Abstract
In the early 1800s, the industrial revolution was powered by fossil fuels as the primary energy resource. As environmental degradation started to be felt, countries began moving toward reduced emissions and carbon-neutral footprints. Subsequently, India also began to make enormous strides in nurturing the tremendous potential of renewable energy. As it has one of the most significant energy-harvesting potentials, solar energy has remained the widely accepted choice for researchers in India. In the last few years, India has witnessed tremendous research and development in solar energy, especially in the field of photovoltaics. Significant research effort has been invested in exploring the new generation of photovoltaic devices as alternatives to traditional silicon (Si)-based solar cells. Among the various new-generation photovoltaic devices, dye-sensitized solar cells (DSSCs) remain very attractive to researchers due to their easy preparation methodology, low toxicity, and ease of production. A typical DSSC is composed of a photoanode, a sensitizer, an electrolyte, and a cathode. Various research groups in India have studied the role of each individual component within DSSCs and performed research and development activities to improve their photovoltaic efficiency. The most important part of a DSSC is the dye, which is actually the source of photoexcited electrons. This topical review will provide an overview of the research efforts undertaken in India to support the optimisation of different components of DSSCs. However, emphasis has been placed on the research activities that support the exploration of different photoactive dyes as alternatives to the N3- and N719-based organometallic dyes.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction to DSSC research
Rapid industrialization and a sharp increase in global energy consumption have created a notable demand for renewable resources in the Indian energy market. In India, coal is still the primary energy-producing fuel, which meets more than 50% of the country's energy demand. Renewable energy resources are considered clean, since they reduce global CO2 emissions. Solar energy remains the most favoured renewable energy source throughout the globe. The rising interest in renewable energy sources has also motivated many Indian researchers to initiate or direct their research activities towards the production of clean energy. In the last decade, many deliberations and discussions have been aired concerning the techno-economic feasibility of solar power and eventually photovoltaic (PV) research has been encouraged by the Government of India [1]. The available PV cells can broadly be categorized into first-, second-, and third-generation solar cells. The first- and second-generation cells comprise Si-based crystalline cells and thin film-based cells, respectively. Third-generation solar cells are mainly based on new technologies, including organic PVs, dye-sensitized solar cells (DSSCs), and perovskite solar cells (PSCs).
Thin-film and Si-based solar cells have long been used due to their high efficiency and stability. However, they possess some critical drawbacks. Solar cells employing Si-based materials are expensive and are not considered environmentally friendly when disposed of in the environment. Moreover, the manufacture of silicon wafers is an exhaustive, energy-consuming, and tedious process [2, 3]. Research and development into economically viable and environmentally friendly non-silicon solar cells is thus very significant.
Among a variety of next-generation solar cells, DSSCs have been extensively studied as an economical and credible alternative to p–n junction PV cells, following the pioneering research led by Grätzel et al in 1991 [4–6]. Prof. Grätzel and his group achieved efficiencies of up to 7% in DSSCs using Ru-based N749 dye and TiO2-based photoanodes. The theoretical photo conversion efficiency (PCE) of DSSCs was estimated to be ∼20%, and the current performance of lab-scale DSSCs has reached ∼12%–14%. The current National Renewable Energy Laboratory (NREL) certified PCE for a DSSC is 12.3% [5]. Following the research activities by Grätzel's group, India also made great strides in developing DSSCs to improve their photovoltaic performance [6].
In this topical review, we summarize the research activities into DSSCs mainly performed by researchers in India. We begin with the operational principles and features of the various components of DSSCs. The research activities performed by various research groups in India into different components of DSSCs are highlighted thereafter. Emphasis is given herein to discussing the explorations of sensitizers that are alternatives to N3- and N719-based organometallic dyes for DSSC applications.
2. Constituents and operating principles of DSSCs
The general configuration of a typical DSSC is presented in schematic figure 1. A DSSC is usually composed of four components, namely a photoanode, a photosensitizer (dye), an electrolyte, and a counter electrode. In order to understand and improve the performance of DSSCs, knowledge about the individual components is important. The general features of these components are discussed in the following sections.
Figure 1. Schematic configuration of a dye-sensitized solar cell.
Download figure:
Standard image High-resolution image2.1. Photoanode
To prepare the photoanode, semiconducting metal oxides (SMOs) with wide bandgaps (such as TiO2 and ZnO) are coated over transparent conducting glass substrates. A photosensitizer, i.e., a dye, is then anchored to the metal-oxide layer. Under exposure to sunlight, the dye becomes photoexcited and injects electrons into the metal-oxide layer. The metal oxide then conducts the electrons from the dye to transparent conductive glass substrates in the anode. The composition, bandgap, morphology, and the coating thickness of SMOs influence the collection and conduction of photoexcited electrons.
2.2. Photosensitizer
A large variety of photosensitizers, including synthetic as well as natural dyes, have been investigated for DSSCs [7]. Synthetic dyes can, again, be classified into organometallic and organic dyes. Organometallic dyes contain transition metals within their structures, whereas organic dyes contain various organic chromophores. The most propitious sensitizers, in terms of efficiency and stability, are the Ru-bipyridyl compounds. These dyes have many advantages, such as stability, absorption of light in the visible range of the solar spectrum, and smooth electron injection and transportation. Even though Ru-based dyes can yield high PCEs, their preparations are multistep and time-consuming. Ru is also a rare metal and thus expensive. Scientists are looking for low-cost but efficient dyes as alternatives to Ru-based organometallic dyes.
The following characteristics are recommended for an efficient dye for DSSC applications.
- (a)The dye should absorb light from the entire spectrum of the visible and near-infrared regions.
- (b)The dye is required to have strong interaction with the metal oxides to be adsorbed on the semiconductor film. Reactive groups, such as carboxylates and phosphates, are introduced to form ester bonds with the metal oxide. This causes the absorbing light and electrons to be excited to the lowest unoccupied molecular orbital (LUMO); they are interposed into the metal oxide's conduction band (CB) without any loss.
- (c)The dye's energy levels should maintain the correct match ((LUMO)dye > (CB)metal oxide; (HOMO)dye < (HOMO)electrolyte) (HOMO stands for highest occupied molecular orbital) with the energy levels of the metal oxide and the electrolyte.
- (d)Dye aggregation should be avoided in order to minimize the excited state's nonradiative decay to the ground state [8].
- (e)The dye needs to have high chemical, heat, and light stability for long periods of operation, which is currently the major challenge for commercialization.
2.3. Electrolyte
Within a DSSC, the electrolyte plays an important role in regenerating the oxidized photosensitizer into its initial state. An electrolyte is usually composed of a solvent, additives, a redox couple, ionic liquids, and cations. A candidate material must have the following properties to be chosen as an electrolyte.
- (a)The redox couple must be capable of efficiently regenerating the oxidized dye.
- (b)The selected electrolyte should have long-term stability.
- (c)The electrolyte must be non-corrosive with respect to the other DSSC components.
- (d)It should permit the speedy diffusion of charge carriers between electrodes.
- (e)The absorption spectra of the chosen electrolyte must not overlap with the dye's absorption spectra.
Liquid, quasi-solid, and solid electrolytes have been studied for DSSC applications. However, the most efficient and low-cost electrolytes remain the iodide/tri-iodide (I−/I3 −)-based liquid-phase redox couple system. The (I−/I3 −) electrolyte system shows slow penetration into SMO films, fast dye regeneration, and low recombination losses for DSSCs. However, the corrosive/toxic nature and relatively high vapour pressure of I−/I3 − often create difficulties for the fabrication of cells. Apart from I−/I3 −-based electrolytes, various other redox couples such as SCN−/(SCN)2, SeCN−/(SeCN)2, Br−/Br3 − are also used for DSSC applications. Nevertheless, a variety of quasi-solid/solid-state electrolytes have been explored by different researchers for DSSC applications.
2.4. Counter electrode
In DSSCs, the counter electrode collects the electrons from the external circuit and catalyses the functioning of redox electrolytes. Usually, thin layers of noble metals, such as Pt, Au, or Ag, are coated onto transparent glass substrates to prepare the counter electrodes for DSSCs. However, researchers have also used low-cost conductive carbonaceous materials to make counter electrodes for DSSCs.
2.5. Transparent conductive substrates
It is shown in figure 1 that to fabricate a DSSC, two transparent and conductive substrates are required. The ingredients of DSSCs are sandwiched between these substrates. For a substrate to be utilized in DSSCs, two features play significant roles.
- (a)The substrate should have more than 80% transparency to permit an optimum amount of sunlight to impinge on the cell's effective area, i.e., it should provide excellent light transmittance.
- (b)For efficient charge transfer and reduced power losses in DSSCs, the substrate should possess high electrical conductivity.
Usually, fluorine-doped tin oxide (FTO), indium-doped tin oxide (ITO), and aluminium-doped zinc oxide (AZO) [9] are used as conductive substrates in DSSCs. The FTO-based cells, however, show the highest efficiencies due to fast charge transportation, as compared to their counterparts, ITO and AZO [10]. Comparisons of the performances of ITO-, FTO-, and AZO-coated glass substrates have been reported elsewhere by Patni et al [9].
2.6. Operating principles
The operating principles of DSSCs are comprehensively described in the literature. Herein, the operating principles of DSSC are further described for the convenience of readers by reference to figure 2, which was originally described by Sengupta et al elsewhere [10]. In DSSCs, light is first absorbed by the dyes which are anchored to the SMO layer. Photoexcited electrons from the dye molecules then reach the CB of the SMO and are eventually transferred to the conductive surface of the working electrode.
Figure 2. Schematic representation of the operating principle of dye-sensitized solar cells [10]. Reprinted from [10], Copyright (2016), with permission from Elsevier.
Download figure:
Standard image High-resolution imageWhen the working electrode is connected to the counter electrode through an external load, the photoexcited electrons reach the counter electrode. The redox electrolyte remains active for the regeneration of the oxidized dye by taking the electrons at the counter electrode. For effective performance of the DSSC, the regeneration of the dye by the electrolyte should be faster than the recombination of the photoexcited electrons. The desired processes, which include the photoexcitation of the dye (path 1), the collection and transportation of photoexcited electrons (path 2–4), the recycling of the redox couple (path 5) and the regeneration of the dye (path 6) are marked in figure 2. The competitive but unfavourable recombination of photoexcited electrons with oxidized dyes or tri-iodides (present in the electrolyte) are also labelled as paths 7 and 8 in figure 2.
Figure 3 represents a kinetic view of electron transfer in both charge transportation (blue arrows) and loss reactions (black arrows). The transportation of photoexcited electrons from the excited dye to the CB of the SMO should be faster (on the order of femtoseconds to picoseconds) than the process whereby the dye decays from the excited state to the ground state (nanoseconds). Listorti et al have reported that the rate of electron injection significantly depends on the electronic coupling between the LUMO of the dye's excited state and acceptor states of the SMO [11]. The restoration of the oxidized dye by the redox couple and the recycling of the redox couple at the counter electrode should be very fast to restrict the loss of photoexcited electrons. Therefore, studies of the dynamics of charge transportation within different junctions are essential in order to understand the performance of DSSCs.
Figure 3. Diagram of the energies and kinetics of photoexcited electrons within DSSCs. Reprinted with permission from [11]. Copyright (2011) American Chemical Society.
Download figure:
Standard image High-resolution image3. Photovoltaic parameters and their estimation
The performance of DSSCs is usually evaluated by the open-circuit voltage (VOC), the short-circuit current (ISC), the fill factor (FF), and the PCE. These parameters are determined from the I–V plot. Figure 4 shows typical I–V plots for photovoltaic devices. Descriptions of these parameters are given below.
Figure 4. Typical current–voltage (I–V) plots for DSSCs.
Download figure:
Standard image High-resolution image3.1. Open-circuit voltage (VOC)
The open-circuit voltage is the measured cell voltage when there is no current flow within the cell. It is the maximum voltage available from a solar cell when the resistance between the working and counter electrodes is infinite. This represents the difference between the redox potential of the electrolyte and the Fermi level of the semiconducting oxide.
3.2. Short-circuit current (ISC)
The short-circuit current is the output current of the cell when the voltage difference between the electrodes is zero. In other words, it is the current obtained from the cell when the load resistance is zero. Usually, it is presented in terms of the short-circuit current density (JSC), which is the ratio between the measured short-circuit current and the active area of the cell.
3.3. The fill factor (FF)
The ratio of the maximum power output (Pm) to the product of the short-circuit photocurrent (ISC) and the open-circuit photovoltage (VOC) is known as the FF. As can be seen from figure 4, the maximum power (Pm) is obtained by the multiplication of Im and Vm that respectively represents the photocurrent and photovoltage corresponding to the maximal power point. It represents the extent of the electrical and electrochemical losses within DSSCs:
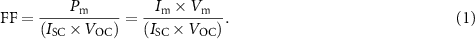
3.4. Photo conversion efficiency (PCE)
The overall power-conversion efficiency (η) measures the efficiency of the conversion of incident light into electrical energy. The PCE is calculated according to equation (2) using the parameters obtained from the I–V plot shown in figure 4:

where JSC, VOC, FF, and Pin are the short-circuit current density, the open-circuit voltage, the fill factor, and the incident power, respectively.
3.5. Incident photon to current conversion efficiency (IPCE)
IPCE, also known as quantum efficiency, is a measurement of the effectiveness of the conversion of light to electrical energy. It describes the generation of electrons with respect to the wavelength of the incident light. It shows the photocurrent of the solar cell when illuminated by monochromatic light. The following equation is used to experimentally estimate the IPCE:

4. Statistics for the worldwide DSSC research and India's contribution
In order to obtain statistics about DSSC research, we searched the Scopus database (www.scopus.com dated 6 May 2021) using the search phrase 'Dye Sensitized Solar Cells' and found the results shown in figure 5. Regarding figure 5(a), it is important to note that the number of Scopus indexed publications on the topic of DSSC up to the year 2000 is insignificant. However, the number of research publications increased significantly thereafter until 2014 and then started to decrease. Currently, the rate at which research articles on DSSCs are being published is falling, since the stability of DSSCs under ambient conditions is a major challenge and researchers have found technical limitations to the commercial viability of DSSCs.
Figure 5. Number of Scopus indexed documents on the topic of DSSCs published worldwide (a) by year (b) by country.
Download figure:
Standard image High-resolution imageFigure 5(b) represents the number of Scopus indexed documents per country, and it is notable that for the last two decades, researchers from India have put significant effort into DSSC research. Figure 6(a) shows the number of Scopus indexed documents from 1991 to 2021. It can be seen that researchers from India published in significant volume on the topic of DSSCs until 2019. However, the recent trend shows that researchers are no longer concentrating on further exploration of DSSCs. This may be because researchers are now more devoted to the exploration of perovskite-based solar cells, which are currently attractive as promising photovoltaic devices. Many organisations in India have studied DSSCs as photovoltaic devices. Figure 6(b) shows the different Indian institutes that have published significant Scopus indexed articles in DSSC research. The Scopus indexed articles published on the topic of DSSCs by researchers from India are shown in figure 6(c). Various funding agencies in India have supported research into DSSCs. Figure 6(d) shows a list of funding agencies acknowledged by various articles indexed in Scopus.
Figure 6. Number of Scopus indexed documents published in India (a) by year (b) by institution (c) by researcher (d) by funding agency.
Download figure:
Standard image High-resolution imageDutt et al have already mapped the research activities into DSSC performed by different research groups in India [12]. Their results reveal that among all the premier institutions in India, the CSIR-Indian Institute of Chemical Technology (CSIR-IICT), the University of Madras, Amrita Vishwa Vidyapeetham, Indian Institute of Technology, Roorkee (IITR), and the Indian Institute of Technology, Bombay (IITB) have contributed significantly to DSSC research.
5. Brief description of worldwide research into the different components of DSSCs
Prior to discussing the research and development activities pursued for different DSSC components in India, brief highlights of the international status of the advancement of DSSC research are described here. A year-by-year analysis by NREL (USA) from 1980 until now interprets the photoconversion efficiency of different types of solar cell, including DSSC, in figure 7. It is important to note that a significant improvement in the efficiency of DSSCs has not been observed within the last two decades. However, PSCs have emerged as promising photovoltaic devices within a short span of only one decade.
Figure 7. Year-by-year status of the efficiencies of different PV technologies. Reproduced with permission from National Renewable Energy Laboratory, Golden, CO.
Download figure:
Standard image High-resolution imageWe will discuss a few relevant research and development activities performed across the globe on the different components of DSSCs in the last five years. As reviewed, worldwide research has mainly concentrated on the development of low-cost, environmentally friendly, stable, and efficient DSSCs.
TiO2 and ZnO remain the most-studied materials for the photoanodes of DSSCs. Promising photovoltaic performances have been obtained using a few TiO2-modified photoanodes, which include SiO2/Ag/TiO2 [13], g-C3N4, and ZnO/TiO2 [14], multilayer TiO2 [15], Ag/TiO2 [16], Nb/TiO2 [17], CuO/TiO2 [18], carbon black-TiO2 [19], etc. Similarly, a few promising ZnO-based photoanodes are Mn/ZnO [20], Cu/ZnO [21], ZnO/TiO2 nanocomposite [22, 23], ZnAl-MMO/graphene [24], ZnO/MWCNT [25], Al/ZnO [26], Li/ZnO [27], Ga/ZnO [28], etc. Most of the aforementioned materials have shown promising electron transport in the photoanode, which enhances the cell efficiency.
Dye molecules are considered the most important component of DSSCs because they produce photoexcited electrons when exposed to light. The most efficient dyes for DSSCs are the ruthenium-based organometallic dyes (N3, N719, N749, etc). The research group of Aghazada et al from EPFL has reported a combination of ruthenium sensitizers and cobalt electrolytes that enhance the efficiency of DSSCs [29]. They have introduced six new cyclometalated ruthenium (II)-based dyes developed through ligand engineering. Some recent studies have incorporated alkyl tetrathienoacene- [30], N-hexylcarbazole- [31], triphenylamine- (TPA) [32], azobenzene- [33], etc, based metal-free sensitizers for DSSC applications. Li et al, in a detailed report on metal-free dyes, reported that the suitable incorporation of heteroaromatic units in dyes/spacers is beneficial in increasing DSSC performance [34]. It has also been reported that superior DSSCs can be fabricated by using dyes with improved wettability [34]. Dyes from natural resources are also very popular as sensitizers for DSSC. Taya et al have investigated the DSSC performances of 11 natural dyes, which were extracted from various flowers, fruits, and leaves, and used as sensitizers for DSSC applications [35]. Recently, Yildiz et al published their work on black mulberry (Morus nigra) and madder (Rubia tinctorum) root-based sensitizers for DSSCs [36].
Electrolytes are another very crucial part of regulating the efficiency of DSSCs. To date, a number of research groups have put their efforts into developing superior redox couple/electrolytes for DSSC. Since the introduction of the iodide/tri-iodide redox couple as an electrolyte by Grätzel and his group, it has remained popular for DSSC applications [4, 37]. A recent report by researchers from Malaysia and Sweden has revealed a polyacrylonitrile-based gel polymer electrolyte which shows superior charge transport characteristics in DSSCs [38]. Çetin et al have reported poly (2-acrylamide-2-methylpropane sulfonic acid/itaconic acid/N,N'-methylenebisacrylamide) (poly (AMPS-co-IA)) hydrogel and its fluorine-, bromine-, chlorine-, and aniline-doped derivatives as a gel polymer electrolyte for DSSC applications [39]. These types of electrolyte are more studied than liquid electrolytes due to their comparatively higher stability and longevity. A few recent articles have reported complex variants such as a poly (ethylene oxide) (PEO)/poly (vinyl alcohol)-based gel polymer electrolyte [40], a polyurethane acrylate with tetrabutylammonium iodide gel polymer electrolyte [41], etc. Han et al developed a unique electrolyte which is easy to use in injection processes due to its liquid nature, but inside the device, it undergoes an in-situ transformation from a liquid to a gel-based substance, showing superior cell efficiency [42]. A quasi-solid-state composite electrolyte [43], a double-layered printable electrolyte [44], a solid-state lamellar-nanostructured polymer electrolyte [45], etc, have also been reported by researchers. These types of efficient electrolyte help to make a good interfacial connection with the counter electrode.
An efficient counter electrode should have minimal charge-transfer resistance and higher current density to facilitate the reduction of the electrolyte. Apart from the most-applied Pt electrode, several other materials are used for the counter electrode. Among the many recently developed cathode materials, some selected materials are disulfide/thiolate/graphene [46], gold [47], mesoporous carbon [48], carbon/silver nanowire/copper nanowire/polyacrylate polymer [49], polyaniline/graphene oxide [50], Cu2Cu1−x Mnx SnS4 [51], NiCo2S4 [52], CoS [53], etc. A few recent reports have also included the use of complex and conductive polymers such as PEDOT:PSS [54], chalcogenides [55], poly-pyrrole [56], etc.
The aforementioned discussion on the international research scenario is important before describing the progress of research into DSSCs in India.
6. Highlight on the research activities in India on different components of DSSC
6.1. Photoanodes
In India, researchers also extensively studied TiO2, ZnO, and their modified counterparts as photoanode materials for DSSC applications. TiO2 and ZnO-based nanowires, nanofibers, nanorods, etc, have already been studied for DSSCs. Table 1 briefly summarizes a few activities carried out by different research groups in India to explore the use of 1D TiO2 and ZnO as photoanodes for DSSCs.
Table 1. 1D ZnO and TiO2 nanostructures as photoanodes for DSSCs explored by Indian researchers.
| Material | Morphology | η (%) | Reference |
|---|---|---|---|
| 1D TiO2 | Nanoparticles | 2.17 | [57] |
| Nanorods | 4.28 | [58] | |
| Nanotubes | 2.38 | [59] | |
| Nanotubes/Nanoflakes | 7 times increase | [60] | |
| Nanoparticles/Nanofibers | 7.89 | [61] | |
| 1D ZnO | Nanowires | 4.7 | [62] |
| Nanorods | 5.105 | [63] | |
| Nanofibers | 2.97 | [64] | |
| Nanotips | 1.29 | [65] | |
| Nanoflake | 2.95 | [66] | |
| Nanoparticles | 3.50 | [67] |
It has been reported that the ZnO-SnO2-based ternary oxide Zn2SnO4 has performed better than its binary counterparts ZnO and SnO2, providing a potential alternative for the development of photoanodes [68]. As an alternative to ZnO, a ZnO-graphene nanocomposite has shown a PCE of 3.17%, i.e. 53% higher than that of bare ZnO-based DSSCs [69]. Hydrothermally prepared ZnO with simple micro-rods and nano-tip-decorated micro-rods have been examined as photoanode materials by Das et al. The surface of the nano-tip-decorated micro-rod, which is uneven and patterned provides a higher PCE than the simple micro-rods. Moreover, they reported that with the inclusion of a thin passivation layer, the PCE is further enhanced [70]. TiO2 has been modified with different components to achieve higher efficiency. For a modified TiO2 photoanode with size-selective N, F, and S co-doped graphene quantum dots (NFS-GQDs), an enhanced PCE of 11.7% was achieved. The incorporation of size-controlled, heteroatom-doped GQDs enriched the efficiency of DSSCs by permitting more optoelectronic operation [71]. A TiO2-graphene 0.25 wt.% nanocomposite has shown an elevated photocurrent density (Jsc) of 18.4 mA cm−2 and an efficiency (n) of 4.69% more than that of pristine TiO2 [72]. The influence of different metallic ion (e.g. Al3+, Nb5+, W6+, Mg2+, Zr4+, Ce4+, etc.) and non-metal (e.g., B, C, N, S, F, etc.) -doped titania and zinc oxide photoanodes for DSSC applications has also been discussed in the literature [73]. The addition of 3 at. % Co2+ into pristine BaSnO3 nanostructures enhanced the PCE to 8.22% [74]. Table 2 summarizes the advantages and drawbacks of TiO2, ZnO, and SnO2 in the preparation of photoanodes for DSSCs. TiO2 is still favoured, as it is cost-effective and non-toxic, with adequate stability. Generally, TiO2 films in the anatase phase are preferred over the rutile phase.
Table 2. Advantages and disadvantages of prominent metal oxides used as photoanodes.
| Metal oxides | Advantages | Disadvantages | References |
|---|---|---|---|
| TiO2 | • Abundant commercial availability, low-cost, biocompatible, non-toxic • Stable n-type semiconductor • Better photostability • Fast electron injection rate | • Limited electron mobility • High recombination | [75] |
| ZnO | • Heterogeneity in ZnO nanostructures • High electron mobility | • Complexation with dyes • Dye-dependent performance | [76] |
| SnO2 | • High electron mobility • Sizeable bandgap | • Lower electron injection • Faster electron recombination • Less adsorption of dye | [77] |
Various studies have also shown that the PCE of TiO2-based DSSCs can be enhanced by the incorporation of particular metal nanoparticles such as gold (Au) and silver (Ag) which can generate plasmonic optical and electrical behaviours in TiO2, owing to their specific localized surface plasmon resonance (LSPR) properties. Recently, Kaur et al reported bimetallic Au and Ag metal nanoparticles embedded in a TiO2 based photoanode for DSSC applications [78]. The embedded-metal TiO2 system facilitates the light harvesting of N719 dye because of the well-matched LSPR absorption band, resulting in a PCE enhancement of 88%, compared to a bare TiO2 photoanode. Parveen et al synthesized TiO2 nanoparticles with embedded Ag by electrochemical deposition from a silver nitrate aqueous solution on a TiO2 film [79]. The authors of that paper reported that the incorporation of plasmonic silver nanoparticles significantly increased the photocurrent density using 'Rose Bengal' dye as a sensitizer. In this case, the surface plasmon resonance effect increased the interaction of light with the dye and actuated charge separation by the establishment of a Schottky barrier between the Ag nanoparticles and the TiO2. Using the same dye, the same group previously reported a TiO2-based photoanode with embedded plasmonic nanoparticles for DSSC applications due to copper being cheaper and more abundant in nature [80]. Excellent PCE enhancement was generated by the intense light absorption due to the LSPR of Cu nanoparticles incorporated into DSSCs. Saravanan et al explained the plasmonic effect by the inclusion of green synthesized silver nanoparticles from the Peltophorum pterocarpum flower into TiO2 photoanodes for DSSC applications [81]. Due to the plasmonic effect, the PCE of the solar cell was increased from 2.83% to 3.62% after the integration of the silver nanoparticles. Solaiyammal et al used Au rather than Ag to develop hydrothermal-route-derived Au@TiO2 core–shell nanostructures as a photoanode component for DSSC applications [82]. The short-circuit current density of a Au@TiO2-based DSSC showed an increase of 32.7% in comparison to a pristine TiO2-based DSSC. This is because the Au@TiO2-based DSSC had a direct coupling between the surface plasmon resonance of the gold nanoparticles and the N719 dye molecules. Since the surface plasmonic resonance had a connection not only with the photoanode but also with the dye, we have also described some other instances of the SPR effect in the upcoming section. Apart from the conventional TiO2, ZnO, and SnO2-based photoanodes, several others have also been progressed by researchers from India (summarized in table 3).
Table 3. Modified photoanodes studied in India for DSSC applications.
| Photoanode material | Synthesis procedure | Other components | Reported efficiency | Indian institute | Author's claim | Reference |
|---|---|---|---|---|---|---|
| ZnO-TiO2 | Prepared by sol-gel spin coating technique, followed by facile hydrothermal technique. | Dye-N719 | NNAsa-1.47% | CSIR-Central Electrochemical Research Institute | More surface area for dye loading with better light absorbance, higher recombination resistance and electron lifetime. | [83] |
| CE-Pt | NWAsa-0.91% | |||||
| CS-FTO glass substrate; Electrolyte-Mixture of 1-methyl-3-propylimidazolium iodide, LiI, iodide, and tertbutyl pyridine | ||||||
| B-GQDs-ZnO nanocomposite a | Synthesized via a microwave reactor-assisted process from bulk B4C crystals. Nano-ZnO was then incorporated. | Dye-N719 | 3.7% | CSIR-Central Electrochemical Research Institute | Increased amount of dye loading, extended optical path length, and fast electron transport properties. | [84] |
| CE-Pt | ||||||
| CS-FTO glass | ||||||
| Electrolyte-LiI and iodine solution in acetonitrile. | ||||||
| α-Bi2O3 | Synthesized by citrate, nitrate gel formation. | Dye-N719 and Eosin Y | N719-0.09% | University of Calicut | Poor dye attachment and back recombination, resulting in poor efficiency. | [85] |
| CE-Pt | Eosin Y-0.05% | |||||
| CS-TCO | ||||||
| Sn-ZnO | Spray pyrolysis technique | CS-ITO-coated glass substrates | 0.74% | Annamalai University | Large surface area for adsorption of dye molecules. | [86] |
a NNA—nano-needle array; NWA—nano-wire array; B-GQD—boron doped-graphene quantum dots.
Double-perovskite oxides, A2LuTaO6 (A = Ba, Sr, Ca) have also been explored as alternatives to TiO2- and ZnO-based photoanodes, since these are highly conductive (nearly 104 times that of TiO2) with a wide bandgap [87].
Doping with different auxiliary components also leads to promising performance for TiO2 and ZnO photoanodes. Ag [88], poly (N-vinyl carbazole) [89], Cu [21], CeO2 [90], Eu [91], Co [64], TiO2-Au nanocomposites [92], and poly iso-thionaphthene [93] provide appropriate doping, which provides better PCEs than undoped photoanodes under similar conditions.
Graphene-based nanocomposite materials display unique efficiency-enhancing properties, such as an electron-conducting layer, a transparent conductive electrode, and a sensitizer for DSSC photoanodes. Graphene‐based materials, such as pristine graphene, graphene oxide, reduced graphene oxide, and graphene quantum dots possess potential properties for various elements of DSSC photoanode functions [94].
6.2. Electrolytes
The I−/I3 − redox couple is studied extensively by researchers in India as an electrolyte for DSSCs. I−/I3 − integrated into a polyaniline/thiourea matrix is also used for solid-state DSSC applications. This optimization has resulted in an elevated open-circuit voltage and short-circuit current density, leading to a significant increase in the PCE [95]. A quasi solid-state DSSC has also been proposed with a stable gel electrolyte employing PEO–poly-ethylene glycol (PEG). Under optimized conditions, the PCE of the gel-state DSSC with NaI is found to be 5.4% at 100 mW cm−2 with an air mass (AM) 1.5G filter. When nanocrystalline TiO2 is used as a filler in the gel electrolyte, the PCE is found to increase by 6.3% [96]. Electrolytes have also been fabricated using liquid (Triiodide) and gel-based polymers (Gelator). The electrolyte frame is composed of polymethyl methacrylate (PMMA), tetrahydrofuran, propylene carbonate, and ethylene carbonate, and uses triiodide as a liquid electrolyte. For a ratio of 8:2 liquid to gelator solution, the DSSC achieved an efficiency of 11.32% [97]. Roy et al developed a series of pyridinium-imidazolium-based tri-cationic ionic crystals to be used as an electrolyte in DSSCs. These compositions were specifically selected for their high conductivity, diffusion coefficient, and thermal stability. The PySCN ionic crystal-based solid electrolyte has showed an enhanced efficiency of 7.7% under 100 mW cm−2 [98].
Polymer and biopolymer electrolytes have also been studied for DSSC applications. A 1-ethyl-3-methylimidazolium dicyanamide-doped solid polymer electrolyte was employed, and promising efficiency was reported [99]. A few other electrolytes which have been studied in India for DSSC applications are summarized in table 4.
Table 4. Various electrolytes developed for enhanced efficiency.
| Electrolyte detail | Synthesis procedure | Other components | PCE | Indian institute | Author's claim | Reference |
|---|---|---|---|---|---|---|
| c-MWCNT containing PEO/polyaniline (PAni) | In-situ polymerization of aniline in a mixture of PEO and c-MWCNT | Photoanode—0.5 wt% Ce3+-doped TiO2 | 4.08% | Tezpur University | Improved ionic conductivity of composite electrolytes and enhanced interfacial contact between electrode and electrolyte. | [100] |
| PAN/CoS electro-spun nanocomposite membrane electrolytes (2 wt.%) | Electrospinning carried out after the normal hydrothermal process, and then soaked in an ionic liquid containing LiI, I2, 4-Tert-butyl pyridine and 1-butyl-3-methylimidazolium iodide in acetonitrile | Photoanode—TiO2 | 7.41% | Centre for Nanoscience and Technology | CoS proved to be an efficient filler, improving ion transport kinetics, and better electrocatalytic activity. | [101] |
| CE-Pt | ||||||
| CS-ITO glass substrate | ||||||
| Dye-N719 | ||||||
| PEO-PEG gelated alkali electrolytes | PEO and PEG were both mixed with electrolyte containing iodine, DMPII, TBP in acetonitrile, and made to completely dissolve. | Photoanode—TiO2 | 5.4% | CSIR—Central Electrochemical Research Institute | High conductivity, comparatively higher than its normal ionic electrolyte. | [96] |
| CS-FTO glass substrate | ||||||
| Dye-N719 | ||||||
| 30 wt % 2-MCP-doped PEO-PMMA-KI-I2 | The electrolyte was made by a solution cast technique. | Photoanode—TiO2 | 3.0% | Thiruvalluvar University | The study revealed that the solvent plays an important role on the conductivity of solid polymer electrolytes. | [102] |
| CS-FTO glass substrate | ||||||
| Dye-N719 | ||||||
| CE-Pt | ||||||
| Guar gum (0.6 g) gel electrolyte | Prepared by continuously stirring LiI, I2, 1-methyl-3-propyl imidazolium iodide, 4-tertbutyl pyridine, and poly (ethylene glycol) in acetonitrile. | Photoanode—TiO2 | 4.96% | National Institute of Technology, Trichy | Improves the conducting properties of the DSSC. Moreover, it benefits from easy fabrication, high efficiency and low cost. | [103] |
| CS-FTO glass substrate | ||||||
| CE-Pt | ||||||
| 2-MCBT doped PVDF-HFP/KI/I2 | Synthesized using a solution cast technique using N, N-dimethyl formamide (DMF) as a solvent. | Photoanode—TiO2 | 3.46% | Thiruvalluvar University | Significant improvement in ionic conductivity and amorphous nature. | [104] |
| CE-Pt | ||||||
| CS-FTO glass | ||||||
| DYe-N3 |
6.3. Counter electrodes (CEs)
Pt-coated FTO/ITO is mainly used as the counter electrode in DSSCs due to its high electrical conductivity, electrolytic activity, and corrosion resistance [105]. Alternatives have also been sought, due to the high cost of platinum CEs. A thin-film nickel-doped molybdenum oxide counter electrode has been studied as an alternative to platinum. It has been reported that 10 mol% Ni doping yielded the optimum efficiency [106]. Carbon-based CEs have also been studied for use in DSSCs, and these provided a high surface area with a porous morphology, leading to many reduction sites and low charge-transfer resistance [107]. Nitrogen-containing tubular carbon with hierarchical pore networks activated by polypyrrole nanotubes (APNT) has also been employed as a CE. APNT shows exceptional superior catalytic activity toward triiodide (I3 −) reduction. An APNT-based DSSC has exhibited a PCE of 6.29%, comparable to that of a standard Pt-based DSSC (6.80%) [108]. It has been reported that MnNx /C, a non-Pt metal catalyst integrated through the high-pressure pyrolysis route, can be effectively used as an alternative to Pt for DSSCs [109].
Table 5 summarizes the different cathodes that have been studied in India for DSSC applications.
Table 5. Different counter electrodes studied in India for DSSC applications.
| Cathode material | Synthesis procedure | Other components | Reported efficiency | Indian institute | Author's claim | Reference |
|---|---|---|---|---|---|---|
| Carbon | Graphitization of sugar free (a commercial sugar substitute substance) at high temperature in flowing argon | CS-FTO glass substrate | 6.72% | Indian Institute of Technology, Bombay | Has a comparable efficiency to those of Pt-based CEs. (6.72% vs 8.19%) | [110] |
| Carbon | Obtained by carbonization of the organic ligand (2-methyl-8-hydroxy quinolinol (Mq)) at 1200 °C in flowing argon gas. | Electrolyte — a mixture of guanidinium thiocyanate, iodine, 4-tert-butylpyridine, 1-methyl-3-propylimidazolium and iodide [PMII] in a mixture of Valero nitrile and acetonitrile. | 4.25% | Indian Institute of Technology, Bombay | Has a comparable efficiency to 5.86% of Pt-based CEs under similar conditions. | [111] |
| Graphene nanoplatelets (GNPs) | The CE was prepared from natural graphite by the simple and low-cost liquid-phase high shear exfoliation method. | Photoanode—TiO2 | 6.23% | Indian Institute of Technology, Bombay | Has a comparable PCE to those of Pt-based CEs. | [112] |
| CS-FTO glass | ||||||
| Electrolyte-LiI, I2, 4-tert butyl pyridine and 1-butyl-3-methylimidazolium | ||||||
| Iodide in acetonitrile | ||||||
| rGO-NiCo2S4 | Nanocomposites of reduced graphene oxide (rGO) and NiCo2S4 with different amounts of GO are synthesized through a one-step solvothermal method. | Dye-N719 | 8.15% | CSIR-Central Electrochemical Research Institute | PCE remarkably higher than that of pristine NiCo2S4 (7.36%), and Pt (7.23%) under similar experimental conditions. | [113] |
| Photoanode—TiO2 | ||||||
| CS-FTO glass substrate | ||||||
| Co-Ni selenide nanoparticles | A nanohybrid comprising Co0.50Ni0.50Se nanoparticles dispersed on graphene nanosheets was synthesized by a simple one-step hydrothermal method. | Dye-N719 | 9.42% | Centre for Nanoscience and Technology | PCE relatively higher than that of DSSC fabricated with Co0.5Ni0.5Se (7.59%) and std. Pt (7.68%) CEs. | [114] |
| Photoanode—TiO2 | ||||||
| CS-ITO glass substrate | ||||||
| Electrolyte-LiI, I2, TBP, and 1-butyl-3-methylimidazolium iodide (ionic liquid) in acetonitrile. | ||||||
| Nanoparticle-embedded carbon nanofibers | Polyacrylonitrile was dissolved in N, N-dimethylformamide. To this, Ni acetate and Fe nitrate were added, and the solution was stirred. Electrospinning was carried out, the product was stabilized, followed by carbonization in a nitrogen atmosphere. | CS-ITO glass plates | 4.7% | Centre for Nanoscience and Technology and Indian Institute of Technology, Bombay | Enhanced electrocatalytic activity, electrochemical stability, charge transfer rate and faster reaction kinetics with high exchange current density. | [115] |
| Photoanode—TiO2 | ||||||
| Dye-Di-tetra-butyl ammonium cis-bis(isothiocyanato) bis(2,2'-bipyridyl-4,4'-dicarboxylato) ruthenium (II) | ||||||
| Palladium selenide | Prepared using a single-source molecular precursor by thermolysis. | Photoanode—TiO2 | 7.45% | Indian Institute of Science, Bangalore | The Pd17Se15 film shows superior activity as the CE for DSSCs. | [116] |
| CS-FTO glass substrate | ||||||
| Dye-N719 | ||||||
| Electrolyte-LiI, I2 in dry acetonitrile along with 1,2-dimethyl-n-propylimidazolium iodide, and 4-tertbutylpyridine | ||||||
| CoNi2S4-RGO nanohybrid | The CE was synthesized using a simple one step hydrothermal method. | Photoanode—TiO2 | 9.22% | National Institute of Technology, Durgapur | The nanohybrid structure provided fast ion diffusion pathways, large accessible surface area, and good chemical and thermal stability. | [117] |
| CS-FTO | ||||||
| Dye-N719 | ||||||
| Electrolyte-I2, LiI, and LiClO4 and dissolved in anhydrous acetonitrile | ||||||
| C/ZnAl-LDH | Pyrolysis of ZnAl-LDH using a glucose solution. | CS-FTO glass substrate | 3.18% | Vellore Institute of Technology, Vellore | The PCE was comparable with those of Pt-based DSSCs (4.62%). | [118] |
| Photoanode—TiO2 | ||||||
| Dye-ruthenizer 535-bis TBA | ||||||
| MoSe2/PANI | Hydrothermally prepared MoSe2 was mixed with PANI nanofibers in deionized water by using an ultra-sonicator and then filtered and dried at 100 °C. | Photoanode—TiO2 | 8.04% | Centre for Nanoscience and Technology | Good electrocatalytic activity, fast electron transport rate with increased current flow. | [119] |
| CS-FTO | ||||||
| Dye-N719 |
7. Description of Indian research into sensitizers used in DSSCs
7.1. Organometallic dyes
Organometallic dyes are composed of transition metals Ru, Os, Ir, etc, and organics. Ru (II) metal always remains a rational preference for DSSC applications for the following reasons.
- (a)Its octahedral geometric structure helps in the smooth addition of ligands.
- (b)The attractive photophysical, photochemical, and electrochemical properties of Ru (II) complexes can be tuned to produce the requisite properties.
- (c)It possesses stable and affordable oxidation states from I to IV.
- (d)It is stable in many solvents. ML2(X)2 is the generic structure of the sensitizer preferred as a dye. M depicts a metal, L, a ligand 2,2'-bipyridyl-4,4'-dicarboxylic acid, and X, a halide, cyanide, thiocyanate, acetylacetonate, thiocarbamate, or water substituent group.
Naik et al developed a dye by incorporating simple aniline-based dyes A1–4 into an N3 dye to co-sensitize a DSSC. These co-sensitizers contain a N, N-dimethylaniline ring as the donor scaffold, which is associated with electron-withdrawing functionalities, viz. barbituric acid (A1), N, N-dimethyl barbituric acid (A2), thiobarbituric acid (A3), and N, N-diethyl thiobarbituric acid (A4) as acceptor/anchoring units. The solar cell they developed achieved a PCE of 7.02% [120].
Kalaiyar et al synthesized a new heteroleptic dual-anchored Ru (II) complex (RNPDA) using a 4-nitro-phenylenediamine (NPD-PC) Schiff base as a ligand. Schiff-based metal complexes have been found to be potential sensitizing materials, due to their photophysical properties [121]. The dye contains an electron-withdrawing group of pyridines and an anchoring unit of the nitro group. As a photosensitizer for DSSCs, it has demonstrated an overall PCE of 3.42%.
7.2. Metal-free dyes
Metal-free organic dyes are also used for DSSCs, and in a few cases, the PCEs obtained from the respective cells have even been analogous to those of Ru-based sensitizers. The benefits of selecting metal-free organic dyes as substitutes for Ru-, Os-, and Ir-based complexes are their very high molar extinction coefficients, easily tuned absorption energies, and comparatively lower cost. Moreover, these are free from toxic and expensive Ru metal. These materials can be used as both sensitizers and co-sensitizers to obtain a broad absorption spectrum [122].
Metal-free organic dyes have mostly been designed to have an electron donor (D), a π-conjugation bridge, and an electron acceptor (A), with a push-pull configuration of D-π-A. The photoinduced intramolecular electron transfer between donor and acceptor is accelerated through the π-electron bridge with a strong polarity effect, and may produce a considerable PCE. The HOMO level can be related to the donor and the π-conjugate; the LUMO level depends on the electron acceptor and hence can be finely tuned to achieve enhanced efficiency. The donor groups are selected from electron-rich halves, such as phenylamine, aminocoumarin, indoline, (di-fluorenyl) TPA, TPAs, and carbazoles. The Π-conjugated groups are chosen from thiophenes, polyenes, and benzothiadiazole. Cyanoacrylic acid, rhodamines, and pyridines are chosen as acceptors.
Tetrathiafulvalene (TTF), a sulfur-containing compound, acts as a strong electron donor and has found significant practical applications. TTF-based donors can be easily tuned and have found considerable success in sensitizing solar cells. Along with TTF, dithiafulvalene (DTF) is also considered to be a suitable electron donor due to its unique charge-transport characteristics. DTFs are electron donors and have consistently provided high efficiencies. Giribabu et al have put together an excellent summary of TTF- and DTF-based donor dyes, and they have achieved promising efficiencies [123].
Further studies have been conducted using N-annulated perylene (NP)-based D-π-A structured sensitizers. These dyes contain TPA derivatives connected to NP, thiophene, and 2-cyanoacetic acid as electron donors, conjugated linkers, and acceptors, respectively. It has been observed that (E)-2-cyano-3-(10-(4-(diphenyl amino) phenyl)-1-(2-ethylhexyl)-1H-phenanthro [1,10,9,8] carbazol-3-yl) acrylic acid (NPS-4) is the best among them [124].
Apart from the D-π-A configuration, D-D-π-A and D-A-π-A have also been studied by researchers in India, and results have been reported for enhanced DSSC PCEs. Metal-free D-A-π-A-type dyes (E1–3) with various acceptor/anchoring groups have been designed and synthesized as useful sensitizers for nanocrystalline TiO2-based DSSCs. All these dyes carry an electron-donating methoxy group as an auxiliary and an indole as the principal donor, cyano-vinylene as an additional acceptor, and thiophene as a π-spacer, whereas cyanoacetic acid, rhodanine-3-acetic acid, and 4-aminobenzoic acid act as acceptor/anchoring moieties, respectively. An E3 based DSSC has achieved an overall PCE of 4.12% under standard global AM 1.5G [125].
N, N'-para-aminobenzoic acid (PABA) based on N, N-dimethyl-4-vinyl aniline carrying 4-amino benzoic acid as an acceptor were used as sensitizers by Naik et al to sensitize a TiO2 photoanode. The N, N-dimethylaniline ring functions as an electron donor in sensitizers, while para-aminobenzoic acid functions as an electron acceptor or anchoring unit. DSSC devices fabricated using this dye under AM 1.5G simulated solar radiation revealed a PCE of 1% [126].
Structural modifications in porphyrin sensitizers have been shown to have a significant effect on the PCE of DSSCs. Various positional alternations of donors and anchoring groups have been reported by Duvva et al to improve the IPCE and PCE values [127].
Chandrasekharam et al developed two D-π-A organic sensitizers, SPSGOD3 and SPSGOD4, which differed in their anchoring points (cyan acrylic acid and rhodanine-3-acetic acid) on a typical donor (N-(9,9-dihexyl-9H-fluoren-2-yl)-9,9-dihexyl-N-phenyl-9H-fluoren-2-amine) and π-spacer (furan). SPSGOD3 showed a maximum IPCE of 79% at 485 nm with a PCE of 6.21%, whereas SPSGOD4 reached a maximum PCE of 67% at 536 nm and a PCE of 3.78% [128]. Table 6 lists the different metal-free dyes explored in India for DSSC applications.
Table 6. Metal-free dyes studied in India for DSSC applications.
| Metal-free organic dye | Dye structure | Reported efficiency | Indian institute | Author's claim | Reference |
|---|---|---|---|---|---|
| E1 (2-cyano-3-(5-((Z)-1-cyano-2-(1-hexyl-5-methoxy-1H-indol-3-yl) vinyl) thiophen-2-yl) acrylic acid) |

| 1.48% | National Institute of Technology, Surathkal, Karnataka | E3 dye was claimed to have the highest photovoltaic performance, both in DFT studies and experiment. | [125] |
| E2 (2-((Z)-5-((5-((Z)-1-cyano-2-(1-hexyl-5-methoxy-1H-indol-3-yl) vinyl) thiophen-2-yl) methylene)-4-oxo2-thioxothiazolidin-3-yl) acetic acid) |

| 0.046% | |||
| E3 (4-((E)-(5-((Z)-1-cyano-2-(1-hexyl-5-methoxy-1Hindol-3-yl) vinyl) thiophen-2-yl) methylene amino) benzoic acid) |

| 4.12% | |||
| MCG1 |
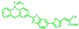
| 4.52% | CSIR-Indian Institute of Chemical Technology (IICT) | Benzothiazole facilitates effective electron transfer from the donor to the anchoring group and lowers the bandgap. | [129] |
| MCG2 |

| 5.73% | |||
| MCG3 |

| 5.71% | |||
| MCG4 |
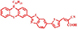
| 6.46% | |||
| SPDGOD3 (3-(5-(4-(bis(9,9-dihexyl-9H-fluoren-2-yl) amino) phenyl) furan-2-yl)-2-Cyano-acrylic acid) |
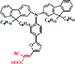
| 6.21% | CSIR-IICT | Two D-π-A organic dyes with different anchoring points showed varying efficiency and varying IPCE at different wavelengths. | [128] |
| SPDGOD4 (2-(5-((5-(4-(bis(9,9-dihexyl-9H-fluoren-2-yl) amino) phenyl) furan-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl) acetic acid) |
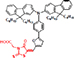
| 3.78% | |||
| 3-{4-[Bis-(4-pyridin-2-ylethynyl-phenyl)-amino]-phenyl}-2-cyano-acrylic acid |
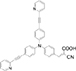
| 5.16% | Indian Institute of Technology, Indore | Reported shorter electron transport time, longer electron lifetime and high charge-recombination resistance. | [130] |
| 3-{4-[Bis-(4-pyridin-3-ylethynyl-phenyl)-amino]-phenyl}-2-cyano-acrylic acid |

| 4.27% | |||
| 3-(4-(diethylamino)phenyl) acrylic acid |
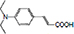
| 1.38% | National Institute of Technology, Trichy | Broadened absorption band with high molar extinction coefficient and negative shift of LUMO level, required for an effective electron injection. | [131] |
| 2-(5-(4-(diethylamino) benzylidene)-4-oxo-2-thioxothiazolidin-3-yl) acetic acid |
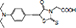
| 0.82% | |||
| 2-(4-(diethylamino) phenyl) benzimidazole5-carboxylic acid |
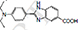
| 0.22% |
7.3. Natural dyes
Natural sources such as fruits, flowers, leaves, and bacteria exhibit different colours and contain numerous pigments that can be extracted and used in DSSCs [132, 133]. The advantages of selecting these natural dyes are their large absorption coefficients in the visible region, copious presence, convenient preparation methodologies, and environmental friendliness [134, 135]. Although they provide much less efficiency than Ru-based dyes due to their selective light absorption, a mixture of natural dyes has been investigated for a possible improvement of their potential [136]. Descriptions of a few of the natural dyes explored by researchers in India are given in the forthcoming discussions.
7.3.1. Chlorophyll
Chlorophyll is one of the most important green-coloured biological pigments and is found in all photosynthetic plants, algae, and cyanobacteria. It is a chelate compound consisting of carbon, hydrogen, oxygen, nitrogen, and magnesium ions. There are four types of chlorophyll: chlorophyll a, chlorophyll b, chlorophyll c, and chlorophyll d. Chlorophyll a and chlorophyll b differ slightly in the composition of a sidechain—they have compositions of C55H72O5N4Mg and C55H70O6N4Mg, respectively. The most efficient is a derivative of chlorophyll 'a' (methyl trans-32-carboxy-pyropheophorbide).
7.3.2. Flavonoids
Flavonoids are the most widespread and physiologically active group of biological pigments present in bryophytes, gymnosperms, angiosperms, and ferns. Their organic structure is based on 15 carbon atoms with 2 phenyl rings, connected by a basic C6–C3–C6 skeleton. They absorb light in the visible region efficiently.
7.3.3. Anthocyanins
Anthocyanins are glycoside salts of phenyl-2-benzopyrilium based on a C15 skeleton, with a chromane ring bearing a second aromatic ring of C in position 2 (C6–C3–C6). Anthocyanin molecules have carbonyl and hydroxyl groups, which can bind to the surface of TiO2 semiconductors. This helps in the excitation and transfer of electrons from the anthocyanin molecules to the CB of TiO2/ZnO.
7.3.4. Carotenoids
Carotenoids or tetraterpenoids are a massive family (over 600 members) of isoprenoids that are found in photosynthetic plants, crustaceans, and bacteria. They can be differentiated by the presence of a C40 hydrocarbon backbone, which is responsible for structural and oxygenic modifications. Their light absorption is achieved by the photo-induced transformation of 'p' delocalized electrons of carotenoid molecules, which forms a singlet chlorophyll state with a slightly higher energy when transferred to the chlorophyll molecules. Chlorophyll's physical structure facilitates this transfer of energy from carotenoids to chlorophyll molecules.
7.3.5. Tannins
Tannins are biological plant pigments, which are present in gymnosperms and angiosperms. Tannins can be classified as hydrolyzable tannins and non-hydrolysable tannins (also known as condensed tannins).
7.3.6. Betalains
Betalains are pigments present in fungi and caryophyllales and can be seen in red to purple colours. Depending on their pigments, betalains can be classified into betacyanins and betaxanthins. Betalains have antioxidant and light-absorbing properties. They are stable in an acidic environment. Table 7 summarizes different types of natural pigments, their categories, and sources.
Table 7. Different types of natural pigments, their categories, and sources.
| Pigment | Category | Source |
|---|---|---|
| Flavonoids [137] | Flavones | Bryophytes |
| Flavonols | Gymnosperms | |
| Flavanones | Angiosperms | |
| Flavanonol | Ferns | |
| Flavanols or Catechins | ||
| Anthocyanins | ||
| Chalcones | ||
| Chlorophyll [138] | Chlorophyll a | Al photosynthetic plants |
| Chlorophyll b | Cyanobacteria | |
| Chlorophyll c | Algae | |
| Chlorophyll d | ||
| Carotenoids [138] | Carotenes | Crustaceans |
| Xanthophyll | Bacteria | |
| Photosynthetic plants | ||
| Tannins [139] | Hydrolysable tannins | Gymnosperms |
| Condensed tannins | Angiosperms | |
| Betalains [138] | Betacyanin | Caryophyllates |
| Betaxanthin | Fungi |
Table 8 summarizes the performance of various natural dyes that have been studied in India for DSSC applications.
Table 8. PCEs of various natural dyes studied in India.
| Natural dye | Efficiency (%) | Reference |
|---|---|---|
| Kenaf Hibiscus | 2.87 | [140] |
| Ivy gourd | 0.08 | [141] |
| Red frangipani flowers | 0.3 | [141] |
| Acalypa godseffia | 4.372 | [142] |
| Epipremnum aureum | 3.362 | [142] |
| Begonia malabarica Lam. (BM) | 1.76 | [143] |
| Melastona malabathricum (MM) | 0.76 | [143] |
| Punica granatum L. (POM) | 1.12 | [143] |
| Anthocyanin (A) from Roselle | 3.73 | [144] |
| Betalain (B) from Spinach | ||
| Chlorophyll (C) from Beetroot | ||
| Henna dye (Lawsonia inermis) (TiO2 based DSSC) | 1.08 | [145] |
| Beetroot dye (TiO2 based DSSC) | 1.3 | [145] |
| Tangerine peel | 0.28 | [7] |
| Rhododendron | 0.57 | [7] |
| Fructus lycii | 0.17 | [7] |
| Marigold | 0.23 | [7] |
| Flowery knotweed | 0.21 | [7] |
| Rose | 0.38 | [7] |
| Lily | 0.17 | [7] |
| Coffee | 0.33 | [7] |
| Broadleaf holy leaf | 0.47 | [7] |
| Red turnip | 1.7 | [7] |
| Khella | 0.05 | [7] |
| Turmeric | 0.03 | [7] |
| Indian jamun | 1.23 | [146] |
| Jackfruit (JDND) | 1.1 | [147] |
| Golden trumpet | 0.40 | [148] |
| Blueberries | 0.80 | [149] |
| Acidified crude indigo (Indigofera tinctoria) | 0.022 | [150] |
| Methanolic extract of dried leaves (Indigofera tinctoria) | 0.114 | [150] |
| Bauhinia purpurea L. | 0.6324 | [151] |
| Hemigraphis colorata (Red Flame) | 0.0065 | [152] |
| Anthocyanin from tomato slurry (TiO2-CuO photoanode) | 2.96 | [153] |
| Saraca asoca | 0.09 | [154] |
| Chlorophyll from Red Amaranth | 0.53 | [155] |
| Betalain from Red Amaranth | 0.23 | [155] |
| Tecomaria capensis | 0.306 | [156] |
| Bauhinia | 0.375 | [156] |
| Kalanchoe | 0.610 | [156] |
| Luffa cylindrica L. | 0.13 | [157] |
Research has also focused on exploring the co-sensitizing effect of two different natural dyes. Co-sensitizing pigments with complementary absorption spectra increases the absorption band. The performance of a betanin-chlorophyll co-sensitized solar cell incorporating betanin and chlorophyll-a has been reported in the literature, which had a PCE of 0.601% for TiO2-based DSSCs [158]. The plasmonic effect of silver nanoparticles (AgNPs) considerably improves the efficiency of the betanin-chlorophyll co-sensitized solar cell, and the plasmon-enhanced betanin-chlorophyll co-sensitized solar cell achieves an improved efficiency of 0.793% compared to the value of 0.612% achieved by its pristine counterpart [159].
Natural dyes have also been extracted from Agaricus bisporus (mushroom), and Citrus limonum (lemon) leaves in a methanol medium, which were employed as photosensitizers by Arulraj et al. They were tested individually and even as a hybrid dye. However, the PCE of the hybrid dye was found to offer little improvement to performance compared to the individual metal-free natural dyes [160].
Roy et al worked on improvements in carotene-based DSSC in the presence of dielectric nanoparticles coupled with a TiO2 mesoporous film. Due to its strong field-effect passivation, screened coulombic attraction, back reflector, and recombination inhibitor properties, lanthanum-doped lead titanate (PLT15) demonstrated improved performance in DSSCs [161]. Roy et al also extracted natural dyes from Malvaviscus penduliflorus in an ethanol medium and studied its performance for DSSC applications [162].
Anthocyanins are promising natural dyes for DSSCs, however the PCE obtained is much lower than that of many synthetic dye-based cells. To improve the PCE of anthocyanin-based cells, two buffer layers made of algal by-products—sodium alginate and Spirulina were introduced by Prabavarthy et al. Their procedure, the extraction of anthocyanins from rose petals using citric acid, achieved enhanced PCE values [163]. Shanmugam et al studied the photovoltaic performances of three types of grass—Hierochloe odorata (HO), Torulinium odoratum, and Dactyloctenium aegyptium. HO-sensitized DSSC was found to show the maximum efficiency. Liquid chromatography-mass spectrometry (LC-MS) investigations have shown that pheophytin is the predominant pigment in HO [164]. Sreeja et al have shown that AgNPs as plasmonic materials improve the efficiencies of betanin dyes for DSSC applications [165]. The same group also studied the photovoltaic performances of betanin-indigo co-sensitized solar cells [166].
Roy et al developed a DSSC solar cell based on a KMnO4-TiO2 composite layer to be used with natural dye extracted from the Mirabilis jalapa flower. Among the different weight percentages of KMnO4 in TiO2, the performance of the 10 wt. % KMnO4-TiO2 composite showed an optimized efficiency of 2.04% [167].
Singh et al developed a mixed dye by employing a Ru-based dye, K-60, and a metal-free D-A organic dye, Y1, and studied its performance in DSSCs. Since it had higher and broader IPCE spectra, the overall PCE of the developed DSSC was found 8.19%, comparatively higher than that only sensitized with K-60 (∼6.26%) [168].
A mixed dye made of chlorophyll and betalain dyes extracted from spinach roots and beet roots, exhibited a higher PCE, because of its wider solar adsorption region than its individual constituents [169]. Sharma et al employed a mixture of N719 and the metal-free dye TA-St-CA, which achieved a PCE of 8.27%, appreciably higher than those of both DSSCs sensitized with N719 (∼5.78%) or TA-St-CA (∼4.45%). Co-sensitizing the dyes with different anchoring points has enhanced overall dye loading and reduced dye aggregation [170]. Metal-free D-(π-A)2-3,3'-(5,5'-(9-hexyl-9H-carbazole-3,6-diyl) bis(thiopene-5,2-diyl)) bis (2-cyanoacrylic acid) (denoted as D) was co-sensitized with N719 metal dye for DSSC application. The N719-D co-sensitized DSSC obtained a significantly enhanced PCE of 7.24% compared to those of the individual N719- (PCE of 5.78%) and D- (PCE of 3.95%) based cells. The higher light-harvesting efficiency and improved charge collection efficiency of the co-sensitized photoanode were attributed to the improved efficiency of the prepared cell [171].
Kumar et al mixed metal-free eosin and coumarin dyes with metal-based N3 and N719 dyes and used them for a TiO2-photoanode-based DSSC which achieved a PCE of ∼5.4% [172].
Donor-acceptor-type metal-free carbazole-based dyes (S1–3) were co-sensitized with a Ru (II) complex (NCSU-10) to broaden the spectral response of co-sensitized devices by Naik et al. The new structure had an electron-rich carbazole unit attached to different electron anchoring groups—4-amino benzoic acid, sulfanilic acid, and barbituric acid. The S1-NCSU-10 co-sensitized DSSC solar cell exhibited an enhanced PCE of 9.55%, compared to that of an NCSU-10-sensitized DSSC (PCE 8.25%) tested under similar conditions [173].
Studies have also shown that the co-sensitization of natural dyes with metal-free synthetic dyes increases the conversion efficiency in DSSCs [174]. Kumar et al developed a mixed dye from Hibiscus sabdariffa and eosin Y for the fabrication of a DSSC with a TiO2 photoanode. An enhanced PCE of 2.02% was reported for the mixed dye-based cells, compared to individual dye-based cells, because of improved light absorption in the higher energy state [175]. PV performance was also reported for D-π-A-structured carbazole-based co-sensitizers with a Ru (II) complex (NCSU-10). The electron anchoring groups were cyanoacetic acid (P1), rhodanine-3-acetic acid (P1–2), barbituric acid (P3), and thiobarbituric acid (P4). The P1 (at a concentration of 0.2 mM) NCSU-10 exhibited the highest PCE of 9.19% [176].
Kalaiyar et al proposed a homoleptic Ru (II) complex [Ru(dabpy)3] Cl2 (RDAB3), which contained an amine-functionalized electron-donating anchoring group. It was further co-sensitized using coumarin-based thiophene and coumarin-based indole. Due to four ancillary amine groups, RDAB3 had a PCE of 3.84% under AM 1.5 solar irradiation. Coumarin co-sensitizers enhanced the PCE to 5.35% under similar experimental conditions [177]. The co-sensitization of a Ru (II) complex (N749) with metal-free indoline dye (D149) achieved a higher PCE (5.40%) than that of an individual N719-based cell [178]. d10 transition-metal di-thiocarbamates containing Zn, Cd, and Hg were synthesized by Yadav et al to form [M(FcCH2EtOHdtc)2] and used as co-sensitizers and co-adsorbents for DSSC applications. A potential performance with an overall PCE of ∼7% was reported using the Zn complex with the N719 dye [179].
As shown by table 8, DSSCs using natural dyes have comparatively lower efficiencies relative to synthetic/commercially based sensitizers. However, there are several scopes for natural dyes which may have directed many researchers to explore these dyes for DSSC applications. Natural dyes are abundant, clean, and low-cost, and can be obtained from a variety of plants available in India. These dyes can easily be extracted from various leaves/fruits/flowers/vegetables utilizing simple extraction procedures based on green solvents. Organic chromophores attached to natural dyes actively function to absorb a wide solar spectrum. Although the efficiencies achieved using natural pigments are still unsuited to large-scale production, the consequences are encouraging and can boost additional studies that aim to search for new natural sensitizers for photovoltaic applications.
8. Opportunities and limitations
Although DSSCs are supposed to be low-cost and efficient photovoltaic devices, they have encountered specific challenges that have restricted their commercial viability. Solar cells require a reasonable PCE as well as long-term stability for sustainability in the commercial market. However, to date, the PCEs obtained from DSSCs under ambient conditions are not promising. Achieving long-term stability in DSSCs also remains a challenge. The primary process involved in DSSCs is the excitation of dye molecules exposed to sunlight. The dyes are usually unsuitable for absorbing the total spectrum of solar light and thus the generation of photoexcited electrons is deficient. The most efficient sensitizers identified to date are Ru-based synthetic organometallic dyes, which are costly and also toxic. The abundant natural dyes which can easily be extracted from plants and vegetables are not promising for producing reasonable PCEs. After the photoexcitation of dye molecules, the next step is the movement of the photoexcited electrons to the CB of the metal oxide, part of the photoanode in DSSCs. Broadly speaking, TiO2- and ZnO-based photoanode materials are popular for DSSC fabrication. Nevertheless, the limited electron mobility, the high recombination of TiO2, and the low chemical stability of ZnO degrade the performance of DSSCs. The electrons from the photoanode subsequently transfer to the counter electrode through the transparent conducting oxides (TCO) substrate and the external circuit. The most popular Pt and carbon-based counter electrodes, with excellent catalytic activity, suffer from a permanent problem of optical transparency along with higher corrosion by the electrolyte and contact resistance to the TCO substrate, resulting in a decrement of the FF. The physical state of the electrolyte, where the regenerative cycle is completed by utilizing the electrons for the redox process, contributes significantly to the performance of DSSCs. The problem associated with the leakage of the liquid-phase electrolyte in DSSCs is often responsible for the degradation of DSSCs. Despite such limitations, DSSCs have paved the way for new sensitizer-based solar cells. Researchers are hoping for a day when the limitations of DSSCs can be overcome by suitable photovoltaic devices. It is encouraging that research activities are now being directed and devoted towards the development of new perovskite-based solar cells, which are showing promising results as an alternative photovoltaic device to traditional Si-based p-n junction solar cells. Here, it is important to mention that the discovery of PSCs was inspired by the operating principle of dye-sensitized solar cells and that they can be considered to be upgraded versions of dye-sensitized solar cells.
9. Summary and conclusions
Dye-sensitized solar cells have been studied extensively throughout the globe for almost the last three decades in order to explore alternative photovoltaic devices to traditional p-n junction-based Si solar cells. The main components of typical DSSCs are the photoanode, the dye, the electrolyte and the counter electrode. Research activities have been individually undertaken for each individual component of DSSCs, with the ultimate goal of improving their PCEs and long-term stability. In order to keep pace with the global research on DSSC, significant efforts have also been exerted in India. Studies have been carried out on all the components of DSSC by researchers from various Indian academic and research institutes. After the enormous research activities devoted to DSSCs, it is felt now that the commercial viability of these photovoltaic devices is challenging, mainly because of their lack of long-term stability. The photoconversion efficiencies of DSSCs are not very promising and are limited to 12%–15% within controlled laboratory conditions. In addition, the maximum efficiencies in DSSCs were obtained using costly Ru-based organometallic dyes. A search for new alternatives began, and researchers have found PSCs, a modified version of DSSCs, to be promising photovoltaic devices. It is nonetheless important to keep in mind that the widespread research into DSSCs actually paved the way for the discovery of PSCs. Researchers from India have contributed significantly to this long journey of studying dye sensitizer-based solar cells. In this review article, an attempt has been made to summarize the research activities performed in India on the different components of DSSCs.
This review started with the importance of DSSCs as a cost-effective, renewable energy source, followed by the history of their discovery, their structural configuration and operating principles. The photovoltaic parameters and their measurements were then discussed for the general awareness of the readers. The statistics in the context of global and Indian research into DSSCs were then analysed using the Scopus database. It is important to mention that India is ranked fourth, just after China, South Korea, and the USA in terms of the number of Scopus indexed documents. Indian institutes and researchers who have contributed significantly to DSSCs were also identified. The next part of this article then highlighted recent advancements in global research into the different components of DSSCs. The last and the main part of the article then discussed the efforts undertaken in India to separately study the photoanode, electrolyte, counter electrode, and photosensitizers for DSSC applications. An attempt was made to discuss the wider spectrum of activities undertaken for each component. However, emphasis was given to discussing the studies performed in India on the different dyes for DSSC applications. This review article can thus provide a comprehensive insight into the DSSC research accomplished in India.
Acknowledgments
This work was supported by a Department of Science and Technology (DST), Ministry of Science and Technology, Government of India funded project under the MI IC#5' Conversion of Sunlight to Storable fuels' issued by the DBT-DST Joint Funding Opportunity, Central Government of India through Mission Innovation Programme DST(DST/TMD(EWO)/IC5-2018/06(G)) (S Roy).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).








