirement of a low temperature and atmospheric pressure for the catalytic transesterification reaction. Furthermore, a faster catalytic transesterification reaction within a short time economically is also possible with homogeneous basic catalysts [50]. In addition, a report shows that basic catalyzed transesterification rates are 4000 times faster than those of acid-catalyzed transesterification [50]. In addition, due to the rapid solubility in methanol and high purity of NaOH, it is favored over KOH in the transesterification for biodiesel production [51]. Furthermore, NaOH is required to be a low quantity compared to KOH in this transesterification [41, 52, 53].
Comparing homogenous basic and acidic catalysts shows that the homogeneous basic catalyst is more effective and has better activity than the homogeneous acidic catalyst due to the better conversion of fatty acids into alkyl ester in the transesterification of biodiesel using the homogeneous basic catalyst. However, it is suggested that the FFA content in waste animal fat be reduced before the biodiesel production from the fat using a homogeneous basic catalyst because the FFA interacts with the catalyst to form soap during the production [51]. Figure 8 shows the related saponification reaction.
Figure 8. Schematic illustration of the saponification reaction of triglycerides.
Download figure:
Standard image High-resolution imageThe performance of the transesterification for biodiesel production is significantly decreased when a homogeneous basic catalyst is used under a feedstock having a high FFA content (that results in saponification during the production) [54]. This saponification degrades the catalyst [55], lowering the biodiesel yield and increasing the cost of production [56]. In particular, glycerol extraction and biodiesel purity are affected by saponification, increasing the cost of treating the basic/alkaline wastewater from the production [57]. In addition, side reactions of the transesterification for biodiesel production that produce undesirable compounds can potentially have a negative impact on the quality of the biodiesel produced [58]. Likewise, the FFA content in the waste animal fats ranging from 5%–30% require essential pretreatment of the fats to avoid a negative impact on biodiesel production [59]. Finally, K/Na traces are also present in the biodiesel produced from the transesterification of waste animal fat using a homogeneous basic catalyst, so the consequently required purification of the biodiesel increases the production cost. However, when the FFA content in the waste animal fat is less than 0.5%, and the acidic values are lower than 1 mg KOH g−1, a homogeneous basic catalyst results in high biodiesel yield and purity in this production [60]. Dias et al [61] used calcium manganese oxide (CaOMnOx ) with 0.8 wt% sodium hydroxide (NaOH) in a molar ratio of 6:1 (CaOMnOx :NaOH) in the transesterification for biodiesel production under a transesterification reaction time and temperature of 0.25–8 h and 60 °C, respectively. Liu et al [62] used radio frequency (RF) heating to produce biodiesel from beef tallow using transesterification under a NaOH homogeneous basic catalyst. A 96% biodiesel yield was achieved in this production under a NaOH concentration of 0.6%, methanol to beef tallow molar ratio of 9:1, RF heating for 5 min, and a transesterification reaction temperature of 20 °C. Likewise, duck tallow [63] 0.28%, lard 0.33% and beef tallow 0.3%–0.9% were successfully converted into biodiesel using transesterification under different homogeneous basic catalysts and methanol [64, 65]. Table 2 illustrates the detailed comparison of biodiesel yield from one-step transesterification using homogeneous alkali catalysts with operating parameters, such as reaction time, reaction temperature and alcohol-to-waste animal fat ratio.
Table 2. A comparison of the one-step transesterification of different waste animal fats used to produce biodiesel.
| Waste animal fat | Production technique | Catalyst (wt/wt% of waste animal fat) | Type of alcohol used | Molar ratio (alcohol:waste animal fat) | Reaction temperature (°C) | Reaction time (h) | Conversion/biodiesel yield (%) | References |
|---|---|---|---|---|---|---|---|---|
| Pork lard | With a homogeneous basic catalyst | Tetramethylammonium hydroxide (TMAH) | Methanol | 6:1 | 65 | 2 | 98 | [66] |
| Beef tallow | With a homogenous basic catalyst | KOH | Methanol | — | 65 | 1.5 | 98 (with 98% purity) | [67] |
| Chicken fat | With a homogenous basic catalyst | NaOH and KOH | Methanol | 6:1 | 25 and 60 | 1–4 | The production of ester increased as the temperature increased. | [68] |
| Beef tallow | With a homogeneous basic catalyst | KOH (1.5) | Methanol | 6:1 | 65 | 3 | 96.4 | [65] |
| Beef tallow | With a homogeneous basic catalyst | KOH (1.5) | Methanol | 6:1 | 60 | 1 | 91 | [64] |
| Beef tallow | With a homogeneous basic catalyst | KOH (0.75–1.75) | Methanol | 3:1–12:1 | 55–60 | 2 | 87.4 | [69] |
| Duck tallow | With a homogeneous basic catalyst | KOH (0.5–3) | Methanol | 3:1–18:1 | 65 | 3 | 97.1 | [63] |
| Chicken fat | With a homogeneous basic catalyst | KOH (1.5) | Methanol | 33.5 cm3:120 g | 30 | 1 | 88.4 | [70] |
| Mutton fat | With a homogeneous basic catalyst | KOH (1.5) | Methanol | 33.5 cm3:120 g | 30 | 1 | 78.3 | [70] |
| Beef tallow | With a homogeneous basic catalyst | NaOH (0.6) | Methanol | 9:1 | 20 | 0.083 | 96.3 | [62] |
| Mixture of soybean oil and pork lard | With a homogeneous basic catalyst | NaOH (0.8) | Methanol | 6:1 | 60 | 1 | 88.6 | [71] |
| Chicken fat | With a homogeneous basic catalyst | KOH (0.34–0.41) | — | 3:1–7:1 | 60 | 1.5 | 95.2 | [72] |
| Beef tallow | With a homogeneous basic catalyst | KOH (0.8) | Methanol | 6:1 | 60 | 2 | 90.8 | [73, 74] |
| Tallow | With a homogeneous basic catalyst | NaOH | Methanol | 6:1 | 60 | 3 | Because of its low pour point, the tallow could not be used in cold conditions. | [75] |
| Pork lard | With a homogeneous basic catalyst | CaMnOx and NaOH | Methanol | 6:1 | 60 | 0.25–8 | — | [61] |
| Beef tallow | With a homogeneous basic catalyst | KOH | Methanol | — | 65 | 1.5 | 95% conversion efficiency | [76] |
Studies have also been conducted on a two-step transesterification to produce biodiesel using acidic or basic catalysts to speed up the transesterification reaction and decrease saponification during the reaction. The first step uses an acidic catalyst to lower the FFA content in the feedstock, while the second step uses a basic catalyst to maximize the biodiesel yield during the transesterification reaction [77]. A detailed comparison of biodiesel yields obtained from two-step processes using homogeneous acid and base catalyst in the first and second step, respectively, with reacting conditions is given below in table 3.
Table 3. A comparison of the two-step transesterification of different waste animal fats to produce biodiesel.
| Waste animal fat | Production technique | Catalyst (wt/wt% of waste animal fat) | Type of alcohol used | Molar ratio (alcohol:waste animal fat) | Reaction temperature (°C) | Reaction time (h) | Conversion/biodiesel yield (%) | References |
|---|---|---|---|---|---|---|---|---|
| Chicken fat | With homogeneous acidic and basic catalysts | H2SO4 and NaOH | Methanol | 7:1 | 45 | 1 | — | [78] |
| Sheep fat | With homogeneous acidic and basic catalysts | H2SO4 and NaOH | Ethanol | 5:1–2.5:1 | 40–70 | 0.5 | 98 (with 98% purity) | [67] |
| Chicken fat | With a homogenous acidic and basic catalysts | H2SO4 or HCl, and KOH | Methanol | 6:1 | 60 | 4 | 87.4 | [68] |
| Pork lard | With homogenous acidic and basic catalysts | NaOH and H2SO4 | Methanol | 6:1 | 45–65 | 5 | 99.6% purity | [79] |
| Tallow | With a homogenous basic catalyst | KOH | Methanol | 6:1 | 50–55 | 1 | 90 | [80] |
| Tallow | With homogeneous acidic and basic catalysts | H2SO4 (1) and KOH (0.35–0.4) | Methanol | 3:1–7:1 | 60 | 1.5 | 94 | [81] |
| Waste animal fat | With homogeneous acidic and basic catalysts | H2SO4 (6–10) and NaOH (0.5–1.5) | Methanol | 35:1 | 62 | 1 | 89 | [82] |
| Chicken fat | With homogeneous acidic and basic catalysts | H2SO4 (25) KOH (1) | — | 1:30 — | 50 30 | 24 1 | 99.01 88.10 | [70] |
| Mutton fat | With homogeneous acidic and basic catalysts | H2SO4 (25) KOH (1) | — | 1:30 — | 60 30 | 24 1 | 93.21 79.70 | [70] |
| Lard | With homogeneous acidic and basic catalysts | Step 1: H2SO4 (2) Step 2: NaOH (1) | — | 1:6 | 65 | — | 66.20 | [83] |
3.1.2. Homogeneous acidic catalysts
The most widely used homogeneous acidic catalysts for transesterification for biodiesel production are HCl, H2SO4, H3PO4, BF3 and FeSO4 [84]. Generally, the homogeneous acidic catalyst is unaffected by the FFA in the bio-oil (or any other feedstock) in this production and can catalyze the FFA and triglycerides simultaneously [85]. However, this acidic catalyst slows down the transesterification, resulting in a prolonged response time for the transesterification reaction to achieve a high biodiesel yield. Consequently, a homogenous acidic catalyst is infrequently used in single-step biodiesel production [86]. On the other hand, the homogenous acidic catalyst is used in the esterification (before the transesterification) in a two-step transesterification for biodiesel production. In essence, a homogeneous acidic catalyst has various drawbacks in FAME and biodiesel production, including the requirement of high alcohol quantities, less catalytic performance, a delayed transesterification reaction rate and the requirement of a high reaction temperature, leading to production equipment corrosion [87, 88]. However, low-cost feedstocks, such as WCO and waste animal fat with a high FFA content, can be used effectively in the transesterification for biodiesel production when a homogeneous acidic catalyst is used in the production [89]. In addition, using a homogeneous acidic catalyst instead of a homogeneous basic catalyst can solve the problem of soap formation during production [90]. In addition, table 4 contains details of the various homogeneous acidic catalysts used in the transesterification for the production of biodiesel from different feedstocks.
Table 4. Various homogeneous acidic catalysts used in the production of biodiesel from different feedstocks.
| Feedstock | Process | Catalyst (wt%) | Alcohol used | Reaction temp. (oC) | Alcohol to feedstock molar ratio | Reaction time (h) | Biodiesel yield | References |
|---|---|---|---|---|---|---|---|---|
| Chicken fat | Transesterification | Concentrated H2SO4 (25–100) | Methanol | 30–60 | 30:1 | 24 | 99 | [70] |
| Mutton fat | Transesterification | Concentrated H2SO4 (25–100) | Methanol | 30–60 | 30:1 | 24 | 93 | [70] |
| Fat | Transesterification | H2SO4 (5–9) | Methanol | 35–65 | 6:1–18:1 | 48 | 89 | [91] |
| Soybean | Transesterification | H2SO4 | Methanol | 65 | 9:1 | 8 | 99 | [92] |
| Tobacco | Esterification | H2SO4 | Methanol | 60 | 18:1 | 0.4 | 91 | [93] |
3.2. Heterogeneous catalysts
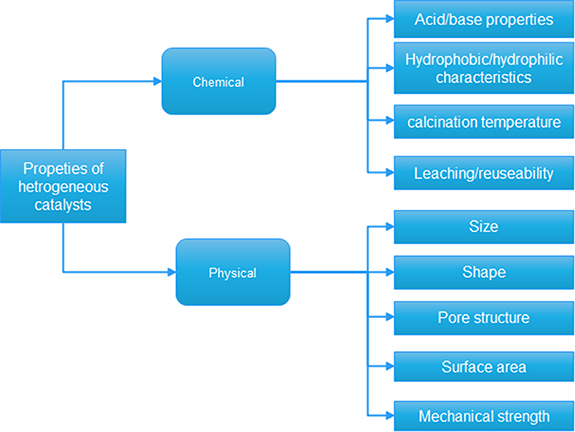
Heterogeneous catalysts have been receiving more attention than homogeneous catalysts in recent years due to their easy accessibility and ability to achieve high transesterification reaction rates and shorter reaction times in biodiesel production using transesterification. Furthermore, since the heterogeneous catalyst is in a dissimilar phase to the transesterification reaction mixture, this catalyst is advantageous in this production [94]. In addition, in this production, a heterogeneous catalyst is easily separable from the product of this transesterification, can be reused several times, eliminates corrosion issues and is suited to use with a high FFA content in the feedstock [54]. Figure 9 shows the characteristics of heterogeneous catalysts. Furthermore, as reported by several researchers, different animal waste fat feedstocks, such as chicken fat, beef tallow, duck tallow and pig fat are used for biodiesel production using transesterification under a heterogeneous catalyst. Palmitic, linoleic and stearic are also major fats in waste animal fat.
Figure 9. Characteristics of heterogeneous catalysts.
Download figure:
Standard image High-resolution image3.2.1. Heterogeneous acidic catalysts
Generally, solid/heterogeneous acidic catalysts, such as metal/mixed/sulfated metal oxides, ion exchange resins, hetero-polyacids and zeolites are commonly used in the transesterification for biodiesel production [95]. Heterogeneous acidic catalysts are less corrosive and non-poisonous, and thus cause few environmental issues [96]. However, when compared to a heterogeneous basic catalyst, a heterogeneous acidic catalyst reacts slowly during biodiesel production but shows promising results under moderate transesterification reaction conditions. Notably, the heterogeneous acidic catalyst requires a high catalyst concentration, a high temperature and an extended time for the transesterification reaction [97]. However, this catalyst meets the specifications of the ASTM standards for biodiesel production [98]. Finally, the heterogeneous acidic catalysts are unaffected by the presence of FFA and water in the feedstock of biodiesel production. In any case, an ideal heterogeneous acidic catalyst must have many accessible active sites, appropriate acid intensity, hydrophobicity and porosity for use in the transesterification for biodiesel production [97]. Heterogeneous acidic catalysts used in transesterification to produce biodiesel from various feedstocks have been classified into four classes: sulfonic acid catalysts, sulfated oxide catalysts, heteropoly acid derivatives and cation exchange resins.
Ngo et al [99] and Kim et al [100] used diphenyl ammonium salts supported onto mesoporous silica SBA-15 and ZrO2 supported metal oxides, respectively, to produce biodiesel from brown greases having a high FFA content (40%). Ngo et al reported that the FFA content in the greases decreased to lower than 1%, and a high grease conversion was achieved in this production under certain conditions. In addition, due to the high content of water and sulfur in the raw feedstock, Kim et al performed esterification of FFA before the transesterification of the greases to achieve a high ester yield. Likewise, Bianchi et al [101] used Amberlyst (A70) as a catalyst in the esterification of lard to reduce the FFA content in the lard. Soldi et al [102] used syndiotactic polystyrene (SPS) catalyst for the production of ethyl ester from refined soybean oil and beef tallow, and this catalyst was effective and resulted in an ethyl ester conversion of 85% and 75% in these feedstocks, respectively. In addition, no ethyl ester was formed in this production under an Amberlyst-15 catalyst and methanol, but the production under the SPS catalyst resulted in a 13% conversion of the respective feedstock [102]. Furthermore, Melero et al [103] demonstrated that a Zr-SBA-15 heterogeneous acidic catalyst enhanced biodiesel production from low-grade waste animal fats using transesterification compared to the same production without a catalyst. Notably, the Zr-SBA-15 catalyst showed admirable reaction stability during the transesterification.
3.2.2. Heterogeneous basic catalysts
Heterogeneous basic catalysts include mostly alkaline oxides, alkaline earth metal oxides, hydrotalcites, metallic salts, anion exchange resins and zeolites [104]. Since alkaline earth metal oxides are inexpensive and have a high basic intensity, they are often used in transesterification for biodiesel production. Generally, a heterogeneous basic catalyst is used when a low FFA content waste animal fat is used in this production [105, 106]. On the other hand, a heterogeneous acidic catalyst is used when a high FFA content (45%) fat is used in the production [99–101, 103]. The heterogeneous basic catalyst can also eliminate problems, such as soap generation, in the production [107]. Furthermore, this catalyst demonstrates a significant performance during the transesterification reaction under mild reaction conditions [108]. As stated previously, a high FFA content feedstock induces saponification during biodiesel production, limiting the glycerol extraction from the biodiesel produced and biodiesel productivity [109, 110]. Notably, the heterogeneous basic calcium oxide (CaO) catalyst is environment-friendly and has a long life and high activity [109]. In addition, the CaO catalyst is made from chicken egg shells, mollusk shells and bones containing a lot of calcium, minimizing the problems with dumping these waste shells and bones [111]. However, mixed metal oxide catalysts have shown a high tolerance towards the FFA and water content in the bio-oil (or any other feedstock) used for biodiesel production under transesterification. Generally, mixed metal oxide catalysts, such as CaTiO3, CaMnO3, Ca2Fe2O5, CaZrO3 and CaO–CeO2 are used in biodiesel production [112, 113]. The different heterogeneous basic catalysts are mixed metal based, transition metal based, alkaline earth metal based, hydrotalcite based and waste-derived catalysts.
Dias et al [105, 114] examined the production of biodiesel from pork lard using transesterification under various heterogeneous basic catalysts, such as calcium iron oxide (CaFeOx ), barium iron oxide (BaFeOx ), calcium zirconium oxide (CaZrOx ), calcium manganese oxide (CaMnOx ), calcium cerium oxide (CaCeOx ) and barium manganese oxide (BaMnOx ). The examination showed that CaMnOx contributed to a good production. In addition, the maximum ester yield in this production was 92.4% at a transesterification temperature and time of 60 °C–70 °C and 4 h, respectively. Furthermore, Huong et al [114] studied the transesterification of fish fat using potassium hydroxide (KOH) and aluminum oxide (Al2O3) as heterogeneous basic catalysts, respectively. In particular, several transesterification conditions were examined in this study to find the conditions for high-quality biodiesel production. The examination showed that an alcohol-to-fat ratio of 6:1 and transesterification temperature and time of 50 °C and 45 min, respectively, resulted in high-quality biodiesel production. Table 5 gives the various heterogeneous acidic and basic catalysts used in the transesterification of waste animal fat to produce biodiesel.
Table 5. Various heterogeneous acidic and basic catalysts used in the transesterification of waste animal fat to produce biodiesel.
| Waste animal fat | Production technique | Catalyst/co-solvent (wt/wt% of waste animal fat) | Type of alcohol used | Molar ratio (alcohol:waste animal fat) | Temperature (°C) | Time (h) | Conversion/biodiesel yield (%) | References |
|---|---|---|---|---|---|---|---|---|
| Lard | With a heterogeneous basic catalyst | Potassium hydroxide (KOH) | Methanol | 4:1 | 65 | 6 | 90 | [101] |
| Poultry fat | With a heterogeneous basic catalyst | Mg–Al hydrocalcite (10) | Methanol | 1:30 | 120 | 8 | 93.00 | [115] |
| Tra catfish fat | With a heterogeneous basic catalyst | KOH/γ-Al2O3 (5–8) | Methanol | 10:1–14:1 | 60 | 1.5 | 92.6 | [114] |
| Beef tallow | With a heterogeneous basic catalyst | KF/CaO–Fe3O4 | Methanol | 3:1–12:1 | 40–65 | 1 | 94 | [116] |
| Pork lard | With a heterogeneous basic catalyst | CaMnOx and CaO (0.6–4) | Methanol | 6:1–24:1 | 60–70 | 4 | 92.4 | [105] |
| Pork lard | With a heterogeneous basic catalyst | CaMnOx (3) | Methanol | 18:1 | 50 | 8 | 92.5 | [61] |
| Mutton fat | With a heterogeneous basic catalyst | MgO–KOH–X; X = 5–20 | Methanol | 11:1–22:1 | 45–65 | 0.33 | 98 | [106] |
| Brown grease | With a heterogeneous acidic catalyst | Mesoporous silica diphenylammonium triflate (15) | Methanol | 15:1 | 95 | 2 | 98 | [99] |
| Brown grease | With a heterogeneous acidic catalyst | ZnO/ZrO2 (0.8 g) | Methanol | 10.5:15 | 200 | 2 | 78 | [100] |
| Lard | With a heterogeneous acidic catalyst | Amberlyst 70 (1.25–10) | Methanol | 4:1 | 65 | 6 | ∼95 | [101] |
| Pork lard | With a heterogeneous basic catalyst | Calcium oxide (CaO) | Methanol | 8:1 | 50 | 0.25–8 | — | [61] |
| Beef tallow | With a heterogeneous acidic catalyst | Sulfonated polystyrene (20 mol %) | Ethanol | 100:1 | 64 | 18 | 75 | [102] |
| Beef tallow | With a heterogeneous basic catalyst | Cs2O/γ-Al2O3 | Methanol | 8:1–12:1 | 55–75 | 2 | 95.5 | [117] |
| Chicken fat | With a heterogeneous basic catalyst | Crab, cockle shells, and their mix of 1:1 of crab:cockle (4.9) | Methanol | — | 65 | 3 | 98.8 | [118] |
a wt% of KOH impregnated over MgO. b In the presence of hexane as a co-solvent. c ml ml−1.
3.3. Enzyme catalysts
In comparison to traditional chemical approaches to biodiesel production, the use of enzyme catalysts in the transesterification for biodiesel production is a relatively new methodology [119]. In particular, the enzyme catalyst helps to overcome the issues with the use of acidic or basic catalysts in this production. In effect, the enzyme catalytic biodiesel production enables the (a) raw materials of biodiesel production having a high FFA content, which are low-cost and low-quality, to be used in the production, (b) avoidance of saponification during production, (c) production of high-quality biodiesel and glycerol (a byproduct), (d) the use of easy production methods, (e) the tolerance to water content in bio-oil (or any other feedstock) and a rise in biodiesel yield in the production and (f) energy-saving due to the transesterification reaction occurring at low temperatures [120].
Generally, biodiesel is produced using lipases (one of the enzyme catalysts), and these catalysts are either homogeneous or heterogeneous, depending on whether they are free or immobilized [121]. Notably, enzymes have high selectivity towards the biodiesel product, help produce high purity biodiesel, and produce no soaps compared to other catalysts used in the transesterification for biodiesel production. In addition, enzymes can catalyze the transesterification of triglycerides and the esterification of FFA [122]. However, the two most significant disadvantages of enzymes are their high cost and the possibility of their deactivation by short-chain alcohols and their derivatives during the transesterification [123, 124]. Normally, the stability of the enzyme catalyst is improved by immobilizing it in its storage and operational circumstances [125, 126]. In particular, a sudden change in temperature or pH has little effect on the immobilized enzyme (like lipase). The immobilization also simplifies the catalyst separation process, prevents contamination of the biodiesel product, and helps produce high-quality biodiesel [127]. Thus, lipases are immobilized on a solid support, allowing these enzyme catalysts to be reused multiple times during transesterification for biodiesel production. As stated previously, the immobilization enables the lipase to be modified to withstand the transesterification conditions and prevents the effects of temperature, pressure, pH and organic solvents on the lipase [121]. In other words, immobilizing enzymes increases their stability and protects them against denaturation [128].
Methanol step-wise addition, replacing methanol with an acyl acceptor (such as methyl acetate or ethyl acetate) or a solvent (such as t-butanol) to increase the methanol solubility, has been followed in the transesterification for biodiesel production [129]. Meanwhile, lipases from a variety of microorganisms, including Candida antarctica, Candida rugosa, Pseudomonas cepacia, Pseudomonas spp., Rhizomucor miehei and immobilized lipases (e.g. Lipozyme RMIM from R. miehei or Novozym 435 from C. antarctica) have been used in this transesterification [130–132].
Nawaz et al [19] used a lipase or basic-catalyzed transesterification to produce methyl or ethyl esters from lard and restaurant grease. In particular, the conversion to methyl and ethyl ester after the third step of this production was 74% and 43%, respectively. Likewise, a lard methanolysis (transesterification using methanol) under a lipase catalyst was carried out by Lu et al [75] across seven cycles, producing more than 80% of FAME or biodiesel without any loss of lipase activity. In particular, the best conditions for converting 1 g of lard in this production were 0.2 g of immobilized lipase as the catalyst, 8 ml of n-hexane as the solvent, 20% of water (based on the lard weight), a methanolysis temperature of 40 °C and a three-step addition of the methanol [75]. Mata et al [73] used two non-commercial supports, an inorganic matrix (niobium oxide, Nb2O5) and a hybrid matrix (polysiloxane–polyvinyl alcohol SiO2–PVA) to covalently immobilize the lipase from Burkholderia cepacia on each of them for ethanolysis (transesterification using ethanol) of beef tallow to produce biodiesel. Notably, the ethanolysis under the two supports resulted in different respective biodiesel yields for the same transesterification reaction time. Furthermore, the lipase PS immobilized on SiO2–PVA resulted in the greatest outcomes in the production of biodiesel from each of the two feedstocks of this production, with a biodiesel yield of more than 89% in a transesterification reaction time of 48 h.
Meanwhile, Moraes et al [76] investigated the enzyme-catalyzed transesterification for the production of biodiesel from beef tallow in a microwave reactor. Notably, a low molar ratio of beef tallow to ethanol (1:6) and a high transesterification reaction temperature (50 °C) maximized this biodiesel production. Huang et al [133] used response surface methodology to optimize the transesterification for the production of biodiesel from lard catalyzed by a combination of Novozym 435 (non-specific) and Lipozyme TLIM (1,3-specific) enzymes. And the best transesterification reaction conditions of this production were 0.04 wt% lipase/oil (w/w), 0.49 wt% Novozym 435/total lipases (w/w), 0.55 wt% tert-butanol/oil (v/v), 5.12 wt% methanol/oil (mol/mol), and a 20 h transesterification reaction time, yielding 97.2 wt% of methyl ester. Table 6 gives the various enzyme catalysts used in the transesterification of waste animal fat to produce biodiesel. In addition, table 7 compares the different catalyst types used in the transesterification for biodiesel production.
Table 6. Various enzyme catalysts used in the transesterification of waste animal fat to produce biodiesel.
| Waste animal fat | Production technique | Catalyst/co-solvent (wt% of waste animal fat) | Type of alcohol used | Molar ratio of alcohol and waste animal fat | Reaction temperature (oC) | Reaction time (h) | Conversion/biodiesel yield (%) | References |
|---|---|---|---|---|---|---|---|---|
| Lard | Lipase catalyzed | Candida antarctica (Chirazyme L-2) | Methanol | 1:1 | 30 | 72 | 74 | [125] |
| Lard | Lipase catalyzed | Candida sp. 99–125 | Methanol | 3:1 | 40–60 | 30 | 87.4 | [134] |
| Beef tallow | Lipase catalyzed | Burkholderia cepacia (Lipase PS) | Ethanol | 12:1 | 50 | 48 | 89.7 | [135] |
| Beef tallow | Lipase catalyzed | Burkholderia cepacia | Ethanol | 6:1 | 50 | 48 | 40.2 | [136] |
| Rendered animal fat | Lipase catalyzed | Mucor miehei (Lipozyme-IM) | Ethanol | 1:1–6:1 | 25–65 | 120 | 27 | [137] |
| Lamb fat | Enzyme catalyzed | C. antarctica (Novozym 435) lipase in SC CO2 (30–50) | Methanol | 3:1–6:1 | 35–60 | 25 | Maximum of 49.2 at a temperature of 50 °C | [138] |
| Lard | Enzyme catalyzed | Immobilized-lipase (Candida sp. 99–125) (20) | — | 1:1 | 40 | 30 | 87.40 | [134] |
| Lard | Enzyme catalyzed | Novozym 435 and Lipozyme TLIM (4) | — | 1:2 | 50 | 20 | 97.20 | [133] |
| Animal fat and fish oil | Enzyme catalyzed | Lipozyme-IM | 1:4 | 1:4 | 25 | 96 | 50 | [137] |
Table 7. Comparison of different catalyst advantages and disadvantages in transesterification for biodiesel production.
| Catalyst types | Advantages | Disadvantages |
|---|---|---|
| Homogeneous basic | No corrosion during the production. Appropriate for bio-oils (or any other feedstock of biodiesel production) with low FFAs and water (H2O) content. Strong activity. Inexpensive and widely available. More active than acidic catalysts. Requires only mild transesterification reaction conditions. | Soap formation during the production. There is no possibility of reusing the catalyst. Requires a lot of washing of the product. Inseparable from the transesterification reactant mixture. High FFAs and water content in the feedstock of the biodiesel production influence the catalyst during the production. The product washing under this catalyst use results in a large amount of wastewater. |
| Homogeneous acidic | Strong activity. No soap formation during the production. Applicable for feedstocks of the biodiesel production having a high FFA content. | Slow transesterification reaction rates. Corrosion during the production. Challenges related to product separation and the non-reusability of the catalyst. |
| Heterogeneous basic | Requires only mild transesterification reaction conditions. Transesterification reaction rate is fast. A high degree of regeneration and reusability of the catalyst. Secure and less expensive. Effluent generation during the production can be minimized. Purification of the biodiesel product is simple. Several times reusability of the catalyst. Less corrosive. | Leaching of active sites of the catalyst is a possibility. Contact of the catalyst with the surrounding air causes catalyst poisoning. Sensitive to FFAs in the feedstock of the biodiesel production and forms soap during the production. Saponification during the production reduces the biodiesel yield and complicates the biodiesel purification. Biodiesel product contamination may result from the catalyst leaching. The catalyst synthesis is expensive. |
| Heterogeneous acidic | Separation of the catalyst from the biodiesel product is simple. Several times reusability of the catalyst. Esterification and transesterification can take place simultaneously in the production. Applicable for feedstocks of the biodiesel production having a high FFA content. FFAs and water in the bio-oil (or any other feedstock of biodiesel production) do not affect the catalyst. Catalyst extraction from the biodiesel product is simple. | The required catalyst concentrations are high. Long transesterification reaction time. Deactivation of the catalyst is a possibility. Requires a high transesterification reaction temperature, long reaction time and high alcohol to bio-oil (or any other feedstock of biodiesel production) molar ratio. The catalyst synthesis is expensive. Biodiesel product contamination may result from the catalyst leaching. |
| Enzyme | Separation of the catalyst from the product is simple. Requires only mild transesterification reaction conditions. Helps produce biodiesel having a high degree of purity. FFAs and water content in the bio-oil (or any other feedstock of biodiesel production) do not affect the catalyst. Preferred for low-grade bio-oils (or any other feedstock of biodiesel production). Several times reusability of the catalyst. High-quality biodiesel products. | Expensive for industry-scale production. Methanol-sensitive and enzyme inactivation. The catalyst synthesis is expensive. Immobilization of the catalyst reduces the catalytic activity. |
4. Quality specifications
Several factors can affect the quality of biodiesel, and the effect can be seen in the chemical and physical characteristics of the biodiesel. Furthermore, in order to be commercialized (table 8), biodiesel must meet the quality requirements defined by organizations such as the ISO and the ASTM 8.
Table 8. Standard specifications for biodiesel fuel (B100) [139].
| Property | Unit | ASTM-D6751-10 standard specification | EN-14214 standard specification |
|---|---|---|---|
| Density (at a temperature of 15 °C) | kg m−3 | — | 860–900 |
| Viscosity (at a temperature of 40 °C) | mm2 s−1 | 1.9–6.0 | 3.5–5.0 |
| Flash point | °C | Minimum of 130 | Minimum of 101 |
| Cetane number | — | Minimum of 47 | Minimum of 51 |
| Sulfur content | mg kg−1 | Maximum of 15 | Maximum of 10 |
| Phosphorous content | mg kg−1 | Maximum of 10 | Maximum of 4 |
| Water content | mg kg−1 | — | Maximum of 500 |
| Acid number | mg KOH g−1 | Maximum of 0.50 | Maximum of 0.50 |
| Free glycerin content | % (mass) | Maximum of 0.02 | Maximum of 0.02 |
| Total glycerin content | % (mass) | Maximum of 0.24 | Maximum of 0.25 |
| Sulfated ash content | % (mass) | Maximum of 0.020 | Maximum of 0.020 |
| Methanol content | % (mass) | Maximum of 0.20 | Maximum of 0.20 |
| Monoglycerides content | % (mass) | — | Maximum of 0.80 |
| Diglycerides content | % (mass) | — | Maximum of 0.20 |
| Triglycerides content | % (mass) | — | Maximum of 0.20 |
| Ester content | % (mass) | — | Minimum of 96.5 |
| Linolenic acid methyl ester content | % (mass) | — | Maximum of 12 |
| Carbon residue content | % (mass) | Maximum of 0.050 | Maximum of 0.30 |
| Polyunsaturated (⩾4 double bonds) methyl ester content | % (mass) | — | Maximum of 1 |
| Iodine value | — | — | Maximum of 120 |
| Oxidation stability (at a temperature of 110 °C) | h | Minimum of 3 | Minimum of 6 |
| Copper corrosion (in a time of 3 h and at a temperature of 50 °C) | Degree of corrosion | Maximum of 3 | Maximum of 1 |
| Total contaminant content | mg kg−1 | — | Maximum of 24 |
| Group 1 metals (Na and K) content | mg kg−1 | Maximum of 5 | Maximum of 5 |
| Group 2 metals (Ca and Mg) content | mg kg−1 | Maximum of 5 | Maximum of 5 |
| Distillation temperature at 90% recovery point | °C | Maximum of 360 | — |
| Water and sediment content | % (mass) | Maximum of 0.05 | — |
5. Properties of biodiesel from different feedstocks
Generally, the quality of biodiesel is affected by the raw materials, including the feedstock, used in biodiesel production and the transesterification and purification processes of the production. The literature on biodiesel properties obtained from the various feedstocks of biodiesel production is summarized in table 9.
Table 9. Comparison of the properties of the biodiesel produced from various feedstocks.
| Property specification | ASTM D6751 limit | EN 14214 limit | Beef tallow | Rapeseed oil | Soybean oil | Neem oil | Chicken fat | Fleshing oil | Poultry fat | Mutton tallow | Lard | Fish oil | Waste cooking oil |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| References | [140] | [140] | [65] | [141] | [141] | [142] | [68, 139] | [143] | [73, 74] | [72] | [144] | [145] | [146] |
| Density at a temperature of 15 °C (kg m−3) | 880 | 860–900 | 872 | 882 | 913.8 | 868 | 883 | 876 | 877 | 882 | 873–877.4 | 881 | 866 |
| Viscosity at a temperature of 40 °C (mm2 s−1) | 1.9–6.0 | 3.5–5.0 | 5.3 | 4.43 | 4.039 | 5.123 | 4.94 | 4.7 | 6.86 | 4.75 | 4.59–5.08 | 4.45 | 4.027 |
| Cetane number | Minimum of 47 | Minimum of 51.0 | 60.36 | 54.4 | 37.9 | — | — | — | — | 59 | — | 52.4 | 66 |
| Iodine number (g of I2/100 g) | — | Maximum of 120 | — | — | 128–143 | — | — | 61 | 78.8 | 40 | 67–77 | 185 | 87.3 |
| Acid value (mg of KOH g−1) | Maximum of 0.50 | Maximum of 0.50 | — | 37 | 0.266 | 0.69 | 0.22 | 0.32 | 0.55 | 0.3 | 0.04–1.13 | 0.3 | 0.38 |
| Pour point (°C) | −15 to −16 | — | 14.3 | −12 | — | 2 | 2 | — | 3 | −5 | 5–7 | — | 9 |
| Flash point (°C) | Minimum of 130 | Minimum of 101 | 156.7 | 170 | 254 | 76 | 171.8 | 174.8 | 172 | — | 143.5–147 | 177 | 170 |
| Cloud point (°C) | −3 to −12 | — | — | −3.3 | 0.9 | 9 | — | — | — | — | — | — | 13 |
| Cold filter plugging point (°C) | Maximum of 5 | — | — | −13 | −4 | 11 | — | 11 | — | — | — | — | 8 |
| Copper strip corrosion (in a time of 3 h and at a temperature of 50 °C) | No. 3 | Class 1 | — | — | 1 ppm | 1 ppm | 1 | — | 1 | — | 1 | — | — |
| Carbon residue content (% (m m−1)) | Maximum of 0.05 | — | — | — | — | — | — | — | — | — | Unmeasurable (um)—0.21 | — | 76.26 (wt/wt%) |
| Methanol content (% (m m−1)) | Maximum of 0.20 | Maximum of 0.20 | 0.1 | — | — | — | 0.01 | — | — | — | — | — | |
| Water content (mg kg−1) | Maximum of 500 | Maximum of 500 | — | — | — | — | 200 | 326.4 | 1201 | — | 184–1100 | — | |
| Sulfur content (mg kg−1) | S15 maximum of 15 and S500 maximum of 500 | Maximum of 10 | — | — | 0.8 | 473.8 ppm | — | — | — | — | — | — | 0 |
| Sulfated ash content (% (m m−1)) | Maximum of 0.02 | Maximum of 0.02 | — | — | <0.005 | <0.005 | — | — | — | — | um—0.002 | — | — |
| Phosphorus content (mg kg−1) | Maximum of 10 | Maximum of 4 | — | — | <0.1 ppm | <0.1 ppm | — | — | — | 16 | — | — | — |
| Free glycerol content (% (m m−1)) | Maximum of 0.02 | Maximum of 0.02 | 0.01 | — | 0.012 | 0 | 0.02 | — | — | — | — | — | — |
| Total glycerol content (% (m m−1)) | Maximum of 0.24 | Maximum of 0.25 | 0.33 | — | 0.149 | 0.158 | 0.19 | — | — | — | — | — | — |
| Monoglyceride content (% (m m−1)) | Maximum of 0.40 | Maximum of 0.70 | 0.13 | — | 0.473 | 0.338 | 0.56 | — | — | — | — | — | — |
| Diglyceride content (% (m m−1)) | — | Maximum of 0.20 | 0.12 | — | 0.088 | 0.474 | 0.09 | — | — | — | — | — | — |
| Triglyceride content (% (m m−1)) | — | Maximum of 0.20 | 0.07 | — | 0.019 | 0 | 0.12 | — | — | — | — | — | — |
| Distillation temperature at 90% recovery point (°C) | Maximum of 360 | — | 307–344 | — | — | — | — | — | — | — | um—352.5 | — | — |
| Oxidation stability at a temperature of 110 °C (h) | Minimum of 3 | Minimum of 8 | — | 7.6 | 2.1 | 7.1 | — | — | — | — | — | — | — |
| Heat of combustion (MJ kg−1) | — | — | — | — | 39.76 | 39.81 | 40.17 | 39.954 | 39.58 | — | — | 40.546 | 38.034 |
6. Conclusion and outlook
Animal fat waste is an excellent prospective feedstock for the generation of high-quality biodiesel since it is readily available and less expensive than vegetable oil. One strategy to limit the impact of biodiesel production on food security while lowering production costs and environmental harm is to use animal fat waste as the feedstock for fuel. A commonly acknowledged method for biodiesel generation is transesterification or esterification of animal fats in the presence of a suitable catalyst. In the past, homogeneous catalysts have been produced on an industrial scale, resulting in faster reaction speeds and higher yields. However, they do have several drawbacks, such as an additional neutralization step, a lengthy purification process, wastewater as a by-product and catalyst non-recyclability. On the other hand, heterogeneous catalysts hold greater promise than homogeneous catalysts due to their ease of removal from the final products and several cycles of reuse, which reduces overall manufacturing costs. However, the development of a highly active and selective heterogeneous catalyst is required to commercialize heterogeneous catalysts. As more research is needed to enhance heterogeneous catalysts made from waste biomass, the affordability and sustainability of the catalyst will be assured, allowing solid catalysts to be commercialized.
More research is needed on biocatalysts because transesterification uses chemical catalysts that could harm the environment. According to numerous reports, the lipase enzyme is an excellent biocatalyst to produce biodiesel and can be utilized commercially once it has been properly tuned for optimal yield. Immobilizing lipase on a nano-catalyst enhances its activity and surface area while also allowing for facile catalyst recovery and reuse over numerous cycles. Due to their greater miscibility with the reactants, which reduces mass transfer restrictions and increases reaction speeds, liquid lipase formulations for biodiesel generation are quickly rising. However, due to its higher cost, biodiesel synthesis utilizing lipase on an industrial scale remains a difficulty. Continuous research is being conducted to enhance the economics of employing enzyme catalysts in industrial processes. On a large scale, enzyme catalysts will soon be able to replace homogeneous catalysts. In short, animal fat waste offers a reliable fuel source for environmentally friendly biodiesel production. Nevertheless, to collect realistic feedstock calls for a complex management system. In addition, biodiesel research necessitates that governments and states create laws that mandate its use and enable its development so that increased demand will result in increased production and financial support for this field. In order to compete with decreasing petroleum prices, tax exemptions in the biodiesel synthesis process may be useful.
Acknowledgments
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government (MOTIE) (20210310100020, production of advanced biofuel from lignocellulosic biomass by a combination of fast pyrolysis and supercritical ethanol upgrading). This work was also supported by the National Research Foundation of Korea (NRF-2021R1A2C3011274) and the Ministry of Environment's waste resource energy recycling professional training project (YL-WE-22-001).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).