Abstract
The development of efficient electrochemical energy storage devices is crucial for future renewable energy management. Aqueous rechargeable batteries (ARBs) are considered to be one of the most sustainable battery technologies due to their low cost, ease of manufacture, high safety and environmental friendliness. However, some tough issues, such as the narrow electrochemical stability window of water, chemical instability of electrode materials, uncontrollable dendrite growth and poor cycling lifespan, severely limit the development of high-energy aqueous batteries with stability and infallible safety. This article mainly summarizes current and future challenges and the advanced science and technology to meet these challenges of various ARBs, such as aqueous Li/Na/K/Mg/Ca/Al/-ion batteries, aqueous flow batteries and photo-responsive batteries. In addition, the potential direction and prospect of the further development of these system batteries are discussed. Finally, given the various technologies and their associated technical challenges, we are motivated to develop a 2022 roadmap on aqueous batteries.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Daxiong Wu1,2, Xiu Li1 and Jianmin Ma1
1 School of Materials and Energy, University of Electronic Science and Technology, Chengdu 611731, People's Republic of China
2 School of Physics and Electronics, Hunan University, Changsha 410082, People's Republic of China
In recent years, the global economy has developed rapidly, and the demand of society for energy is also increasing. In addition, traditional fossil energy is rapidly being exhausted, and its use has a huge impact on the global ecological balance [1]. Therefore, many countries have begun to develop some clean and renewable energy resources with minimal or no harm to the environment, such as wind energy, solar energy, geothermal energy, tidal energy, etc [2]. However, most renewable energy is intermittent and volatile, and an effective large-scale energy storage/conversion system is required to smoothly integrate energy with the grid. Rechargeable ion batteries, with many advantages, such as high round-trip efficiency, environmentally friendly operation, adjustable power and energy output, and simple maintenance have been considered to be the most competitive high-energy storage system [3].
Currently, commercial lithium-ion batteries (LIBs) occupy a dominant position in the field of energy storage due to their high energy density, good cycle stability and high energy efficiency, which bring great convenience to our lives [4]. However, traditional organic electrolytes are highly toxic and inflammable and have great potential safety hazards. Compared with organic electrolytes, aqueous electrolyte has the advantages of low cost, easy manufacturing, high ionic conductivity and safety [5]. Therefore, aqueous rechargeable batteries (ARBs) have become a hot topic in scientific research and industrial development in recent years. To date, ARBs have been mainly based on different metal ions, including alkali metal ions (Li+, Na+ and K+) and multivalent metal ions (Zn2+, Mg2+, Ca2+ and Al3+, etc) [6]. However, for practical applications of ARBs, the energy density and lifetime still need to be further improved. Their energy density is mainly limited by the narrow electrochemical stability window (ESW) (⩽1.23 V) of the aqueous electrolyte, beyond which H2O will decompose into H2 and O2 [3]. In addition, the choice of electrode materials in aqueous electrolyte is also severely limited.
The roadmap summarizes the current state of various kinds of ARBs including aqueous Li/Na/K/Mg/Ca/Al/-ion batteries, aqueous flow batteries and photo-responsive batteries. Each section of this paper focuses on the current and future challenges faced by ARBs, as well as the use of advanced scientific knowledge to address these challenges and improve the electrochemical performance of batteries. We have invited leading researchers of various ARBs to weigh in on these issues and provide their views of and prospects for these fields. Finally, we hope this roadmap can provide a timely perspective on the design principles and roadmap for the practical applications of next-generation reliable ARBs.
2. Aqueous lithium-ion batteries
Xiaoyu Liu and Jin Yi
Institute for Sustainable Energy & Department of Chemistry, Shanghai University, 99 Shangda Road, Shanghai 200444, People's Republic of China
Status
To overcome the drawbacks of organic electrolytes, such as poor ion conductivity, safety hazards and environmental concerns, aqueous lithium-ion batteries (ALIBs) are employed as promising alternative power sources due to their safety advantage, lower cost and good power performance. The first ALIB assembled with a LiMn2O4 cathode, a VO2 anode and an electrolyte of 5M LiNO3 solution was reported in 1994. The average operating voltage of ALIBs is 1.5 V with a higher energy density (∼55 Wh kg−1) than Pb-acid batteries (∼30 Wh kg−1). Since 1994, a great deal of research work has been devoted to the development of various electrode materials. However, different to the electrode materials in organic electrolyte systems, the redox potential of electrode materials in aqueous systems should be within or near the electrolysis potential of water, and electrodes beyond this range cannot work normally. In recent years, great achievements have been made in improving the performance of LIB batteries through strategies such as surface coating, structure and composite material and electrolyte optimization, etc, but they are still limited by their low voltage, low energy density and short cycle life [7–11].
Current and future challenges
The employment of electrode materials in ALIBs is limited by the narrow electrochemical window dependent on the hydrogen/oxygen evolution potentials [12, 13]. Theoretically, a lithium-reservoir cathode with a higher potential and a lithium-accommodating anode with a lower potential can be coupled within the hydrogen/oxygen evolution potential range to assemble a high-voltage ALIB. Figure 1(a) illustrates the dependence of the hydrogen/oxygen evolution potential on the pH value of the aqueous solution, and several electrode candidates possibly suitable for ALIBs. In general, electrode materials with an operating potential within 3–4 V versus Li/Li+ (such as LiCoO2, LiMn2O4 and LiFePO4) can be used as cathode materials in ALIBs. Therefore, electrode materials with an operationing potential within 2–3 V versus Li/Li+ seem capable as anode candidates free of hydrogen evolution [4, 14]. However, the following reaction (R1) could take place in ALIBs due to the existence of O2:
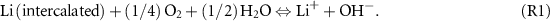
The equilibrium voltage of the above reaction can be calculated as follows (equation (1)):

Figure 1. (a) Several electrode candidates possibly suitable in ALIBs. (b) Typical self-discharge curves of LiTi2(PO4)3 at pH 13 with/without O2. (c) Typical charge/discharge curves of ALIBs based on LiTi2(PO4)3 and LiFePO4 in 1 M Li2SO4 with pH 13 and the absence of O2. (d) Cycling life of ALIBs based on LiTi2(PO4)3 and LiFePO4 at various pH and with/without O2 [18]. (a)–(d) Reprinted with permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature Chemistry [20], Copyright (2010).
Download figure:
Standard image High-resolution imageAccording to equation (1), the equilibrium voltage reaches 3.44 V even at pH = 14 and the value increases gradually with the pH declining. As mentioned above, the operation potential of the anode materials for ALIBs is usually below 3.0 V versus Li/Li+. Therefore, the lithium-intercalated anode can be oxidized via reaction (R1) when the ALIBs are operated in air, hindering the reversible electrochemical redox behavior. Therefore, it is of great importance to eliminate oxygen in the ALIB system [15].
In respect of the cathodic reaction in ALIBs, the proton intercalation mechanism is a complicated issue. There is consensus that protons in the aqueous electrolyte may enter the cathode material along with the lithium ions, and the proton intercalation behavior is highly related to the crystal structure of the cathode. In general, layered cathode materials, such as LiCoO2 and LiNi1/3Mn1/3Co1/3O2, are prone to H+ insertion at deep delithiated state. Nevertheless, proton intercalation is scarcely observed in olivine-structured LiFePO4 and spinel-structured LiMn2O4 materials. On the other hand, the pH value has an impact on the proton behavior, and the proton intercalation potential can be controlled by regulating the pH value of the aqueous electrolyte. By these means, stable lithium intercalation can be achieved in a basic electrolyte for layered cathode materials (LiCoO2: pH > 11 and LiNi1/3Mn1/3Co1/3O2: pH > 11). The structural stability of electrode materials in aqueous solution should also be taken into account. The decomposition of LiFePO4 occurs under strong alkaline conditions, which can be alleviated by surface carbon modification. In addition, vanadium oxides (LiV2O5, LiV3O8,VO2) are soluble in aqueous electrolytes, especially at nano-scale size with large surface area. Therefore, reasonable structural design as well as surface coating strategies are necessary to enhance the structural stability of electrode materials in ALIB systems.
Side reaction is another challenge for ALIBs. Based on the hydrogen/oxygen evolution potential, the ESW of an aqueous electrolyte is thermodynamically confined as 1.23 V, and further enlarged to 2.0 V due to kinetic effects. The expanded voltage window is conductive to delivering higher reversible capacities due to larger utilization of the electrode materials. However, the hydrogen/oxygen evolution reaction still occurs at deep charging state on the negative/positive electrodes, especially with a large overpotential. As a result, the side reaction leads to the variation of pH values near the electrode, which is detrimental to the structural stability of electrode materials [16, 17]. Notably, the side reaction of electrolyte decomposition in organic-based lithium-ion batteries can generate a protective interlayer on the electrode surface, hindering the successive side reaction process. In contrast, the side reaction of electrolyte decomposition in ALIBs only generates gas products, and the absence of a protective interlayer leads to repeated electrolyte decomposition upon cycling. Therefore, the operating voltage window of ALIBs should be carefully modulated by various strategies such as employing the electrolyte additive to suppress the side reaction [2].
Advances in science and technology to meet challenges
Xia's group reported the typical self-discharge process of LiTi2(PO4)3 anode in an aqueous electrolyte (figure 1(b)), in which the active material in the deep-lithiated state is chemically oxidized by O2 and H2O according to reaction (R1). After modulating the pH value of the electrolyte and eliminating the oxygen, the ALIB assembled with a LiTi2(PO4)3 anode and a LiFePO4 cathode in a Li2SO4 electrolyte exhibits reversible electrochemical redox behavior (figure 1(c)). Impressively, this kind of ALIB shows stable cycling performance with an ultra-high capacity retention rate of over 90% during 1000 cycles at 6 C, and 85% capacity is still retained under quite a low current of 0.125 C for 50 cycles (figure 1(d)), demonstrating its great potential as a high-safety, low-cost and long-life power source [18].
Suo et al [19] reported a kind of 'water-in-salt' electrolyte with ultra-high concentration of LiTFSI (>20 M). Due to the formation of a dense interphase on the anode and the reduced activity of water, the ESW of this electrolyte has been expanded to about 3 V (figure 2(a)). Different to the single interaction between Li+ and water oxygen in dilute solutions, the Li+ solvation sheath in the 'water-in-salt' electrolyte involves the intimate interaction between Li+ and TFSI− anions, leading to the formation of a protective LiF layer on the anode stemming from the TFSI− reduction (figure 2(b)). Therefore, the full ALIB assembled with a LiMn2O4 cathode and a Mo6S8 anode in the electrolyte of 21 M LiTFSI exhibits impressive cycling stability with capacity retention of 68% after 1000 cycles at 4.5 C and of 78% after 100 cycles at 0.15 C, superior to the performance of other full AZIBs (figure 2(c)). Design concepts combine electrolyte optimization and in situ construction of functional interphase provides guidance to meet the challenges in aqueous batteries. An anode–electrolyte interface is produced by the reduction of TFSI−, which is composed of LiF. Fortunately, both water and TFSI− can be reduced by the solid-electrolyte interface (SEI). Consequently, the LiFePO4//Mo6S8 battery engineered with the 'water-in-salt' electrolyte can operate in a temperature range from −20 °C to 55 °C, showing high coulombic and rate ability [20]. Moreover, TFSI− anion ions can remain steady in aqueous solution, delivering a wide ESW [21]. It also contributes to stabilization of the electrolyte for high electrochemical battery performance. In addition to the LiTFSI, LiNO3 is also developed to construct a 'water-in-salt' electrolyte, which can enlarge the ESW to 2.55 V [22]. It is impressive that the polymer-like chain of (Li+(H2O)2)n is produced by the self-assembly into linear aggregation between H2O and Li+. The hydrogen bonding is replaced by the interaction between Li+ and O of H2O, presenting a kinetic contribution during the desolvation process. A 'water-in-bi salt' electrolyte has also been developed by Yamada et al to construct an ALIB with energy density >130 Wh kg−1 [23]. TFSI− and  (BETI) possess a large volume to reduce H2O concentration in the electrolyte, which can interact with Li+ to form the solvation structure. Thus, a wide ESW of 3.8 V is exhibited and the LiCoO2//Li4Ti5O12 as well as LiNi0.5Mn1.5O4//Li4Ti5O12 batteries show high voltage (∼2.3−3.1 V).
(BETI) possess a large volume to reduce H2O concentration in the electrolyte, which can interact with Li+ to form the solvation structure. Thus, a wide ESW of 3.8 V is exhibited and the LiCoO2//Li4Ti5O12 as well as LiNi0.5Mn1.5O4//Li4Ti5O12 batteries show high voltage (∼2.3−3.1 V).
Figure 2. (a) Electrochemical stability window of LiTFSI–H2O electrolytes. (b) Effect of LiTFSI concentration on ion–ion and ion-solvent interactions together with SEI formation in aqueous electrolyte. (c) Electrochemical performance of active electrodes and various aqueous Li-ion batteries. (a)–(c) From [21]. Reprinted with permission from AAAS.
Download figure:
Standard image High-resolution imageConcluding remarks and prospects
In the pursuit of stable electrode materials with high energy efficiency and long cycle life, cheap electrolyte solutions with expanded electrochemical windows, and feasible battery assembly technologies, ALIBs will show growing potential as a promising energy storage system with low cost, high safety and appropriate energy densities. In the 'water-in-salt' or 'water-in-bi salt' electrolyte, the ESW can be enlarged, resulting from the inhibition of water decomposition. Moreover, the solvation structure of Li+ is improved, which can deliver the kinetic contribution to promote ion transport during the desolvation process. In particular, in the LiTFSI super-concentration aqueous electrolyte, a SEI of LiF is produced through the reduction in TFSI−, which can further protect the electrode and electrolyte. However, effort is also being devoted to developing a new ALIB system for practical applications.
Acknowledgment
The authors acknowledge support from the National Natural Science Foundation of China (Grant Nos. 21805182 and 22075171).
3. Aqueous sodium-ion batteries
Próspero Acevedo-Peña1 and Edilso Reguera2
1 CONACYT-Instituto Politécnico Nacional, CICATA Legaria, 11500 Mexico City, Mexico
2 Instituto Politécnico Nacional, CICATA Legaria, 11500 Mexico City, Mexico
Status
Aqueous energy storage devices possess some attractive advantages compared to their organic counterparts, such as security, low cost, not requiring a controlled atmosphere, environmental friendliness, etc. They have all fuelled active research in the the pursuit of progress of ARBs. Although, the most commonly employed rechargeable batteries are lithium-ion batteries, the considerably larger natural abundance and uniform geographic distribution of sodium compared to lithium, makes sodium an alternative to assembling large-scale stationary devices attractive for incorporation into the electric grid. Water is the most universal and environmentally friendly solvent in an electrolyte. This makes aqueous sodium-ion batteries (ASIBs) an attractive option for energy storage. In recent years, research in aqueous ASIBs for large-scale energy storage systems has rapidly developed (figure 3(a)). However, as with ALIBs, their narrower thermodynamic voltage window and lack of suitable electrode materials hinder their development. Figure 3(b) displays the ESW and redox potentials of anode/cathode materials in aqueous electrolytes for ASIBs with different pH values [24]. It is worth noting that the H2 evolution potential and O2 evolution potential have a strong dependence on pH values. In order to avoid water decomposition, the choice of electrode material is critical [4]. Therefore, over the past several decades, the focus has been on cathode/anode material development to improve the performance of ASIBs, including Mn-based oxides, polyanionic compounds, Prussian blue analogs (PBAs) and a few inorganic materials. In addition, a lot of research work has also been devoted to electrolyte design [25]. Although the cyclic stability of ASIBs has improved, their output voltage and energy density are insufficient for practical applications due to the limited capacity of electrode materials and narrower thermodynamic voltage window of aqueous electrolyte.
Figure 3. Number of researches for ASIBs/APIBs. Data were collected from Scopus in April 2022 (a). Stable electrochemical window in aqueous electrolyte with different pH values and redox potential for electrode materials for ASIBs (b). Reprinted from [27], Copyright (2020), with permission from Elsevier.
Download figure:
Standard image High-resolution imageCurrent and future challenges
Figure 4 shows some of the materials reported as electrodes for ASIBs as well as the ESW of different electrolytes. The main drawback of aqueous batteries is the narrow ESW of water, which is 1.23 V. However, ESW is normally larger due to kinetic issues. Developing strategies to widen the ESW is vital to assemble ASIBs with a higher specific energy [26]. The ESW has been enhanced through several approaches such as replacing the conventional stainless-steel current collector with other materials according to their low electrocatalytic activity towards oxygen evolution reaction, e.g. Ti or the hydrogen evolution reaction (HER), e.g. Al. These metals rapidly form a compact passive layer that considerably hinders the kinetics of the charge transfer reaction. However, the most attractive alternative to enhance the ESW of water is through developing water in salt electrolytes (WiSEs), which can enhance the ESWby up to 3.0 V [27]. Another advantage of the WiSE is the lower oxygen solubility, which is responsible for the side reaction that causes the self-discharge of the electrodes to their decomposition. The main issue with WiSE is the cost of the salts and the stability of WiSE at low temperatures. Consequently, WiSEs are currently being formulated using low-cost salts such as perchlorates and acetates. The stability at low temperatures has also been enhanced with the addition of organic additives to the WiSE. Mixing different salts has also been shown to be an alternative in order to reduce the cost of the electrolyte and maintain a wide ESW.
Figure 4. Comparison of the ESW in the presence of different salts and the potential of the redox processes of materials reported for ASIBs.
Download figure:
Standard image High-resolution imageAnother big challenge that needs to be overcome to assemble devices for real applications, is ensuring electrode material stability during long-period cycling at different current rates. Hexacyanoferrates are the most commonly employed material for positive electrodes. This family of materials offers great versatility due to the fast kinetics of the redox processes and the possibility of easily tuning its electrochemical behavior with the composition [28]. The enhancement of the electrochemical stability of PBA during the galvanostatic charge–discharge (GCD) cycling can be achieved by controlling the degree of crystallinity, and the number and nature of vacancies.
The diversification of materials for negative electrodes is another challenge to increase the performance and expand the portfolio of ASIBs. Titanium phosphate, TiP, the most commonly employed material for the negative electrode, is normally reported to display a rapid capacity decay during cycling due to the interfacial pH increment caused by the parasitic reactions [29, 30]. Several strategies have been employed to improve its stability, such as forming a passive layer of TiO2 or Al2O3 or the passivation of the electrode by forming an SEI layer in the presence of some salts, such as NaOTF, in the electrolyte. However, the ASIBs assembled using this material normally show voltages ranging from 0.6–1.4 V [26]. The hexacyanochromates and polyimides are two attractive families of materials suitable for negative electrodes. The hexacyanochromates are also members of the PBAs. However, the redox potential of these coordination compounds is either outside or very close to the lower limit of the ESW in conventional electrolytes [31]. The use of WiSE has opened up the opportunity to use manganese hexacyanochromate as a negative electrode, reaching a voltage difference larger than 2.0 V when coupled with manganese hexacyanoferrate [32]. Although polyimides are well known as isolators, these polymers might exhibit reversible electrochemical processes that make them suitable candidates for negative materials in ASIBs [33]. The synthetic route of these materials is less laborious than for TiP, and polyimides also show sluggish kinetics for water reduction reaction, which is advantageous for this application. Hexacyanochromates are normally obtained by co-precipitation at room temperature. The redox processes in these two families of materials can be easily tuned through the composition. The electrochemical response of polyimides can be altered by changing the aromaticity of the imide structure and with the electron-withdrawing groups attached to the aromatic imide system [34]. In the case of hexacyanochromates, the pi-back bonding promoted by the CN bridges allows one to alter the redox processes by changing the polarizing power of the external transition metal employed to precipitate the solid [31]. There is a huge opportunity to develop new ASIBs with engineered characteristics through exploring these families of materials.
The Ragone plot in figure 1 shows that not only the specific energy of the ASIBs needs to be improved, but also the specific power of these devices. Extensive research in material science is needed to engineer electrode materials to behave as extrinsic insertion pseudo-capacitors, in which the redox processes (with well-defined current peaks) are almost independent of the diffusion of sodium ions in the solid. In this way, the assembled ASIBs will display high rate capability with flattened E versus t curves during the GCD characterization, warranting higher specific energy and power.
Advances in science and technology to meet challenges
The enhancement of the ESW of water will increase the number of materials for negative and positive electrodes, with a larger potential gap between them, ensuring devices with an improved specific energy. This will augment the affordability of ASIBs for large-scale applications. The establishment of strategies for in silico design of electrode materials is the most attractive route for proposing new materials. Guo et al proposed some design rules of material for positive electrodes for ASIBs, and they used them to screen several materials [35]. The workflow involves the evaluation of synthesizability, electrochemical stability, aqueous stability and the Na migration barrier. A similar strategy appears suitable for the in silico design of new materials for positive electrodes, which are the ones that currently have the least number of candidates.
Strategies are still needed to minimize the current collector corrosion to offer ASIBs high stability and long lifespan. This is a relevant issue for real applications considering the higher corrosivity of aqueous electrolytes compared to their organic counterparts.
Concluding remarks and prospects
In summary, ASIBs are currently an engaging research topic with a huge number of opportunities, but the practical application of these devices depends very much on overcoming the challenges associated with the improvement of their performance and the preservation of low costs to certainly ensure the permeation of ASIBs for energy storage in a diverse spectrum of technologies. There are currently several approaches with promising results. However, it is also important to (a) assemble devices with high voltage and capacity but with large energy efficiency, (b) evaluate the long-term cycling stability of the device at different currents, (c) test the chemical stability of all the components of the battery and (d) ensure the performance of the device at temperatures that might be experienced in operation.
Acknowledgment
The authors gratefully acknowledge the financial support from CONACYT Projects A1-S-9877 and LN-315800.
4. Aqueous potassium-ion batteries
Daxiong Wu1,2 and Jianmin Ma1
1 School of Materials and Energy, University of Electronic Science and Technology, Chengdu 611731, People's Republic of China
2 School of Physics and Electronics, Hunan University, Changsha 410082, People's Republic of China
Status
With the rapid development of global society and the exhaustion of traditional fossil energy, renewable energy and technology are constantly being updated, and the demand for large-scale energy storage systems is more imminent [36]. Undoubtedly, the electrochemical storage of rechargeable alkaline metal batteries is one of the most effective methods. Based on the consideration of safety, environmental protection, cost and stability, alkali metal-ion intercalation ARBs are a promising technology for grid-scale energy storage and intermittent power supply. ALIBs have been widely studied, but they generally exhibit substandard cycling stability, and the natural abundance of lithium is relatively low, so the manufacturing cost is high. Although the natural abundance of sodium is high, the water-system sodium-ion battery has the problem of low capacity. Potassium (K) has an overall advantage due to its much higher natural abundance than lithium and has a lower standard redox potential (−2.93 V) than its sodium (Na) counterpart (−2.71 V on a standard hydrogen electrode) [37, 38]. In addition, the Stokes radius of solvated K ions is the smallest compared to that of lithium ions and sodium ions. Meanwhile, in a water system alkaline metal battery, K-ion-based electrolyte shows higher ion transport characteristics, which results in the K-ion intercalated electrode displaying ultra-high magnification performance [39]. Therefore, aqueous potassium-ion batteries (APIBs) are a potentially feasible technology. However, the larger ionic radius of K+ (0.138 nm) than Li+ and Na+ limits the selectivity of electrode materials, which probably causes structural distortions and instabilities of electrode materials during the repeated charging/discharging process. In addition, to ensure the stability of the aqueous electrolyte, the insertion/extraction potentials of the electrode materials are unable to go beyond the range of H2 evolution potential and O2 evolution potential. Therefore, the development of desertion/insertion-type electrode materials is hindered. Thus, there is still a long way to go in seeking electrode materials for APIBs. To date, only a small selection of cathode materials have been proposed for APIBs, including PBAs, oxides, organic materials and V-based oxides (figure 5) [40], which can meet the electrochemical window of aqueous electrolytes. However, the anodes of APIBs are very limited to mainly organic compounds, such as poly(anthraquinonyl sulfide) and 3,4,9,10-perylenetetracarboxylic diimide anodes. Fortunately, full APIBs can achieve a high energy density of 80 Wh kg−1, better than most ASIBs and some commercialized energy storage technologies (lead-acid battery and Ni–Fe battery, table 1), but there is still a very large gap between commercial LIBs and ALIBs [25].
Figure 5. Stable electrochemical window in aqueous electrolyte with different pH values and redox potential of electrode materials for APIBs. Reproduced from [26] with permission from The Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageTable 1. Comparison of ALIBs, ASIBs, APIBs and other commercialized electrochemical battery types.
| Battery type | Systems | Electrolyte | Average voltage | Energy density (Wh kg−1) | Status |
|---|---|---|---|---|---|
| Li-ion battery | C//LiFePO4 | 1 M LiPF6 | 3.3 | 145 | Commercialized |
| Lead-acid battery | 5 M H2SO4 | 2.0 | 35 | Commercialized | |
| Ni–Fe battery | 2 M KOH | 1.2 | 40 | Commercialized | |
| Ni–MH battery | 6 M KOH | 1.35 | 100 | Commercialized | |
| ALIBs | Cn//LiBr, LiCl [41] | 21 M LiTFSI + 7 M LiOTf | 4.2 | 460 | Bench scale |
| LiMn2O4//Li4Ti5O12 [42] | 2.55 | 120 | Bench scale | ||
| LiMn2O4//Li4Ti5O12 [43] | 42 M LiTFSI + 21 M Me3EtN ‧ TFSI | 2.55 | 145 | Bench scale | |
| LiNi0.5Mn1.5O4//Li4Ti5O12 [44] | 3.2 | 165 | Bench scale | ||
| LiMn2O4//Li4Ti5O12 [45] | 2.4 | >160 | Bench scale | ||
| ASIBs | Na3MnTi(PO4)3//Na3MnTi(PO4)3 [46] | 1 M Na2SO4 | 1.4 | 40 | Bench scale |
| NaTi2(PO4)3//Na2CuFe(CN)6 [47] | 1 M Na2SO4 | 1.4 | 48 | Bench scale | |
| NaMnHCF//NaTiOPO4 [48] | 9 mol kg−1 NaOTF + 22 mol kg−1TEAOTF | 1.74 | 71 | Bench scale | |
| NaMnHCF//KMnHCC [49] | 17 M NaClO4 | 1.7 | 58 | Bench scale | |
| NaCoHCF//NTP [50] | 1 M Na2SO4 | 1.33 | 67 | Bench scale | |
| APIBs | KFeMnHCF//PTCDI [37] | 22 M KCF3SO3 | 1.2 | 80 | Bench scale |
| K-FeHCF//KTP/C [51] | 21 M KCF3SO3 | 1.5 | 47.3 | Bench scale | |
| KFHCF//β-PTCDA [52] | 30 M KFSI | 0.9 | 41 | Bench scale | |
| PNTCDA//FeHCF [53] | Saturated KNO3 and K2SO4 solution | 0.72 | 46.9 | Bench scale |
Current and future challenges
However, the aqueous K-ion battery still suffers from serious challenges. For example, the large ionic radius of K ions severely restricts the insertion/extraction in the charging and discharging process, resulting in large deformation and irreversible pulverization of the electrode material during the cycle [54]. In addition, conventional aqueous electrolytes have a very narrow ESW (1.23 V). Beyond this voltage window, water splitting occurs, leading to hydrogen evolution and HERs. This greatly limits the maximum output voltage of the aqueous K-ion battery, resulting in substantially lower energy density and an unnecessary restriction on the choice of electrode materials [55]. Therefore, the lack of suitable electrode materials and electrolytes with wide ESW is the biggest obstacle to the current and future development of the aqueous K-ion battery.
Advances in science and technology to meet challenges
At present, the electrode materials of the aqueous K-ion battery mainly include layered transition metal oxides [56, 57], Prussian blue and its analogs [36, 37, 54, 58]. The large ionic radius of potassium ions leads to the distortion and instability of the structure, which can be resolved by selecting layered electrode materials with large layer spacing. In addition, electrode materials with stable structures that can buffer the insertion and extraction of K ions have been explored. Charles and his colleagues reported structural water-engaged potassium-intercalated disordered vanadium oxide nanosheets. Benefiting from the synergistic effects of both structural water and highly disordered nature, the electrode achieved superior high capacity with 183 mAh g−1 in half cells at 5 mVs−1 and excellent capacity retention (100%) after 5000 cycles in full cells (figures 6(a) and (b)) [56]. Wang et al synthesized an open-framework structured K2FeII [FeII(CN)6] ‧ 2H2O nanocube with good structural integrity, fast kinetics and high K-ion content together with the two accessible one-electron redox processes rendering high capacity and outstanding cycle life. In recent years, there has also beenmuch research work to deal with the issue of narrow ESW for aqueous K-ion batteries, such as water-in-salt electrolytes, water-in-bisalt electrolytes, hydrate-melt electrolytes, DMF/H2O hybrid electrolytes and polymer gel electrolytes [55, 59, 60]. Water-in-salt electrolytes are obtained by minimizing the number of free water molecules and hence suppressing the reactivity of water. Chen et al proposed a K bis(fluorosulfonyl)imide (KFSI)-based water-in-salt electrolyte with a broader stable electrochemical window of 3.97 V without hydrogen/oxygen evolution (figure 6(c)) [52]. The assembled aqueous K-ion full battery in this electrolyte shows great rate performance with 92% of its original capacity even at a high rate 25C and long lifespan with a high capacity retention of 89% after 1000 cycles at 12.5C (figure 6(d)) [52]. Hu et al proposed an Fe-substituted Mn-rich Prussian blue cathode, an organic anode and a 22 M KCF3SO3 water-in-salt electrolyte. Due to the mitigation of phase transitions by Fe substitution, the cathode obtained a high capacity retention (70%) at 100C over 10 000 cycles. The battery obtained a high energy density of 80 Wh kg−1 and overlength cycling stability over 2000 cycles at 20C. Furthermore, the assembled 11 mAh pouch cells can operate well at rates of 0.1–20C in a wide temperature range (−20 °C–60 °C) [37].
Figure 6. Local structure of vanadium oxide nanosheets obtained from the refinement of x-ray and neutron PDFs (a) and the average gravimetric electrode capacity through the 5000 cycles (b). (a), (b) Reproduced from [58]. CC BY 4.0. Linear voltammetry curves recorded at 10 Mv s−1 in various electrolytes (c) and the cycling performance of a full battery (d). (c), (d) Reproduced from [54] with permission from The Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageConcluding remarks and prospects
The aqueous K-ion battery is one of the most promising large-scale energy storage devices. In recent years, although aqueous K-ion batteries have displayed significant achievements, more effort is still required to achieve further progress before practical application. The structural stability of electrode materials is the main reason for the rapid capacitance decay. Therefore, it is necessary to explore more effective solutions to expand the potential window and inhibit the parasitic water-splitting reaction and to develop more stable and highly reversible electrode materials. Research on APIBs is still in the exploratory stage, and more effort is needed to achieve practical applications. For the next stage of the development of APIBs, combined with more accurate and high-end in situ characterization techniques and conclusive theoretical calculations/simulations, we will further understand the electrochemical reaction mechanism of APIBs. In addition, it is also very meaningful to explore new electrode materials or optimize the electrolyte through additives to expand the output voltage and energy density of APIBs. In conclusion, optimization of factors, such as reaction mechanism, electrode materials, electrolytes, electrochemical window of water, oxygen evolution/hydrogen evolution and possible corrosion of current collectors, is still the focus in the next stage of APIB research.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Grant No. 11675051).
5. Aqueous Mg-ion batteries
Kai Zhu
Key Laboratory of Superlight Materials and Surface Technology, Ministry of Education, College of Materials Science and Chemical Engineering, Harbin Engineering University, Harbin 150001, People's Republic of China
Status
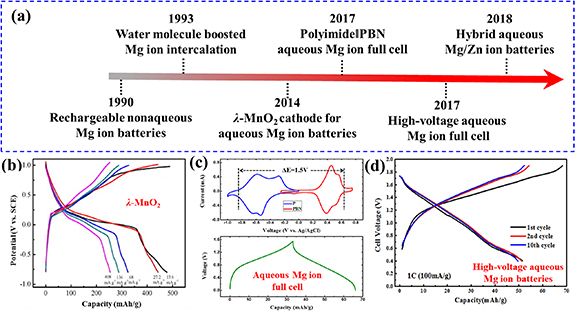
Rechargeable Mg-ion batteries as multivalent metal-ion batteries display unique advantages, such as high theoretical volumetric capacity (Mg: 3833 mAh cm−3 versus Li: 2046 mAh cm−3), low reduction voltage (−2.37 V versus Standard Hydrogen Electrode), high safety and abundant Mg resources. The breakthrough and development of aqueous Mg-ion batteries are summarized in figure 7(a). Since the first model of rechargeable Mg||Mgx CoOy batteries proposed in 1990 [61], tremendous efforts such as electrolyte design and cathode material exploration, have been put into the development of non-aqueous Mg-ion batteries. Unfortunately, the electrochemical performance of non-aqueous Mg-ion batteries is still hindered by unsatisfactory de-solvation at electrolyte–electrode interfaces and the oxidation layer on the Mg anode. Although Novak and Desilvestro presented that the water molecules could facilitate the Mg2+ insertion process in 1993 [62], rechargeable Mg-ion batteries with aqueous electrolytes received less attention due to corrosion and dissolution of the Mg anode. Recently, Yuan et al first reported that the λ-MnO2 presents typical Mg2+ storage behavior in the aqueous electrolyte using a three-electrode system (figure 7(b)) [63]. Later on, various Mn-based materials, such as Birnessite MnO2 [64] and tetragonal spinel MgMn2O4 [65], were also developed as capable cathode materials for Mg-ion batteries. Meanwhile, the development of anode materials, such as carbon-based polyimides [66] and V-based oxides [67], promises high-capacity Mg-ion full cells. Therefore, rechargeable aqueous Mg-ion batteries have attracted a lot of attention. Many typical aqueous Mg-ion full cells have been designed and fabricated, such as polyimide||Na1.4Ni1.3Fe(CN)6 ‧ 5H2O with 1 M MgSO4 electrolyte (energy density of 45 Wh kg−1) [68], PPMDA||Li3V2(PO4)3 with 4 M Mg(TFSI)2 electrolyte (power density of 6.4 Kw kg−1) [69] and FeVO4||Mg-OMS-1 (magnesium octahedral molecular sieves) with 1 M MgSO4 electrolyte (energy density of 70 Wh kg−1) [70] (figures 7(c) and (d)). In addition, more novel Mg-based aqueous energy storage systems are proposed, for example, the Li/Na/Zn–Mg hybrid batteries and Mg-ion battery capacitor.
Figure 7. (a) Timeline of developments of aqueous Mg-ion batteries. (b) Charge–discharge profiles of λ-MnO2 cathode. Reprinted from [65], Copyright (2014), with permission from Elsevier. (c) Cyclic voltammetry curves of polyimide and Na1.4Ni1.3Fe(CN)6 ‧ 5H2O (upper) and charge–discharge profiles of polyimide||Na1.4Ni1.3Fe(CN)6 ‧ 5H2O full cell (down). Reprinted with permission from [70]. Copyright (2017) American Chemical Society. (d) Charge–discharge profiles of PPMDA||Li3V2(PO4)3. Reprinted with permission from [71]. Copyright (2017) American Chemical Society. https://pubs.acs.org/doi/10.1021/acscentsci.7b00361. Further permission related to the material excerpted should be directed to the ACS.
Download figure:
Standard image High-resolution imageCurrent and future challenges
The development of aqueous Mg-ion batteries is still in its infancy. The primary challenge is the lack of capable Mg2+-host materials that can provide good structural stability and high capacity during repeated ion storage. On the cathode side, although spinel and layered Mn-based oxides show good Mg-ion storage behavior, they still suffer from poor conductivity, sluggish Mg-ion diffusion and potential Mn dissolution. Meanwhile, different types of cathode materials are expected to promote the cycling performance and rate ability of aqueous Mg-ion batteries with high energy density. Regarding the anode, it is still a challenge to develop a satisfactory Mg-ion host with low redox potential to pair with the cathode, which restricts the design and fabrication of the Mg-ion full cell. Simultaneously, the mechanism of the Mg-ion insertion and extraction process in these electrode materials requires an intensive investigation. In addition, similar to other aqueous metal-ion batteries, aqueous Mg-ion batteries also face issues such as narrow potential windows, electrolyte decomposition, and side reaction.
Advances in science and technology to meet challenges
Understanding the energy storage mechanism in the Mg2+ host in favor of the design of novel electrode materials and fabrication of advanced Mg-ion storage systems. Advanced ex situ and in situ characterization, such as special aberration corrected transmission electron microscopy (TEM), in situ x-ray diffraction and in situ Raman spectroscopy, could provide atomic-level perception and detailed phase evolution information of the active materials during the Mg2+ intercalation and extraction process. Moreover, density functional theory computation can be carried out to investigate the Mg-ion storage properties, and the high-throughput screening method can be applied to predict the potential capable Mg-ion host. In addition, the choice of the electrode materials can be expanded by constructing a Mg-ion storage system beyond the traditional 'rocking-chair' battery, such as metal-ion hybrid batteries and battery capacitors. Soundharrajan et al reported rechargeable aqueous magnesium–zinc hybrid batteries using a MgMn2O4 cathode, Zn anode and ZnSO4 + MgSO4 hybrid electrolyte, which displayed an energy density of 370 Wh kg−1 at a power density of 70 W kg−1 (figure 8(a)) [71]. Meanwhile, in the dual-ion hybrid system, dual-ions, such as Li+/Mg2+ and Na+/Mg2+, could co-intercalate into the capable hosts, such as TiO2-B and Li4Ti5O12, leading to enhanced cycling performance [72, 73]. In addition, Zhang et al designed and assembled a Mg-OMS-2/graphene||activated carbon system, in which a Mg-OMS-2/graphene cathode serves as a (de)intercalation host and activated carbon presents a typical electric double-layer capacitance [74] (figure 8(b)). The capacitor battery showed a remarkable energy density of 46.9 Wh kg−1. These works demonstrate the potential application of existing electrode materials for advanced Mg-ion storage systems.
Figure 8. Illustration of (a) an aqueous Mg–Zn hybrid battery. Reprinted with permission from [73]. Copyright (2018) American Chemical Society. (b) Mg-ion battery capacitor. Reprinted with permission from [76]. Copyright (2017) American Chemical Society. Rate ability of (c) MgMn2O4/rGO composite. Reproduced from [77] with permission from The Royal Society of Chemistry. (d) Birnessite MnO2 (inset: illustration of hydrated Mg2+ insertion). Reprinted with permission from [66]. Copyright (2015) American Chemical Society. (e) MgMn2O4 hollow spheres (inset: TEM image of MgMn2O4). Reproduced from [79] with permission from The Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageThe electrochemical performance of the electrode is extremely affected by the electronic conductivity and ion diffusion ability. To overcome the poor electron conductivity of MgMn2O4, Liu et al introduced a reduced graphene oxide (rGO) conductive framework to construct the MgMn2O4/rGO composite, which promoted the charge transfer and the Mg2+ diffusion. Thus, MgMn2O4/rGO composite exhibited an enhanced capacity of 211 and 140 mAh g−1 at 50 and 1000 mA g−1, respectively (figure 8(c)) [75]. Meanwhile, crystal structure modulation can reduce the electrostatic interaction and enhance the Mg-ion diffusion dynamics. Nam et al demonstrated that the shielding effect of crystal water on the electrostatic interaction between Mg2+ and Birnessite MnO2 hosts, resulting in good cycling performance (62.5% capacity retention after 10 000 cycles at 2 A g−1) and rate performance (figure 8(d)) [64]. Zhang et al optimized the Mn and Fe ratios in the spinel MgFex Mn2−x O4 to improve the Mg-ion diffusion coefficient, leading to an enhanced rate ability [76]. In addition, the design of micro/nanostructure is also an effective method to improve the electrochemical performance of the Mg-ion host. The tetragonal-spinel MgMn2O4 hollow spheres assembled by countless nanocrystals present a discharge capacity of 261 mAh g−1, stable cycling ability and remarkable rate performance (figure 8(e)) [77].
Electrolytes play a crucial role in the electrochemical stability and performance of batteries, especially aqueous batteries. Anion optimization can enhance the Mg-ion (de)intercalation capability of the host materials. The electrolyte additive should also attract more attention to inhibit the dissolution of transition metals, such as Mn, in electrode materials. In addition, the application of the superconcentration electrode is a promising approach to enlarge the working voltage window and improve the electrochemical stability. Wang and Xia demonstrated that 4 M Mg(TFSI)2 electrolyte could provide a stable working voltage window of −0.9–1.1 V versus Ag/AgCl [9]. Due to the expanded electrochemical window, Li3V2(PO4)3 and poly pyromellitic dianhydride can serve as the cathode and anode, respectively. The assembled Mg-ion cell exhibited remarkable capacity retention of 92% after 6000 cycles.
Concluding remarks and prospects
Benefiting from low cost and high safety, aqueous Mg-ion batteries are expected to meet the requirements of the next-generation large-scale energy storage system, which has received great attention. In the past decade, aqueous Mg-ion batteries have made steady progress. However, they still have a long way to go before commercialization. Fortunately, the proven success of Li-ion batteries and advanced characterization techniques can shorten the time of development of practical aqueous Mg-ion batteries. Based on the in-depth understanding of the Mg-ion storage mechanism and behavior, more Mg-ion host materials with favorable kinetics and desirable power/energy density could be explored. The electrode materials with pseudocapacitive behavior should be paid more attention due to the fast charge transfer and excellent cycling stability. Meanwhile, aqueous electrolyte optimizations, such as Mg-salt selection, additives, and concentration regulation would be essential for achieving high-performance batteries with wide voltage windows and operating temperature. In addition, the assessment of the self-discharge, battery (pack) configuration and recycling should be performed before mass production. We hope this review can help to boost the development of aqueous Mg-ion batteries, in which challenges and opportunities coexist.
6. Aqueous Ca-ion batteries
Duan Bin
School of Chemistry and Chemical Engineering, Nantong University, Nantong, Jiangsu 226019, People's Republic of China
Status
Among various multivalent ion batteries, using Ca2+ ion as a charge carrier has gained much attention due to their relatively low reduction potentials (−2.87 V versus SHE) comparable to that of lithium, high abundance and volumetric capacity (2073 mAh ml−1) [78–80]. Therefore, rechargeable Ca-ion batteries (CIBs) that can transport more electrons than monovalent ions are expected to become one of the next generation of important energy storage devices. In early CIBs, the surface oxidation passivation layer inhibited the electroplating and stripping of calcium metal, which makes it difficult for calcium ions to be deposited in traditional electrolytes. In 2016, Ponrouch et al reported that calcium in organic electrolytes can be electroplated and stripped at appropriate temperature (75 °C–100 °C) [78]. However, the non-aqueous CIBs limit the reversible plating and stripping of metallic Ca to high temperature over 75 °C [2]. In 2019, Lang et al developed a highly reversible room-temperature Ca-ion-based hybrid battery via a tri-ion strategy, which involves the transport of Ca2+, Li+ and  ions. This optimized CIB improves the diffusion kinetics of calcium ions withoutstanding rate capability [81]. In spite of these advantages, metal Ca is easily contaminated by a small amount of water when using the aqueous electrolyte. In this regard, replacing metal Ca with an intercalated anode is feasible for developing aqueous Ca-ion batteries (ACIBs).
ions. This optimized CIB improves the diffusion kinetics of calcium ions withoutstanding rate capability [81]. In spite of these advantages, metal Ca is easily contaminated by a small amount of water when using the aqueous electrolyte. In this regard, replacing metal Ca with an intercalated anode is feasible for developing aqueous Ca-ion batteries (ACIBs).
Current and future challenges
Current research into ACIBs is still in its infancy. The oxide layer on the surface of metal calcium makes it difficult to directly act as an electrode like metal zinc in aqueous zinc batteries. The large ionic radius of Ca2+ (100 pm) causes most electrode materials to have poor ion diffusion kinetic properties, making it difficult to achieve theoretical capacity and ideal rate performance. In addition, the incompatibility between electrode material and inorganic salt solution is another key factor for the development of ACIBs. Therefore, selecting reversible intercalation electrode materials is very important to study the electrochemistry of ACIBs.
Advances in science and technology to meet challenges
Due to the reversible coordination reaction, many organic compounds with carbonyl groups are being considered as potential electrode materials, not only in alkali-metal ion (Li+, Na+, K+) batteries, but also in batteries based on multivalent ions such as Zn2+, Mg2+, Ca2+ [66, 82]. In 2017, Yao and co-workers reported a fast and highly reversible polyimide poly[N,N'-(ethane-1,2-diyl)-1,4,5,8-naphthalenetetracarboxiimide] (PNDIE) anode, with its discharge capacity mainly produced from the Ca-ion coordination to the C=O group within the conjugated aromatic molecules (figure 9(a)) [83]. When coupled with a copper hexacyanoferrate (CuHCF) cathode, a rocking-chair type ACIB was designed, as illustrated in figure 9(b). The electrochemical behavior of the PNDIE anode in 2.5 M Ca(NO3)2 solution exhibited distinct redox peaks at −0.44/−0.19 and −0.65/−0.45 V (versus Ag/AgCl), which could contribute to the discharge capacity of 183 mAh g−1 (figure 9(c)). In addition, the Ca0.3CuHCF cathode (the pre-intercalation of CuHCF) presents a visible redox peak at 0.83/0.80 V, corresponding to capacity of 65 mAh g−1. As a result, the full ACIBs could exhibit a specific capacity of 40 mAh g−1 at a current density of 1C and an outstanding capacity retention of 88.0% at 10C after 1000 cycles. Following this work, Mitra and co-workers developed an in situ polymerized polyaniline (PANI) anode to work in 2.5 M Ca(NO3)2 electrolyte, which involves the doping/de-doping of  at the anode [84]. As shown in figure 9(e), the PANI anode delivers a reversible specific capacity of 123 mAh g−1 at a current density of 0.15 A g−1 in the potential range of 0.4 to −0.5 V (versus Ag/AgCl). However, the full ACIBs coupled with the calcium-based copper hexacyanoferrate (Cax
CuHCF) cathode only displays an average output voltage of 0.6 V due to the high intercalation potential of the PANI anode. To improve the stability of the organic electrode, Cang et al prepared poly(3,4,9,10-perylentetracarboxylic diimide) (PPTCDI) organic material supported on porous SBA-15 to use as the anode in ACIBs (figure 9(f)) [85]. Due to the large specific surface and stable structure, the PPTCDI/SBA-15 composite anode exhibited a high specific capacity of 199 mAh g−1 at 0.1 Ag−1 and a high cycling stability (98% capacity retention over 100 cycles) in 1 M Ca(NO3)2 electrolyte (figure 9(g)). Considering Ca2MnO4 as a cathode, the full ACIBs can work in a wide voltage range from 0 to 1.8 V while achieving a high energy density of 130. 6 Wh kg−1.
at the anode [84]. As shown in figure 9(e), the PANI anode delivers a reversible specific capacity of 123 mAh g−1 at a current density of 0.15 A g−1 in the potential range of 0.4 to −0.5 V (versus Ag/AgCl). However, the full ACIBs coupled with the calcium-based copper hexacyanoferrate (Cax
CuHCF) cathode only displays an average output voltage of 0.6 V due to the high intercalation potential of the PANI anode. To improve the stability of the organic electrode, Cang et al prepared poly(3,4,9,10-perylentetracarboxylic diimide) (PPTCDI) organic material supported on porous SBA-15 to use as the anode in ACIBs (figure 9(f)) [85]. Due to the large specific surface and stable structure, the PPTCDI/SBA-15 composite anode exhibited a high specific capacity of 199 mAh g−1 at 0.1 Ag−1 and a high cycling stability (98% capacity retention over 100 cycles) in 1 M Ca(NO3)2 electrolyte (figure 9(g)). Considering Ca2MnO4 as a cathode, the full ACIBs can work in a wide voltage range from 0 to 1.8 V while achieving a high energy density of 130. 6 Wh kg−1.
Figure 9. (a) Illustration of the reversible intercalation mechanism of the PNDIE electrode and (b) schematic illustration of the aqueous Cax CuHCF/PNDIE device. (c) CV curves of the PNDIE anode and Ca0.3CuHCF cathode in 2.5 M Ca(NO3)2. (a)–(c) Reproduced from [85]. CC BY 4.0. (d) Obtained PANI-coated carbon cloth and (e) the selected GCD curves at the current density of 0.15 A g−1. (d), (e) Reprinted with permission from [86]. Copyright (2020) American Chemical Society. (f) Preparation procedure of PPTCDI/SBA-15 composite. (g) GCD curves in different cycles at 0.1 A g−1. (f), (g) [87] John Wiley & Sons. © 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Download figure:
Standard image High-resolution imageIn a recent work, Han et al used 5,7,12,14-pentacenetetrone (PT) as an organic crystal anode material and a high-voltage KCoFe(CN)6 ‧ xH2O cathode for ACIBs (figure 10(a)) [86]. When tested in 1 M CaCl2 electrolyte, the CV curves of the PT anode showed two reduction peaks (at −0.3 and −0.66 V (versus Ag/AgCl)) and three oxidization peaks (at −0.47, −0.38 and −0.2 V (versus Ag/AgCl)), corresponding to the multiple electron transfer of Ca2+ insertion and extraction process (figure 10(b)). Compared with the previous organic electrode, the PT anode displayed a superior rate performance. As shown in figure 10(c), the PT anode exhibited a high specific discharge capacity of 150.5 mAh g−1 at 5 A g−1. Even at the highest current density of 100 A g−1 (6.2 s a cycle), it could still achieve a large discharge capacity of 86.1 mAh g−1. In addition, Tang et al investigated the effect of electrolyte concentration on the aqueous Ca-ion storage of a Ca0.4MnO2 cathode [87]. A 1 M Ca(NO3)2 aqueous electrolyte exhibits weak acidity and a narrow stability window of 1.95 V. However, an 8.37 m (mol kg−1 solvent) saturated Ca(NO3)2 aqueous solution could expand the ESW and restrain the HER and oxygen evolution reactions (OER) due to the high solubility of Ca(NO3)2 salt (figure 10(d)). When using saturated Ca(NO3)2 aqueous solution including 10 wt% polyvinyl alcohol (PVA), the PVA aqueous gel also gives rise to a wide electrochemical window up to 2.6 V in Ca0.4MnO2 cathode (figure 10(e)). Meanwhile, this perturbation of the water hydrogen bond network by water–PVA interaction can further reduce the activity of the water solvent, thereby suppressing the diffusion of the polysulfide into the water from the sulfur anode. The novel aqueous gel electrolyte indeed has a significant effect on the electrochemical behavior of electrode materials in ACIBs.
Figure 10. (a) ACIBs based on a PT anode and KCoFe(CN)6 ‧ xH2O cathode. (b) CV curves of a PT anode in 1 M CaCl2 solutions. (c) GCD curves of a PT anode at different current density. (a)–(c) Reproduced from [88]. CC BY 4.0. (d) Electrochemical stability window and (e) the redox voltages of a Ca0.4MnO2 cathode in different electrolytes. (d), (e) Reproduced from [89]. CC BY 4.0.
Download figure:
Standard image High-resolution imageConcluding remarks and prospects
Like other aqueous battery systems, a full ACIB should be assembled by a low potential intercalated anode and a high potential calcium-source cathode within the potential range of HER and OER. To date, reversible Ca2+ storage behavior in aqueous electrolytes has only been investigated for some organic anodes, PBAs and Ca-based oxide cathodes.
7. Aqueous Al-ion batteries
N Melzack and R G A Wills
Energy Technology Research Group, University of Southampton, Highfield, Southampton SO17 1BJ, United Kingdom
Status
Aqueous aluminum intercalation batteries, using abundant electrode materials provide the possibility of high rate, safety, low-cost energy storage, and are non-toxic. There is also an established recycling process for aluminum, which offers a potential environmental advantage for these batteries [88]. In theory, the ionic radius of Al is slightly smaller than that of Li, leading to a high charge density with no increase in the physical size of the electrode. In addition, Al has a high density (2.7 g cm3 @25 °C), which leads to a volumetric energy density of almost four times that of lithium, 8.04 Ah cm−3 and 2.06 Ah cm−3, respectively.
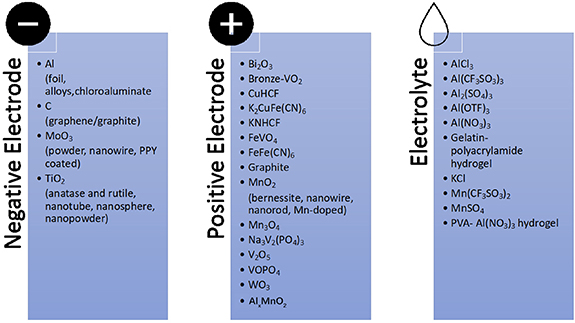
Aqueous Al-ion batteries are in a pre-commercial stage with various half-cell and full-cell batteries being demonstrated in a laboratory environment. Figure 11 shows that from a single paper published in 2012 [89], there were 77 total publications by the end of 2020. Based on the initial positive KCuFe [CN]6 (CuHCF) and negative TiO2 electrode materials, many half-cell materials have now been reported in the literature [90]. Figure 12 provides an overview of the electrode materials and electrolytes currently reported (Negative electrodes: Al [91], C [92], TiO2 [89] and MoO3 [93]; Positive electrodes: Bi2O3 [94], bronze-VO2 [95], CuHCF [96], K2CuFe(CN)6 [97], KNHCF [98], FeVO4 [99], FeFe(CN)6 [100], graphite [101], MnO2 [102], Na3V2(PO4)3 [103], V2O5 [104], VOPO4 [105], WO3 [106] and Alx MnO2 [107]; Electrolytes: AlCl3 [108], Al(CF3SO3)3 [105], Al2(SO4)3 [106], Al(NO3)3 [109], gelatin-polyacrylamide hydrogel [110], KCl [111], Mn(CF3SO3)2 [112], MnSO4 [113] and PVA–Al(NO3)3 hydrogel [114]). We suggest that higher specific capacities (above 200 mAh g−1) are achievable using vanadium-based electrodes,. although the highest capacity example only reported a cycle lifetime of 20 cycles. Graphite-based electrodes have a reported capacity of 157 mAh g−1 along with a promising cycle lifetime of 3500 cycles [92]. PBAs are favored positive electrodes, with good cycle lifetime but reduced specific capacity compared to other positive electrode materials when put into a full cell.
Figure 11. Cumulative publications in the academic literature between 2012 and 2020, showing key half-cell developments towards the first operational battery in 2018 [90].
Download figure:
Standard image High-resolution imageFigure 12. Summary of electrode materials and electrolytes.
Download figure:
Standard image High-resolution imageThe use of AlCl3-based electrolytes gives good cycle life for TiO2-containing electrodes with the addition of KCl increasing cycle life further. Hydrogel electrolytes are reported to increase the cycle lifetime substantially, with the PVA–Al(NO)3 electrolyte combined with aluminum and PBA electrodes providing just over 500 cycles [115]. Gelatin-polyacrylamide hydrogel has the highest cycle life reported for a full cell, 2800, with 13.8% capacity fade [110]. Half-cell vanadium-containing electrodes reported between 12–1000 cycles [95, 99, 116], while half-cell MoO3 [93, 117] electrodes had cycle lives of 350–400.
Current and future challenges
Two key challenges arise for aqueous Al-ion chemistry:
Capacity limitations of intercalation electrodes–the high charge density means it is difficult for Al3+ to lose its coordinating ligands in the solvated state, and while intercalating, the Al3+ ions can distort the lattice structure of electrodes. The former negates the advantage of the small ionic radius of Al3+, while the latter can reduce the lifetime significantly as the lattice distortions accumulate with each cycle, causing structural damage within the electrode.
ESW of water—aqueous electrolytes are often safer than ionic liquids or non-aqueous electrolytes, due to their low flammability and ease of handling [118]. Water is low-cost, widely available and does not require complex manufacturing or storage (compared to other electrolytes). However, there is the concern about a narrow ESW of water of about 1.23 V. Beyond this point, electrolysis of water may take place liberating hydrogen and/or oxygen at the negative and positive electrodes, respectively. This would limit the energy density of cells constructed in this manner and offer challenges in suppressing the parasitic hydrolysis reaction. Other limiting factors, not bespoke to aqueous electrolytes, also apply. Corrosion of electrodes is likely in protic electrolytes, while dendrite formation on electrodes is more likely in alkaline electrolytes. Of course, these both limit the lifetime and discharge voltage obtained over time [118].
Advances in science and technology to meet challenges
The use of graphite is more common in non-aqueous Al-ion batteries. However, two studies have recently used them as the positive electrode within an aqueous cell [92, 119]. The multi-layer graphite (as discussed in [119]) showed an intercalation/de-intercalation of Al3+ between layers. However, the expansion of the lattice during insertion led to cracking of the structure and hence a short cycle lifetime. When looking at graphene with the addition of carbon nanoparticles, a capacitive storage mechanism is suggested, which may lead to a longer cycle lifetime (reportedly 0% fade over 3500 cycles) [90], but potentially a higher self-discharge.
Bismuth oxide (Bi2O3) was investigated in 2020 by Nandi and Das [94]. Initial discharge capacities in a full cell with an Al-ion negative electrode showed very high values (1130 mAh g−1 @ 1.5 A g−1), and ∼99% Columbic efficiency. However, this dropped significantly to 103 mAh g−1 within 20 cycles, and remained stable for the following 50, showing no additional signs of capacity fade.
Although WO3 electrodes show poor Coulombic efficiency of approximately 80% [106] over cycling in both 1 M AlCl3 and 0.5 M Al2(SO4)3, the capacity increases over cycling time, to around double the initial capacity seen. With no significant structural changes seen in the electrode after 100 cycles and the main mechanism is assumed to be intercalation/deintercalation of Al3+.
Material selection, doping and architecture will be key to further developing aqueous Al-ion systems. Nanostructuring of electrode materials decreases diffusion lengths and increases electrolyte/electrode contact, allowing higher rate cycling.
Regardless of the low ESW, there is still high demand for aqueous electrolyte development. The potential ionic storage of these electrolytes is two orders of magnitude higher than that of organic non-aqueous electrolytes, enabling high power capability. However, with diluted aqueous electrolytes, additive formulations and electrolysis-suppressing electrode materials, the electrolytes can have an ESW of 2 V or more. Polymer and gel-type electrolytes are still in their development stage and offer the advantage of a high ESW, with lower flammability. Aqueous WiSEs in which salt is saturated also have the potential for higher ESWs. The high charge density of Al3+ may also minimize the decomposition of aqueous electrolytes, helping to widen the practical potential of the electrolyte. It is possible that if a double layer is formed, Al3+ could allow for a higher charge accumulation on the surface of the electrode giving capacitive storage effects in addition to intercalation.
Concluding remarks and prospects
The development of aqueous electrolyte batteries is justified by the possibility of high rate capability (>1 A g−1 or >1 W g−1), intrinsic safety, low toxicity and potentially low-cost storage devices. Aluminum's abundance, pre-existing production industry and recyclability make it a sustainable option.
Secondary Al-ion batteries have attracted increased attention in the last 5 years, with the exact charge storage mechanisms remaining complex and ill-defined for many electrodes. This is clearly a space with the potential for growth, with better elucidation of reaction mechanisms and refinement of electrode material design.
There have been few studies of supercapacitors with aqueous Al-ion technology. However, many of the secondary batteries display pseudo-capacitive behavior. It is expected that when the exact charge storage for some electrodes (such as TiO2) is determined, the role of Al-ion battery technology will align to applications traditionally reserved for supercapacitors.
Acknowledgments
This work was funded by UKRI under an STFC Studentship and the EPSRC Faraday Training Grant EP/S514901/1.
8. Aqueous flow batteries
Jianhang Huang
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Life Science, Zhejiang Normal University, Jinhua 321004, People's Republic of China
Status
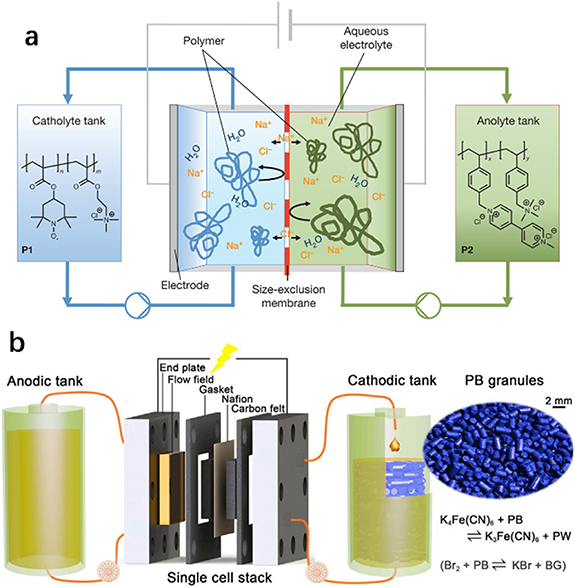
Due to the unique advantages of decoupled power and energy and high scalability, the flow battery has long been considered a competitive candidate for large-scale energy storage. Generally, redox species in a flow battery are dissolved or suspended in electrolytes. The electrolyte containing the species with the lower redox potential is called the negative electrolyte (negolyte or anolyte), whereas the counter electrolyte containing the species with the higher redox potential is called the positive electrolyte (posolyte or catholyte). During operation, the electrolytes are pumped through an electrochemical cell equipped with an inert electrode, in which the negolyte and posolyte are separated by a membrane to avoid mixing of different redox materials in the negolyte and posolyte, but allows the passage of inert ions to retain high ion conductivity (figure 13(a)). In this unique structure, power and energy are decoupled. The energy density of a flow battery is not only controlled by the potential difference between the negolyte and the posolyte, but also by the electrolyte volume and concentration of redox materials. On the other hand, the power density is adjusted by scaling the reaction area of the electrodes [120, 121]. Compared to flammable organic solvents, the aqueous flow battery exhibits higher safety, ionic conductivity, environmental compatibility and economic efficiency, which is more suitable for large-scale energy storage. The first flow batteries patented by Kangro in 1949 were aqueous flow batteries, which were based on redox species of chromium, iron, titanium and chlorine. The most well-developed flow battery, i.e. the all-vanadium flow battery, also uses aqueous electrolyte. The above examples strongly demonstrate the superiority of the aqueous flow battery. To date, a significant amount of redox chemistry for the aqueous flow battery has been developed. Redox materials in the flow battery can be categorized into inorganic redox materials (vanadium-based, zinc-based, iron-based, polysulfide-based, polyoxometalate-based flow battery) and organic redox materials (viologen-based, quinone-based, 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO)-based, nitrogen-centered heteroaromatic-molecule-based flow battery).
Figure 13. (a) Illustration of flow battery components. Modification of (b) electrolyte. Reprinted from [124], Copyright (2018), with permission from Elsevier. Modification of (c) an electrode. Reprinted from [125], Copyright (2017), with permission from Elsevier. Modification of (d) a membrane. Reprinted with permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature Energy [126], Copyright (2021).
Download figure:
Standard image High-resolution imageCurrent and future challenges
Inorganic redox materials have a long research history, and some flow battery systems are now in the demonstration stage, such as the all-vanadium redox flow battery (AVFB), zinc–bromine flow battery and iron–chromium flow battery (FCFB). As the most developed aqueous flow battery system, AVFB effectively minimizes cross-contamination by using the same element in both the anolyte (V2+/V3+) and catholyte (VO2+/VO2
+). However, further commercialization of AVFB is hindered by the high chemical cost of vanadium, low thermal stability of pentavalent vanadium, toxicity of vanadium species, poor solubility and redox kinetics. Furthermore, for other soluble metal redox chemistries, such as Fe2+/Fe3+ and Cr2+/Cr3+, they are easy to hydrolyze and usually need to have ligand added to form a stable coordination compound. The zinc-based flow battery with deposition/dissolution mechanism possesses some advantages derived from the zinc anode, such as low price, low potential (−0.76 V versus SHE in mild acidic/neutral electrolyte, −1.26 V in alkaline electrolyte) and environmental friendliness, which has attracted significant attention in recent years. Nonetheless, the problems accompanying the zinc anode, such as hydrogen evolution, formation of dendrites and passivation layers, cannot be ignored. In particular, in alkaline electrolyte zinc dendrite formation is serious, as this is the most important factor for battery degradation. Furthermore, the limited areal deposition capacity places strict constraints on the energy density of the zinc flow battery. Although polysulfide redox materials show high solubility, are low-cost and abundantly available, serious crossover and sluggish kinetics result in rapid capacity fade. In addition, halogen species, such as Br−/Br and  , are usually used as catholyte materials. However, Br shows serious toxicity and corrosivity, and I2 is insoluble and must combine with I− to form soluble
, are usually used as catholyte materials. However, Br shows serious toxicity and corrosivity, and I2 is insoluble and must combine with I− to form soluble  , which leads to low utilization of I species. In addition to inorganic materials, organic redox materials are characterized by large earth abundance, high structural designability and environmental friendliness. However, it should be noted that most organic materials display poor solubility and chemical/electrochemical stability.
, which leads to low utilization of I species. In addition to inorganic materials, organic redox materials are characterized by large earth abundance, high structural designability and environmental friendliness. However, it should be noted that most organic materials display poor solubility and chemical/electrochemical stability.
Advances in science and technology to meet challenges
The above-mentioned challenges of various redox chemistry can be summarized as the following points: low stability of active materials (such as precipitation of pentavalent vanadium, hydrolysis of Fe and Cr species, oxidation of organic materials), low reaction kinetics, low solubility, dendrite formation for metal anode, parasitic reactions (such as hydrogen and oxygen evolution), cross-contamination between anolyte and catholyte. Researchers have made a tremendous effort to meet these challenges. The modification of electrolyte is a common strategy to mitigate low stability, such as optimization of anions in electrolytes, additives and pH values. Roe et al [125] reported that 1 wt% H3PO4 + 2 wt% ammonium sulfate could increase the induction time for precipitation of pentavalent vanadium. In addition, the improved stability may be due to the synergistic effect of ammonium and phosphate (figure 13(b)) [122]. For the low kinetics, most investigations focus on the modification of electrode materials. The most common method is the functionalization of carbon-based materials via surface treatment, including treatment by strong acid/base and heat and decoration with catalysts (figure 13(c)) [123, 126].The zinc-based flow batteries are challenged by HER, electrode passivation and dendrite formation. In addition to the optimization of electrolyte, the membrane always plays a key role in the improvement of flow batteries. Yuan et al [127] prepared a membrane with high mechanical stability to effectively avoid zinc dendrite. In addition to the resistance to dendrite, the primary role of the membrane is to restrain cross-contamination between the anolyte and catholyte. Furthermore, different types of membranes have been developed to match various kinds of flow batteries, such as cation exchange membrane, anion exchange membrane and size-exclusion membrane. Modifications of the membranes become an effective means to further improve membrane performance. Li and Lu [124] developed a charge-reinforced ion-selective membrane by coating polyvinylidene fluoride and Ketjen black carbon on Nafion membrane. The high hydrophobicity of polyvinylidene fluoride assists to alleviate water migration caused by osmosis pressure and the Ketjen black carbon can absorb polysulfide/polyiodide effectively, which traps and accumulates negatively charged anions, and finally electrostatically repels active anions in the electrolyte and reduces cross-contamination (figure 13(d)). For organic active materials, low solubility and stability are the main problems in the way of development [66]. Generally, the charged product of anolyte is easy to oxidize by oxygen, and some organic materials are subject to chemical and/or electrochemical decomposition during redox. In order to mitigate the above problem, reasonable design of organic molecules, such as adding functionalization group and/or polymerization, are considered as effective mitigation methods. Huskinson et al [128] introduced two sulfo-groups into the anthraquinone to obtain 9,10-anthraquinone-2,7-disulfonic acid with high solubility up to 1 M. Janoschka et al [129] designed a polymer consisting of a redox-active group and another group enhancing water solubility. The proposed polymer flow battery not only shows improved cycle stability but also allows the use of cheap filter membranes due to the high steric hindrance of polymer materials (figure 14(a)).
Figure 14. (a) Illustration of a flow battery using polymer as active materials. Reprinted with permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature Energy [131], Copyright (2015). (b) Illustration of a redox-targeting flow battery, in which PB is Prussian blue, and PW is Prussian white. Reprinted from [132], Copyright (2019), with permission from Elsevier.
Download figure:
Standard image High-resolution imageConcluding remarks and prospects
Aqueous flow batteries have received more and more attention in recent years. Although large progress on electrolytes, electrodes and membranes has been achieved, there is still a long way to go to reach large-scale application. Novel redox chemistry, mass transport characteristics and design of battery structure should also be heeded for further development of the aqueous flow battery. For example, semi-solid active materials for flow batteries are proposed due to their high energy density. Yan et al [131] proposed a slurry flow battery that employed homogeneously dispersed polymer particulates as active materials (polyhydroquinone as cathode material and polyimide as anode material). The semi-solid slurry with uniform dispersion of redox materials is able to certainly break the solubility limit and even make it feasible to use insoluble materials in the flow battery. For the innovative structure of the flow battery, a redox-mediated flow battery (also called a redox-targeting flow battery) was proposed recently [130, 132], in which solid electrode materials are charged/discharged by dissolved redox mediators in the flow battery (figure 14(b)). The battery voltage and power capability are determined by soluble redox mediators. However, the energy capacity is determined by the chemical reactions between soluble redox mediators and solid electrode materials. Compared to the traditional flow battery, the condensed state of solid materials endows a much higher energy density to the redox-mediated flow battery.
Acknowledgment
The authors gratefully acknowledge funding support from the National Natural Science Foundation of China (Grant Nos. 21805126 and 22179119).
9. Photo-responsive battery
Xiaotong Wang1, Xiaofeng Lin2 and Dingshan Yu1
1 Key Laboratory for Polymeric Composite and Functional Materials of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou 510275, People's Republic of China
2 School of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou 510006, People's Republic of China
Status
To overcome the ever-increasing energy crisis and adverse environmental issues, the massive utilization of solar energy has become one of the most appealing options due to its abundant, ecofriendly and economic nature as well as low running cost. Despite great progress having been made in the development of popular solar cells so far, which plays an increasingly significant role in power grids, solar cells can merely transduce light energy into instant electricity for immediate use with the lack of energy storage capability. In addition, sufficient utilization of solar energy is still restricted by its intrinsic intermittency. In this regard, harvesting solar energy into electrochemical rechargeable batteries is an attractive solution, which not only achieves the large-scale utilization of unlimited and low-cost solar energy, but also helps reduce the input and/or enhancement of the electric energy output of conventional rechargeable cells. How to realize highly effective integration of solar energy into various rechargeable cells is still a rather challenging task. In this context, photo-responsive batteries that integrate solar harvesting, energy conversion and storage functionalities in one system have emerged. Based on the integration degree of electrodes, it can be divided into the two-chamber three-electrode system and the one-chamber two-electrode system, both of which in principle integrate the light-responsive module and the basic battery module into one system (figure 15). The basic battery module is often employed as a charge storage/release unit and can be replaced with many different types of batteries, such as lithium-ion battery, sodium-ion battery, lithium-oxygen battery, lithium-sulfur battery, metal-air battery, flow battery, etc, depending upon the actual requirements [133]. As the core component of a photo responsive battery, the photo-responsive module can be used as an extra single electrode or integrated into the working electrode of the battery module. Among various responsive systems, photo-responsive air/O2-based batteries (e.g. Zn/Li-air/O2 batteries) are particularly attractive due to their high theoretical energy density. In particular, the corresponding OER/oxygen reduction reaction (ORR) during the charging/discharging process can be effectively coupled with the sunlight [134]. In available photo-responsive air/O2-based systems, light energy can be directly stored during charging to afford different photocharging rechargeable cells while boosting discharge performance via photoelectric effects in some systems. These photo-responsive systems not only favor energy conversion and/or storage for enhanced energy efficiency, but also endow traditional cells with some intelligent functions for self-powered electronics/optoelectronics/sensors. Regardless of the type and device architecture of cells, photo-responsive air/O2 batteries follow a similar operation principle: the photoactive components in photoelectrodes (PEs) can produce electron–hole pairs via the photoelectronic effect upon light exposure, which makes it possible to modulate the electrochemical reactions during charge/discharge by coupling photogenerated electrons and/or holes. In this a manner, the light energy can be transduced and/or stored without affecting the working cycles of other battery components.
Figure 15. Illustration of the different type of photo-responsive system.
Download figure:
Standard image High-resolution imageDespite some advances in photo-responsive batteries, it still remains a great challenge to develop advanced and low-cost solar-powered rechargeable cells with high overall energy efficiency, highly compact configurations and excellent compatibility between different battery modules. In this perspective, we present a brief summary of photo-responsive air/O2-based batteries.
Current and future challenges
A major challenge for photo-responsive batteries is to maximize the utilization of solar energy. How to convert the light energy into a beneficial factor for the battery module by the photo-responsive module is a key issue. The overall energy efficiency of a photo-responsive system is a key indicator of effective solar energy utilization, which is closely linked to the photoelectronic conversion efficiency and energy conversion/storage efficiency of the whole system. Taking a typical photo-responsive Zn-air battery as an example, the PE plays a central role in realizing high overall efficiency and high power/energy density for the whole system, which not only undertakes the light capture and conversion function, but also requires high electrochemical activity corresponding to the charging and discharging processes along with excellent stability under light. This imposes stringent requirements on the PE materials and narrows down the material choices significantly. Research on photo-responsive batteries is still in its infancy, and most efforts have focussed on the design of PEs. The exploitation of the photovoltaic/photocatalytic/photothermal effect to obtain a direct/indirect performance enhancement is a topic of great interest to researchers. Nevertheless, the performance of PE still does not meet the application requirements, especially in scenarios with high power requirements, and their light harvesting/conversion efficiency is still at a low level.
Stability is another critical concern for photo-responsive battery systems. In addition to unstable active electrode materials for normal battery electrodes and especially PEs, volatile and corrosion-sensitive liquid electrolytes can also result in inferior long-term stability under light exposure. Therefore, exploring durable, compatible electrolytes and photo-/electrode materials with excellent photostability and electrochemical stability simultaneously is crucial to the overall stability. In particular, designing solid-state photo-responsive batteries with solid-state electrolytes with excellent photo-/thermal stability could be an appealing option to realize a highly stable system. In addition to PE design, the negative effects induced by light need to be paid more attention in future efforts. In practical applications, it is not enough to merely emphasize the effect of light on the PE, and the light effect on all battery modules should be carefully considered.
Only a small fraction of studies are concentrated on photo-responsive batteries compared to conventional batteries. Many detailed measurement parameters and conditions in currently available studies have not been standardized. This necessitates a useful standard to rationalize the battery performance output and discuss future trends. Furthermore, the compatibility and highly effective integration of various battery modules as well as the running cost of the entire system are also of significance for device commercialization. Finally, integrating photo-responsive batteries with light-induced effects and other different stimuli responsiveness, such as pressure and moisture, will be an intriguing research direction.
Advances in science and technology to meet challenges
Current research regarding photo-responsive batteries as exemplified by photo-responsive air/O2-based systems mainly focusses on the exploration and utilization of photocatalytic/photovoltaic/plasmonic effects with the aim to reduce the electric energy consumption of the charging process and enhance the energy output of the discharge process via the conversion/storage of light energy. Thus, seeking suitable PE materials and rational PE design has long been the bottleneck issues in this emerging field. Generally, an ideal PE should have good light harvesting ability, high carrier separation efficiency, excellent photo-electrochemical activity and high stability. For air/O2 electrodes, the energy-level structure of the photo/oxygen electrode plays a crucial role in boosting the oxygen reaction process via photoexcited electrons or holes in such a battery [135]. In addition, sufficient utilization of the light energy and the separation efficiency of the photogenerated carriers should also be considered when selecting PE materials. To date, only a few metal oxide/polymer semiconductors and their composites/heterojunctions have been explored as PEs for various photo-responsive air/O2 batteries, showing enhanced charge and/or discharge performance with boosted energy efficiency by absorbing solar energy.
PEs are key to photo-charging performance. However, to date, the slow redox kinetics of PEs has immensely limited the development of the photo-responsive battery. Wedege et al proposed a single TiO2 to protect Si photocathode neutral and semi-organic solar redox flow batteries with an unbiased solar conversion efficiency of 1.6% and electrolyte energy density of 3.9 Wh l−1 from 0% to 95% SOC [136]. It has been proved that single-photon deviceshave high solar-to-chemical conversion efficiency without polarizing charges. Bae and colleagues [137] used a C–Si optical electrode with buried p–n junction to achieve backside lighting without parasitic light loss while developing a single-photon photocharging device with a high solar-to-chemical conversion efficiency of over 9.4% for a redox flow cell system. This design principle provides efficient conversion efficiencies for further development of solar rechargeable redox flow batteries. In addition, single semiconductors, such as g-C3N4, TiO2, Co3O4, BiVO4 and α-Fe2O3, have been regarded as efficient, stable and integrated photocathode materials for photo-responsive air/O2-based batteries, since photogenerated electrons and holes from these semiconductor PEs can be employed to modulate the electrochemical reactions that occured in air/O2-based batteries. g-C3N4 has been exploited as a photocatalyst and an ORR catalyst simultaneously, which endows a photo-responsive Li–O2 battery, with an ultralow charging voltage of 1.9 V. Upon photo-charging, the photo-voltage generated on the g-C3N4 PE compensates for the cell's charge voltage, resulting in an energy-conversion efficiency of 142% [138]. BiVO4 and a-Fe2O3 are also utilized as photocathodes under illumination to decrease the charge voltage of Zn-air batteries by 0.5–0.8 V [139]. Proper band structure has been identified as a key factor for the charging performance by comparing BiVO4 and α-Fe2O3 PEs in Zn-air batteries that produce charge voltages of 1.20 and 1.43 V, respectively. Given that the light responsiveness can be introduced into the anode or cathode for air/O2-based batteries, a dual-PE photoelectrochemical air battery, composed of a TiO2 photoanode for OER and polyterthiophene (pTTH) photocathode for ORR, displays simultaneously improved charge and discharge performance by direct photoelectric effect with an ultrahigh discharge voltage of 1.90 V and ultralow charge voltage of 0.59 V [140]. This arises from the additional solar energy that can be transduced into electric energy during discharge and chemical energy during charge. It is well known that the optical absorption of most semiconductors is limited to the UV spectrum region, only occupying 4% of the solar spectrum. To expand the light harvesting from UV to the visible light region, a low-band-gap C4N polymer has been introduced as the PE to modulate oxygen catalysis. The C4N-enabled Zn-air battery shows a low charge voltage of 1.35 V under visible light, corresponding to a high energy efficiency of 97.78% [141]. This PE can also be applied in visible-light-responsive polymer-air cells for improved device performance.
The above-mentioned PE materials based on single semiconductors, such as TiO2 and g-C3N4, often suffer from inferior response in the visible-light range arising from their wide band gap and high carrier recombination rate, thereby restricting their photoelectric conversion performance. To promote the separation of photogenerated carriers and expand the light absorption range for maximizing the conversion/storage of light energy in batteries, coupling two different semiconductors into suitable heterojunctions with desirable energy-level structures is an attractive solution. In this regard, a few heterojunctions, such as N12P5/N-doped carbon nanotubes (N-CNTs), TiO2/Fe2O3 and Au/C3N4 with nitrogen vacancies (Au/NV–C3N4), have been developed as bifunctional photo-/electrocatalysts for photo-involved air/O2-based batteries, which can not only effectively constrain the energy loss during charge, but also tune the output energy of the system during discharge. For example, creating properly engineered N12P5/N-CNT p–n heterojunctions with N12P5 and N-CNT as OER and ORR active sites can accelerate the electron–hole migration across p–n heterojunctions during charge/discharge processes of Zn-air batteries, thus boosting the battery performance enabled by an additional built-in electric field [142]. Upon light illumination, the N12P5/N-CNT-enabled Zn-air battery displays obviously improved charge/discharge potential and consequently increased energy efficiency. In addition, coupling TiO2 with narrow-band-gap Fe2O3 can efficiently regulate the electronic structure of TiO2 and improve the photoelectrochemical performance. The formed TiO2/Fe2O3 heterojunction PE can afford superior light-harvesting ability and electron–hole separation rate, thus endowing a photo-involved Li–O2 battery with ultralow overpotential of 0.19 V and good cyclic stability [143]. It is believed that the photo-generated electrons boost the oxygen reduction during discharge, while the photo-generated holes expedite the Li2O2 decomposition during charge. This heterostructure engineering provides an appealing pathway towards the effective utilization of solar energy to enhance the round-trip efficiency of air/O2-based systems by decreasing the overpotential during charge/discharge. Alternatively, coupling semiconductors with suitable plasmonic metal can also enable low electron–hole recombination and broadened light harvesting, which together with the unique plasmon-enhanced effect can synergistically boost the photoelectrochemical performance. Rationally designed plasmonic heterojunction, such as Au/NV–C3N4, can be applied as oxygen cathodes to enhance the light utilization efficiency and further accelerate the cathode reaction kinetics of the photoresponsive Li–O2 battery [144]. Under visible light, the discharge voltage of the Au/NV–C3N4 cathode is increased to 3.16 V along with the reduced charge voltage to 3.26 V, corresponding to an ultrahigh round-trip efficiency of 97.0%. The plasmon-triggered hot electrons in Au and the prolonged carrier life on Au/NV–C3N4 also result in good rate capability and cycling stability of batteries. This opens up many new opportunities towards the development of semiconductor heterojunction PE for visible-light-responsive air batteries with plasmonic enhancement effect. In addition to optoelectronic and plasmonic effects, the photothermal effect can also be utilized to modulate the electron and ion transport by local temperature rise to improve the electrochemical performance of batteries with the assistance of light energy, which represents an alternative option to fully exploit the potential of solar energy in rechargeable batteries.
Cycle stability is also a crucial concern for various battery systems. Most current photo-responsive batteries are mainly based on liquid electrolyte. Solid-state electrolytes possess better photo/thermal stability and safety with respect to traditional liquid electrolytes. In this regard, developing photoresponsive solid-state cells could be a promising solution to realize optimally stabilized systems. Succinonitrile has been introduced as a solid-state electrolyte into the photo-involved Li–O2 battery with ZnS@CNTs as the photocatalyst and oxygen electrocatalyst simultaneously [145]. The obtained battery yields a lower photo-enhanced charge potential (2.08 V) compared to that in the dark (4.09 V), which results in an efficient energy saving of 50%. Significantly, the charge potential remains rather stable during the cycling test (50 cycles), superior to previous photo-involved Li–O2 cells equipped with liquid electrolytes. Notably, the performance of solid-state batteries can be further improved via photothermal effect due to the higher ion conductivity of solid-state electrolytes at relatively high temperatures.
Concluding remarks
Photo-responsive batteries that combine photochemical and electrochemical energy conversion and storage with the aid of solar energy as represented by photo-responsive air/O2-based cells have shown great promise in the large-scale utilization of solar energy in a more efficient and economic manner. They not only serve as an energy-sufficient source to mitigate the energy storage concern of popular solar cells and energy density concern of electrochemical batteries, but also afford some intelligent functions, such as self-powered sensors, which are fit for the next generation of intelligent optoelectronics/electronics/sensors. Despite some advances in the development of innovative PE materials and the achievement of basic battery module designs, research on the photo-responsive battery is still in its early stage. The key challenges that still confront us lie in maximizing the performance metrics of these integrated photo-responsive systems by optimizing some key parameters including the overall energy efficiency, long-term stability and energy/power density. Continuous future efforts should be devoted to the development of highly efficient electrode/PE materials with excellent photostability and electrochemical stability as well as durable and compatible electrolytes for realizing high energy conversion/storage efficiency, large capacity and overall stability of systems simultaneously. Furthermore, exploring compatible strategies for highly efficient integration of photoactive modules and energy storage modules while reducing the overall cost of the system is also crucial for pushing technological commercialization forward. We do believe that photo-responsive batteries will have a bright future for large-scale solar energy utilization, and more exciting studies on such intriguing batteries will appear in the near future.
Acknowledgments
We gratefully acknowledge financial support from the National Natural Science Foundation of China (Grant No. 51973240), the Natural Science Foundation of GuangDong Province (Grant No. 2020B1515420001) and the Guangdong Basic and Applied Basic Research Funding-Regional Joint Fund for youth project (Grant No. 2020A1515110823).
Data availability statement
No new data were created or analyzed in this study.