Abstract
Since its successful isolation in 2014, two-dimensional black phosphorus (BP) has triggered considerable interest ffrom physicists, chemists and material scientists. Benefitting from the unique structural and physicochemical properties, BP has been explored in various applications including photoelectric, biological and electrochemical fields. Besides, BP also shows great potential as a promising electrode material and electrocatalyst in energy storage and electrocatalytic applications, and tremendous progress has been made in these electrochemical fields in recent years. Here, this review highlights the recent experimental and theoretical progress of BP-based electrodes and electrocatalysts. The latest recent advances of BP-based functional materials in energy storage applications including lithium-, magnesium- and sodium-ion batteries, lithium–sulfur batteries and supercapacitors, are presented in detail. Further, the emerging electrocatalytic applications of BP for hydrogen evolution reaction, oxygen evolution reaction and nitrogen reduction reaction are systematically reviewed with achievements and challenges. Finally, we offer brief personal comments on the existing challenges and prospective outlook on the basis of current research progress.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Serious energy crises and environmental pollution problems of traditional non-renewable energy resources have raised an urgent requirement for clean, environmentally friendly and sustainable energy storage facilities. Nowadays, researchers are striving to develop various advanced energy storage and conversion technologies, such as rechargeable batteries [1, 2], supercapacitors [3, 4], fuel cells and metal-air batteries [5, 6], etc. The energy storage performance and conversion efficiency of these devices strongly depend on the morphology and electrical properties of active materials as well as the electrochemical reaction progress in the devices. Rechargeable batteries mainly include lithium-ion batteries (LIBs), lithium–sulfur batteries (LSBs), magnesium-ion batteries (MIBs) and sodium-ion batteries (SIBs) [7, 8]. The electrochemical performance of these batteries is primally related to the cathode and anode materials. In term of fuel cells and metal–air batteries, oxygen evolution reaction (OER) is a critical electrochemical reaction progress and determines the energy conversion efficiency [5, 9, 10]. However, OER generally suffers from seriously sluggish reactivities and requires efficient electrocatalysts to accelerate reaction [11, 12]. Besides, electrocatalytic hydrogen evolution reaction (HER) and nitrogen reduction reaction (NRR) are very important parts of energy conversion applications, but still face serious kinetically sluggish issues [13–15]. Therefore, developing advanced electrode materials and high-efficiency electrocatalysts is very urgent and desirable. The successful exfoliation of graphene in 2004 has attracted considerable efforts into the exploration of other two-dimensional (2D) materials and their potential applications. As we know, layered 2D materials possess unique structural and electronic advantages, such as high hardness, tunable electronic and electrochemical properties, as well as an ultra-large specific surface, etc. These excellent properties render 2D materials as promising candidates in widespread applications ranging from electrode materials of batteries and electrocatalysts for OER, HER and NRR. At present, a series of anode/cathode materials and electrocatalysts have been successfully designed by carefully screening from existing 2D layer materials [16–19], such as a 2D C3N allotrope as an anode material for LIBs [20], N-doped graphene (NG) electrocatalysts for OER [5, 21], and antimonene nanosheets (NSs) for HER [22].
In recent years, BP has become a rising-star 2D material and inspired many theoretical and experimental investigations in various fields owing to unique structural and electronic characteristics. The discovery of BP can be dated back to 100 years ago. In 1914, Bridgman achieved great success in the pioneering research of BP through the conversion of the white phosphorus into a BP single crystal at a high temperature of 200 °C and high pressure of 1.2 GPa [23]. However, severe synthesis conditions limited further investigations. Until 2014, Zhang et al firstly reintroduced few-nanometers-thick BP NSs as field-effect transistors with excellent performance [24], which reignited the renaissance of BP. Since then, BP is emerging as a promising elemental 2D material in nanophotonic and nanoelectronics applications. It is noted that BP is the most stable and least reactive form compared to other allotropes of red, white and violet phosphorus. BP is composed of puckered honeycomb layer structures stacked through van der Waals (vdW) interactions (figure 1). Similar to the preparation of graphene from graphite, the single- and few-layer BP can also be obtained by micromechanical and liquid exfoliation methods [25, 26]. It possesses a highly anisotropic structural property with different bond angles and lengths along the armchair and zigzag directions (figure 1), which results in anisotropic electronic, thermal conductivity and optical properties [24, 27, 28]. Importantly, BP exhibits a thickness-depend tunable bandgap ranging from ∼0.3 to ∼2.0 eV with decreasing thickness from bulk to monolayer BP (phosphorene). This widely tunable direct bandgap is challenging for layered transition metal dichalcogenides (TMDs) and the intrinsic direct-semiconductor character of phosphorene differs from the metallic nature of graphene with a zero band-gap that limits its potential application in the semiconductor field. Additionally, BP as a p-type semiconductor possesses a remarkable hole carrier mobility of ∼1000 cm2V−1s−1, superior to TMDs [24]. These advanced features offer BP the unprecedented opportunity to bridge the gap of practical application between graphene and TMDs. As expected, BP has been widely utilized in optoelectronic devices [29–31], thermoelectricity [32, 33], photocatalytic hydrogenation [34, 35] and so on [36]. Moreover, a large specific surface area with more active sites and high theoretical capacity as the Li/Na-ion batteries anode also render BP as an ideal candidate for electrocatalyst and novel electrode material. Nowadays, there has been a great deal of theoretical and experimental works on BP-based electrode materials and electrocatalysts. Considering the urgent need for clear energy in modern society, it is necessary to make a detailed review on the recent achievements of BP-based energy storages and electrocatalytic applications, as well as the current challenges and some constructive ideas for the future development of these energy applications, which will provide meaningful guidance for researchers to further explore more excellent electrode materials and electrocatalysts.
Figure 1. Schematic structures of three-layer BP: (a) perspective side view, (b) top view and (c) side view.
Download figure:
Standard image High-resolution imageIn this review, the research progress of BP-based functional materials in energy storage as well as electrocatalytic applications are summarized, aiming to provide an outline of this emerging 2D material in the energy field. Firstly, we will discuss the recent research on BP-based energy storage devices, including LIBs, LSBs, MIBs, SIBs, and supercapacitors. In the following context, a detailed description of recent developments of BP-based electrocatalysts for HER, OER and NRR is presented. Finally, we will propose an outlook of the challenges and opportunities of BP in these research fields.
2. Energy storage applications
Emerging energy storage devices mainly contain LIBs, LSBs, MIBs, SIBs and supercapacitors, as shown in figure 2. Recently, BP has shown outstanding performance as a potential candidate for these devices, as shown in table 1. In this chapter, we will review and describe in detail the development and problems of BP in these energy storage devices for the past few years.
Figure 2. A summary of potential applications for electrochemical energy storages of BP. Reproduced from [3] with permission of The Royal Society of Chemistry. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature Materials [6] © 2011. Reprinted with permission from [37]. Copyright (2014) American Chemical Society.
Download figure:
Standard image High-resolution imageTable 1. Summary of electrochemical performance of BP composites for LIBs, LSBs, SIBs and SCs applications.
| Applications | Materials | Synthetic method | Capacity or capacitance | Cycling (after n cycles) | Reference |
|---|---|---|---|---|---|
| LIBs | BP-Carbon | High energy mechanical milling (HEMM) | 2010 mAh g−1 at 0.1 A g−1 | More than 600 mAh g−1, n = 100 | [48] |
| BP-Graphite | HEMM | 2786 mAh g−1 at 0.2 C | 2270 mAh g−1 at 0.2 C, n = 100 | [52] | |
| BP nanosheets | Sublimation-induced transformation | 630 mAh g−1 at 0.2 A g−1 | 1683 mAh g−1 at 0.2 A g−1, n = 100 | [53] | |
| BP–Graphene (GO) oxide composite | Spark plasma sintering | 1013.3 mAh g−1 at 0.1 A g−1; 415 mAh g−1 at 10 A g−1 | 725 mAh g−1 at 0.5 A g−1, n = 200 | [55] | |
| BP-G hybrid paper | Vacuum filtration | 920 mAh g−1 at 0.1 A g−1 | 80.2% capacity retention at 0.5 A g−1, n = 500 | [57] | |
| G-BPGO sandwiched film | Vacuum filtration | 1633 mAh g−1 at 0.1 A g−1 | 1401 mAh g−1 at 0.1 A g−1, n = 200 | [58] | |
| E-BP/PEDOT | In situ polymerization | 1408 mAh g−1 at 0.1 A g−1 | 77.6% capacity retention at 0.1 A g−1, n = 100 | [103] | |
| BP/RP hybrid | Sonochemical method | 696 mAh g−1 at 0.05 A g−1; 260 mAh·g−1 at 2 A g−1 | 491 mAh g−1 at 0.05 A g−1, n = 100 | [114] | |
| Ni2P@BP | Sonication-assisted exfoliation and solvothermal | 1196.3 mAh g−1 at 0.1 A g−1 | 743.7 mAh g−1 at 1 A g−1, n = 1000 | [115] | |
| LSBs | BP-carbon nanofiber | Ultrasonic approach and vacuum filtration | 1262 mAh g−1 at 0.2 C; 985 mAh g−1 at 1 C | 660 mAh g−1 at 1 C, n = 500 | [59] |
| BP-coated separator | Vacuum filtration deposition | 930 mAh g−1 at 0.4 A g−1 | 800 mAh g−1 at 0.4 A g−1, n = 100 | [65] | |
| SIBs | Sandwiched BP-G hybrid | Solution mixing | 2440 mAh·g−1 at 0.05 A g−1 | 83% capacity retention at 0.05 A g−1, n = 100 | [98] |
| Few-layer BP | Electrochemical exfoliation | 1878.4 mAh g−1 at 0.1 A g−1; 591 mAh·g−1 at 1.5 A g−1 | 603.3 mAh g−1 at 1.5 A g−1, n = 100 | [100] | |
| BP/Ketjenblack–multiwalled carbon nanotubes | High energy ball milling | 2119 mAh g−1 at 0.2 A g−1 | 1826.9 mAh g−1 at 0.416 A g−1, n = 50 | [101] | |
| BP-polyaniline (PANI) | Liquid–liquid interfacial method | ∼200 mAh g−1 at 0.5 A g−1 | Almost 200 mAh g−1 at 0.5 A g−1, n = 50 | [102] | |
| E-BP/PEDOT | In situ polymerization | 1397 mAh g−1 at 0.1 A g−1 | 67.4% capacity retention at 0.1 A g−1, n = 100 | [103] | |
| 4-NBD-BP/RGO hybrid | Solvothermal reaction | ∼660 mAh g−1 at 1 A g−1 | 650 mAh g−1 at 1 A g−1, n = 200 | [105] | |
| BP/Ti3C2Tx MXene | Vacuum freeze drying process | 535 mAh g−1 at 0.1 A g−1 | 343 mAh g−1 at 1 A g−1, n = 1000 | [106] | |
| SCs | BP nanoflakes | Liquid-phase exfoliation | 13.75 F cm−3 at 0.01 V s−1 | 15.5% and 28.2% capacitance decay, n = 10000 and 30000 | [111] |
| BP/G films | One-step mask assisted simplified fabrication | 37.5 F cm−3 at 0.005 V s−1 | [112] | ||
| BP/Polyaniline (PNAI) | In situ oxidative polymerization | 354 F g−1 at 0.3 A g−1 | 96% capacitance retention, n = 175 | [113] |
2.1. Lithium-ion batteries (LIBs)
Compared with other commonly commercial batteries, Li-ion rechargeable batteries have been undoubtedly the most successful electrochemical energy storage devices, which can reversibly convert the chemical energy into electricity. During the past few decades, LIBs have expanded in all aspects of people's life and production owing to several important advantages, such as high energy density, light weight, high power density and low self-discharging [1, 38]. LIBs usually consist of three primary components including a cathode (positive electrode), anode (negative electrode) and electrolyte. The electrochemical performance of LIBs is closely linked to the anode and cathode materials. In recent decades, the anode material has attracted wide research interests because of its direct contribution to energy density of LIBs. Nowadays, graphite is generally used as the anode material of commercial LIBs as its good electroconductivity, low lost and large abundance. LIB-based graphite anodes and LiCoO2 cathodes can achieve the practical energy density of ∼150–190 Wh kg−1, and such a moderate energy density value cannot meet the needs of market for high energy demand. In order to achieve higher energy density, considerable research efforts have been made to design new anode materials by screening from novel 2D layer materials, including graphene-based material, metal chalcogenides, and Si, etc. In addition, theoretical and experimental researchers have also devoted extensive effort to utilize BP as the anode material of LIBs during past decades.
The intrinsic puckered layer structure endows BP with sufficient interstitial space for Li insertion and extraction. Theoretical calculations have demonstrated that the atomistic lithiation process of BP-based LIBs was similar to that of commercial graphite, in which Li showed a columnar intercalation mechanism and tended to locate in different 2D layers [39]. Owing to the unique structural and physical properties of BP, numbers of theoretical calculations have proved that 2D BP shows excellent potential as a desirable anode candidate towards high-performance LIBs [40–42]. It was found that the Li atom was able to bind strongly with phosphorus atoms with significant electron transfer, finally leading to the formation of a cationic state [43, 44]. In addition, the ultrahigh diffusivity of Li along the zigzag direction due to the shallow energy barrier 0.08 eV was estimated to be 102 and 104 times faster than that on MoS2 and graphene at room temperature, indicating the great possibility of ultrafast charge and discharge [43]. Further, Li intercalation in 2D BP could induce semiconducting-to-metallic transition, which gave rise to good electrical conductivity and was ideal for an electrode [43, 44]. Theoretical calculations further demonstrated that lithiation and de-lithiation cycles had a little influence on the structure of monolayer phosphorene with a small volume change of only 0.2% and good reversibility had also been demonstrated owing to the good self-recovery of monolayer phosphorene after the removal of the Li atoms [44–46]. Elemental phosphorus could react with three Li to form Li3P compounds, giving rise to a high theoretical specific capacity of 2596 mAh g−1 for BP-based LIBs [47], which was larger than that of commercial graphite anode materials. Zhang et al also demonstrated that the theoretical specific capacities of monolayer and double layer phosphorene were calculated to be 432.79 and 324.59 mAh g−1, respectively [44].
In 2007, Park et al first reported BP and BP-carbon based anodes of LIBs and they suggested that BP showed a reversible charge capacity of 1279 mAh g−1 with the first cycle efficiency of 57% [48]. They also found that the charge capacity of the BP-carbon anode was 1814 mAh g−1 and the first cycle efficiency was as high as 90%. Subsequently, Sun et al demonstrated that the electrochemical performance of BP-based anodes strongly depended on pressure and temperature [47]. It was found that at 4 GPa and 400 °C, BP obtained from white phosphorus showed the highest first discharge and charge capacities of 2505 and 1354 mAh g−1, respectively. When pressure and temperature increased to 4.5 GPa and 800 °C, the charge capacities of BP obtained from red phosphorus (RP) achieved the high value of 2649 and 1425 mAh g−1. Theoretical calculations suggested that intrinsic point defects in phosphorene, such as vacancy and stone-wales defects could block ultrafast migration of lithium, so preparing defect-free phosphorene in the experiment was highly critical for the performance of LIB application [49]. In addition, some researchers focused on performing the strategic modification of the BP/carbon mixture for anode materials [50, 51].
However, successful applications of BP-based anodes have also been faced with a series of great challenges of rapid capacity fading as well as loss of electrical contact, which mainly originated from large volume change during the cycling process [47, 52–54]. For example, Park et al had found that in the case of BP and BP-carbon based anodes, large volume expansion and structure degeneration existed, taking place from P to Li3P, finally leading to the degeneration of energy capacity to 200 mAh g−1 after 30 cycles [48]. It was also observed that because of the serious side reactions from large specific surface area and volume expansion (∼300%), the few-layer BP as the anode for LIBs showed a negligible reversible specific capacity of 210 mAh g−1 with a low first-cycle coulombic efficiency of 11.5% [55]. To better understand the volume expansion progress and failure mechanisms, Xia et al employed the in situ transmission electron microscopy (TEM) to study the electrochemical lithiation and de-lithiation progress in the BP anode [54]. They found that, upon lithiation, the BP anode underwent anisotropic volume expansion accompanied by obvious phase change from orthorhombic BP to amorphous Lix Py compounds. And they disclosed that the irreversibility and poor cycling performance of the BP anode was mainly associated to the de-lithiation process rather than the lithiation one because of the sudden pulverizing of BP during discharging.
To address this challenge of poor cyclic performance, Cui et al fabricated BP nanoparticle (NP)-graphite composites by employing BP as starting materials in a high energy mechanochemical reaction process [52]. They demonstrated that the stable P–C bonds during lithium insertion/extraction were attributed to excellent electrical connection between P and C. The initial discharge capacity of LIB based on BP-carbon anodes could reach to 2786 mAh g−1 at 0.2 °C and the capacity retention still kept 80% after 100 lithium insertion/extraction cycles, indicating excellent cycle life. In addition, the heterostructure design of BP and other 2D materials is also an effective way to improve the performance of LIBs. Theoretical reports have demonstrated that the phosphorene/graphene heterostructure could effectively enhance the cycle life of LIBs owing to ultrahigh stiffness by prohibiting the distortion of pristine phosphorene after the lithium insertion [56]. This property has been further proved by several experimental studies [55, 57]. Chen et al demonstrated a paper-like flexible LIBs electrode material by combining exfoliated BP NSs and highly conductive graphene sheets (figure 3(a)) [57]. Such BP/graphene (BP/G) composite was synthesized by a mixture of 80% BP NS powder and 20% few-layer graphene power. According to their reports, the BP/G hybrid electrode displayed very high specific capacity of 920 mAh g−1, which was much higher than that of individual BP NSs (180 mAh g−1) and pure graphene (435 mAh g−1) (figure 3(c)). Importantly, the hybrid electrode also showed long cycle life with the capacity retention of 80.2%, low capacity decay rate per cycle of 0.04% and average coulombic efficiency of 100% over 500 cycles (figure 3(d)). These excellent properties primarily were attributed to the good contact of BP NSs and graphene, appropriate charge-transport pathway and short diffusion distance of lithium ions, etc. Zhang et al proposed that compared with pure few-layer BP, BP-graphene composites (figure 3(e)) prepared by a spark plasma sintering (PG-SPS) as the anode material of LIBs could improve greatly the first-cycle coulombic efficiency (60.2%), and specific capacity (1306.7 mAh g−1 in figure 3(f)) with excellent air stability and long-term cycling life of 91.9% retention after 800 cycles at 10 A g−1 (figure 3(h)) [55]. The construction of the sandwich structure anode is also an effective pathway to acquire the good performance of LIBs. The BP/G hybrid sandwiched film anode has been successfully synthesized by staking two layers of graphene sandwiched between the BP/G composite. LIBs based on this sandwiched file anode exhibited superior cycle performance with a reversible capacity of 1401 mAh g−1 after 200 cycles at a current density of 100 mA g−1 [58].
Figure 3. (a) Photograph of the flexib BP-G hybrid paper. (b) Cross-section view SEM image of the BP-G hybrid paper. (c) The second galvanostatic charge/discharge profiles of the BP nanosheet, graphene paper, and BP-G paper electrodes at a current density of 100 mA g−1 within a potential window of 0.001–3 V, and their rate performance at different current densities. (d) Coulombic efficiency and cycling stability of the BP-G hybrid electrode at 500 mA g−1 after 500 cycles. [57] John Wiley & Sons (e) Cross-section SEM images of PG-SPS and PG (PG was obtained by annealing the mixture of BP and graphene oxide) electrodes on Cu foil. (f) The first galvanic charge/discharge profiles of BP, PG, and PG-SPS at 100 mA g–1 current density and rate capabilities of PG and PG-SPS electrodes at different current densities. (g) Cycling life of PG and PG-SPS electrodes based on volumetric capacities. (h) Cycle stability of PG-SPS electrode at high current densities of 500 mA g–1 and 10 A g–1 [55] John Wiley & Sons.
Download figure:
Standard image High-resolution image2.2. Lithium-sulfur batteries (LSBs)
With ever-growing energy needs, developing batteries with high specific energy and high power is urgent. Abundant elemental sulfur in nature will facilitate the development of low-cost and environmental-friendly batteries, which makes the development of LSBs with great commercial value [59]. Nowadays, a large number of scientists are working on exploiting novel LSBs due to its high theoretical energy density of 2600 Wh kg−1, which is obviously superior to Li-ion technology [2, 6]. In spite of these potential advantages and extensive efforts, the practical use of LSBs is still suffering from several drawbacks, such as the low conductivity of sulfur and various discharge products, inducing high overpotentials; the dissolution of polysulfides intermediates into electrodes during electrochemical progress, leading to the severe loss of active materials and 'shuttle effect'; and the large volume expansion of the S cathode upon lithiation/de-lithiation cycles (up to 80%), resulting in capacity fading [60–63]. To address these issues, trapping the sulfur species in the electrodes of LSBs is considered as an effective solution to increase electrode conductivity, suppress the polysulfide products, and reduce the volume change.
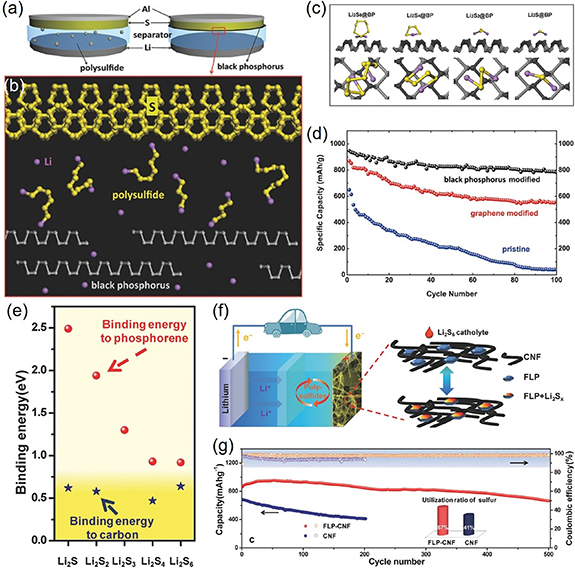
Recently, BP has proved to exhibit great potential as a promising cathode material for LSBs to solve the above problems. Firstly, Zhao et al theoretically explored the adsorption and diffusion of various lithium polysulfide (Li2Sx ) species on phosphorene during different lithiation/de-lithiation states to examine the trapping capacity for polysulfide intermediates [64]. Computational results showed that, compared with carbon materials, phosphorene could interact with polysulfide intermediates with moderate adsorption energy, suggesting monolayer BP could act as a prospective anchoring material to trap polysulfide intermediates. Li2Sx species exhibited ultrahigh diffusivity on the phosphorene surface with a small diffuse barrier, and the charge transfer from polysulfide species to phosphorene induced smaller bandgaps of the hybrid materials than pristine phosphorene, indicating improved electrical conductivity. In the experiment, Cui et al employed a BP-coated separator by introducing a uniform BP layer on to the conventional polypropylene separator to trap the polysulfide species in LSBs [65], as shown in figure 4(a). The BP coating layer showed high electron conductivity and the side facing the sulfur-based electrode could effectively bind with polysulfide intermediates to suppress their diffusion through the separator (figure 4(b)). First-principle calculations suggested that the binding energies between the BP surface and polysulfides/sulfides were larger than those on the graphene layer, indicating better trapping capacity for polysulfides (figure 4(c)). With such a functional BP-coated separator, LSBs achieved the much higher initial specific capacity of 930 mA h g−1 and improved cycle performance. The BP-modified cell still retained a capacity of 800 mA h g−1 after 100 cycles (figure 4(d)) with the high retention rate of 86%, which were much higher than the graphene-based LSBs with 66% retention rate [65]. The strong trapping performance of BP for polysulfide species in LSBs has since been observed by Li et al [59], and they also performed the theoretical calculations to examine the binding strength of lithium polysulfide adsorption on the phosphorene surface. As shown in figure 4(e), polysulfide Li2Sx species shows higher binding energies ranging from 0.92 to 2.49 eV than those on the carbon-based surface, indicating better effectiveness on the phosphorene surface to trap the polysulfides than the conventional carbon-based surface [66]. In addition, Li et al successfully prepared the FLP–CNF electrode by incorporating liquid-exfoliated few layer phosphorene NSs (FLP) into the carbon nanofiber (CNF) through the ultrasonic approach (figure 4(f)) [59]. Experimental measurement results demonstrated the FLP-CNF electrode possessed a specific capacity of 1262 mA h g−1 at 0.2 °C, which was much higher than the pure CNF electrode with initial average discharge capacity of 944 mA h g−1. Moreover, the FLP–CNF electrode showed excellent cycling stability and high coulombic efficiency than the CNF electrode. After 500 charge/discharge cycles, the FLP–CNF electrode still exhibited a specific capacity of ∼600 mA h g−1 with only 0.053% capacity fade per cycle, while the capacity fade per cycle for the CNF electrode without BP was about 0.25% over only 200 cycles (figure 4(g)). This significant difference clearly indicated that the introduce of FLP into CNF could greatly improve the electrochemical stability of the LSBs. Theoretical capacity analysis further revealed that the FLP–CNF electrode displayed a much greater sulfur utilization than the baseline CNF electrode (figure 4(g) inset). Authors also found other advantages of the presence of phosphorene, such as lowering the polarization and accelerating the redox reaction. The application of 2D BP has opened up new avenues to further achieve the high-performance of LSBs.
Figure 4. (a) Schematic configuration of the Li–S cell employing commercial separator and the BP-coated separator. (b) Operating principle of the BP coating layer in Li–S battery. (c) The side and top views of molecular models of the interaction between BP and Li2S8, Li2S4, Li2S2, and Li2S. (d) Reversible lithiation capacity and coulombic efficiency for the first 100 galvanostatic cycles of Li–S batteries with BP-modified, graphene-modified and conventional separators. [65] John Wiley & Sons. (e) Calculated binding energies between lithium polysulfides and phosphorene (red), and carbon network (blue). (f) Schematic of the FLP-CNF electrode for the Li–S battery. (g) Cycling stability and coulombic efficiency of FLP–CNF and CNF electrodes and the inset showed utilization of sulfur. [59] John Wiley & Sons.
Download figure:
Standard image High-resolution image2.3. MIBs and SIBs
Rechargeable magnesium-ion batteries have also gained considerable interest in recent years, owing to its high volumetric capacity of 3868 mA h g−1 than LIBs, high melting point of 649 °C and low cost, etc [67–69]. The increase of energy density mainly originates from the bivalent nature of magnesium atom [67]. Moreover, abundant natural resources and high-safety features of metallic magnesium endowed the MIBs with good practicability [70]. However, the practical application of MIBs is still limited by several serious problems, such as the incompatibility of the anode and electrolyte, the lack of high capacity/voltage cathode and proper anode, and the narrow electrochemical window of electrolyte [71–73]. In addition, the high charge and large radius of Mg2+ induced the strong interaction with other ions in the cathode material, leading to the slow diffusion of Mg2+ [69]. In spite of these challenges, researchers are devoting more effort to exploit new electrolytes, anode and cathode materials to achieve high-performance MIBs.
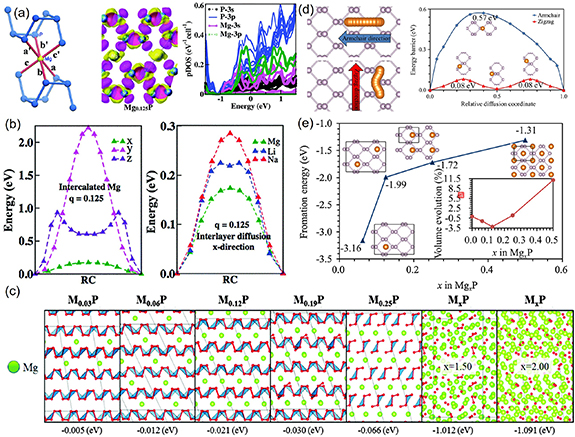
A number of studies have shown that 2D BP could be a promising anode material for MIBs. Banerjee and Pati theoretically explored the diffusion kinetic of Mg-ions and storage potential of BP as anode in MIBs [74]. They suggested that the BP-matrix anode could exhibit a low potential of 0.15 V and a high specific capacity of 1730 mA h g−1. The synergistic interaction between Mg-ions and a covalent P atom effectively reduced the diffusion barrier of Mg (figure 5(b)) and further optimized anodic voltage, which could help solve the bottleneck in MIBs. Using first-pristine calculations, Hembram et al described the magnesiation progress in BP-based MIBs [39]. Authors reported that the magnesiation progress showed a columnar intercalation mechanism and the BP with the insertion of Mg-ions exhibited the hardening property (figure 5(c)). Sibari et al observed a semiconductor-to-conductor transition after the complete adsorption of Mg-ions, which was vital for improving the electroconductivity of the electrode [75]. Moreover, based on density functional theory, Jin et al explored the adsorption and diffusion of Mg atoms on the monolayer BP and its structural stability with the increase of Mg content [76]. The results showed that Mg atoms preferred to absorb on the monolayer BP surface, and exhibited anisotropy diffusion behavior with a small barrier of 0.08/0.57 eV along zigzag and armchair directions, indicating fast charging/discharging potential (figure 5(d)). Importantly, monolayer BP still maintained good structural stability for the Mg0.5P compounds with volume expansion of about 11% (figure 5(e)). These advantages of theoretical predication have clearly shown great potential and prospects for 2D BP as an anode material for MIBs, which points toward experimental research on BP-based batteries.
Figure 5. (a) Octahedral coordination number of Mg-ions at a low charge state a-phase; charge density difference and the projected density of state for Mg0.125P. (b) Energy profiles for interlayer diffusion in the a-phase: Mg-diffusion along x, y and z-directions and Li, Na, Mg-diffusion along the x direction. Reproduced from [74] with permission of The Royal Society of Chemistry. (c) Insertion mechanism in BP with the concentration increase of Mg and the number represented the formation energy. Reproduced from [39] with permission of the PCCP Owner Societies. (d) Diffusion paths along armchair/zigzag direction and the energy barrier profile of Mg diffusion on monolayer BP. (e) The formation energies as a function of Mg concentration. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Journal of Materials Science [76] © 2016.
Download figure:
Standard image High-resolution imageAt present, LIBs are most widely used high-performance energy storage devices, but it also faces the critical problem of limited natural resources and the high cost of lithium. SIBs are becoming promising alternatives to LIBs as next-energy storage devices owing to the low cost, natural abundance and friendly environment of sodium resources [37, 77–79]. The working principles of SIBs are similar to those of LIBs, that is sodium ions are shuttled between cathode and anode electrodes through the sodium-ion electrolyte during charge and discharge cycles [80]. However, compared to lithium ions (0.76 Å), the larger ionic radius of sodium (1.06 Å) results in slow electrochemical reaction kinetics and huge volume change of electrode materials in the charging and discharging process [81, 82]. Recent reports have presented that SIBs based on several cathode materials including layered sodium-transition-metal materials NaxMO2 (M = Fe, Mn, Co, Ni, etc), polyanionic compounds and their analogs, exhibited comparable high performance to their LIBs counterparts [83–85]. Therefore, the major scientific challenge for achieving a competitive SIBs devise is to develop new anode materials with high energy density, prolong cycling life and high specific capacities.
In recent years, people have devoted great effort to searching for appropriate anode materials for SIBs. The development of anodes for SIBs has been predominately based on conventional carbon materials, such as graphite, carbon nanospheres and nanowires, amorphous carbon, etc [79, 86–88]. In addition, Na–M alloys (M = Si, Ge, Sn, etc), the porous and layered oxide material and MoS2 have also been demonstrated as promising anode materials [82, 89–91]. However, SIBs based on these anode materials showed low specific capacity and limited cycle life.
BP has been potentially very attractive as the anode material in SIBs because of its unique layered structure, theoretical high specific capacity and suitable electrochemical properties [92, 93]. Numerous theoretical investigations have demonstrated that 2D BP could be a promising anode for high-performance SIBs [39, 94–97]. Liu et al reported Na atoms could be well stabilized on the surface of monolayer BP without forming clusters, indicating that monolayer BP was able to accommodate the storage of sodium ions [95]. Using first-principles calculations, Kulish et al systematically investigated the Na adsorption property, theoretical specific capacity and Na diffusion behavior on monolayer BP [94]. The calculations results indicated that the clusters of Na atoms were not easy to form at low Na concentration due to the favorable Na–phosphorene interaction. The Na diffusion on the surface of 2D BP was fast with a small energy barrier of 0.04 eV, indicating high charge/discharge rates in practical SIBs. And the semiconductor-to-metal transition at high Na concentration was beneficial for electronic conductivity of SIBs. Moreover, BP showed good structural integrity and mechanical stability upon Na insertion [94]. According to Hembram et al, the sodiation mechanism of BP in SIBs was similar to that in LIBs, which was an intercalation progress that occurred at low Na concentrations and an alloying progress that gradually dominated with the increase of Na concentrations. Particularly, Na showed a planar intercalation mechanism and was inclined to localize in the same layer [39, 97].
In the experiment, using in situ TEM and ex situ x-ray diffraction techniques, Cui et al also demonstrated a two-step sodiation mechanism of intercalation and alloying [98], which agreed with the before-mentioned theoretical results (figure 6(a)). In the intercalation progress, the sodium ions were first inserted between the 2D BP layers along the puck channels. The intercalation of sodium ions expanded the interlayer distance of the 2D BP layer and did not degrade the host structure, indicating high reversibility upon cycling. Then further sodiation in the alloy reaction led to the formation of Nax P species, finally transforming into the Nax P phase (figure 6(a)), which had been proved to be primarily responsible for the high specific capacity of BP. Subsequently, Nie et al also investigated transport pathways of sodium ions inside 2D BP by using in situ TEM and complementary density functional theory [99]. The results showed at the early stage of sodiation, sodium ions preferred to migrate along the puck channels of [100] direction originating from the anisotropic diffusion property (figure 6(b)), which was consistent with experimental results of Cui et al [98]. They also confirmed the high electrochemical performance of 2D BP in half-cell SIBs with good rate capability, good cycling stability and high capacity. Ji et al have suggested that few-layer 2D BP could be directly utilized as an anode in SIBs and deliver superior sodium-storage performance with a high capacity of 1968 mAh g−1 at a current density of 100 mA g−1 [100]. However, the practical application of BP as anode materials still is hindered by several drawbacks, such as low electroconductivity, sluggish reaction kinetics and anisotropic volumetric expansion. The alloy progress of the two-step sodiation mechanism was always associated with serious mechanical and structural fracturing with a large volume expansion (∼500%), which led to significant capacity fade upon cycling [98].
Figure 6. (a) Two-step sodiation mechanism of BP including schematics before sodiation, the first step of sodium-ion intercalation and the second step of alloy reaction. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature Nanotechnology [98] © 2015. Sodium transport in 2D BP with (b) contact interface normal to the [100] direction and (c) parallel the [100] direction with respect to the sodium source (reprinted with permission from [99]. Copyright (2016) American Chemical Society), respectively. (d) Digital photographs of N-methyl-2-pyrrolidone dispersions of graphene, phosphorene and a mixture of graphene and phosphorene. (e) Structural evolution of the sandwiched hybrid phosphorene–graphene structure during sodiation. (f) Reversible desodiation capacities for the first 100 galvanostatic cycles of various phosphorene–graphene electrodes with different C/P mole ratios. (g) Reversible coulombic efficiency and desodiation capacity for the first 100 galvanostatic cycles of the phosphorene–graphene anode tested under different currents. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Nature Nanotechnology [98] © 2015.
Download figure:
Standard image High-resolution imageTo address these challenges, many researchers have explored effective strategies to engineer the BP anode compositions to improve the electroconductivity and accommodate the volumetric change, including alloying or hybrids of BP [101, 102], surface engineering of BP [103], and enhancing interlayer coupling [51, 104–106]. Cui et al reported a 2D BP-graphene hybrid materials as anode for SIBs with a few 2D BP layers sandwiched graphene layers [98] (figure 6(d)). They fabricated a variety of hybrid materials with different compositional C/P ratios and the galvanostatic cycling suggested that samples with C/P ratios of 2.78:1 and 3.46:1 showed similar capacity retention upon 100 cycles. This sandwiched phosphorene-graphene anode has several advantages, as follows: (a) the graphene layer could effectively accommodate the anisotropic expansion of phosphorene layers during the alloy progress by providing an elastic buffer. (b) The graphene also could enhance the electrical conductivity of the material and facilitate the electron transport generated in the redox reaction of phosphorene. (c) This sandwich hybrid material showed a high specific capacity of 2440 mAh g−1 with the capacity retention of 83% at a current density of 0.05 A g−1 (figures 6(f) and (g)). Additionally, Xu et al fabricated nanostructured BP/ketjenblack-multiwalled carbon nanotubes composite (BPC) as anode materials for SIBs by high energy ball milling [101]. Such a BPC composite exhibited a high coulombic efficiency of 91.1% and reversible capacity of 2011.1 mAh g−1 with excellent cycling performance of ∼1700 mAh g−1 upon 100 cycles at 13 A g−1. Recently, researchers reported a phosphorene/MXene hybrid anode by utilizing the high capacity of phosphorene and high elasticity and conductivity of Ti3C2TX MXene. The introduction of Ti3C2TX MXene not only facilitated the diffusion of both sodium and electrons, but also buffered the structural expansion of phosphorene. The x-ray photoelectron spectroscopy analysis demonstrated that the fluorine terminated Ti3C2F2 MXene could enhance the coulombic efficiencies and cycling performance by forming the fluorine-rich solid electrolyte interphase film. Consequently, this phosphorene/Ti3C2F2 hybrid anode delivered a high reversible capacity of 535 mAh g−1 at 0.1 A g−1 and outstanding cycling performance with a capacity retention of 87% at 1 A g−1 after 1000 cycles [106]. Utilizing surface engineering of BP, Zhang et al demonstrated a PEDOT-functionalized few-layer BP by homogeneously depositing 3, 4-ethylenedioxythiophene (PEDOT) nanofibers on surface-modified BP NS [103]. This unique functionalization with PEDOT nanofibers could achieve a continuous conductive network with enhancing charge transfer kinetics and super surface wettability with electrodes. As a result, the surface engineering of BP exhibited improved reversible specific capacities of 1597 mAh g−1 for SIBs compared to that of exfoliated few-layer BP with specific capacities of 345 mAh g−1.
2.4. Supercapacitors (SCs)
In the field of energy storage, supercapacitors are another important energy-storage device with attractive advantages, such as high-power density, ultrafast charging/discharging rate and longer cycle life as compared to other conventional energy-storage systems [3, 4]. According to different charge storage mechanism, supercapacitors can be classified into two types including a electrical double layer capacitor and pseudocapacitors. The high-performance of supercapacitors mainly depends on the morphology and intrinsic properties of electrodes, such as high strength, high surface area and high electric conductivity. Generally, carbon materials of graphene and metal chalcogenides are used as effective electrodes for supercapacitors [107–110]. Recently, 2D BP has also been considered as a competitive electrode candidate for supercapacitor application. Hao et al successfully fabricated flexible all-solid-state supercapacitors (BP-ASSA) by restacking few-layer liquid-exfoliated BP NSs in supercapacitors (figure 7(a)) [111]. As a result, the BP-ASSA devices achieved outstanding electrochemical energy storage performance and delivered a high stack capacitance of 45.8 F g−1 (13.7 F cm−3) at the scan rate of 0.01 V s−1 with excellent mechanical flexibility and electrochemically stability (28.2% capacitance recession over 30000 cycles and 15.5% over 10000 cycles) (figure 7(b)). Subsequently, Xiao et al demonstrated one-step mask-assisted simplified manufacturing of high-energy micro-supercapacitors (PG-MSCs) in ionic liquid, based on the interdigital hybrid electrode patterns by stacking few-layer high-quality BP NSs and graphene (figure 7(c)) [112]. The as-fabricated PG-MSCs exhibited remarkable areal capacitance of 9.8 mF cm−2, energy density of 11.6 mWh cm−3 and volumetric capacitance of 37.0 F cm−3 (figure 7(d)). Additionally, this hybrid electrode also showed excellent flexibility and stable performance with slight capacitance vibration under different bending states. The high electrochemical and mechanical performance of PG-MSCs was primarily attributed to the synergistic effect and complementary combination of phosphorene and graphene. First, the puckered BP NS offered a fast transport path and more ionic accommodation, and effectively prohibited the restacking of graphene NSs. In the meantime, graphene sheets provided high-speed electron conductivity network and high-strength mechanical skeleton. Second, the simplified fabrication progress efficiently prevented the oxidation of phosphorene and solvent pollution. Third, the intrinsic mechanical flexibility and atomic thickness of 2D NSs helped to obtain the strong interfacial interaction between graphene and phosphorene, as well as the substrate and electrode film. In addition, other researchers reported a high-performance hybrid electrode material (BP/PANI) for the pseudocapacitor by combining with a few-layer BP NSs and polyaniline (PANI) [113]. The electrochemical performance of obtained BP/PANI hybrid material outperformed individual pure BP and PANI, with high specific capacitance of 354 F g−1, prolong cycling stability and high rate capacity. These outstanding electrochemical performances principally originated from the synergistic effect between PANI and BP NSs, which not only supported a large growth area for PNAI, but also offered more accessible sites of electrolyte ions to the PNAI active materials. Interestingly, a BP and RP hybrid material had also been synthesized for supercapacitor application [114]. The BP/RP hybrid material successfully achieved the high specific capacitance of 60.1 F g−1 and long cycling life with 83.3% capacity retention after 2000 cycles. To summarize this chapter, we make a table (table 1) to clearly display electrochemical performance of above BP composites for LIBs, LSBs, SIBs and SCs applications.
Figure 7. (a) Schematic illustration for fabrication process of the BP-ASSP devices. (b) Cycle stability of an LE-BP-ASSP device electrodes based on liquid-exfoliated BP nanoflakes in the alternate flat and bent configurations. [111] John Wiley & Sons. (c) Schematic illustration of the fabrication process of PG-MSCs. (d) Areal capacitance and volumetric capacitance of PG-MSCs and G-MSCs at different scan rates. Reprinted with permission from [112]. Copyright (2017) American Chemical Society.
Download figure:
Standard image High-resolution image3. BP-based electrocatalysts
In addition to the great potential value in energy storage applications, very recently, researchers have started to explore the electrocatalytic performance of BP-based materials as effective electrocatalysts for various energy-related chemical reactions, such as HER, OER and NRR, as shown in figure 8. In this chapter, we will review and discuss systematically the performance and mechanism of BP-based electrocatalysts in these catalytic reactions.
Figure 8. The summary of potential electrocatalytic applications of BP.
Download figure:
Standard image High-resolution image3.1. Hydrogen evolution reaction (HER)
Hydrogen (H2) has been regarded as the ideal clear-energy source owing to its high energy density, easy storage and environmental-friendliness. Among various preparation methods, electrochemical HER is a clear and facile approach for the mass production of hydrogen. The HER is generally kinetically sluggish and needs appropriate electrocatalysts to boost its rate [116]. Therefore, designing and developing of high-performance and cost-effective electrocatalysts for HER is very urgently need. Recently, BP has been proved to exhibit great potential as high-performance HER electrocatalyst. The potential catalytic effects of BP for HER was identified firstly by Pumera and co-workers in 2016 [117]. They investigated the electrochemical properties toward HER of basal- and edge-plane BP, and observed significantly different catalytic performance. Compared to basal-plane BP with the onset potential of −1.13 V vs standard hydrogen electrode (SHE), the edge-plane one exhibited a more promising catalytic activity with the onset potential of 0.55 V due to the metallic characteristic. Although BP has shown electrochemical catalytic potential for HER, the catalytic activity of a pure one is very low. Nowadays, heteroatoms doping, hybrid composing with other materials, interfaces engineering and functionalizing, have been demonstrated as effective strategies to improve the electrochemical activity towards the HER of BP.
3.1.1. Heteroatoms doping and hybrids
Theoretical calculations have suggested that doping no-metal heteroatoms including C, N, S and O into BP lattice could flexibility tune the binding strength of H* with active sites on BP, thus greatly enhancing HER catalytic performance [118]. Among these heteroatoms, the embedded O atoms provided optimal H* binding strength, indicating great potential as the HER catalyst. Besides no-metal doping, various metal atoms have been introduced into BP to improve the HER performance. Yu et al have successfully synthesized various transition metal-doped phosphorene including Co, Mn and Ni by a facile and rapid electrochemical strategy (figure 9(a)) [119]. The embedded dopants generated redistribution of charge, electronic band transformation and occupation state, giving rise to improved electroconductivity and plentiful active sites. Consequently, compared to the bare phosphorene, metal-doped materials presented enhanced HER activities and electrocatalytic stability. Particularly, Co-doped phosphorene exhibited the highest catalytic activity with an onset overpotential of 0.133 V and a potential 0.294 V at 10 mV cm−2 (figure 9(b)). In addition, a new kind of hybrid of PtRu nanoclusters (NCs) and BP NSs has been developed [120]. This hybrid NCs/BP materials demonstrated outstanding HER catalytic activity with 88.5 mV cm−2 at −70 mV in alkaline conditions, which greatly surpassed that of commercial Pt/C. DFT theory suggested that the excellent activity derived from the strong electronic interaction between PtRu NCs and BP, which effectively accelerated the dissociation of water and optimized the H* binding strength.
Figure 9. (a) Schematic diagram illustrating the experiments to explore the effects of Co+ and TBA+. (b) HER polarization curves and (c) Tafel plots of BP(Co), BP(Mo), BP(Ni), Pt/C, and pristine phosphorene. [119] John Wiley & Sons. (d) Schematic illustration of the solid exfoliation of bulk BP into nanosheets and deformation charge density of adsorption configuration of CO(NH2)2 on BP nanosheets. (e) Voltammetry and (f) Tafel plots of bulk BP, milled BP, NH2-BP nanosheets, glassy carbon (GC) and Pt for the electrocatalytic HER. Reproduced from [121] with permission of The Royal Society of Chemistry. (g) Schematic illustration for construction of EBP@NG. (h) HER polarization curves of EBP@NG and other reference catalysts. (i) Calculated HER free energy diagrams of on BP and BP-NG. Reprinted with permission from [122]. Copyright (2019) American Chemical Society.
Download figure:
Standard image High-resolution image3.1.2. Functionalizing
Chen et al reported a NH2-functionalizd few-layer BP NSs by employing a new green and facile solid-exfoliation method of bulk BP based on CO(NH2)2-asisisted ball milling (figure 9(d)) [121]. The fabricated NH2-BP NSs showed improved electrocatalytic HER activity with low overpotentials of 290 mV vs SHE at a current density of −10 mA cm−2 and a Tafel slope of 63 mV dec−1 (figures 9(e) and (f)), which were significantly superior to those of pristine bulk BP, indicating a prospective metal-free HER catalyst. Authors also suggested that this improved HER catalytic performance was mainly attributed to more exposed active sites and dangling bonds and efficient charge transfer. Xia et al suggested that the CN-group could not only passivate the edges of BP nanostructures, but also optimize the band edge positions of valence and conduction band of BP to further improve performance because of larger electronegativity than P atoms. This CN-group supported that the BP quantum dot (QD) electrode showed electrocatalytic HER performance with a low overpotential of 148 mV at −10 mA cm−2 superior to pure BP, as well as remarkable electrocatalytic stability [123]. Recently, Feng and co-author also pointed out, based on DFT calculations, that introducing NH2 and OH functional groups could enhance the HER catalytic activity of BP, especially for plane and zigzag edge sites [118]. Another first-principles simulations demonstrated that the defective functionalizing including atomic vacancies and edges also played a critical role in activating the HER reaction [124]. The empty and localized defective states were compensated by adsorbed H* species, which led to an appropriate hydrogen adsorption strength comparable to platinum.
3.1.3. Interfaces engineering
Regulating the electronic properties by interfaces engineering is a promising strategy to design effective and highly active electrocatalyst towards HER. Zeng et al demonstrated a MoS2–BP interfaces by depositing MoS2 flakes on BP NSs [125]. Due to the higher Fermi energy of BP than that of MoS2, effective electron tended to transfer from BP NSs to MoS2 flakes through heterojunction contact in MoS2–BP interfaces, which led to the accumulation of electrons on MoS2 in the MoS2–BP heterostructure with a narrower band gap than that of pristine MoS2 flakes. The as-synthesized MoS2–BP interfaces showed excellent HER catalytic performance with an overpotential of 85 mV (at 10 mA cm−2), which was much lower than individual metallic or edge-oriented MoS2 and BP NSs. The MoS2–BP heterostructure exhibited a high exchange current density of 0.66 mA cm−2 that was 22 times higher than MoS2. Moreover, this hybrid interfaces catalyst achieved remarkable electrocatalytic stability. Recently, Dai et al designed a metal-free 2D/2D heterostructure EBP@NG as effective HER catalysts by rationally coupling few-layer exfoliated black phosphorus (EBP) with NG (figure 9(g)) [122]. Benefiting from the well-designed interface engineering, the synergistic effect of EBP and NG not only significantly improved the ambient stability of EBP but also modulated the electronic configuration of individual components to promote their electrocatalytic activities. Because of a higher Fermi level of NG relative to EBP, direct interlayer electron transfer enriched the electron density of BP and thus optimized the absorption/desorption of H*, finally achieving highly active HER with ultralow overpotential at 10 mA cm−2 and excellent durability (figure 9(h)). Another representative example was the Ni2P nanocrystals-BP heterostructure (Ni2P@BP) reported by Yan et al, which could strongly suppress the stacking of BP and the agglomeration of Ni2P, resulting in more exposed active sites for HER [115]. As a result, this specially designed Ni2P@BP heterostructure showed enhanced charge carrier concentration of 1.37 × 1020 cm−3 and electrical conductivity of 6.25 × 104 S m−1. As a result, the Ni2P@BP electrode exhibited excellent electrocatalytic activity with an overpotential of 107 mV at 10 mA cm−2 and a small Tafel slope of 38.6 mV dec−1, as well as good chemical stability. Soon afterwards, Zhang et al also reported a new hybrid catalyst through in-situ grown of Ni2P NPs on 2D BP to improve the HER activity [126]. Apart from MoS2, NG and Ni2P, other HER electrocatalytic materials through hybrids or interfaces engineering have also been investigated, such as MoSe2-BP [127] and BP/Co2P [128], etc. A summary of the BP-based electrocatalysts mentioned above towards HER is shown in table 2.
Table 2. Summary of HER performance of BP-based electrocatalysts.
| Materials | Electrolyte | Overpotential (V) at current density of 10 mA cm−2 | Tafel slope (mV dec−1) | Reference |
|---|---|---|---|---|
| Edge-BP | 0.5 M H2SO4 | 0.55 (onset) | [117] | |
| Basal-BP | 0.5 M H2SO4 | 1.13 (onset) | [117] | |
| BP(Co) | 0.5 M H2SO4 | 0.294 | 107 | [119] |
| BP(Mn) | 0.5 M H2SO4 | 0.522 | 116 | [119] |
| BP(Ni) | 0.5 M H2SO4 | 0.691 | 112 | [119] |
| Bare BP | 0.5 M H2SO4 | 0.740 | 182 | [119] |
| PtRu NCs/BP | 1 M KOH | 0.022 | 19 | [120] |
| NH2-BP | 1 M KOH | 0.29 | 63 | [121] |
| BP/PB PN | 0.5 M H2SO4 | 0.148 | 79 | [123] |
| MoS2–BP | 0.5 M H2SO4 | 0.085 | 68 | [125] |
| EBP@NG (1:4) | 1 M KOH | 0.125 | 76 | [122] |
| Ni2P@BP | 0.5 M H2SO4 | 0.107 | 38.6 | [115] |
| Ni2P/BP | 0.5 M H2SO4 | 0.185 | 81 | [126] |
| MoSe2–BP | 0.5 M H2SO4 | 0.38 | 97 | [127] |
| BP/Co2P | 0.5 M H2SO4 | 0.34 (100 mA cm−2) | 62 | [128] |
| 1 M KOH | 0.336 (100 mA cm−2) | 72 | ||
| BP QDs/MXene | 1 M KOH | 0.19 | 83 | [129] |
3.2. Oxygen evolution reaction (OER)
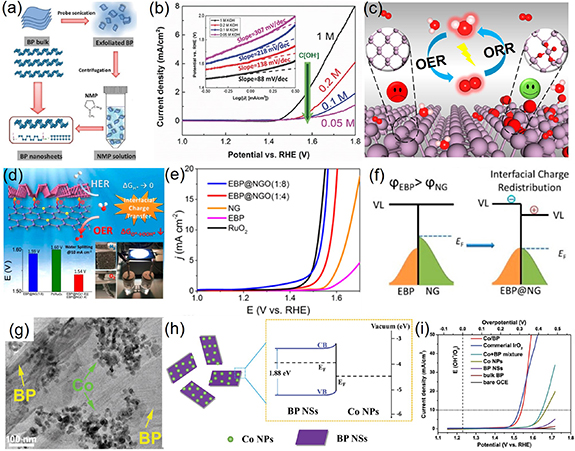
The OER is a critical reaction progress for various energy storage and conversion technologies, such as fuel cells, water-splitting cells and metal-air batteries [11, 130]. However, similar HER, OER also suffers from sluggish reaction kinetics and requires high-efficiency catalysts to accelerate reaction [12, 131]. Because of high cost and limited natural resource of precious metal-based catalysts [11], various 2D metal-free layered materials have been extensively examined as electrocatalysts for OER, such as heteroatom-doped graphene [5, 21, 132, 133] and TMDs [134], etc. On the basis of intrinsic structural and electronic properties, apart from HER, BP has also been identified as a promising application in catalyzing OER. In 2016, Wang et al developed a novel thermal-vaporization transformation strategy to directly grow BP thin-film on Ti foil (BP–Ti), and investigated their electrocatalytic potential for OER for the first time [10]. The as-prepared BP–Ti exhibited highly advanced OER activity with an onset potential of about 1.48 V in 0.1 M KOH solution, which was comparable with most commercial metal oxide-based catalysts. To further improve the OER electrocatalytic performance, BP was also grown on a carbon nanotube network (CNT) interconnected matrix (BP-CNT), showing better conductivity and dispersion. Importantly, BP-CNT delivered more excellent electrocatalytic OER activity with the onset potential of 1.49 V, which only required 1.6 V to obtain a current density of 10 mA cm−2, close to commercial RuO2 (1.59 V) and IrO2 (1.57 V). Soon afterwards, in view of restricted active sites on the bulk crystal structure of BP, Qi and co-workers prepared few-layer BP NSs by the facile liquid exfoliation method and investigated the OER electrocatalytic performance (figure 10(a)) [9]. Compared to the bulk BP, BP NSs greatly improved the electrocatalytic activity with favorable OER onset-potential of 1.45 V and Tafel slope of 88 mV dec−1 (figure 10(b)). Importantly, the OER performance showed the strong layer-thickness dependence. BP NSs with different thickness could be attained by controlling the centrifugation rate. With the reduction of the thickness of BP NS, the OER activity exhibited gradually enhanced tendency. The improved electrocatalytic performance was primarily attributed to the lager specific surface area and more active sites owing to the reduced thickness of BP NSs. Atomistic understanding the OER catalytic mechanism was crucial for further designing and improving the catalytic activity of BP-based electrocatalysts. Theoretical calculations have demonstrated that OER catalytic activity strongly relied on the local oxidation of the BP surface, which could effectively tune the binding strength of reaction intermediates especially for O*, thus changing the OER electrocatalytic activity (figure 10(c)). The higher local oxidation degree corresponded to the better OER performance [135].
Figure 10. (a) Schematic illustration of solution-phase exfoliation route to prepare few-layer BP nanosheets. (b) Polarization curves with Tafel plots in the inset of BP nanosheets in the KOH electrolyte in different concentrations. [9] John Wiley & Sons. (c) Schematic diagram of the influence of oxidation degree on OER activity of BP nanosheets. Reprinted with permission from [135]. Copyright (2019) American Chemical Society. (d) Schematic diagram of EBP@NG towards bifunctional HER and OER activities. (e) OER polarization curves of EBP@NG and other reference catalysts. (f) Illustration of interfacial charge redistribution between EBP and NG. Reprinted with permission from [122]. Copyright (2019) American Chemical Society. (g) TEM images and (h) energy band diagrams of the Co/BP nanohybrids. (i) Linear sweep voltammetry curve of Co/BP nanohybrids and other reference catalysts. [139] John Wiley & Sons.
Download figure:
Standard image High-resolution imageThe good catalytic performance of pristine BP nanosheets inspired more researchers to further explore and improve the OER catalytic activity and environmental stability of BP-based electrocatalysts. Similar HER, element doping, rational interface engineering and hybrids with other materials are also considered as effective strategies to enhance the OER catalytic activity of BP NSs.
3.2.1. Element doping
Recently, sulfur (S)-doped BP NSs, grown using high-pressure synthesis followed by liquid exfoliation, have been proved to possess much better OER catalytic performance compared to pristine NSs [137], as indicated by the small Tafel slope of 75 mV dec−1, which was favorable for OER kinetics reaction. The S-doped BP NSs also displayed excellent ambient stability without degradation after six days of exposure to the environment. DFT calculations indicated that the OER activity of BP mainly arose from the intrinsic defects and doped S atoms played a critical role in improving OER activity and stability. Tellurium (Te) of the same group-VI as S have also been introduced into the BP to improve the OER activity [138–140]. Yan et al have successfully doped a uniform and mass of Te atoms into BP NSs by liquid exfoliation, and demonstrated that Te-doped BP NSs exhibited improved catalytic activity with onset-potential of 1.49 V, compared to the undoped one of 1.63 V [140]. Subsequently, Zhu et al theoretically investigated the OER electrocatalytic origin of Te-doped phosphorene by DFT calculations and suggested that the synergetic effects between doped Te atoms and intrinsic defects could tune the catalytic activity within a wide range [139]. The cluster of Stone-Wales defect decorated by one Te atom exhibited the best OER catalytic performance.
3.2.2. Interface and hybrids engineering
In terms of interface engineering, as mentioned in the HER section, a novel metal-free EBP@NG heterostructure had been successfully synthesized and achieved improved bifunctional HER and OER activities with outstanding durability (figure 10(d)) [122]. The synergy of EBP and NP endowed EBP@NG with a low cell voltage of 1.54 V (at 10 mA cm−2), smaller than that of integrated Pt/C@RuO2 compound of 1.6 V (figure 10(e)). Hybrids of BP NSs with other metal materials also attracted wide attention. In 2018, Feng et al reported a Co/BP NSs nanohybrids by in situ growth of Co NPs on BP NSs based on a facile solvothermal reduction path (figure 10(g)) [136]. Through rational engineering the metal Co-semiconductor BP interface, effective electrons will transfer from BP NSs to Co NPs owing to the higher fermi level of BP than that of metallic Co (figure 10(h)), finally resulting in the equilibrium of fermi level and increased electrical conductivity. As a result, the prepared Co/BP NSs achieved remarkable OER performance, with an overpotential of 0.31 V at 10 mA cm−2 and outstanding electrocatalytic stability in alkaline solution (figure 10(i)), indicating great potential as the candidate of commercial IrO2. Recently, a hybrid of BP NSs modified with Au NPs (BP/Au) synthesized by a one-pot hot solution method was also considered as a suitable candidate for OER electrocatalysts [141]. BP/Au realized outstanding OER electrocatalytic performance with an ultra-low onset-potential of 1.36 V and the Tafel slope of 68.1 mV dec−1. Theoretical simulation uncovered that the loading of Au NPs induced the semiconductor-to-metal transformation, leading to a faster carrier mobility of BP/Au. The improved carrier mobility and catalytic activity primally originated from the coupling effect between the metal and local semiconductor owing to the electrons transfer from Au NPs to the surface of BP. Apart from above-mentioned materials, researchers also focused on other potential BP-based catalysts decorated by hybrids or interfaces engineering, such as Co3O4@BP [142], BP/Co2P [128], Co(OH)2/BP [143], decorated Ni3N|Ni2N|BP [144], etc.
In addition to the BP NSs, BP QDs have also shown great potential as high-performance OER electrocatalysts. Recently, the phosphorene QDs functionalized with N-containing groups (FPQDs) by a simple electrochemical exfoliation strategy was for the first time reported by Prasannachandran and co-workers [145]. Because of the electronegativity difference between P and N atoms, the N-functionality effectively induced charge separation, which in turn enhanced the adsorption strength of OH− on the P atoms, finally achieving improved OER performance than pure BP QDs. The as-prepared FPQDs showed efficient electrocatalytic activity with a potential of 1.66 V (10 mA cm−2) and excellent stability. Soon afterwards, 2D MXene-supported BP QDs was synthesized by loading BP QDs on the Mxene substrate through simple vdW self-assembly [129]. In this case, the large surface area and abundant active sites of the BP QDs guaranteed the high-performance OER activity. On the other hand, the MXene substrate effectively confined the BP QDs and prevented them from aggregation. The MXene NSs further provided the excellent structural robustness and electroconductivity. These favorable advantages rendered BP QDs/MXene as a bifunctional electrocatalyst with outstanding synergy in the HER and OER. Particularly, OER possessed a low overpotential of 360 mV and a Tafel slope of 64.3 mV dev−1, which were largely lower than those of their individual BP QDs and MXene NSs. In addition, Batmunkh et al developed a facile and efficient strategy to prepare high-quality BPQDs using a microwave method [146]. They demonstrated that the as-prepared BPQDs without any supporting could be used as an efficient electrocatalyst with an overpotential of 450 mV (10 mA cm−2). Furthermore, the integrated CoOx–BPQDs catalyst exhibited advanced OER electrocatalytic performance with lower overpotential 360 mV compared to traditional CoOx of 480 mV and a low Tafel slope of 58.5 mV dec−1, as well as remarkable stability. A summary for OER performance of these BP-based electrocatalysts are listed in table 3.
Table 3. Summary of OER performance of BP-based electrocatalysts.
| Materials | Electrolyte | Overpotential (V) at current density of 10 mA cm−2 | Tafel slope (mV dec−1) | Reference |
|---|---|---|---|---|
| BP-Ti | 0.1 M KOH | 0.25 (onset) | 91.52 | [10] |
| BP-CNT | 0.1 M KOH | 0.37 | 72.88 | [10] |
| Few-layer BP | 1 M KOH | 0.23 (onset) | 88 | [9] |
| EBP@NG | 1 M KOH | 0.265 | 89 | [122] |
| BP/Co2P | 1 M KOH | 0.517 (100 mA cm−2) | 78 | [128] |
| S-doped BP | 1 M KOH | 0.41 | 75 | [137] |
| Te-doped BP | 1 M KOH | 0.26 (onset) | [140] | |
| Co/BP | 1 M KOH | 0.31 | 61 | [136] |
| Co3O4@BP | 1 M KOH | 0.4 | 63 | [142] |
| Co(OH)2/BP | 1 M KOH | 0.276 | 57 | [143] |
| Ni3N|Ni2P|BP | 1 M KOH | 0.247 | 79 | [144] |
| N-BP QDs | 1 M NaOH | 0.43 | 48 | [145] |
| BP QDs/MXene | 1 M KOH | 0.36 | 64.3 | [129] |
| CoOx-BP QDs | 1 M KOH | 0.36 | 58.5 | [146] |
3.3. Nitrogen reduction reaction (NRR)
Synthesis of ammonia (NH3) from earth-rich nitrogen (N2) is all-important for a series of applications in various fields [147–149], such as pharmaceutical, fertilizer and energy storage industry, etc. Owing to harsh production conditions including high-temperature, high-pressure and huge consumption of energy for conventional Haber–Bosch process [150, 151], electrocatalytic NRR has become an alternative and promising strategy for the mass production of NH3 [14, 15, 110]. However, NRR usually suffers from the slow kinetics because of the difficulty of breaking N ≡ N insert bond and the vigorous competition of HER [14, 152], searching for efficient electrocatalysts with high activity and selectivity for NRR is very urgent.
In addition to the effectively catalyzing OER and HER, BP has also attracted wide attention as an idea for nonmetallic electrocatalysts towards NRR. Very recently, inspired by the concept of 'like dissolves like', Zhang and co-workers for the first time demonstrated that few-layer BP NSs (FL–BP NSs) could be used as superior metal-free electrocatalysts for NRR (figures 11(a)–(d)) [153]. Under ambient conditions, the well-exfoliated FL–BP NSs exhibited a high faradaic efficiency of 5.07% in the acidic aqueous solution, and achieved a high NH3 yield rate of 31.37 μgh−1mg−1 cat (figure 11(e)). DFT calculations suggested that the active center of FL-BP NSs were zigzag and diff-zigzag type edges owing to the active orbital and electrons, which could enable selective electrocatalysis of N2 to NH3 through an alternating HER pathway (figure 11(f)).
Figure 11. (a) Atomic representation, (b) SEM image, (c) EM images and (d) HRTEM image and SAED pattern of BP. (e) Faradaic efficiency and yield rate of NH3 at various potentials. (f) Diagram of the possible reaction mechanisms for electrochemical reduction of N2 to NH3 at the zigzag edge of few-layer BP nanosheets. [153] John Wiley & Sons. (g) Low- and (h) high-magnification TEM images of BP@SnO2−x nanotube. (i) Faradaic efficiencies of BP@SnO2−x nanotubes at different potentials, and comparison of NH3 yields of BP@SnO2−x nanotubes, SnO2−x nanotubes and BP QDs. [154] John Wiley & Sons.
Download figure:
Standard image High-resolution imageDownsizing of bulk BP to QDs generally generates more active sites than bulk ones due to larger surface areas. However, BP QDs are inclined to aggregate, which will inevitably lead to the loss of active sites. Moreover, the poor electrical conductivity of QDs is not conducive to charge transport during electrocatalysis progress. To resolve these issues, Liu et al reported firstly an BP@SnO2−x nanotubes (figure 11(g)) [154]. In this case, SnO2−x nanotubes acted as an electrochemically active and conductive matrix. Then, the QDs were stably attached on the SnO2−x nanotubes through a self-assembly progress. Benefitting from the synergistic effect between BP and SnO2−x , BP@SnO2−x hybrid electrocatalyst achieved a high ammonia yield of 48.87 μgh−1mg−1 cat and Faradaic efficiency of 14.6% (at −0.4 V vs RHE) (figure 11(i)).
The remarkable NRR electrocatalyst activity in the experiment simulates more theoretical research into further understanding the atomistic catalytic mechanism and improving the NRR catalytic performance of BP-based electrocatalysts. Based on the principle of 'Lewis acid pair', Shi and co-workers theoretically demonstrated a metal-free boron (B)-decorated BP electrocatalyst for NRR and proposed a new mechanism for the activation of N ≡ N bond by the pull-pull effect between N2 molecule and Lewis acid sites [155]. First-principle calculations suggested that the doubly B-doped BP possessed excellent NRR catalytic performance and para-doped one exhibited highest activity with an ultra-low onset-potential of 0.19 V. Subsequently, a series of single Mo-center catalysts supported on N-doped BP were proposed as efficient electrocatalyst toward NRR [156]. DFT calculations suggested that the Mo1N3 structure could chemically adsorb N2 and showed the optimal catalytic activity with an ultralow NRR overpotential of 0.02 V, indicating the efficient catalyst of NRR at ambient conditions. And the produced NH3 molecule could be fast removed on the Mo1N3 with a small energy barrier of 0.56 eV and the reaction intermediates showed good stability. Furthermore, these Mo-centered single-atom catalysts were proved to be more selective to NRR over the competing HER. In addition to the doping heteroatom into BP lattice, Liu et al reported a series of single-atom catalysts with transition metals (Fe, Mn, Cr, Mo, W, V and Nb) anchored on the BP surface [157]. DFT calculations suggested that W-supported BP could be used as a promising catalyst for the NRR, with good thermodynamic stability, high electrocatalyst activity and selectivity. The favorable reaction mechanism of W@BP (W atoms adsorbed on the BP surface) and W-BP (W atoms substituted surface P atoms) were the enzymatic pathway with low reaction onset-potential of 0.46 V and hybrid pathway with the onset-potential of 0.42 V, respectively, indicating highly effective activity towards NRR. The remarkable catalytic performance of W-anchored BP originated from the WP3 active center, which served as an electron adaptor to activate N2 through donating electrons and adjusting charge transfer during the NRR progress. These theoretical studies provided a meaningful guidance to construct BP-based electrocatalysts towards NRR and opened new prospective strategies to further improve their catalytic activity.
4. Conclusion and perspective
In this review, we have provided a summary of recent progress of BP in energy storage and electrocatalytic applications, as shown in figure 12. In addition to reviewing the development of pure bulk BP, NSs, and QDs as effective electrode materials for various ion-batteries and supercapacitors, as well as electrocatalysts for HER, OER and NRR, we also discussed some current effective strategies to enhance the electrochemical performance of BP-based functional materials, including doping heteroatoms, interface engineering, hybridization and surface functionalization, etc. Benefitting from the attractive structural and electronic properties, such as high carrier mobility, anisotropic dispersion and large surface area, extensive experimental and theoretical research has demonstrated that BP-based functional materials could serve as promising electrode materials and electrocatalysts in energy storage and conversion applications. Thus far, many advanced devices and outstanding improvements have been achieved in 2D BP. Despite significant development having been made in these fields, fundamental investigations on BP for energy storage and electrocatalytic applications are still in its early stage with an ongoing progress, and considerable efforts are needed to solve tricky challenges and exploit new applications.
Figure 12. Scheme summary showing various energy storage and electrocatalytic applications of BP-based materials.
Download figure:
Standard image High-resolution imageThe atomistic mechanism investigations are all-important for enhancing the electrochemical performance of BP-based electrodes and electrocatalysts, which can provide rational guidance for next-generation devices. However, most of the current studies usually focus on the fabrication, advanced characterization, performance examination of BP-based materials, in-depth mechanistic investigations for enhanced electrochemical performance and catalytic origin are still lacking. The atomic- and molecular-level understanding of the working process of electrodes and active origin of electrocatalysts are instructive for achieving the full potential of BP-based devices. At present, state-of-the-art first-principles calculations are one of the most valid approaches to study the atomistic mechanism. It not only can explain the experimental results reasonably at atomic level, but can also rationally predict the electrochemical performance of modified BP-based materials. Hence, the integration of experimental studies and advanced theoretical simulations is urgent, which will help people better understand the physicochemical origin of BP-based electrodes and electrocatalysts.
Long-term stability under ambient conditions is also a significant factor for electrochemical performance of energy storage devices and electrocatalysts, which is urgently needed but remains a serious challenge for BP-based hybrid materials. The BP surface suffers from air instability due to the presence of a lone-pair of electrons, which can strongly react with oxygen dissolved in water. This will destroy the original atomic configuration, leading to the rapid degradation of conductivity and other excellent electrical properties. Generally, a few reliable passivation strategies have been developed to present the BP from oxygen/moisture degradation, such as electrostatic functionalization, liquid–phase surface passivation, covalent surface modification, etc. However, current effective methods have not been considered on the scalable process. In addition, the production of few-layer BP with a large size and high quality is the premise of all energy applications from the purpose of practical implementation. Currently, the popular scotch-tape technique, liquid-phase and electrochemical exfoliation, chemical vapor deposition (CVD) are limited to research laboratories and their BP yield is still too low to meet large-scale commercial demand. Moreover, the liquid–phase exfoliations inevitably introduce some intrinsic and extrinsic defects in the BP lattice that will adversely impact the electrochemical performance, and the high-quality production of BP through the CVD method usually requires a suitable substrate. The electrochemical properties of BP strongly depend on the number of layers, so the preparation of BP with tunable layers is also very important. In addition, the synthesis progress of BP-based functional electrodes and electrocatalysts has also been just at the laboratory level and is hard to scale up, limiting viability of these new material. Therefore, developing new highly competitive techniques to revolutionize the fabrication of BP-based functional devices is also a significant research topic in the future.
The successful realization of 2D BP significantly enriches the family of 2D materials. Intrinsically superior structural and electronic properties render BP-based functional materials as promising candidates for energy-related devices. However, the full potential of these BP-based devices is yet to be explored due to the existing great challenges. Continuous efforts in combination with theoretical simulations and experimental investigations should be made to solve these issues. In spite of the development of BP-based materials in the target field, it is also envisaged tocontinuously expand the multifunctional application of them in two or more fields. On the basis of enough basic knowledge and evolving experimental techniques, many advanced BP-based functions and novel devices will gradually come into our lives in the coming years.
Acknowledgments
This work is supported by the National Science Foundation of China with Grant Nos. 12104385, 11974105 and 11634001, the National Basic Research Programs of China under Grant No. 2016YFA0300900, Hunan Key Laboratory of Two-Dimensional Materials (No. 2018TP1010), and the Program for Changjiang Scholars and Innovative Research Team in University (IRT_17R91).
Data availability statement
No new data was created or analysed in this study.