Abstract
The COVID-19 pandemic has revealed the need of novel diagnostic technologies for rapid and accurate virus detection. In the European CONVAT project, a point-of-care nanophotonic biosensor is being developed for the direct, fast and specific identification of severe acute respiratory syndrome coronavirus 2 from both human patient samples and animal reservoirs. The technology will provide a quantitative detection of the viral load and it can be implemented in decentralized settings to improve the early diagnosis and clinical management of patients as well as coronavirus environmental monitoring to prevent future outbreaks.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In December 2019, a new coronavirus was identified as the cause of a disease outbreak (COVID-19) originated in Wuhan, China. The virus, known as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), cause an upper respiratory tract infection with common mild symptoms like cough, fever and breathing difficulties for the majority of the infected people, but it can also trigger inflammatory complications (e.g. pneumonia, multiple organ dysfunction syndrome), requiring intensive care assistance and, unfortunately, leading to patient's death. In March 2020, the World Health Organization (WHO) declared the COVID-19 outbreak a pandemic and, to date (October 2020), it has infected more than 44.5 million people globally, with more than 1 million deaths [1, 2].
Given the health and social emergency with the dramatic spread of COVID-19 pandemic, rapid, accurate, and sensitive diagnostic techniques to promptly provide an accurate virus detection are required to enable an early infection identification, to improve patient management, and to stop and control the disease transmission. In fact, reliable and early diagnosis of COVID-19 has become one of the major challenges in the correct management of the pandemic. Current diagnostic techniques mainly rely on polymerase chain reaction (PCR) tests [3, 4]. PCR assays consist in detecting and identifying the specific genomic material (RNA) of the virus via enzyme-mediated amplification of targeted genes. Most of the approved COVID-19 PCR kits target specific sequences like RdRp, E, N or ORF1ab genes, corresponding to highly conserved regions of the virus genome. PCR tests provide the required sensitivity and specificity together with clinical robustness, but the relatively long time to result (between 2 and 6 h) and the need of samples transport to specialized laboratories, delays overly the diagnosis results and hampers the massive population screening [5]. New PCR-related modalities have been proposed, such as the loop-mediated isothermal amplification or the rolling circle amplification, as well as emerging CRISPR-based techniques seeking for a point-of-care (POC) genomic assay, although their rapid implementation in clinics is still complex [6–8]. Rapid antigen diagnostic tests based on lateral flow assays (LFAs) are a good alternative, offering fast detection (15 min approximately). These immunochromatography strips detect viral antigens, mostly the N protein, via a sandwich assay. However, they usually present low sensitivity and reliability, especially when the viral load is low [9, 10]. Additionally, serological assays are also being used as complementary diagnosis technique, either with conventional techniques (chemiluminiscence or enzyme-based immunosorbent assay) or in the LFA format. This blood-based analysis detects the human antibodies generated upon infection, therefore they cannot be employed as early diagnostics but rather as retrospective and confirmatory approach.
Strong worldwide efforts are pursued to surpass this bottleneck by developing reliable, fast and user-friendly diagnostics tests than can be employed at the point-of-need [9, 11]. Biosensor technology is one of the most well prepared to tackle this challenging goal. Biosensors are integrated systems that combine specific biorecognition agents (e.g. antibodies, enzymes, or DNA strands) with a transducer (i.e. electrochemical, optical, or mechanic) in such a way that when the targeted analyte interacts with the bioreceptor, a series of physicochemical changes produced on the transducer can be translated into readable and measurable signals for quantitative analysis. Several biosensor platforms have been produced in the last decades for virus analysis, demonstrating highly sensitive and selective detection in a few minutes directly from clinical samples [12–14]. Among them, photonic biosensor technologies have gained significant attention, owing to their versatility, label-free monitoring, and outstanding sensitivity. Moreover, photonic biosensors present capabilities to be multiplexed and miniaturized, meeting the requirements of POC testing [15]. Thereby, label-free nanophotonic biosensors have been positioned as potential solution for the development of new technologies for SARS-CoV-2 detection and rapid COVID-19 diagnosis [16, 17].
The CONVAT project is one of the first large research projects funded by the H2020 European Union Framework program (H2020-SC1-PHE-CORONAVIRUS-2020) to fight COVID-19. The main expected contribution is the early diagnosis and clinical management of patients infected with COVID-19 by introducing a POC label-free nanophotonic biosensor for the direct, fast and specific identification of SARS-CoV-2 without requiring complex equipment. CONVAT employs an innovative design of an evanescent-wave nanophotonic sensor based on silicon photonics interferometric technology, which has been previously demonstrated for the direct detection of tumor biomarkers and other pathogens with exceptional sensitivities [18–21]. In this article, we report on the novel COVID-19 diagnostic approaches and the benefits that POC nanophotonic biosensors may enable, as envisioned in the project, together with the impact and perspectives for such technology in the boost of global healthcare.
2. Nanophotonic biosensor technology to combat COVID-19: the CONVAT approach
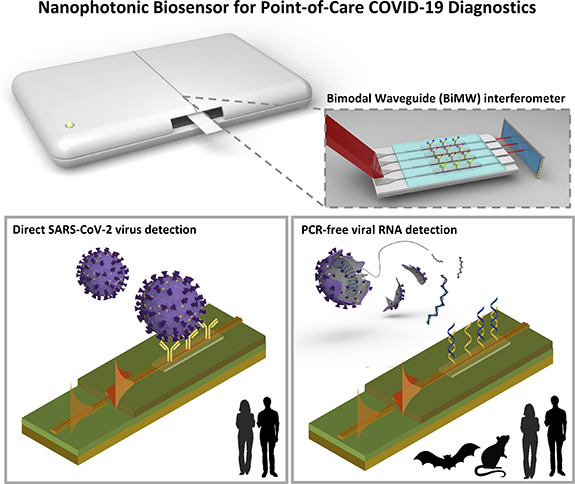
A summary of the CONVAT approach for COVID-19 diagnostics is illustrated in figure 1. The main idea is to develop an integrated POC biosensor device for accurate detection and identification of the SARS-CoV-2 virus in a few minutes and in decentralized settings. Two diagnostic assays are developed within the same platform: (a) direct virus detection via antigen recognition, for the rapid infection detection and population screening; and (b) viral genomic identification via hybridization assay, for diagnostic confirmation. Additionally, the genomic assay may be multiplexed thereby allowing for rapid discrimination of different respiratory viruses (e.g. human coronaviruses, influenza, etc) or different coronavirus genera (i.e. alpha-coronavirus, beta-coronavirus, etc). The latter is particularly interesting for the application of the CONVAT POC biosensor for environmental screening, that is, for monitoring and characterization of coronaviruses in reservoir animals, like bats or rodents, which could prevent from future pandemic outbreaks.
Figure 1. Summary of CONVAT approach for diagnostics and surveillance of COVID-19. A point-of-care biosensor device based on the bimodal waveguide (BiMW) interferometric technology will be applied for two integrated strategies: the direct detection of whole intact SARS-CoV-2 viruses for early diagnosis and population screening (left), and for PCR-free viral RNA identification for both specific diagnosis confirmation and coronavirus surveillance in animal reservoirs (right).
Download figure:
Standard image High-resolution image2.1. The bimodal waveguide (BiMW) interferometer
Photonic biosensors are systems that seize different light-based phenomena for the fast detection and quantification of biomarkers. These devices have become critically important as an efficient tool for environmental control and clinical health diagnostics. Among the different photonic biosensors, silicon photonics biosensors based on evanescent-wave sensing principle offer undisputed advantages such as robustness, reliability, high sensitivity and low power consumption [22]. Principal advantages of this technology include the small footprint and high scalability of the sensors, made possible via semiconductor fabrication. These characteristics make this technology ideal for multiplexed analysis, where diagnostic value improves with increasing panel size. Additionally, the real-time monitoring expedites assay development and enables rapid direct observation of biological interactions, unlike the endpoint-based detection methodologies in many standard techniques [23–26].
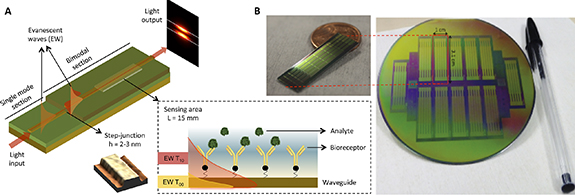
In the CONVAT project we employ a nanophotonic biosensor based on interferometric bimodal waveguides (BiMWs) (figure 2). The working principle of a BiMW biosensor is based on the interferometric design, using a single straight waveguide where light from a coherent source operating at visible wavelengths is coupled. The BiMW sensor has a first waveguide zone exhibiting single-mode behavior, where only the fundamental mode is propagating. After some distance, the core thickness of the waveguide is increased and the light modes are splited in fundamental and first order. Both modes keep travelling through till the output of the sensor chip. Due to the modes having different velocities and penetrations of their evanescent fields into the medium, they produce an interference pattern at the exit of the waveguide, which is related to the changes induced by any interaction event happening on the sensor surface. This distribution corresponds to the phase variation of the light between the two modes, which can be measured off the sensor chip with a sectional photodiode (see figure 2(A)). However, in the BiMW biosensors is important to consider the existence of a sinusoidal dependence between the phase variation and the wavelength. This problem of the periodic sensor response and the resulting phase ambiguities, could be solved by modifying the phase difference between the two propagating modes, altering their effective indices through small variations of the propagating light wavelength. In that line, we introduced a simple and cost-effective all-optical phase modulation technique that does not require any additional fabrication processes or external equipment [27]. This configuration provides an excellent sensitivity of detection with a resolution of 10−8 refractive index units.
Figure 2. (A) BiMW biosensor working principle. The inset figure represents the sensing area of the device, where the two evanescent waves corresponding to the fundamental (yellow) and the first mode (red) of the light are illustrated. (B) Photographs of the BiMW biosensor chip with 20 independent sensors and its corresponding wafer fabricated with silicon microelectronics techniques.
Download figure:
Standard image High-resolution imageThe BiMW sensor chip is fabricated using standard silicon microelectronics technology. The design of the BiMW technology involves waveguides of micro/nanodimensions, which are essential to control the electromagnetic evanescent field interacting with the molecules in the biorecognition event. Each sensor consists of a silicon nitride (Si3N4) waveguide covered with a silicon oxide (SiO2) layer used as cladding. These materials have been selected due to their high refractive index contrast essential for a highly sensitive transducer based on waveguides. During the process fabrication, a Si3N4 layer (RI 2.00 and 350 nm thickness) was deposited using low-pressure chemical vapor deposition on a thermally grown SiO2 buffer layer (RI 1.46, 2 μm thickness). To ensure single-mode behavior in the longitudinal direction in the visible, a nanometric height rib waveguide (4 μm width × 2 nm height) was formed by buffered HF etching through a photoresist mask patterned by conventional photolithography. The structure was covered by a SiO2 cladding layer (RI 1.48 at 633 nm) deposited by plasma-enhanced chemical vapor deposition. Finally, in the bimodal section, a portion of the cladding is removed defining a sensing area (15 × 0.05 mm2) where the evanescent field of the guided light can probe the surrounding dielectric environment. For the biosensing evaluation, the BiMW interferometric biosensor is conditioned with a microfluidic flow cell fabricated with an optically transparent polymer (i.e. polydimethylsiloxane, PDMS) for the sample introduction and flowing over the sensor surface. Each silicon wafer processed contains 12 sensing chips (30 × 10 mm2), and each chip includes an array of 20 BiMW interferometric sensors (figure 2(B)). The simplicity of the design and fabrication of the BiMW interferometers makes them more attractive for mass production as compared to their counterparts, the Mach–Zehnder and the Young interferometers.
This robust BiMW biosensor system has been demonstrated previously for the ultrasensitive detection of clinical biomarkers and pathogens in human fluids for medical diagnostic of infectious diseases. For example, this biosensor was employed for the detection of several relevant bacteria pathogens, as for the detection of spontaneous bacterial peritonitis, being capable of detecting Escherichia coli at extremely low concentrations (Limit of Detection, LOD: 4 CFU ml−1) in human ascitic fluid using a direct immunoassay [18], with a time to result of only 25 min and with no need of any sample purification. In addition, it was recently shown the detection of Pseudomonas aeruginosa (LOD sim 30 CFU ml−1) and methicillin-resistant Staphylococcus aureus (MRSA), both of them considered as two of the most prevalent bacteria associated with nosocomial infections. This approach enables the specific identification of the resistant pathogen (MRSA) and its differentiation from methicillin-susceptible S. aureus [28]. Besides, it was developed an ultrasensitive methodology for the detection of genes associated with the multidrug-resistance found in Gram-negative bacteria as E. coli without using PCR amplification. Also, it was applied for the detection of genes encoding several β-lactamase enzymes able to hydrolyze a broad spectrum of beta-lactams. As a proof-of-concept, the BiMW biosensor was used to detect two genes of the unamplified genomic DNA directly extracted from bacteria commonly found in samples of patients attended at the hospital. All the steps took 30 min, achieving a LOD of only 5.8 aM [29].
2.2. BiMW biosensor for intact SARS-CoV-2 detection
The possibility to directly detect the presence of viruses in the respiratory tract fluids is a clear sign of early infection, and if it can be done in a rapid and simple way wherever it is needed, it would allow for massive screening of the population, enabling efficient identification of infected COVID-19 patients that could be readily controlled, treated if required, and isolated to stop the disease spread. Furthermore, rather than a positive/negative response, if the diagnosis can provide information about the viral load, it would drastically improve the management of patients with a high infection degree, which could be closely monitored for treatment and assistance to minimize disease complications. This is the main diagnosis strategy proposed in CONVAT.
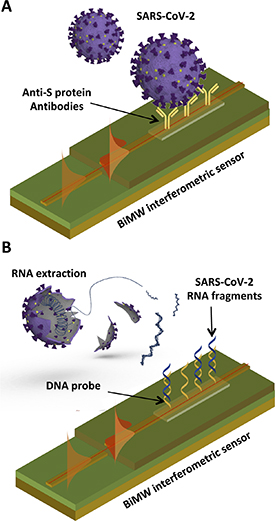
In order to capture and detect whole intact viruses from a sample, the BiMW sensor surface is modified with specific receptors targeting external antigens of the virus, such as the Spike (S) protein of the SARS-CoV-2 (figure 3(A)). Once the sample is introduced in the biosensor, the virus particles are captured by the receptors onto the sensor surface, generating an interferometric signal that is recorded in real time. The sensor response is directly proportional to the concentration of viruses in the sample, therefore providing an accurate quantification of the viral load in the patient. Two factors are critical for realizing an accurate and reliable biosensor: the selection of the bioreceptor and its immobilization on the sensor surface. The selection of the bioreceptors is of paramount importance as they confer the required affinity as well as the specificity to the assay. Antibodies are the prevailing biorecognition elements used in bioassays development. These molecules, naturally generated in our body during an immune response, can be produced in vitro in large quantities for virtually any type of antigen—namely, proteins, peptides, or small molecules—and they have demonstrated the greatest affinities and specificities for the targeted antigens. Monoclonal antibodies are the most widely employed, but several varieties and derivatives can also be produced via genetic engineering, such as recombinant chimeric antibodies or antibody fragments (i.e. nanobodies), which may enhance the analytical sensitivity and specificity [30]. On the other hand, aptamers (i.e. oligonucleotide sequences able to capture and detect specific antigens through structural conformation recognition) have emerged as possible alternative to antibodies. These bioreceptors offer advantages such as the high stability and robustness, and the synthetic production and purification at relatively low cost; but, obtaining a high-affinity and high-selectivity aptamer for certain antigens involves a complex and tedious process that not always results in the desired features as compared to antibodies [31–33]. Along with the availability and suitable selection of high-quality bioreceptors, another step that must carefully study and optimize is the surface biofunctionalization. An optimum biorecognition interface depends on the correct orientation and density of the bioreceptors, to maximize antigen-recognition efficiency and minimize steric hindrance effects, and it should also prevent from non-specific binding of other molecules or compounds that might be present in the sample matrix [34–36]. This is especially relevant since the adsorption of non-specific components may generate false-positive signals. To achieve so, it is necessary to apply well-controlled surface chemistry strategies that enable a robust immobilization of bioreceptors and evaluate the assay conditions to effectively reduce possible background signals while enhancing specific target detection.
Figure 3. (A) Illustration of CONVAT strategy for intact SARS-CoV-2 detection with the BiMW biosensor. (B) Illustration of CONVAT strategy for viral genomic analysis with the BiMW biosensor.
Download figure:
Standard image High-resolution imageFinally, the BiMW biosensor can be applied for the COVID-19 analysis in different patient samples, such as nasopharyngeal, nasal, or saliva. The presence of virus particles has been confirmed in all these upper respiratory tract fluids and it represents an opportunity for facilitating the population screening [37]. Although nasopharyngeal swabs are nowadays the preferred option in clinics, it may result disturbing for some patients, like children, and it requires specialized personnel for appropriate sampling. Nasal fluids or saliva could be a good alternative for non-invasive sample obtaining and could ideally be performed by the patients themselves, which would also greatly aid in minimizing the infection risk for sanitary personnel. Nonetheless and particularly for saliva, it is necessary to evaluate first the large variability of components and virus levels as well as the different strategies for sample collection. The CONVAT project is foreseen to fulfill both laboratory and clinical validation of the technology with COVID-positive and negative samples that are currently being collected in different hospitals around Europe (Italy, France and Spain). Given the outstanding analytical sensitivity of the BiMW biosensor demonstrated for pathogen detection (below 10 CFU ml−1), the clinical sensitivity is expected to be superior to 95% with 98%–99% specificity, if optimal bioreceptors are accomplished within the project timeline.
2.3. BiMW biosensor for viral genomic analysis
Although antigen-based assays can be used for selective screening and detection of COVID-19 infection, the relatively slight differences in the viral proteins between different virus types may lead to cross-reactivity issues with the bioreceptors. Also, these proteins are prone to mutate and change structural conformation very fast, so antibodies might lose affinity or selectivity if they are not re-designed accordingly. Fortunately, the genomic sequence of a biological entity is unique and exclusive, and some of the regions are usually conserved for longer times. Therefore, the detection and identification of the viral genomic material (RNA) leads to the most specific analysis type. In this regard, the CONVAT project envisions such analysis as a confirmatory diagnostic test, which could be performed in only 30 min as time to result and employing the same biosensor device. Label-free photonic biosensors can perform specific genomic detection via hybridization assays without the need of PCR amplification and reaching a comparable sensitivity. In fact, the BiMW biosensors have demonstrated detection limits in the aM range for direct measurement of RNA from clinical samples [19, 20]. The assay is simple, and it only requires previous RNA extraction and fragmentation from the virus, which can be easily done directly upon sample collection. When the sample is introduced in the biosensor, the specific target gene fragments are captured by complementary single-stranded DNA probes previously immobilized on the sensor surface, forming a duplex chain, and leading to real-time signals directly proportional to the number of viruses (figure 3(B)).
To further exploit the capabilities of the nanophotonic BiMW technology, CONVAT aims at developing a multiplexed genomic biosensor assay. By functionalizing the multiple sensor waveguides with different DNA probes, we can detect and identify various viruses in the same sample and assay. We could target different RNA genes of the same SARS-CoV-2—enhancing the selectivity of the assay—or include specific genetic regions for the identification of other beta-coronaviruses (e.g. SARS-CoV, MERS-CoV) or alpha-coronaviruses (e.g. hCoVs, responsible for common cold in humans). It could be also possible to include probes for the detection of influenza viruses and discriminate in one rapid assay whether the patient suffers COVID-19 or flu, for example. An interesting added value proposed in CONVAT is the utilization of the POC BiMW genomic biosensor for environmental monitoring, that is, for the analysis and identification of different coronaviruses in reservoir animals like bats or rodents. The routine control of alpha- or beta-coronaviruses in animals could greatly help in the early identification of alarming mutated or evolved viruses, which could eventually infect humans. The availability of a POC biosensor that enables a simple and rapid screening will simplify the monitoring tasks, deliver timely warnings, and prevent the outbreak of future pandemics. The project will validate the new BiMW biosensor for both, clinical analysis of human patient samples collected in different European hospitals and the analysis of bats and rodent feces samples, which are also being collected in different locations worldwide. Based on previous works proving the excellent BiMW biosensor sensitivity, the innovative and rapid viral genomic assay is expected to offer performances comparable to standard PCR-based assays, which are around 98%–100% sensitivity and specificity.
3. Expected impact
The trend of miniaturization, cost-effectiveness and rapidness of diagnostic devices for an improved and massive infectious virus screening is undeniably a global public health ambition. A major driver for POC biosensor development is the ability to diagnose infectious diseases at sites with limited infrastructure without the requirement to transport the clinical sample to a centralized facility. It is expected that CONVAT could provide a COVID-19 POC technology as integrated solution for complete, accurate, and reliable virus analysis.
The outstanding levels of sensitivity demonstrated by the BiMW biosensor anticipate an excellent performance of the novel POC diagnostic device, with detection limits in the range of 102–103 viruses ml−1 for the direct and rapid SARS-CoV-2 virus screening. Taking into account that common viral loads for COVID-positive patients are found in the range of 105–107 viruses ml−1, this value is a good indicator that the CONVAT technology might be able to diagnose even asymptomatic cases with relatively low viral loads. Regarding the genomic assay, the ability to directly detect minute concentrations (aM-fM levels) of RNA in only 30 min in a straightforward manner would greatly increase the accuracy of COVID-19 diagnostics in a massive population screening. Furthermore, the multiplexing potential could serve in clinics to drastically reduce the amount of serial analysis needed for disease diagnosis, enabling the direct identification of the specific infectious virus in less than 1 h.
Finally, it is important to remark the large versatility of the nanophotonic BiMW biosensors. The device can be considered as a general-purpose instrument to be implemented in hospitals, laboratories, and primary care centers, and it could be used for routine diagnostic analysis of numerous diseases and medical conditions. The BiMW sensor chip can be easily adapted for the specific assay of different protein or nucleic acid biomarkers, and also for the detection and identification of different pathogens, including viruses and bacteria. The mass production of the chips with conventional silicon microelectronics fabrication techniques will ensure a low-cost system, which is expected to be below 15 €/assay considering the complete and fully operative biosensor cartridge, and the on-going advances in system's connectivity, automation and remote control, and data analysis and interpretation will be key for realizing that pioneering POC diagnostic biosensor urgently demanded by the clinics and the society.
Acknowledgments
The work is funded by the H2020 Research and Innovation Programme of the European Commission (H2020-SC1-PHE-CORONAVIRUS-2020, Project No. 101003544). The CONVAT consortium is formed by the ICN2 (Professor Laura M. Lechuga, Spain), the University of Barcelona (UB, Professor Jordi Serra-Cobo, Spain), the Aix-Marseille University (AMU, Professor Remi Charrel and Professor Bruno Coutard, France), and the National Institute for Infectious Diseases Spallanzani (INMI, Professor Antonino di Caro, Italy). The ICN2 is funded by the CERCA programme of the Generalitat de Catalunya. The ICN2 is supported by the Severo Ochoa Centres of Excellence programme, funded by the AEI (Grand No. SEV-2017-0706).