Abstract
A biofuel cell that can generate electricity using only water is expected to be used as a new energy harvester for an emergency power supply. A new 4-series/4-parallel structured paper-substrate biofuel cell was prepared using a fuel supply paper preloaded with glucose and phosphate buffer salts. When a power generation test was conducted by supplying water to the fuel-preloaded paper, the paper-based biofuel cell produced an output approximately 90% (0.84 mW) of that obtained by supplying a phosphate buffer containing glucose as the electrolyte. The open-circuit voltage was 2.1 V, and an LED could be powered by simply supplying water to the cell without using a booster circuit.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In recent years, efforts have been made to install sensors everywhere toward a smart life and smart society utilizing the Internet of Things. Consequently, it is important to supply power to operate the various component systems, including sensors. In order for a user to continually employ a sensor with minimal interaction, it is necessary to provide a stable power supply to drive the sensor according to the application. For this reason, there are high expectations for energy harvesting technologies that can obtain electric power from environmental sources such as light, vibrations, and heat for the sustainable use of various sensors and their controlling systems.
Organic (bio) molecules or macromolecules (e.g. juice containing sugar and fructose, liquor containing ethanol, paper, rice, lactic acid in sweat, and sugar in urine) are abundant in our daily lives and contain extremely high amounts of energy, making them suitable sources for energy harvesting. Many researchers are exploring the possibility of energy harvesters, called biofuel cells, that can directly convert these organic molecules into electric power as a new power source for sensor devices [1–16].
Biofuel cells can be composed solely of organic substances such as enzymes, paper, and carbon, and they can be used for a power source of wearable device [17–19]. For example, a wireless transmission device can be driven by the biofuel cell to transfer data. Because it is made of paper, carrying the cell would not burden the user. For this reason, paper-based biofuel cells (PBFCs) have been attracting worldwide research attention in recent years [17–25].
We have developed high-power PBFCs [17, 18, 26–28] and showed that printing porous carbon on paper to increase the effective surface area improves the power density per cell. In addition, because biofuel cells do not require a separator, they can be connected in series and parallel by printing the wiring on a paper substrate. For example, a 4-series/4-parallel array biofuel cell has exhibited an actual output of 1 mW [18].
The paper biofuel cells reported previously require the supply of an aqueous solution containing a fuel, which is highly effective for monitoring body fluids. On the other hand, for use as an emergency power supply as described above, we aimed to generate electricity with only water or a solution that does not contain fuel by pre-drying a paper with a fuel supply.
Small batteries that use magnesium and silver/silver chloride electrodes to generate electricity from water or body fluids have been reported as a similar power source [29, 30]. Although magnesium batteries can be an important tool, the merit of biofuel cells, by comparison, is that they are more easily disposed of because they can be manufactured using only organic substances.
Therefore, in this study, we prepared a new PBFC array in which glucose is preloaded as the fuel. For this purpose, we designed a paper-based 4 series/4 parallel array consisting of paper, carbon, enzymes, and organic mediators. The PBFC pre-charged with glucose showed approximately 90% of the output (0.84 mW) of the cell supplied with a solution containing the fuel. It was also possible to drive an LED without a booster circuit.
2. Experimental
A water-repellent-treated Japanese paper (Gasenshi Izumo, Keynote Planning, 120 μm thickness) was used as the printable substrate, which has a printable smooth surface and good oxygen permeability. One side of the paper surface was coated with a water repellent (Hajix, Comens, Japan), the principal component of which is silicon nanoparticles.
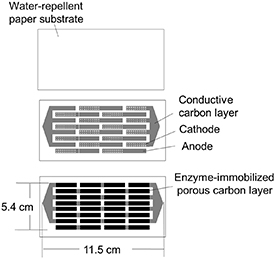
The PBFC was composed of bioanode and biocathode components, in which porous carbon electrodes were constructed using porous carbon inks. A conductive carbon layer (5 mm × 10 mm) was printed using conductive carbon ink (JELCOM CH-10, Jujo Chemicals) onto the water-repellent Japanese paper, followed by curing at 120 °C for 30 min. To allow for oxygen supply from the paper to the porous carbon layer, the biocathode conductive carbon layer was designed with 25 pores (0.5 mm diameter).
Furthermore, a porous carbon ink was printed on the conductive carbon layer by mixing 440 mg of MgO template carbon (Toyo Tanso), 110 mg of polyvinylidene fluoride (Kureha), and 3 ml of isophorone (Wako Pure Chemical Industries), then dried at 45 °C for 30 min.
Figure 1 shows a schematic of the manufactured electrodes. The electrode surface area of the porous carbon reaction component was 20 mm × 5 mm. The array number of the PBFCs is expressed as (number of cells connected in series) × (number of cells connected in parallel).
Figure 1. Fabrication process of a PBFC array.
Download figure:
Standard image High-resolution imageThe biocathode was prepared by applying 20 μl of a bilirubin oxidase (BOD from Myrothecium sp., Cat. No: BO-3, 1.2 U mg−1, Amano Enzyme) solution (1 U μl−1) containing 0.01% Triton X-100 onto the UV/ozone-pretreated porous carbon layer. For glucose oxidation on the bioanode surface, 20 μl of a glucose oxidase (GOx from Aspergillus niger, Cat. No: 599-04681, 100 U mg−1, Wako, Japan) solution (20 U μl−1, dissolved in phosphate buffer solution at pH 7.0) and 20 μl of a saturated tetrathiafulvalene solution in methanol were added via syringe to the porous anodic carbon layer and pretreated with UV/ozone for 15 min.
For output evaluation, the solution was supplied to the electrode by placing the Japanese paper on the electrode via the method shown in figure 2, and 1 M phosphate buffer (pH 7.0) containing 0.1 mol dm−3 glucose was added dropwise to the paper.
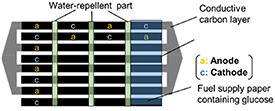
Figure 2. Schematic illustration of the fuel-preloaded 4-series/4-parallel PBFC.
Download figure:
Standard image High-resolution imageWe also developed a fuel-preloaded PBFC using glucose-containing paper. First, the Japanese paper was water-repellent to prevent short-circuiting in the series direction [18]. Then, a 0.1 mol dm−3 glucose solution was added dropwise to each part of the water-repellent Japanese paper, after which the paper was dried at 100 °C for 30 min. A fuel-preloaded PBFC was prepared by fixing the glucose-containing paper on the anode and cathode using water-repellent tape. Evaluation of the fuel-preloaded PBFC was performed after charging 500 μl of ultrapure water on each glucose-containing paper and allowing it to stand for 1 min at 25 °C with a humidity of 50%.
3. Results and discussion
Arraying is a method used in solar cells that can yield large outputs by combining cells and modules. In the case of solar cells, the cells are independent and connected in series and in parallel using silver wiring underneath. Owing to the substrate specificity of the employed enzyme, biofuel cells do not strictly require a separator between the anode and cathode fuels. Therefore, we created a PBFC array using a screen-printing method that allows us to freely design the shape of the thick-film electrodes to be suitable for manufacturing biofuel cells.
Figure 2 shows a schematic diagram of the operating principle of the array PBFC. In a conventional PBFC [18], the fuel solution is supplied independently to individual cells. In this study, to supply fuel more efficiently and reliably, a paper fuel supply layer was placed in the cells in series. In this case, when the fuel solution is supplied to a part of the paper fuel supply layer, the fuel is uniformly supplied to each cell by the paper capillary phenomenon. This can prevent situations where the series/parallel structure is not maintained because fuel is not supplied to some of the cells, which can occur in conventional cells.
The output of the prepared PBFC array was evaluated by the two-electrode method, and the fuel was supplied by adding an electrolytic solution containing dissolved glucose to the paper. The measurement conditions were a scanning potential range of open-circuit voltage to 0 V, scanning speed of 1 mV s−1, and a 1 mol dm−3 phosphate buffer solution (pH 7.0) containing 0.1 mol dm−3 glucose as the electrolyte.
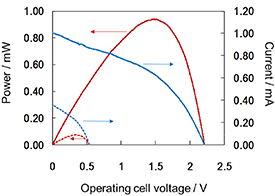
Figure 3 shows the current–voltage and output curves of the PBFC arrays (1 × 1 and 4 × 4). In the 1 × 1 cell, an open-circuit voltage of 0.57 V and a maximum output of 0.064 mW were obtained. For the 4 × 4 cell, the open-circuit voltage was 2.21 V and the maximum output was 0.94 mW. These are approximately the same as those in our previous report [18]. These results confirmed that the wearable PBFC array (4 × 4) can output approximately 1 mW even with a non-metal PBFC.
Figure 3. Current–potential (blue) and power–potential (red) curves of the single (dotted lines) and 4-series/4-parallel (solid lines) PBFCs.
Download figure:
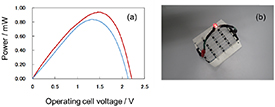
Standard image High-resolution imageNext, a paper containing 1 mol dm−3 phosphate buffer (pH 7.0) with 0.1 mol dm−3 glucose was placed on the electrode, ultrapure water was added dropwise to the PBFC array (4 × 4), and the output was evaluated (figure 4(a)). When only the electrolyte without fuel was dropped onto the glucose-containing paper, an open-circuit voltage of 2.1 V and a maximum output of 0.84 mW were obtained. Therefore, when the glucose-containing paper was used, approximately 89% of the output was obtained compared with when the fuel was supplied in the form of a glucose solution dropped on the paper, and it was possible to generate electricity by supplying only the electrolytic solution. We also succeeded in powering an LED without a booster circuit (figure 4(b)).
Figure 4. (a) Power–potential curves of the 4-series/4-parallel PBFC with a glucose-containing fuel solution supplied to a non-fuel-preloaded cell (red line) and with water supplied to a fuel-preloaded cell (blue line). (b) Image of an LED powered by the 4 × 4 PBFC with preloaded fuel.
Download figure:
Standard image High-resolution imageThe power produced remained constant for approximately 2000 s at a fixed voltage of 1.5 V, and then gradually decreased to 0 mW at 6000 s with a single application of water. The decrease in output might be largely due to the evaporation of the water (figure S1 (available online at stacks.iop.org/JPENERGY/3/016001/mmedia)).
4. Conclusions
We created a fuel-charged biofuel PBFC using paper as a substrate. The produced PBFC showed an output of approximately 0.94 mW cm−2 by supplying 0.1 mol dm−3 glucose. In addition, by using a supply paper charged with fuel, the output was approximately 90% upon supplying only water compared with the output obtained by supplying a fuel solution. As a flexible and thin printable PBFC, this apparatus has the potential to drive a power-saving wireless transmission device. Therefore, it is expected to be applicable as a tag for sensors and as a new energy harvester for wearable devices. In addition, we think it would be good to use it as toys for children. Because biofuel cells are highly safe, they are thus suitable for use in toys when sprinkled with water.
Acknowledgments
This work was partially supported by JST-ASTEP Grant Number JPMJTS1513, JSPS Grant Number 17H02162, the Private University Research Branding Project (2017–2019) from the Ministry of Education, Culture, Sports, Science and Technology, and the Tokyo University of Science Grant for President's Research Promotion.
We would like to thank Editage (www.editage.com) for English language editing.