Abstract
Chemical looping air separation (CLAS) is a promising technology for oxygen generation with high efficiency. The key challenge for CLAS is to design robust oxygen sorbents with suitable redox properties and fast redox kinetics. In this work, perovskite-structured Sr1-xCaxFe1-yCoyO3 oxygen sorbents were investigated and demonstrated for oxygen production with tunable redox properties, high redox rate, and excellent thermal/steam stability. Cobalt doping at B site was found to be highly effective, 33% improvement in oxygen productivity was observed at 500 °C. Moreover, it stabilizes the perovskite structure and prevents phase segregation under pressure swing conditions in the presence of steam. Scalable synthesis of Sr0.8Ca0.2Fe0.4Co0.6O3 oxygen sorbents was carried out through solid state reaction, co-precipitation, and sol-gel methods. Both co-precipitation and sol-gel methods are capable of producing Sr0.8Ca0.2Fe0.4Co0.6O3 sorbents with satisfactory phase purity, high oxygen capacity, and fast redox kinetics. Large scale evaluation of Sr0.8Ca0.2Fe0.4Co0.6O3, using an automated CLAS testbed with over 300 g sorbent loading, further demonstrated the effectiveness of the oxygen sorbent to produce 95% pure O2 with a satisfactory productivity of 0.04 gO2 gsorbent−1 h−1 at 600 °C.
Export citation and abstract BibTeX RIS
1. Introduction
Oxygen, as the second largest industrial gas based on annual consumption, is widely utilized in steel, chemical, medical, and oil & gas industries. The global oxygen market has exceeded $38 billion in 2017, and is projected to reach $48 billion by 2023 [1]. At present, a majority of oxygen is produced by the cryogenic distillation technology due to its ability to produce high purity oxygen (i.e. 99+%) at large scales. However, the cryogenic air separation is both energy and capital intensive, requiring ∼0.24 kWh to produce 1 kg O2 [2]. Such energy consumption corresponds to merely ∼25% thermodynamic 2nd law efficiency [3]. Many research efforts have been devoted to alternative technologies for oxygen production. Currently, pressure swing adsorption (PSA) is being used commercially for small scale oxygen production [4]. PSA is based on selective adsorption of nitrogen over oxygen on a zeolite sorbent surface, due to surface charge on sorbent surface and nitrogen being more polarizable comparing to oxygen. The technology, however, is less efficient than cryogenic air separation and oxygen purity is generally limited (< 95%) [5].
Chemical looping air separation (CLAS) represents an alternative air separation technology with the potential of being significantly more efficient [6–9]. CLAS is based on the redox chemistry of oxide based sorbent materials, which reversibly absorb and desorb oxygen under oxygen partial pressure and/or temperature swings. When exposed to low oxygen partial pressure (PO2), the lattice oxygen in the oxide releases to form gaseous oxygen, while the oxide is reduced. By introducing air, the reduced oxide absorbs oxygen into its lattice structure and regenerates to oxidized form. Many oxides have been investigated as oxygen sorbents for CLAS. Based on thermodynamics, transition metal oxides such as Mn2O3, Co3O4, and CuO have equilibrium PO2 in the range of 0.001–1 atm at below 1000 °C, matching with CLAS conditions from a practical standpoint [7]. Owing to the high oxygen capacity and fast redox kinetics, CuO based oxygen sorbents were the most extensively studied for CLAS [10–13]. It is known that both CuO and Cu2O have a relatively low Tamman temperature (i.e. <550 °C). Meanwhile, the typical operating temperature of using CuO for CLAS is above 800 °C due to kinetic limitations. As such, sintering of the CuO based oxygen sorbent represents an important challenge. Generally, support materials are used to disperse CuO to improve redox kinetics and mechanical strength, and inhibit sintering during redox cycling at high temperatures [14–20]. For example, 50 wt% CuO/SiO2 exhibits oxygen capacity of 4.7 wt% and average redox rate of ∼0.46% min−1 at 850 °C [21]. By adding 20–40 wt% MgO in CuO/SiO2, the stability of the resulted oxygen sorbent significantly improved for 41 redox cycles at 900 °C [22]. Other supports such as ZrO2 and MgAl2O4 have also been reported to improve the stability of CuO for CLAS [23–26]. Comparing to monometallic oxide, a number of mixed oxides have been demonstrated to have superior performance due to balanced redox properties and faster redox kinetics [27, 28]. For example, mixed Co-Fe oxides supported on perovskite support exhibit oxygen capacity of 3.4–4.2 wt% and average redox rate of ∼0.2 wt% min−1 at 850 °C [29]. Nonetheless, CLAS sorbents composed exclusively of first row transition metal oxides generally requires > 800 °C operating temperature due to kinetic limitations. From process efficiency and capital cost standpoints, sorbents that are capable of operating at lower temperatures are highly desirable.
Perovskite based oxygen sorbents have the advantage of reversibly absorb and desorb oxygen at significantly lower temperatures (e.g. ≤ 600 °C), owing to their structural flexibility to accommodate significant amount of oxygen vacancies [30–36]. For example, La0.1Sr0.9Co0.9Fe0.1O3-δ has a wide range of oxygen vacancy depending on temperature and oxygen partial pressure [37, 38]. By oxygen partial pressure swing between air and helium at 600 °C, ∼1 wt% oxygen capacity was achieved for oxygen production [39]. Recently, SrFeO3 based oxygen sorbents have received many research interests, owing to its large oxygen capacity and low operating temperature [40–42]. The versatile structure of SrFeO3 offers many opportunities to introduce dopants at A and/or B site to further tuning the redox property [43–45]. By co-doping of Ca and Co at A and B sites respectively, oxygen partial pressure swing can be operated between 0.05 and 0.2 atm to achieve 1 wt% oxygen capacity at 400 °C–500 °C based on our recent study [46]. The SCFC sorbent is very robust and stable for up to 2000 cycles in the presence of 1% H2O. However, for practical air separation application, it is critical to have sorbent with fast redox kinetics to improve oxygen productivity and reduce reactor size. Furthermore, sorbent stability in concentrated steam (as the purge gas) needs to be established, as steam purging is required to release oxygen out from the sorbent.
In this work, we performed detailed studies on Sr1-xCaxFe1-yCoyO3-δ (SCFC) based oxygen sorbents in terms of the effects of A/B site dopant levels on redox rate and oxygen carrying capacity, effect and scalability of various synthesis methods, and performance testing at industrially relevant operating conditions. Comprehensive oxygen partial pressure swing studies coupled with sorbent characterization elucidated the effect of cobalt doping on redox rate and steam resistance of the oxygen sorbents. Three preparation methods including solid state reaction, co-precipitation, and sol-gel methods have be explored for scalable synthesis of SCFC oxygen sorbents. The effect of synthesis methods on sorbent structure and performance have been investigated. The optimized SCFC composition, i.e. Sr0.8Ca0.2Fe0.4Co0.6O3-δ, was used to prepare sorbent at kilogram per batch scale, which is then used for performance evaluation at a large scale testbed to process 1 standard liter of air per min. The process conditions such as cycle structure and pressure have been comprehensively studied to establish sorbent performance (i.e. oxygen productivity and oxygen purity) as a function of operating conditions.
2. Experimental section
2.1. Materials preparation
Perovskite based oxygen sorbents Sr1-xCaxFe1-yCoyO3-δ (SCFC) were prepared via co-precipitation (CP), sol-gel (SG) and solid-state reaction (SSR) methods. For a typical synthesis of Sr0.8Ca0.2Fe0.9Co0.1O3-δ by co-precipitation method, 16.93 g of strontium nitrate (Sr(NO3)2, Sigma-Aldrich, 99+%), 4.72 g of calcium nitrate tetrahydrate (Ca(NO3)2.4H2O, Sigma-Aldrich, 99+%), 36.36 g of iron nitrate nonahydrate (Fe(NO3)3.9H2O, Sigma-Aldrich, 98+%) and 2.91 g of cobalt nitrate hexahydrate (Co(NO3)2.6H2O, Sigma-Aldrich, 98+%) were dissolved in 200 ml of deionized water. After that, 30 ml of 28% ammonium hydroxide solution (NH4OH, Sigma-Aldrich, 99.99+%) was added to the above solution under stirring. The resulted mixture was heated at 100 °C to evaporate the water. The precipitate was dried at 80 °C in oven for 16 h, followed by calcination at 1000 °C in air for 8 h. The sample was denoted as SCFC8291-CP. For preparing Sr0.8Ca0.2Fe0.4Co0.6O3-δ sample by solid-state reaction method, metal precursors including 11.81 g of strontium carbonate (SrCO3, Sigma-Aldrich, 98+%), 2.0 g of calcium carbonate (CaCO3, Sigma-Aldrich, 99+%), 3.19 g of iron oxide (Fe2O3, NOAH Technologies, 99.9%), and 4.82 g of cobalt oxide (Co3O4, Sigma-Aldrich, 99.5%) were mixed in a Teflon cup and ball milled by zirconia beads at 250 rpm for 8 h using an all-direction planetary ball mill (Columbia International, CIT-XBM4X-2.0L). The sample was then collected and pressed to form pellets, followed by calcination at 1100 °C for 8 h to form perovskite structure. The sample was denoted as SCFC8246-SSR. The procedure of preparing sol-gel sample was similar as previously reported [46].
2.2. Characterization
The structure of prepared oxygen sorbents was characterized by x-ray powder diffraction (Rigaku SmartLab) operated at 44 kV and 40 mA. The sample was scanned between 10 °C and 80 °C with a step size of 0.05o and residence time of 3 s/step. The morphology and elemental composition of SCFC oxygen sorbents were analyzed by scanning electron microscopy (SEM, JEOL JSM-6010LA) coupled with energy dispersive x-ray spectroscopy (EDX, Oxford). Surface area of SCFC oxygen sorbents were studied by krypton adsorption-desorption analysis at 77 K using a Micromeritics ASAP 2020 instrument.
2.3. Chemical looping air separation experiments
The oxygen capacity of SCFC oxygen sorbents were measured by thermal gravimetric analysis (TGA, TA Instruments SDT Q600). In a typical experiment, 50 mg of oxygen sorbent was loaded into an alumina pan, which was placed on the beam holder in TGA. The sample was firstly heated to 700 °C in 20% O2/Ar and isotherm for 10 min, followed by cooling to 400 °C. Then the flow gas was switched between Ar and 20% O2/Ar automatically using Labview controlled gas panel. The reduction step in Ar was lasted for 6 min, while the oxidation step in 20% O2/Ar was 4 min. The sample was tested at between 400 °C and 700 °C at an interval of 50 °C. At each temperature, the redox cycle was performed twice. Under fast oxygen partial pressure swing mode, the redox cycle was performed at 500 °C, 550 °C, and 600 °C with reduction time of 2 min in Ar and oxidation time of 1 min in 20% O2/Ar. The stability of oxygen sorbent under dry condition was investigated by redox cycling between Ar and 20% O2/Ar at 550 °C for 100 cycles. Steam resistance of oxygen sorbent was studied by oxygen partial pressure swing between Ar and 20% O2/Ar at 500 °C for 1000 cycles in the presence of 10% H2O. For each cycle, the reduction time is 2 min, while the oxidation time is 1 min. Large scale evaluation of SCFC8246 oxygen sorbent was performed at Thermosolv LLC using an automated CLAS testbed with 334 g sorbent loading. Air was flowed through the testbed at 1 standard liter per min during oxidation step, while steam was used to sweep oxygen out from the sorbent during reduction step.
3. Results and discussion
As a scalable synthesis method for mixed oxides, co-precipitation was first explored to prepare the Sr1-xCaxFe1-yCoyO3-δ sorbents. The effect of Ca doping at A site of perovskite structure on oxygen capacity was investigated first, by varying Ca doping from 10%–30% (defined as Ca/(Ca + Sr) × 100%). Shown in figure 1, the oxygen capacity follows a volcano shaped correlation with temperature. For example, the 20% Ca doped SCFC8291 has an oxygen capacity of 0.20 wt% at 400 °C under oxygen partial pressure swing between Ar and 20% O2 (table 1). The oxygen capacity doubles to 0.45 wt% at 450 °C, and further increases to the maximum of 1.14 wt% at 600 °C. After that, the oxygen capacity drops to 1.09 and 0.96 wt% at 650 and 700 °C, respectively. The initial increase in oxygen capacity is attributed to increased redox kinetics with temperature. At higher temperature, the thermodynamics limits the maximum attainable oxygen capacity, as evidenced by decreasing in the weight of oxidized SCFC with temperature (figure S1 (stacks.iop.org/JPENERGY/2/025007/mmedia)). After increasing Ca loading from 10% to 20%, the oxygen capacity increases significantly, particularly at 500 °C–700 °C. For example, at 600 °C, the SCFC8291-CP has an oxygen capacity of 1.14%, which is nearly double that of SCFC9191-CP sample. However, further increasing Ca loading to 30% (i.e. SCFC7391-CP) only leads to decrease in oxygen capacity (figure 1 and table 1). Doping of Ca causes distortion of perovskite structure and creates oxygen vacancies even in the presence of 20% O2, which possibly leads to smaller oxygen capacity observed [46].
Figure 1. Effect of Ca loading on oxygen capacity of SCFC-CP oxygen sorbents under oxygen partial pressure swing between Ar and 20% O2 at 400 °C–700 °C.
Download figure:
Standard image High-resolution imageTable 1. Oxygen capacity of SCFC-CP oxygen sorbents with various Ca and Co loading under oxygen partial pressure swing between Ar and 20% O2 at 400 °C–700 °C.
| Oxygen capacity (wt%) | |||||||
|---|---|---|---|---|---|---|---|
| Oxygen Sorbent | 400 °C | 450 °C | 500 °C | 550 °C | 600 °C | 650 °C | 700 °C |
| SCFC8291-CP | 0.20 | 0.45 | 0.73 | 1.00 | 1.14 | 1.09 | 0.96 |
| SCFC8282-CP | 0.28 | 0.55 | 0.78 | 0.95 | 1.00 | 0.92 | 0.81 |
| SCFC8246-CP | 0.15 | 0.40 | 0.75 | 0.81 | 0.72 | 0.56 | 0.38 |
| SCFC9191-CP | 0.23 | 0.43 | 0.50 | 0.56 | 0.58 | 0.60 | 0.56 |
| SCFC7391-CP | 0.02 | 0.55 | 0.80 | 0.88 | 0.88 | 0.81 | 0.68 |
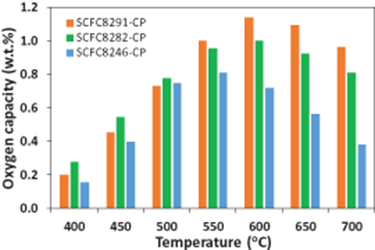
The effect of cobalt is also examined by comparing the oxygen capacity at 400 °C–700 °C. As illustrated in figure 2, all the oxygen sorbents with various cobalt loading demonstrate a similar volcano shaped trend with respect to operating temperature. The maximum oxygen capacity occurs at 550 °C–600 °C for cobalt doping ranging from 10% to 60%, as a result of balance between kinetic and thermodynamic effects. At 600 °C, the oxygen capacity decreases from 1.14% to 0.72% by increasing Co loading from 10% to 60%. The redox kinetics of SCFC8291-CP and SCFC8246-CP samples were further investigated by pressure swing between Ar and 20% O2 at 500 °C, 550 °C, and 600 °C. The cycle time is shortened to 3 min to avoid reaching equilibrium weight during pressure swing operation. Shown in figure 3 and table 2, the oxygen capacity of SCFC8291-CP is 0.45, 0.55, and 0.62 wt% at cycling temperatures of 500 °C, 550 °C, and 600 °C, respectively. In comparison, the oxygen capacity of SCFC8246-CP sorbent is notably higher at 0.58, 0.75 and 0.69 wt% under 500 °C, 550 °C, and 600 °C. Further examining the redox kinetics of SCFC8291-CP and SCFC9246-CP sorbents shows that Co increases redox rates of SCFC sorbents. For instance, the average redox rate (calculated as oxygen capacity per cycle divided by cycle time) of SCFC8246-CP at 500 °C is 0.20 wt% min−1, which is 33% higher than that of SCFC8291-CP sorbent. Similarly, faster redox kinetics of SCFC8246-CP is also observed at higher temperature (i.e. 550 °C and 600 °C). Owing to its fast kinetics, the bed size factor (BSF), defined as amount of sorbent required to produce 1 ton of oxygen per day, is 556 lbs TPD−1 O2 for SCFC8246-CP at 550 °C. Small BSF is critical for practical applications, as it reduces the reactor size and the amount of sorbents used.
Figure 2. Effect of Co loading on oxygen capacity of SCFC-CP oxygen sorbents under oxygen partial pressure swing between Ar and 20% O2 at 400 °C–700 °C.
Download figure:
Standard image High-resolution imageFigure 3. Oxygen partial pressure swing between Ar and 20% O2 at 500 °C, 550 °C, and 600 °C for (a) SCFC8291-CP and (b) SCFC8246-CP oxygen sorbents. A typical redox cycle at 550 °C for (c) SCFC8291-CP and (d) SCFC8246-CP with 1 min of oxidation and 2 min of reduction.
Download figure:
Standard image High-resolution imageTable 2. Average redox rate and bed size factor for SCFC8291-CP and SCFC8246-CP oxygen sorbents operated under oxygen partial pressure swing mode between Ar and 20% O2.
| SCFC8291-CP | SCFC8246-CP | |||||
|---|---|---|---|---|---|---|
| 500 °C | 550 °C | 600 °C | 500 °C | 550 °C | 600 °C | |
| Oxygen capacity (wt%) | 0.45 | 0.55 | 0.62 | 0.58 | 0.75 | 0.69 |
| Average redox rate (% min−1)a | 0.15 | 0.18 | 0.21 | 0.20 | 0.25 | 0.23 |
| Bed size factor (lbs/TPD O2)b | 906 | 758 | 672 | 706 | 556 | 604 |
aAverage redox rates is calculated as maximum oxygen capacity divided by cycle time. bBed size factor is determined as pounds of sorbents required to produce 1 ton of oxygen per day.
The stability of the prepared oxygen sorbents was investigated by pressure swing between Ar and 20% O2 at 550 °C for extended cycles. Shown in figure 4, both SCFC8291-CP and SCFC8246-CP oxygen sorbents are stable up to 100 redox cycles. For the SCFC8291-CP sample, the oxygen capacity at the 1st cycle is 0.47 wt%, which gradually increases to 0.55 wt% at 10th cycle. After that, the oxygen capacity stabilizes at 0.56 wt% till 100th cycle. The initial increase in first 10 cycles is due to its slow redox kinetics, requiring several redox cycles to stabilize. As SCFC8246-CP has a much higher redox kinetics, the initial oxygen capacity is 0.77 wt%, which is nearly unchanged during the 100 redox cycles.
Figure 4. Stability of (a) SCFC8291-CP and (b) SCFC8246-CP oxygen sorbents under oxygen partial pressure swing between Ar-20% O2 at 550 °C for 100 cycles.
Download figure:
Standard image High-resolution imageSteam resistance of the prepared sorbents was also studied by oxygen partial pressure swing for 1000 cycles in the presence of 10% steam. After the test, the spent sorbent is collected and tested for oxygen partial pressure swing between Ar and 20% O2 from 400 to 700 °C. As shown in figure 5, there is notable drop in capacity for spent SCFC8291-CP sorbent, particularly at 450 °C–700 °C. For example, the fresh SCFC8291-CP sorbent has an oxygen capacity of 0.73 wt% at 500 °C, which decreases to 0.59 wt% after 1000 redox cycles in 10% steam. For SCFC8246-CP sorbent, it is interesting to note that the oxygen capacity of spent sample increases at 400 °C–500 °C and decreases at 550 °C–700 °C. The highest oxygen capacity of spent SCFC8246-CP sorbent is 0.81 wt%, which is the same as that of fresh sample. However, the operating temperature of the highest oxygen capacity for spent SCFC8246-CP shifts to lower end after 1000 cycles test in 10% steam. This suggests that the SCFC8246-CP sorbent is stable for operating at low temperature range in the presence of steam.
Figure 5. Oxygen capacity of (a) SCFC8291-CP and (b) SCFC8246-CP oxygen sorbents before and after 1000 redox cycles in 10% H2O.
Download figure:
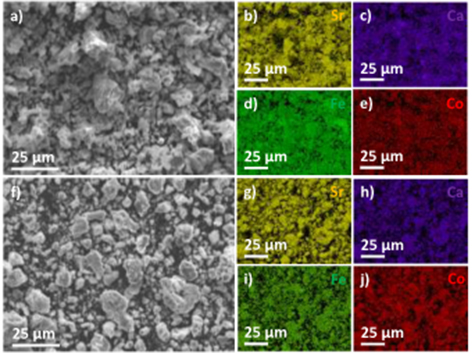
Standard image High-resolution imageThe structures of fresh/spent SCFC8291-CP and SCFC8246-CP sorbents were characterized by XRD (figure 6). For the fresh and spent SCFC8291-CP and spent SCFC8246-CP sorbents, the major peaks at 32.3–32.9, 39.9–40.5, 46.5–47.1, 58.0–58.6, 68.2–68.8, and 77.6–78.3° correspond to (110), (111), (200), (211), (220), and (310) planes of cubic phase of SrCoO3 (PDF 04-019-5710). For SCFC8291-CP sorbent, an additional peak appears at 33.8°, which could be attributed to (101) plane of hexagonal phase Ca(OH)2. SEM images of spent samples are similar with broad size distribution of 0.5–18 μm (figure 7). However, EDX mapping shows that segregation of Ca was observed for SCFC8291-CP sample, which is consistent with XRD peak of Ca(OH)2 phase in spent SCFC8291-CP sample (figures 7 and S2). In comparison, the elemental distributions are quite uniform for SCFC8246-CP sample at sub-micrometer level. The phase integrity of SCFC8246-CP sorbent explains its better steam resistance under extended redox cycles comparing to SCFC8291-CP sample.
Figure 6. XRD patterns of fresh/spent SCFC8291-CP and SCFC8246-CP oxygen sorbents.
Download figure:
Standard image High-resolution imageFigure 7. SEM images and EDX mapping of spent (a)-(e) SCFC8291-CP and (f)-(j) SCFC8246-CP oxygen sorbents.
Download figure:
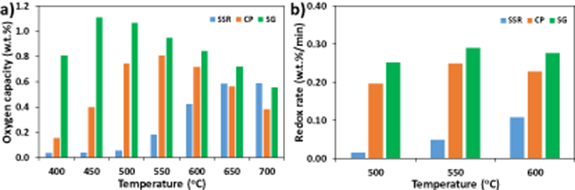
Standard image High-resolution imageAfter confirming the fast kinetics and steam resistance of SCFC8246 sorbent, the sample was also prepared by other scalable methods. For example, solid state reaction method (SSR) was used to prepare SCFC-SSR (i.e. solid state reaction method) at 20 g/batch scale, while a large scale sol-gel method (SG) was used to prepare SCFC8246-SG at kg/batch scale. As depicted in figure 8(a) and table 3, preparation methods have noticeable effect on the oxygen capacity of the resulted oxygen sorbents. The oxygen capacity follows the trend of SG > CP > SSR from 400 to 600 °C. Particularly, the sol-gel sample has oxygen capacity above 1 wt% at 450 °C–500 °C. The low temperature air separation is also evidenced by its easy oxygen release at ∼400 °C (figure S3). The redox kinetics of the three oxygen sorbents were further examined by fast pressure swing between Ar and 20% O2 at 3 min/cycle. Shown in figure 8(b), the redox kinetics follow the same trend as their oxygen capacities, as SCFC8246-SG has the highest average redox rate. For example, the average redox rate of SCFC8246-SG at 500 °C is 0.25% min−1, which is nearly 20 times higher than that of SCFC8246-SSRsample, and 25% higher than that of SCFC8246-CP sorbent. The fast redox kinetics of the SCFC8246-SG sample prepared by sol-gel method results in its superior oxygen capacity under pressure swing condition.
Figure 8. (a) Oxygen capacity and (b) average redox rate of SCFC8246 sorbents prepared by solid state reaction method (SSR, 20 g/batch), co-precipitation method (CP, 20 g/batch), and large scale sol-gel method (SG, 1 kg/batch). Oxygen capacity was measured by pressure swing between Ar (6 min) and 20% O2 (4 min) at 400 °C–700 °C. Average redox rate was measured by oxygen partial pressure swing between Ar (2 min) and 20% O2 (1 min) at 500 °C–600 °C.
Download figure:
Standard image High-resolution imageTable 3. Oxygen capacity of SCFC8246-SG, SCFC8246-CP and SCFC8246-SSR oxygen sorbents under oxygen partial pressure swing between Ar (6 min) and 20% O2 (4 min)at 400 °C–700 °C.
| Oxygen capacity (wt%) | |||||||
|---|---|---|---|---|---|---|---|
| Oxygen Sorbent | 400 °C | 450 °C | 500 °C | 550 °C | 600 °C | 650 °C | 700 °C |
| SCFC8246-SSR | 0.03 | 0.03 | 0.05 | 0.18 | 0.42 | 0.58 | 0.59 |
| SCFC8246-CP | 0.15 | 0.40 | 0.75 | 0.81 | 0.72 | 0.56 | 0.38 |
| SCFC8246-SG | 0.81 | 1.11 | 1.07 | 0.95 | 0.84 | 0.72 | 0.55 |
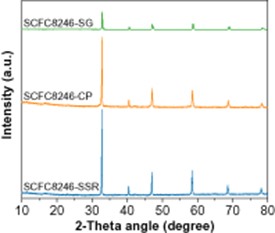
In order to understand the difference in oxygen capacity and redox kinetics of SCFC8246 samples prepared by the different methods, the structure and morphology of the three sorbents were characterized in detail. From the XRD patterns (figure 9), all three samples shows the perovskite structure similar as SrCoO3 (PDF 04-019-5710). However, the peak intensity increases following the trend of SG < CP < SSR. Based on the broadening of (110) peak, the crystal sizes of SCFC8246-SG, SCFC8246-CP, and SCFC8246-SSR are determined as 45, 51, and 60 nm, respectively. The decreased crystallite size of SCFC8246-SG sample may lead to faster diffusion rates for oxygen ions via crystalline boundaries and therefore speeding up the redox kinetics. The faster redox kinetics in turn increases oxygen capacity under limited reduction and oxidation durations.
Figure 9. XRD patterns of SCFC8246-SG, SCFC8246-CP and SCFC8246-SSR oxygen sorbents.
Download figure:
Standard image High-resolution imageFrom SEM characterization, the morphology of SCFC8246-SG and SCFC8246-CP sorbents are similar, consisting of sub-grains with size of 2–9 μm (figure 10). The distribution of Sr, Ca, Fe, and Co elements are very uniform at sub-micrometer level. As a comparison, the SCFC8246-SSR sample has a much wide size distribution of sub-grains from 2 to 18 μm. Furthermore, segregation of Co phase was also observed from EDX mapping. It is known that Co doping is critical to improve oxygen diffusion in SrFeO3 based perovskites. The large particle size and segregation of Co phase is likely to have caused the observed slow redox kinetics and low oxygen capacity of SCFC8246-SSR sorbent prepared by solid state reaction method. It is noted that all the three SCFC oxygen sorbents prepared by different methods have a relatively low specific surface of ∼1 m2 g−1 (table S1), owing to thermal treatment at ≥1000 °C during sample preparation.
Figure 10. SEM images and EDX mapping for (a)–(e) SCFC8246-SG, (f)–(j) SCFC8246-CP and (k)–(o) SCFC8246-SSR oxygen sorbents.
Download figure:
Standard image High-resolution imageThe most promising SCFC8246-SG sorbent was further investigated for chemical looping air separation at a large scale testbed. The testbed has a solids inventory of 334 g, capable of handling 1 l air per min as the feed. The testing conditions such as pressure, and cycle structure (i.e. absorption time, vent time, desorption time, and close time) were studied systematically. Using cycle structure of 30/5/15/1 (i.e. 30 s absorption/5 s vent/15 s desorption/1 s close), the oxygen productivity is 0.002 g of O2 per gram of sorbent per hour at 0 psig. After increasing the operating pressure to 15 or 25 psig, the oxygen productivity significantly increases to ∼0.02 gO2 gsorbent−1 h−1 (figure 11). The high oxygen pressure provides the driving force to oxygen absorption, resulting in fast kinetics and high oxygen capacity. Increasing absorption/desorption time also improves oxygen productivity. For example, the oxygen productivity doubles as 0.04 gO2 gsorbent−1 h−1 by increasing absorption and desorption time to 120 and 60 s, respectively. It is noted that produced oxygen purity also improves at high operating pressure and longer cycling time. At 15 psig, 95% oxygen can be produced at 0.04 gO2 gsorbent−1 h−1, corresponding to a bed size factor of ∼1 ton sorbent for producing 1 ton oxygen per day, which is close to the requirement for practical implementations for sorbent based air separation systems. In practical operation of chemical looping air separation, oxygen rich gas can be circulated to purge the residue gas in the sorbent bed to further improve oxygen purity to above 99%. Further increasing absorption pressure to 25 psig, the oxygen purity lowers from 95% to 90%, while the oxygen productivity drops to ∼0.02 gO2 gsorbent−1 h−1. It is noted that the purging time is 5 s, which may leads to unpurged residue air in the reactor at high absorption pressure and results in reduced oxygen purity and oxygen productivity. We note that significantly higher oxygen purities can be anticipated in a larger-scale multi-channel system where enriched oxygen gas can be recycled as a purge gas similar to the commercial PSA based H2 production processes. Moreover, higher linear velocities in larger systems can also decrease mass transport resistance for the redox reactions of the sorbents, leading to smaller bed size factor. Mass transfer limitation is likely to be responsible for the difference between the bed size factor predicted by small scale kinetic studies (556 lbs TPD−1 O2 with a less active CP sorbent) and that from the testbed study.
Figure 11. Large scale testing of SCFC8246-SG sorbent for chemical looping air separation. Label in the figure shows the cycle structure of absorption time/vent time/desorption time/close time.
Download figure:
Standard image High-resolution image4. Conclusions
In summary, we have demonstrated scalable synthesis of Sr1-xCaxFe1-yCoyO3-δ (SCFC) oxygen sorbents with fast redox kinetics and robust steam resistance for chemical looping air separation. Doping of calcium at A site increases oxygen capacity, while cobalt dopant at B site of SCFC perovskite is important to improve the sorbent redox rate and stability. By increasing Co loading (i.e. Co/(Co + Fe) × 100%) from 10% to 60%, the average redox rate was enhanced by more than 30%. Furthermore, Co improves the stability of perovskite structure and inhibits Ca segregation, particularly under extended redox cycles in the presence of steam. Both co-precipitation and sol-gel methods are feasible for large scale synthesis of SCFC8246 oxygen sorbents with desirable structure, uniform elemental distributions, and small sub-grain sizes, which are critical to achieve high oxygen capacity and fast redox kinetics. Large scale evaluation of SCFC8246 sorbent demonstrate the efficient production of high purity oxygen with a bed size factor of ∼1 ton sorbent/TPD O2.
Acknowledgments
This work was supported by the U.S. Department of Energy (Award No. FE0031521), National Science Foundation (CBET-1510900), and the North Carolina State University Kenan Institute for Engineering, Technology, and Science. The authors acknowledge the use of the Analytical Instrumentation Facility (AIF) at North Carolina State University, which is supported State of North Carolina and the National Science Foundation.