Abstract
Artificial photosynthesis, converting solar energy to renewable fuels and valuable chemicals, shows a high potential for addressing the exhaustion of fossil fuels and the greenhouse effect. The superior optoelectronic properties of metal halide perovskites (MHPs) make this emerging family of materials promising candidates for efficient solar-to-fuel conversion. However, the issue of stability has been the main obstacle for MHPs based photocatalysis. In this work, we emphasize the major bottleneck that hinders the application of MHPs for photocatalytic solar-to-fuel conversion. After outlining the unstable factors for MHPs based photocatalysis, we analyse recent works in related fields and provide a critical review of approaches to improving the stability of MHPs for the photocatalytic H2 evolution reaction and CO2 reduction reaction. We conclude by proposing possible directions for the development of stabilizing MHPs towards efficient and cost-effective solar-to-fuel conversion.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Artificial photosynthesis, mimicking nature's process to convert solar energy into storable and portable fuels, presents a promising solution to the global energy and environmental crisis [1]. Particularly, H2 production from the photocatalytic water splitting reaction provides renewable H2 resources and the photocatalytic CO2 reduction may enable a closed carbon cycle with zero emissions [2]. Those applications only apply if a high solar conversion efficiency is achieved by using stable and low-cost photocatalysts. Since the pioneering work of photoelectrochemical water splitting on TiO2 in 1972, intensive effort has been devoted to finding new materials and improving efficiency, but the solar conversion efficiency is far from the requirement (e.g. the solar to hydrogen (STH) conversion efficiency, of at least 10%) for practical applications [3, 4]. To achieve high solar conversion efficiency, a photocatalyst should possess excellent optoelectronic properties, including intensive and broad light absorption, efficient charge separation and transfer, proper redox capability for the aimed reactions, and a long operating lifetime. However, most photocatalysts, such as TiO2, BiVO4, CdS, Ta3N5, and g-C3N4, cannot satisfy all of those requirements [3]. Various strategies have been developed to overcome the shortcomings of these photocatalysts, yet progress is slow, which motivates the photocatalysis community to explore new photocatalysts with desirable properties.
A new family of metal halide perovskites (MHPs) with superior optoelectronic properties, including a high absorption coefficient, high charge carrier mobility, and long charge carrier diffusion length, appears as a promising candidate for photoelectronic applications [5, 6]. For instance, the power-conversion-efficiency (PCE) of MHPs based solar cells has experienced a leap from 3.81% (2009) to 25.7% (2021) over the past decade [7, 8]. Such an exciting advance also promotes the development of MHPs based photocatalysts (MHPPs), aiming at low-cost and high-efficiency solar-to-fuel conversion. Significant progress has been achieved following the first report of photocatalytic H2 production using CH3NH3PbI3 (MAPbI3) in 2016 [9, 10]. Typically, the STH conversion efficiency of MHPPs has reached 1.09% [11]. To deliver their promise as a high-efficiency and low-cost photocatalyst, a prerequisite is long-term stability in the operational environment. Therefore, the intrinsic and photocatalysis-induced instability of MHPP is the major challenge facing the photocatalysis community [12]. Improving the stability of MHPPs is of great importance and urgency, while relevant studies are rare.
In this progress report, we survey the existing literature and highlight the most pressing stability problems facing MHPPs. This review begins with a brief introduction about the unique properties of MHPs and the advantages of MHPPs for photocatalytic applications. Next, the major stability concerns for MHPPs are analysed based on a general photocatalytic environment, including structure, water, oxygen, heat, and light. Then we provide a critical discussion of the proposed strategies for improving the stability of MHPPs. A brief perspective on the future development of stable MHPPs, such as the stability test, the understanding of deactivation mechanisms, and the design of new photocatalytic systems is also presented. This timely review will guide the design of stable MHPPs and pave the way toward economic and efficient solar-to-fuel conversion.
2. Structure and properties of MHPPs
The family of MHP semiconductors share a common formula of ABX3, which takes the crystal structure of calcium titanate (CaTiO3). Typically, the A site of MHPs can be different cations (organic/inorganic), the B position is occupied by a metal cation, and X is a halide anion (figure 1(a)) [13]. The ionic crystal and semiconducting features endow MHPs with unique photoelectronic properties. Taking MAPbX3 as an example, the bandgap can be easily varied by adjusting the type or molar ratio of halides on the X site (figure 1(b)) [14]. Meanwhile, Pb-derived p–p electronic transitions from the valence band (VB) to the conduction band (CB) of MAPbI3, together with symmetric structure and direct bandgap, result in a high optical absorption coefficient (105 cm−1) [15]. The defect-tolerance nature of MHPs leads to a long carrier diffusion length, reaching over 100 µm for a single crystal [16]. Furthermore, MAPbI3 presents ambipolar carrier mobility with similar effective mass for electrons and holes, and photo-excited charge carriers migrate like free carriers due to the low exciton binding energy [17, 18]. These outstanding photoelectronic properties make MHPs potential candidates for photocatalytic applications, of which the advantages will be discussed along with the photocatalytic process.
Figure 1. (a) Typical crystal structure of perovskites. (b) Bandgap alignment of MHP together with redox potentials of some reactions. Reprinted with permission from [12]. Copyright (2020) American Chemical Society. (c) Schematic illustration of the basic three-step photocatalytic process on semiconductor-based photocatalysts. [19] John Wiley & Sons. Wiley-VCH Verlag GmbH&Co KGaA, Weinheim.
Download figure:
Standard image High-resolution imageThe photocatalytic process can be divided into three basic steps based on the band-structure theory, namely (a) light absorption, (b) charge separation and transfer, and (c) redox reactions (figure 1(c)) [19]. Under light illumination, only photons with energy larger than the bandgap of semiconductors can excite electrons from the VB to the CB. In other words, the narrower the bandgap, the greater percentage of the sunlight spectrum can be converted to charge carriers. For instance, the theoretical PCE limit of perovskite with a bandgap of 1.6 eV is 30.14% [20]. The readily adjustable bandgap and high optical absorption coefficient of MHPs promise a superior efficiency of MHPPs. The photo-generated excitons then separate into electrons and holes and migrate to active sites, during which charge recombination occurs in a timescale of 10−15s – 10−6s [21]. The low exciton binding energy, high carrier mobility, long charge diffusion length, and suppressed charge recombination of MHPs benefit the population of charge carriers reaching active sites. Finally, only surviving charge carriers at active sites can participate in redox reactions. The CB maximums of MHPs are negative enough to trigger the water/CO2 reduction reactions (figure 1(b)). The slow reaction rate (10−6 s – 10−3 s) limits the utilization of charge carriers, while defects in MHPs may act as active sites to lower the energy barrier and accelerate redox reactions [21, 22]. Therefore, the outstanding electronic and optical properties of MHPs not only deliver high PCE for photovoltaics but also demonstrate a high potential for application in photocatalytic reactions, because photoactive materials used in both photovoltaics and photocatalysis share some similar mechanisms of light absorption, and charge separation and migration.
3. Instability constraints of MHPPs
The excellent optoelectronic properties of MHPPs make this family promising candidates for solar-to-fuel conversion. However, the instability of MHPPs under a photocatalytic environment is a major bottleneck that limits their applications. The decomposing mechanisms of MHPs based solar cells have been deeply investigated and understood, which have been summarized in some recent reviews [23–25]. Nevertheless, the operating environment of photocatalysis is different from that of photovoltaic applications. An analysis of how the photocatalytic environment challenges the stability of MHPPs is critical. Here we set a generic photocatalytic condition, for which the photocatalytic reaction is conducted in the gas/liquid phase under air pressure, at room temperature (no additional heating) with sunlight irradiation [26]. Thus, the unstable constraints facing MHPPs mainly include water, light, oxygen (O2), and light-induced heat, in addition to the structural instability of MHPs.
3.1. Structure instability
There is no doubt that MHPPs must maintain their photoactive structure during the synthetic and photocatalytic processes. The structural stability of ABX3 structured MHPs can be predicted according to the Goldschmidt tolerance factor (equation (1)), t:

where RA, RB, and RX represent the radiuses of the A cation, B cation, and X anion, respectively [27]. Theoretically, perovskite structures can be formed with t between 0.8 and 1, of which ideal cubic or tilted octahedra perovskite structures are favoured. Furthermore, the effective radius is introduced to calculate the tolerance factor of a multi-component perovskite (Ax A'1−x )B(Xy X'1−y )3, as shown in equations (2) and (3) [6]. RA(eff) and RX(eff) can be used to calculate the tolerance factor t based on equation (1):


3.2. Water instability
The hydrolysis of MAPbI3 is demonstrated by equations (4)–(7) [25]. The key point is the hydrogen bond connecting organic and inorganic units in the MHPs, which is heavily influenced by high polar solvents, especially water. Combined with the hydrophilic nature of MAPbI3, water can easily adsorb and penetrate the crystal structure, resulting in degradation [28]. Therefore, all-inorganic perovskites, by replacing organic cations with Cs+, show potential for photocatalytic gas-phase/non-aqueous reactions, due to improved moisture tolerance [29, 30]. As for photocatalytic water splitting and CO2 reduction reactions, water is a generally used reactant, so the gas-phase reaction may be more suitable for MHPPs compared to the liquid one.
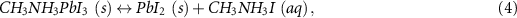



3.3. Illumination instability
The stability of materials under prolonged light illumination is the prerequisite for photocatalytic applications, while MHPPs are unfortunately fragile. The impact of light shows in two aspects. One is the ion distribution and segregation, owing to the light-induced built-in electric field [23]. Such an effect may lead to phase segregation and decomposition with prolonged light exposure. The other is the photochemical decomposition of metal halides under light irradiation [31, 32]. Taking PbI2 as an example, photo-excited electrons reduce Pb2+ to Pb0 and holes oxidize I− to I2. Furthermore, the time-scale gap between fast photo-response (10−15 s – 10−6 s) and slow redox reactions (10−6 s – 10−3 s) results in the accumulation of photo-generated charge carriers, which will accelerate the decomposition of MHPPs [33]. Thus, the efficient extraction of charge carriers from MHPPs is essential to suppress photochemical instability [34].
3.4. Oxygen instability
The oxidation of MHPs is mainly the photooxidation in the presence of oxygen and light because MHPs have been demonstrated to be stable in dry air under dark conditions [35]. Oxygen molecules adsorbed onto the surface trap photo-excited electrons from MHPs and form a highly reactive superoxide O2 −, which in turn decomposes MHPs [36]. Here defects, especially iodide vacancies, play an important role. Iodide vacancies with a similar volume to oxygen molecules, favour oxygen adsorption/diffusion and act as active sites for the photooxidation reaction [37, 38]. To restrain oxygen-induced photooxidation of MHPs, it is critical to remove O2 from the photocatalytic system and lower the density of iodide vacancies in MHPs.
3.5. Thermal instability
Temperature has a great impact on the crystal phase and structure of MHP, even though studies of most MHPs films using thermogravimetric analysis have demonstrated no mass loss at a temperature lower than 65 °C [23]. The phase transition from tetragonal to cubic was reported to occur for MAPbI3 at 54 °C – 57 °C [39]. More importantly, heat can speed up the degradation of MHPs caused by other factors. For instance, the combination of water and heat leads to the rapid decomposition of MAPbI3 [35]. To avoid the thermally caused decomposition of MHPs, the use of a cooling system is important to keep the temperature of the photocatalytic system at a proper level.
Notably, these unstable factors are not isolated but interact with each other. The impact of one factor on the stability of MHPPs may be aggravated or alleviated by another factor [23]. For instance, a recent understanding of the photooxidation of MAPbI3 perovskite demonstrates an intricate relationship among those factors [40]. A dry photooxidation pathway without water and a water-accelerated photooxidation process have been identified. It is found that water (1% relative humidity) can even double the decomposition rate of MAPbI3 at 25 °C compared to a water-free environment. However, the promoting impact of water is weakened at increased temperature, due to the variation of activation energies for those two pathways. Such complex effects further enhance the difficulty of stabilizing MHPPs for photocatalytic applications.
4. Recent progress in improving the stability of MHPPs
Due to the fragile nature of MHPs, various strategies have been developed to improve the stability of MHPPs under operational environments, including the engineering of the composition, structure, surface, and reaction environment. All inorganic MHPs have shown better stability compared to organic counterparts, due to the easy attack upon organics by water [29]. Moreover, two-dimensional (2D) MHPs have demonstrated enhanced moisture stability, which is attributed to the structure-induced unique physiochemical properties, such as bond lengths, surface terminations, and charge transfer distance [41–44]. Therefore, the construction of 2D or mixed 2D/3D all-inorganic MHPPs may further enhance their stability for photocatalytic applications. Nevertheless, a delicate design of appropriate strategies is required for specific photocatalytic applications, owing to the different reaction mechanisms and operational environments. Here the measured stability of MHPPs for the photocatalytic H2 evolution and photocatalytic CO2 reduction reaction (RR) are summarized and analysed. Furthermore, typical examples are presented to demonstrate some effective approaches, such as MHP-saturated halogen-acid solutions, surface passivation, improved intrinsic stability, low-polarity solvents, hydrophobic interaction, and encapsulation. Interestingly, the improved stability has mostly been accompanied by a corresponding enhancement of the conversion efficiency. This may be explained by the efficient extraction of photo-excited charge carriers from MHPPs, which has been demonstrated to improve photostability [34]. It is worth noting that the stability of MHPPs has been improved significantly, while the underlying mechanisms have not been fully understood.
4.1. Photocatalytic H2 production
Since the first application of MHPPs in photocatalytic H2 evolution, tremendous effort has been devoted to the development of MHPPs for highly efficient and stable solar to H2 conversion [9, 10, 45]. The composition of MHPPs and corresponding photocatalytic reaction conditions are summarized in table 1. The photocatalytic H2 evolution performance of most MHPPs is evaluated under visible light (λ > 420 nm), possibly due to the instability of MHPs under UV light irradiation [23]. Considering the relatively low percentage (<5%) of UV light in the sunlight spectrum, the removal of UV light should not decrease the solar conversion efficiency significantly [46]. The temperature of reaction solutions ranges from 5 °C to 40 °C, which is usually controlled via cooling systems. However, the impact of temperature on the stability of MHPPs is important, yet has received negligible attention. Most photocatalytic H2 evolution measurements with MHPs are conducted in an inert-gas-filled system without the involvement or evolution of O2. Thus, the O2 induced deterioration of MHPs is not a problem here. Noticeably, few reports present the photocatalytic overall water splitting from water in liquid/gas phases, of which the stability is attributed to the unique composition of the MHPs [47, 48]. Therefore, water, light and photochemical stability are the major concerns facing MHPPs for solar to H2 conversion. A few strategies have been developed to improve the stability of MHPPs, of which the details will be discussed below.
Table 1. Summary of the photocatalytic H2 evolution activity from MHPPs.
| Photocatalyst | Reaction environment | Light source | Temp. (°C) | Measured stability (h) | References |
|---|---|---|---|---|---|
| MAPbI3 | HI-H3PO2 aqueous solution | >475 nm | 25 | 160 | [10] |
| MA3Bi2I9 | HI-H3PO2 aqueous solution | >400 nm | 15 | 70 | [49] |
| Cs2AgBiBr6 | HBr-H3PO2 aqueous solution | >420 nm | 5 | 80 | [50] |
| Pt/DA3BiI6 | HI-DAI-H3PO2 aqueous solution | White LED lamp | 6 | 16 | [51] |
| CoP/MAPbI3 | HI-H3PO2 aqueous solution | >420 nm | — | 15 | [52] |
| MoS2/MAPbI3 | HI-H3PO2 aqueous solution | >420 nm | 25 | 90 | [53] |
| Pt/CsPbBr3−x Ix | HI-H3PO2 aqueous solution | >420 nm | 15 | 50 | [44] |
| Cs2AgBiBr6/N-C | HBr-H3PO2 aqueous solution | >420 nm | — | 24 | [54] |
| MA3Bi2I9/DMA3BiI6 | HI-H3PO2 aqueous solution | >420 nm | 15 | 100 | [55] |
| Pt/Ta2O5/MAPbBr3 | HBr-H3PO2 aqueous solution | >420 nm | 25 | 9 | [56] |
| Cs2AgBiBr6/RGO | HBr-H3PO2 aqueous solution | >420 nm | 5 | 120 | [57] |
| Pt/TiO2/MAPbI3 | HI-H3PO2 aqueous solution | >420 nm | 25 | 12 | [58] |
| Pt/MAPbBr3−x Ix | HI/HBr-H3PO2 aqueous solution | >420 nm | 15 | 30 | [59] |
| MoS2/Cs2AgBiBr6 | HBr-H3PO2 aqueous solution | >420 nm | — | 500 | [60] |
| MoS2/MAPbI3 | HI-H3PO2 aqueous solution | >420 nm | 25 | 200 | [11] |
| Ni3C/MAPbI3 | HI-H3PO2 aqueous solution | >420 nm | 25 | 200 | [61] |
| Pt/Cs2SnI6 | HI-H3PO2 aqueous solution | >420 nm | 25 | 24 | [22] |
| Pt/TiO2/EtbtBi2I10 | HI-H3PO2 aqueous solution | Full spectrum | — | 20 | [62] |
| MA+/MAPbI3 | HI-H3PO2 aqueous solution | Full spectrum | — | 4 | [63] |
| Mo3S13 2−/K-MAPbBr3 | HBr aqueous solution | >420 nm | 5 | 200 | [64] |
| Pt/g-C3N4/Cs3Bi2Br9 | TEOA aqueous solution | 300–800 nm | — | 7 | [65] |
| Pt/g-C3N4/DMASnBr3 | TEOA aqueous solution | 300–800 nm | — | 7 | [66] |
| DMASnI3 | Pure water | Full spectrum | 10 | 20 | [47] |
| MA2CuCl2Br2 | Water vapour | Full spectrum | 40 | 50 | [48] |
MA = CH3NH3 +; DA = (CH3)2NH2 +; DMA = CH3NH2CH3 +; N–C = nitrogen doped carbon; RGO = reduced graphene oxide; TEOA = Triethanolamine.
4.1.1. MHP-saturated halogen-acid solutions
In 2016, Nam and co-workers firstly proposed a dynamic equilibrium of MAPbI3 in HI aqueous solution, realising photocatalytic H2 production from HI splitting with MAPbI3 [10]. The working mechanism of dynamic equilibrium is that the dissolution and reprecipitation of MAPbI3 in the saturated solution occur at the same rate (figure 2(a)). Here the concentration of I− and H+ is essential to achieve dynamic equilibrium. A relatively high I− concentration (–log[I−] < −0.4) is necessary to guarantee the dissolution of PbI3 − in aqueous solutions, while the acidity of aqueous solutions should be maintained at a certain level (pH < −0.5) to suppress the formation of hydrates. The bandgap alignment of MAPbI3 is capable of hydrogen reduction and iodine oxidation (figure 2(b) and equations (8) and (9)). Here the timely reduction of I3 − to I− is necessary to maintain the long-term stability of this process. The decreased concentration of I− will affect the dynamic equilibrium of the saturated MAPbI3 solution. Moreover, the dark brown colour of I3 − will impede the light absorption of MAPbI3, leading to a decreased H2 generation rate. A reduction agent H3PO2 is usually added to reduce I3 − to I− and stable H2 evolution activity is achieved for up to 160 h (figure 2(c)).


Figure 2. (a) Schematic illustration of the dynamic equilibrium of the MAPbI3 powder in saturated HI aqueous solution. (b) Bandgap alignment of the photocatalytic HI splitting reaction. (c) Long-term H2 evolution activity of the photocatalytic HI splitting reaction on MAPbI3. Reproduced from [10], with permission from Springer Nature. (d) Cycling photocatalytic H2 evolution activity of the 20% MoS2/Cs2AgBiBr6 (CABB) photocatalyst under visible-light illumination. Reprinted from [60], Copyright (2022), with permission from Elsevier.
Download figure:
Standard image High-resolution imageInspired by this pioneering work, the potential of MHPPs with various components has been explored for photocatalytic halogen-acid splitting and significant progress has been achieved [9, 67]. Particularly, 500 h long cycling stability is achieved in a similar system with MoS2/CABB (Cs2AgBiBr6) as the photocatalyst (figure 2(d)) [60]. The long-term photocatalytic stability of MoS2/CABB is attributed to the intrinsic stability of all-inorganic perovskite (Cs2AgBiBr6) and the efficient consumption of photo-generated charge carriers with proper co-catalysts (MoS2). The stable photocatalytic halogen acid splitting with MHPs demonstrates the potential of MHPPs for efficient and cost-effective H2 production.
4.1.2. Surface passivation
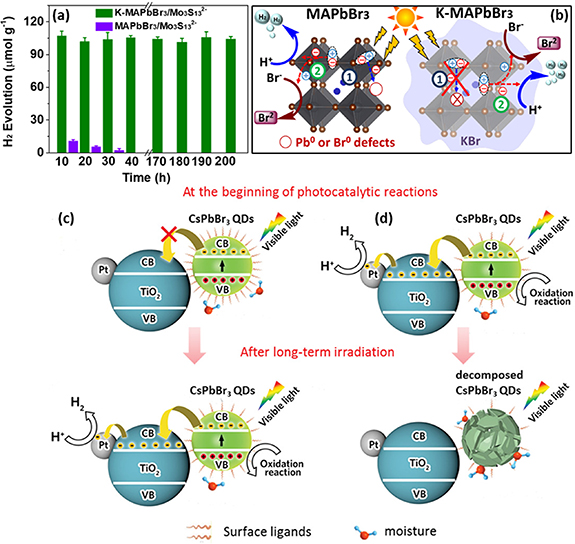
To further improve the stability of MHPPs in halogen-acid solutions, surface passivation, a widely used strategy for materials engineering, has been explored [68]. A light-and-solution treatment is proposed to improve the moisture stability of MAPbI3 via surface modification [63]. Saturated MAPbI3 HI solution is dropped on MAPbI3 power, followed by Xe lamp illumination. The photocatalytic reaction between I2 and H2O results in the in-situ formation of a hydrophobic MA+ layer around the MAPbI3 surface, effectively protecting MAPbI3 crystals from moisture. The MA+ passivated MAPbI3 shows a 2.24 times H2 evolution rate compared to that of the original MAPbI3, but the long-term stability is not evaluated. Meanwhile, the post-treatment of MAPbBr3 with potassium ions (K+) leads to the generation of a KBr layer on the surface of MAPbBr3 together with K+ dopants in the crystal structure [64]. After loading with a proper co-catalyst (Mo3S13 2−), the K-MAPbBr3/ Mo3S13 2− photocatalyst exhibits stable H2 evolution for up to 200 h without any reduction agents (figure 3(a)). The long-term stability is attributed to the suppression of Pb0 and Br0 defects in MAPbBr3 crystal by K dopants together with the KBr surface layer (figure 3(b)). Here the low temperature (5 °C) may also benefit the stability of the K-MAPbBr3/Mo3S13 2− photocatalyst. Recently, some alkaline earth metal dopants (Mg, Ca, Sr, Ba) have been predicted to suppress the oxygen-induced deterioration of MHPs [69]. It is worth noting that the stability of MHPs in halogen-acid solutions is based on the dynamic equilibrium between MHPs crystals and the MHP-saturated solution. The stability of such surface passivating layers and how they influence the dynamic equilibrium of MHPs crystals in halogen-acid solutions needs to be understood thoroughly.
Figure 3. (a) The recycling test of the photocatalytic H2 evolution with different catalysts. (b) The proposed mechanism of the H2 evolution activity on MAPbBr3 and K-MAPbBr3. [64] John Wiley & Sons. © 2020 The Authors. EcoMat published by John Wiley & Sons Australia Ltd, on behalf of The Hong Kong Polytechnic University. (c) Mechanism illustration of the surface ligand engineering on CsPbBr3 QDs/Pt-TiO2 complex with more and (d) less ligand density on the surface of CsPbBr3 QDs. [70] John Wiley & Sons. Wiley-VCH Verlag GmbH&Co KGaA, Weinheim.
Download figure:
Standard image High-resolution imageApart from the external passivating layers, MHPs quantum dots (QDs) possess internal surface passivation, namely surface ligands [71]. Surface ligands derived from the synthesis of MHP QDs are usually hydrophobic and insulative, which can protect MHP QDs from moisture, but hinder charge transfer between MHP QDs and other components. To address this problem, rational control of surface ligands on CsPbBr3 QDs was reported to achieve a good balance between charge transfer and stability (figures 3(c) and (d)) [70]. Notably, CsPbBr3 QDs experience a partial loss of surface ligands during the photocatalytic process, which should be taken into consideration for ligands control. When combined with platinum decorated TiO2 (Pt-TiO2), the well-controlled CsPbBr3/Pt-TiO2 photocatalyst presents continuous H2 evolution under visible light in water-and-methanol vapour for up to 160 h. This strategy demonstrates the possibility of stabilizing MHPs in polar solvent vapour for gas-phase photocatalytic reactions via delicate surface engineering.
4.1.3. Improved intrinsic stability
The high acidity of halogen acid limits the combination of MHPs with other photocatalysts/co-catalysts because most metal oxides/hydroxides suffer from poor stability in acid solutions [72]. Thus, the desire for intrinsically stable MHPs in pure water continues to motivate the exploration of materials. Encouragingly, DMASnI3 (DMA = CH3NH2CH3 +) was reported to be stable in water with reversible bandgap narrowing, during which no phase transformation is detected (figures 4(a) and (b)) [47]. The outstanding water stability makes DMASnI3 a possible candidate for photocatalytic overall water splitting. A constant H2 evolution rate is detected among four cycles for DMASnI3 when dispersed in pure water under light irradiation (figure 4(c)). However, DMASnI3 partially decomposed into SnI4 during this process, due to photochemical oxidation. MA1−x DMAx SnBr3 and DMASnBr3, with similar compositions, also present excellent water stability [66, 73]. The DMASnBr3/g-C3N4 complex demonstrates stable photocatalytic H2 evolution activity with TEOA as the electron donor and Pt as the co-catalyst for the H2 evolution reaction. Other water-stable MHPs include Cs3Bi2Br9, (3-ethylbenzo [d] thiazol-3-ium)4Bi2I10 (EtbtBi2I10), and MA2CuCl2Br2 [48, 62]. When applied to vapour-phase photocatalytic water splitting, both H2 and O2 are detected for the MA2CuCl2Br2 photocatalyst with stability for up to 50 h. Here the O2 evolution on MA2CuCl2Br2 without any co-catalysts is unexpected because self-photooxidation rather than O2 evolution usually occurs on most metal halides based photocatalysts, even though the VB edge is more positive than the oxidation potential of water [74]. The active site on MA2CuCl2Br2 for the O2 evolution reaction should be further investigated.
Figure 4. (a) Photos of the reversible transformation of DMASnI3 at 80 °C in air. (b) XRD patterns of DMASnI3 powder exposed in DI water for 16 h. (c) The recycling test of the photocatalytic H2 evolution with DMASnI3 as the catalyst. [47] John Wiley & Sons. Wiley-VCH Verlag GmbH&Co KGaA, Weinheim.
Download figure:
Standard image High-resolution image4.2. Photocatalytic CO2 reduction reaction
Compared to the H2 evolution reaction, the CO2 reduction reaction (CO2 RR) is a bit more complicated, due to the multi-step and multi-electron involved procedure [75]. The dissolution of CO2 is challenging in a liquid-phase reaction, while the adsorption and activation of CO2 by photocatalysts are challenging in a gas-phase reaction. Meanwhile, water is usually added as a proton source for CO2 hydrogenation. Various approaches have been developed to address those challenges [9]. As shown in table 2, inorganic MHPs with improved stability are the most popular candidates adopted for the photocatalytic CO2 RR, due to the absence of volatile and hygroscopic organic cations [76]. Organic solvents with low polarity instead of halogen-acid solutions are used for the photocatalytic CO2 RR, possibly due to the low solubility of CO2 in aqueous solutions with high acidity. Water droplets as a reactant are added to those organic solvents and water vapour is usually introduced into the gas-phase reaction system. The operating temperature can be easily controlled for the liquid phase reaction using a cooling system, while it is difficult for the gas phase reaction to manage the temperature fluctuation during long-term measurements. Light-induced heat together with moisture has been demonstrated to accelerate the degradation of MHPs [23]. How to solve this problem is of great importance to maintaining the long-term stability of MHPPs for the photocatalytic CO2 RR.
Table 2. Summary of the photocatalytic CO2 reduction reaction from MHPPs.
| Photocatalyst | Test environment | Light source | Temp. (°C) | Measured stability (h) | References |
|---|---|---|---|---|---|
| Cs2AgBiBr6 | Ethyl acetate | Full spectrum | — | 6 | [77] |
| Cs2AgBiBr6 | Ethyl acetate | 405 nm | — | 6 | [78] |
| CsPb(Brx /Cl1−x )3 | Ethyl acetate | Full spectrum | 20 | 9 | [79] |
| CsPbBr3 | Ethyl acetate | >380 nm | — | 30 | [80] |
| CsPbBr3/GO | Ethyl acetate | Full spectrum | — | 12 | [81] |
| Ag/Cs2AgInCl6 | Ethyl acetate | Full spectrum | — | 9 | [82] |
| Ti3C2/CsPbBr3 | Ethyl acetate | Full spectrum | 25 | 9 | [83] |
| CsPbBr3 | Ethyl acetate/water | Full spectrum | — | 8 | [84] |
| BP/CsPbBr3 | Ethyl acetate/water | Full spectrum | — | 30 | [85] |
| [Ni(terpy)2]2+/CsPbBr3 | Ethyl acetate/water | >400 nm | 25 | 16 | [86] |
| C60/CsPbBr3 | Acetonitrile/water | >420 nm | — | 16 | [87] |
| RGO/CsPbBr3 | Ethyl acetate/water | >420 nm | — | 60 | [88] |
| CsPbBr3/g-C3N4 | Acetonitrile/water | >420 nm | 20 | 6 | [89] |
| CsPbBr3/Bi2WO6 | Ethyl acetate/water | >400 nm | 25 | 8 | [90] |
| CsPbBr3/UiO-66(NH2) | Ethyl acetate/water | >420 nm | 5 | 12 | [91] |
| Core/shell CsPbBr3/GDY-Co | Acetonitrile/water | >400 nm | — | 36 | [92] |
| Core/shell CsPbBr3/TiO2 | Ethyl acetate/water | Full spectrum | — | 30 | [93] |
| Cs2AgBiBr6/g-C3N4 | Ethyl acetate/methanol | Full spectrum | — | 12 | [94] |
| CsPbBr3-BF4/Co | Ethyl acetate/isopropyl alcohol | >400 nm | 25 | 8 | [95] |
| Re(CO)3Br(dcbpy)/CsPbBr3 | Toluene/isopropyl alcohol | >420 nm | — | 15 | [96] |
| Cs3Sb2I9 | CO2, water vapour | >420 nm | — | 9 | [97] |
| CsPbBr3 | CO2, water vapour | >400 nm | 25 | 30 | [42] |
| CsAgCl2 | CO2, water vapour | Full spectrum | — | 5 | [98] |
| Cs2AgBiI6 | CO2, water vapour | >420 nm | — | 3 | [99] |
| Cs3Bi2(BrI)9 | CO2, water vapour | >420 nm | — | 10 | [100] |
| CsPbBr3−x Acx | CO2, water vapour | >400 nm | — | 6 | [101] |
| Ni:CsPbCl3 | CO2, water vapour | Full spectrum | — | 6 | [102] |
| Fe:CsPbBr3 | CO2, water vapour | Full spectrum | — | 3 | [103] |
| FAPbBr3/Ti3C2 | CO2, water vapour | Full spectrum | — | 3 | [104] |
| Cu/RGO/Cs2AgBiBr6 | CO2, water vapour | Full spectrum | — | 12 | [105] |
| Cs3Bi2I9/BiWO6 | CO2, water vapour | >400 nm | — | 18 | [106] |
| g-C3N4/CsPbBrx Cl3−x | CO2, water vapour | >420 nm | — | 24 | [107] |
| Core/shell CsPbBr3/ZIF | CO2, water vapour | Full spectrum | — | 18 | [108] |
| Cu/RGO/CsPbBr3 | CO2, water vapour | Full spectrum | — | 12 | [109] |
| CsPbBrI2/melamine foam | CO2, water vapour | Full spectrum | — | 42 | [110] |
| CsPbBr3/melamine foam | CO2, water vapour | Full spectrum | — | 104 | [111] |
| α-Fe2O3/amine-RGO/CsPbBr3 | CO2, water vapour | >420 nm | 25 | 40 | [112] |
4.2.1. Low-polarity solvents
The structure of most MHPs is easily decomposed when exposed to high-polarity solvents (e.g. H2O), thus solvents with low polarity and high solubility of CO2 are widely selected for the photocatalytic CO2 RR in the liquid phase. The polarity and CO2 solubility of generally used solvents have been summarized in table 3. The stability of inorganic MHPs in various solvents has been investigated by taking CsPbBr3 as an example [80]. CsPbBr3 decomposes immediately in methanol, but the colour and crystal structure of CsPbBr3 remain unchanged after storing in toluene, ethyl acetate (EAC), isopropyl alcohol (IPA) and acetonitrile (ACN) for 50 d, demonstrating the stability of CsPbBr3 in low-polarity solvents (figures 5(a) and (b)). Then, the performance of CsPbBr3 as the photocatalyst for the CO2 RR was evaluated in different solvents. Interestingly, the solvent not only influences the productivity but also the product selectivity of the photocatalytic CO2 RR (figure 5(c)). Only CO is detected in toluene and ACN, while CO, CH4 and H2 are generated in EAC. This phenomenon may be attributed to the proton concentration in the solvent and the absence of protons suppresses proton-involved reaction pathways, such as the formation of H2 and CH4 [80, 113]. Moreover, CsPbBr3 showed the highest photo-conversion-efficiency and cycled stability (5 cycles, 30 h) in EAC (figure 5(d)). These results indicate that polarity and CO2 solubility of solvents should be considered when selecting proper solvents for the CO2 RR with MHPPs.
Figure 5. (a) XRD patterns of CsPbBr3 after the CO2 photoreduction in various solvents for 6 h. (b) Photos of CsPbBr3 powders in various solvents with saturated CO2. (c) The CO2 photoreduction performance of CsPbBr3 in various solvents. (d) Long-term photocatalytic CO evolution activity of CsPbBr3 in ethyl acetate with CO2 refilled per 6 h. Reproduced from [80] with permission from the Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageTable 3. Summary of the stability of CsPbBr3 in a series of solvents for CO2 photoreduction reaction [80].
| Solvent | Polarity | CO2 solubility (mM) | Stability |
|---|---|---|---|
| Toluene | 2.4 | 97.4 | Yes |
| Ethyl acetate (EAC) | 4.3 | 241.0 | Yes |
| Isopropyl alcohol (IPA) | 4.3 | 86.1 | Yes |
| Acetonitrile (ACN) | 6.2 | 270.0 | Yes |
| Methanol (MeOH) | 6.6 | 138.6 | No |
4.2.2. Hydrophobic interaction
Even though all-inorganic MHPs exhibit improved moisture tolerance, the durability of MHPPs when exposed to a high-humidity environment is a question. Thus, the hydrophobic interaction is utilized to further protect the MHPPs from water [109, 112]. The stability of Cs2AgBiBr6 has been evaluated and no crystal structure changes were detected under relatively high humidity (55%), long-term light irradiation and heat treatment (figure 6(a)) [77]. When applied to the photocatalytic CO2 reduction reaction, the performance of the third cycle is much lower compared with the first cycle (figure 6(b)) [105]. However, after combining with copper-loaded reduced graphene oxide (Cu-RGO), not only the reaction rate but also the cycling stability are improved significantly (figure 6(c)). It is found that Cs2AgBiBr6 keeps stable with a relative humidity of 60% – 70% for two days and then decomposes to Cs3Bi2Br9, Cs(OH)2, and AgBr. However, no phase change is detected for the Cu-RGO/Cs2AgBiBr6 composite exposed under the same environment after 8 d. The improved stability of the Cu-RGO/Cs2AgBiBr6 composite is attributed to the hydrophobic feature of the RGO, which can effectively protect Cs2AgBiBr6 from moisture. Controlling the humidity to a low level may be helpful to stabilize MHPs for photocatalytic applications.
Figure 6. (a) XRD patterns of Cs2AgBiBr6 after different treatments. [77] John Wiley & Sons. Wiley-VCH Verlag GmbH&Co KGaA, Weinheim John Wiley and Sons. (b), (c) Recycling performance of the CO2 photoreduction reaction on Cs2AgBiBr6 and Cu-RGO/Cs2AgBiBr6, respectively. Reproduced from [105] CC BY 3.0. (d) Schematic illustration of the CsPbBr3 decorated on melamine foam (MF). (e) Contact angles of CsPbBr3 anchored MF (MF/CsPbBr3) and pristine MF. (f) Long-time CO and CH4 evolution from CO2 photoreduction reaction on MF/CsPbBr3. [111] John Wiley & Sons. Wiley-VCH Verlag GmbH&Co KGaA, Weinheim.
Download figure:
Standard image High-resolution imageA similar mechanism is proposed for the melamine foam (MF) supported MHPs [110, 111]. The contact angles of water for MF/CsPbBr3 and MF are 108.3° and 0.0° respectively, demonstrating the hydrophobic character of MF/CsPbBr3 (figures 6(d) and (e)). Organic solvent dimethyl sulfoxide (DMSO) is used for the growth of CsPbBr3 particles on the framework of MF. It is found that the DMSO-induced rough surface of MF enhances the surface hydrophobicity of MF. MF/CsPbBr3 presents long-term stability, with product yields not showing an obvious decrease after continuous reaction for 104 h (figure 6(f)), which is attributed to enhanced water resistance.
4.2.3. Encapsulation
UV light, heat, and moisture are inevitable elements in the photocatalytic H2 evolution reaction and CO2 RR. Thus, the encapsulation of MHPPs is an easy solution to think of, which is also widely used on MHPs for other applications [6]. Polymers, metal oxides and metal-organic frameworks have been covered on the surface of MHPs to form core/shell structures and protect MHPs during the photocatalytic process [92, 93, 108]. Encouragingly, excellent stability has been achieved for the photocatalytic CO2 RR (figure 7). Electron-transfer-layer and hole-transfer-layer materials used in solar cells are possible candidates for shell layers [6]. However, unlike the charge-transfer dominant solar cells, photocatalytic activities with redox reactions are complicated. Here some suggestions are proposed when screening possible materials to encapsulate MHPs for photocatalytic applications: (a) the material must be stable during the photocatalytic process; (b) the protecting layer should guarantee the efficient charge transfer between MHPs and reactants; (c) the material should not impede the photocatalytic reaction, for example, by shielding light, the adsorption of reactants and the desorption of products; (d) proper methods should be deliberately designed to make a uniform surface cover of MHPs without destroying the MHPs. Very recently, an in situ photocatalyzed synthesis is proposed to grow polymer brushes on the surface of CsPbBr3 nanocrystals [114]. CsPbBr3 nanocrystals not only act as the photocatalyst but also provide tethered initiators for the growth of polymers, resulting in a CsPbBr3/polymer core/shell structure. These polymer brushes stabilize CsPbBr3 nanocrystals in acetone/ethanol/water and UV irradiation conditions for up to 7 d. However, the charge conductivity of the polymer is critical for photocatalytic applications, yet is not identified in this work.
Figure 7. (a) Diagram for the synthesis of core/shell CsPbBr3@GDY and the corresponding redox reactions. (b) Long-term stability test. Reprinted with permission from [92]. Copyright (2020) American Chemical Society. (c) Illustration of the core/shell CsPbBr3/TiO2 structure and corresponding reaction mechanism. (d) The recycled CO2 photoreduction measurement of CsPbBr3 NCs/a-TiO2(20) with CO2 refilled per 3 h. [93] John Wiley & Sons. Wiley-VCH Verlag GmbH&Co KGaA, Weinheim. (e) Diagram for the preparation of core/shell CsPbBr3/ZIFs and the corresponding CO2 photoreduction process. (f) Recycling measurement of CsPbBr3/ZIF-67 with CO2 refilled per 3 h. Reprinted with permission from [108]. Copyright (2018) American Chemical Society.
Download figure:
Standard image High-resolution image5. Conclusion and outlooks
MHPs present high potential as photocatalysts for efficient, economic, and renewable solar-to-fuel conversion, thanks to their outstanding photoelectronic properties. The ionic crystal structure of MHPs is a double-edged sword though, which endows unique optoelectronic properties but brings a fragile nature to the materials. Some emerging strategies, such as compositional engineering, surface passivation, and encapsulation, indicate the possibility of stabilizing MHPs for photocatalytic applications, yet there is a long way to go before real applications commence. It is worth noting that the operational stability of MHPPs is equally important as that of the solar conversion efficiency in new photocatalyst development. Some suggestions for further designing stable and high-performing MHPPs are described below.
The long-term operating stability of photocatalysts plays a key role in determining the cost of solar fuels [6]. Therefore, it is important to improve the stability of MHPPs, in addition to the efficiency enhancement. However, it is remarkable that the stability tests slow down the process. For example, 1000 h (12 h d−1, ∼1/4 year) testing is challenging for the photocatalysis community, while this time window is too short for a potential real practice, e.g. 1 year operation. Fortunately, tremendous effort has been devoted to revealing the accelerated degradation mechanisms, thus improving the stability of MHPs based photovoltaic devices [6, 23]. The experience of solar cells can be taken as a reference even though they have different working mechanisms and operating environments. For instance, accelerating lifetime tests of MHPs based solar cells should be recommended for photocatalytic applications, which is crucial to getting rapid feedback and understanding the deactivation mechanisms.
Since the operating environment varies with different photocatalytic reactions, it will probably not be possible to set standards for stability tests. However, a guideline should be developed for the characterization of catalysts after testing. Currently, x-ray diffraction (XRD) patterns, scanning electron microscopy (SEM) images and light absorption spectra are the major indicators of a catalyst's stability [12]. Notably, there are no observable changes in a photocatalyst's product output rate and XRD patterns, SEM images, and bandgap absorption spectra do not mean that there are no photocatalysis-induced changes at all. Photovoltaic studies report significant changes in MHPs under illumination, such as surface reconstruction, ion migration, and halide segregation, which cannot be easily detected by XRD and SEM [23]. Comprehensive characterizations should be conducted to identify structural changes in MHPPs after long-term testing. X-ray photoelectron spectroscopy, and element analysis should be used to investigate the chemical states, molar ratios, and distribution of all elements. Interestingly, many recent reports demonstrate that in-situ formed defects or reconstruction of catalysts during the catalytic process benefit the catalytic activity [115–117]. Therefore, we should go beyond the stereotype that photocatalytic-induced changes are adverse, which might be beneficial and deserve thorough understanding. Furthermore, the construction of MHPs thin films on substrates and the photoelectrochemical characterization may provide a better understanding of the deactivation mechanisms.
The understanding of the deactivation mechanisms of MHPPs not only contributes to improving the stability of existing photocatalysts but also guides the development of new photocatalysts and photocatalytic systems. Organic cations on the A site have been demonstrated to be responsible for the water instability, thus compositional engineering should be a promising strategy to improve the intrinsic stability of MHPPs. Meanwhile, water is one of the major unstable factors for MHPs but might not be an indispensable element for some photocatalytic applications. For example, the replacement of H2O with H2 as the proton source for the photocatalytic CO2 hydrogenation should relieve the moisture instability of MHPPs.
The outstanding optoelectronic properties of MHPs promise a superior efficiency for solar fuel production, while stability is the major bottleneck facing photocatalytic applications. The development of stable MHPPs is still in its infancy stage, and persistent effort is essential to make such an advanced technology emerge earlier.
Acknowledgments
The authors would like to acknowledge the support from the Australian Research Council via the Australian Laureate Fellowships, Discovery Project, and Discovery Early Career Research Award.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).