Abstract
This study examines the fly ash from Soc Son municipal waste power plant (SMPP) and suggests ways to repurpose it to reduce its environmental impact. Fly ash from the Soc Son waste power plant has a gray color, spherical particles with a 5–103 μm diameter, and a high carbon and heavy metal content. Bermorite crystals can absorb and release heavy metals, making monitoring secondary pollutants during incineration crucial. The EDX analysis of fly ash from the Soc Son waste power plant revealed that it was predominantly contaminated with metal elements, with the highest percentage of calcium. The EDX was able to detect heavy metals in incinerator fly ash. The concentration of Zn in the fly ash exceeded QCVN 07:2009/BTNMT standards, indicating the high amounts of some elements that may be hazardous to the environment and human health. Using the SEM/EDX and XRF, the fly ash from the Soc Son landfill power plant was analyzed and discovered that it exceeds permissible limits for dangerous heavy elements. The most common inorganic elements are Ca, followed by Zn, Pb, Cd, and Ag. Fly ash is classed as hazardous waste due to its high concentration of heavy metals, which results from the combustion of municipal solid waste that has not been separated. Vietnam fights municipal solid waste incinerator fly ash production. Some nations stabilize fly ash to remove harmful components and use it in buildings. Stabilized fly ash makes unfired construction bricks and cement manufacturing components and combining fly ash with inorganic trash protects the environment.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Increases in population and rapid urbanization, especially in developing countries, are mostly responsible for the rise of municipal solid waste (MSW). As a result, there has been increased waste production, higher consumption rates, and a lack of waste management infrastructure to handle the growing amount of MSW. Management of MSW is essential for the timely and secure disposal of waste produced by homes and businesses. The primary techniques for managing MSW are landfilling, composting, and incineration [1]. Landfilling is a common practice selected for its practicality and affordability. It has the potential to manage vast amounts of waste effectively and economically. By limiting waste and lowering odors and vectors, landfills can assist in lessening the environmental effect of waste [2]. However, leachate poisoning of groundwater, greenhouse gas emissions and the potential spread of disease-causing organisms are some of the adverse environmental effects of landfills. Composting is a practical method to substitute conventional landfilling for managing MSW. Composting can save landfill costs, help local economies, and decrease the quantity of non-biodegradable waste in landfills [3]. Further investigation into composting as a management technique for MSW is essential, as it holds the potential to serve as a viable alternative to traditional landfilling methods. Composting is an effective way to handle trash that contains a lot of perishable organic material and costs less than incineration. Composting, however, could be more effective for managing non-perishable organic and inorganic materials. While selecting a waste management strategy for various forms of waste, this limitation of composting should be considered.
In recent years, waste power technology has become more popular as a viable way to handle the rising amounts of MSW generated worldwide [4]. Similar to incineration, this technology significantly reduces the volume and amount of solid waste and, as a result, eliminates the need for landfilling. According to Tabasová et al (2012) [5], waste-to-energy technology can improve energy security and lower emissions of pollutants. Waste power technology does, however, have significant disadvantages. The production of air pollutants such as carbon dioxide, nitrogen oxides, and sulfur dioxide could significantly impact the environment. The combustion process produces a sizable amount of solid incineration leftovers, such as bottom ash, fly ash, and boiler ash.
As a byproduct of the incineration process, fly ash is created in municipal solid waste power plants. This dangerous chemical has high concentrations of several different components [5, 6]. Fly ash, however, may be used in new ways, such as in place of cement [7]. The nature of the feedstock, which typically comprises 85%–90% municipal solid waste and 10%–15% sewage sludge waste, determines the unique qualities of fly ash produced by municipal solid waste incineration [8].
Vietnam has long since issued a set of legal laws on waste management and launched numerous useful programs for pollution prevention and treatment after realizing the detrimental effects of environmental contamination. Nonetheless, managing waste, especially domestic solid waste, is still a challenge in Vietnam, and pollution from household solid waste is still a significant situation in most of the country's localities. In 2021, Hanoi city, which has a total area of about 335,000 hectares and a population of around 7.5 million, produced about 7,500 tons/day of municipal solid waste, of which 5,388 tons per days were generated in Son Tay town and 12 other urban districts and 2,127 tons/day in 17 suburban communities [9].
Hanoi aggressively pushed forward municipal solid waste power projects in 2019. Soc Son municipal reliable waste power plant in Hanoi, which is part of the Nam Son integrated waste treatment complex, will apply the Waterleau mechanical grate incinerator technology (from Belgium) to incinerate waste and recover energy to generate electricity using three steam turbine generator sets, including two 30 MW and one 15 MW turbine generator set, to burn waste and recover energy to generate electricity, resulting in total electricity output of 75 MW at the Nam Son integrated waste treatment complex in Nam Son commune, Soc Son district, Hanoi, Vietnam. The municipal solid waste power technology and mechanical grate incinerator, have been imported from Europe. The slag and fly ash from the incineration, acid reaction byproducts collected from the bottom of the reaction tower, coarse dust particles in the exhaust flow, and dust particles collected from the bag filter are all examples of the project's byproducts.
Based on the facts provided, it can be seen that there will be a considerable amount of fly ash emitted from this plant. Numerous studies have previously analyzed and assessed the characteristics of fly ash from thermal power plants. However, the recent implementation of household waste reuse for a new waste-to-energy plant in Vietnam has not been thoroughly evaluated for the fly ash emissions from this specific type of operation. Notably, municipal solid waste in Vietnam is not pre-sorted before being utilized as raw material for waste-to-energy plants. Hence, the emitted fly ash might exhibit distinct characteristics from fly ash from coal-fired power plants. Analyzing and evaluating the features of fly ash from the Soc Son waste power plant will provide additional insights into the characteristics and properties of such fly ash in developing regions, particularly in Southeast Asia. This is the primary objective of our study. Additionally, two analytical methods, SEM/EDX and XRF, are employed to analyze the components of fly ash to assess its composition comprehensively. These two analyses will complement each other, providing a more comprehensive understanding of the fly ash composition. A potential reuse method for the fly ash is to use it as an ingredient for making cement or other building materials. In order to ensure that municipal solid waste power technology is used for the environment safely and efficiently, it is crucial to examine the fly ash characteristics of the Soc Son waste power plant and advise acceptable reuse directions.
2. Materials and methods
2.1. Diagram of the Soc Son waste plant's location and technology
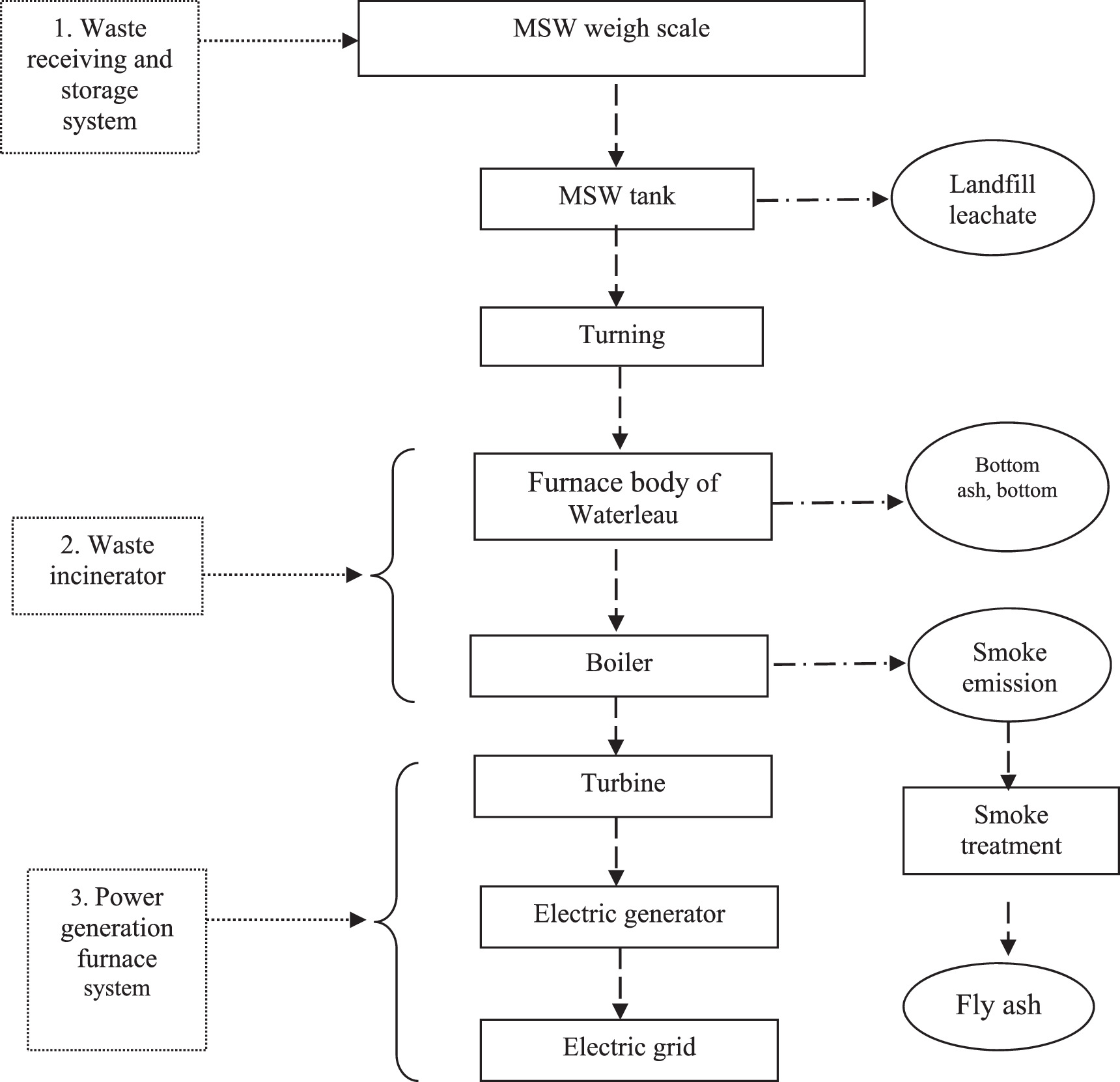
The Soc Son municipal solid waste power plant (SMPP) is at 21°32'36' N, 105°83'70' E in the Nam Son waste treatment complex, Nam Son commune, Soc Son district, Hanoi City, Vietnam. Five incinerators with comparable capacity and technology make up the plant. Figure 1 depicts an incineration system for industrial and operational procedures. The system is made up of several different parts, including a waste receiving and storage system, a waste incinerator, and a power generation furnace (which includes a gas supply system to the grate, an ignition and auxiliary combustion system, an oil supply system, and a smoke system), a wind and air dryer, an incinerator (which includes a grate and furnace body), and a waste heat use system (comprising a boiler, turbine, generator, and other ancillary parts).
Figure 1. Schematic of waste incineration technique of the SMPP used to produce power.
Download figure:
Standard image High-resolution imageThe daily processing volume or capacity, waste type processed, and operational capacity: The facility was built to handle municipal solid waste for Hanoi City, Vietnam. With five furnaces that can each hold 800 tons, the plant has a daily capacity of 4,000 tons. The kind of fly ash emission, the quantity released, and the location of the emission. There are two types of fly ash produced by the waste power plant: (1) fly ash that has been shaken off of the dust filtration system and is not stabilized or chemically bound - the strong connection or attachment between atoms in a molecule (Chelate); and (2) fly ash that has been stabilized and resembles animal dung. The type being investigated for this study are the unstabilized and unchemically bonded version. The amount of fly ash released is roughly 1.8% of the input waste, which translates to 14–15 tons of fly ash per day for each 800-ton furnace.
2.2. Sampling and analysis
The fly ash is a solid sample taken from the ash storage facility or the fly ash silo outlet before it is injected into the giant bag. To avoid contamination from the outside, the sample is separated into smaller sections, stored in individual zip bags, set up in trays, and put in a dehumidifier. In June and July of 2022, samples are taken and examined four times, which corresponds to the frequency of shake-downs of the bag filter system.
The sampling site, sampling technique, sampling duration, analytical variables, and the employed analytical techniques: Particle size, scanning electron microscopy (SEM), energy dispersive x-ray spectroscopy (EDX), elemental mapping data and x-ray fluorescence (XRF), are among the analytical parameters used to assess the composition of fly ash.
The SEM/EDX analysis method for fly ash samples involved a series of meticulous steps to obtain SEM images, EDX data, and mapping, ensuring comprehensive characterization of the sample. Firstly, the fly ash sample was finely ground and sieved through a mesh with a pore size of <9.5 mm (0.374 inches) to ensure uniform particle size distribution, thereby enhancing the accuracy of measurements and analysis. This step was crucial in preparing the sample for subsequent analysis, as it facilitates consistent interaction with the electron beam during SEM imaging and EDX analysis. Subsequently, the fly ash undergoes sterilized drying at a temperature of 600 °C for 2 days to ensure the removal of any moisture or volatile components, while avoiding alteration of the composition of substances in the fly ash. This controlled drying process is essential for preserving the sample's integrity and ensuring accurate SEM imaging and EDX analysis. After drying, the solid-phase fly ash sample was weighed and utilized for SEM and EDX imaging using a Hitachi 8100 SEM device combined with a Bruker SDD (EDX) detector. The measurements were conducted using a 25 kV electron beam at a distance of 1.5 mm. Parameters such as angle size, magnification, and resolution were carefully adjusted to obtain accurate and high-quality images, ensuring detailed visualization of the sample's surface morphology and elemental distribution. The surface images of the fly ash were collected by scanning the surface with an electron beam, allowing for the visualization of microstructural features and elemental composition. These images were processed and optimized to enhance resolution and detail, providing valuable insights into the surface characteristics of the fly ash sample. The measurement device operates automatically and provides a table of elemental composition in the fly ash (percentage) and an x-ray diffraction pattern of the sample. This comprehensive analysis enables the identification and quantification of elements present in the fly ash and the determination of its crystallographic structure through x-ray diffraction. Moreover, the Mapping feature on the EDX device was adjusted, with a measurement time of 2 h per sample, allowing for the comprehensive elemental mapping of the fly ash sample. The automatic run determines the entire range of elements in the measured sample and selects image export, providing spatially resolved elemental distribution information.
The x-ray fluorescence (XRF) analysis method for fly ash samples involves several key steps to determine the sample's elemental composition accurately. Firstly, the fly ash sample was finely ground to achieve a homogeneous size distribution, ensuring representative analysis. This step was crucial in preparing the sample for subsequent analysis, as it facilitates uniform exposure to the x-ray excitation source and enhances the accuracy of the results. Following the grinding process, the finely ground fly ash sample was pressed into tablets with a diameter of approximately 1cm. This tablet formation standardizes the sample presentation and ensures consistent interaction with the x-ray excitation source during analysis. The tablet formation process was essential for maintaining sample integrity and facilitating reproducible results. Subsequently, the prepared tablets were placed into an x-ray fluorescence instrument, specifically the Element Eye JSX-1000S EDXRF/JEOL-US, USA. This instrument was equipped with the capability for automatic analysis, allowing for efficient and precise determination of the elemental composition of the fly ash sample. During the XRF analysis, the instrument utilized x-rays to excite the atoms in the sample, leading to the emission of characteristic x-rays. The x-rays emitted from the instrument were directed at the sample, resulting in the excitation of inner orbital electrons in the sample's atoms. As the inner orbital electrons return to their original positions, characteristic x-rays are emitted. The energy and intensity of the detected x-rays were then utilized to quantify the elemental composition of the fly ash sample. The instrument provided automatic analysis results, typically in the form of a table indicating the concentrations of various elements present in the fly ash sample.
3. Results and discussion
3.1. Physical properties
3.1.1. * Characterizationof fly ash from the Soc Son waste power plant: general morphology and composition
Gray is the initial color of the raw fly ash produced by the Soc Son municipal solid waste power plant. The color of fly ash depends on its chemical and mineralogical components, according to Michael Rafalowski's study, gray color is mainly due to the high carbon content, while brownish color is caused by excess lime and partially high iron concentration [10]. Fly ash's gray color might well be caused by the plant's current flue gas treatment technology, which combines nitrogen removal in selective non-catalytic reduction (SNCR) furnace, acid removal by semi-dry technique, dry lime injection, activated carbon adsorption and bag filter. With a processing capacity of 800 tons/day and a need for 0.32 tons of activated carbon/day, the Soc Son waste power plant has been operated on a basis in June and July 2022 [9].
Figure 2 indicates that the morphology of the fly ash samples from the SMPP was observed at magnifications ranging from 1,000 to 100,000 based on the findings of the initial round of SEM examination. The fly ash's initial physical form was a homogeneous, fine powder, with a minor amount of it slightly agglomerating due to air dampness. The majority of the fly ash particles, which make up more than 70% of the total, were spherical in shape and ranged in size from 5 to 103 μm, according to SEM images of the particles. Particles more significant than 65 μm in diameter or asymmetrical crystal formations made up the remaining 30%. Fly ash from waste incineration has a rough, porous surface with a high specific area, which makes it easier for heavy metals produced during the waste incineration process to condense on it. Kanhar and colleagues (2020) demonstrated that fly ash is a composite material consisting of various components, including reactants, non-reactive compounds, and condensates [11]. The results were in line with Dias-Ferreira et al (2002) [12] study on incinerator fly ash, where they found that 50% of the particles had a surface area and porosity greater than 63 μm. According to Yibo Zhang's research, the fly ash's particle size was smaller than 75 μm after being dried for 24 h at 105 °C in an oven [13]. Incineration is a popular thermal treatment technique that can cause heavy metals in hazardous waste to form firmly bonded crystalline and glassy forms, according to investigations by Zhang et al (2022) [12]. Despite the heat treatment procedure, certain heavy metals in municipal waste, with high vapor pressure and low boiling temperatures, may still volatilize, leading to secondary contamination. Lead, mercury, and cadmium are a few examples of heavy metals with relatively low boiling points that can evaporate at temperatures utilized for burning. They may consequently be transported into fly ash. Also, as the waste is being burned, the heavy metals may combine with other chemicals to create new compounds that may be discharged into the atmosphere or become heavily entrapped within the fly ash's crystal structure. One of the secondary products of combustion is tobermorite (Ca5Si6O16(OH)24H2O) crystal, specifically [14, 15]. Reports indicate that incineration generates secondary contaminants, such as tobermorite crystals, which can absorb heavy metals. However, these crystals may also release heavy metals when exposed to acidic environments. In order to reduce the risk of secondary contamination, care must be taken even if incineration is an efficient way to manage hazardous waste. In particular, it is crucial to carefully monitor the creation and release of secondary pollutants such as tobermorite crystals in order to mitigate the risk of secondary contamination during the incineration of hazardous waste.
Figure 2. SEM image of fly ash from Soc Son waste power plant.
Download figure:
Standard image High-resolution imageThis discovery aligns with earlier research that has also characterized fly ashes originating from waste incineration facilities. Minteer et al (2003) [16] extensively analyzed fly ash samples from two municipal solid waste incinerators in China and concluded that these ashes' chemical composition and leaching behavior classified them as hazardous waste. Their examination also revealed the presence of spherical, elongated, and needle-like particles within the fly ash samples. The primary outcome of this investigation is that the fly ash resulting from solid waste incineration exhibits distinct morphology and composition traits that facilitate the accumulation of heavy metals. This observation corresponds with previous research on fly ashes from waste incineration sites and underscores the critical need for effective waste management strategies to address these hazardous materials appropriately.
3.1.2. * Evaluation of fly ash composition through analysis of EDX results
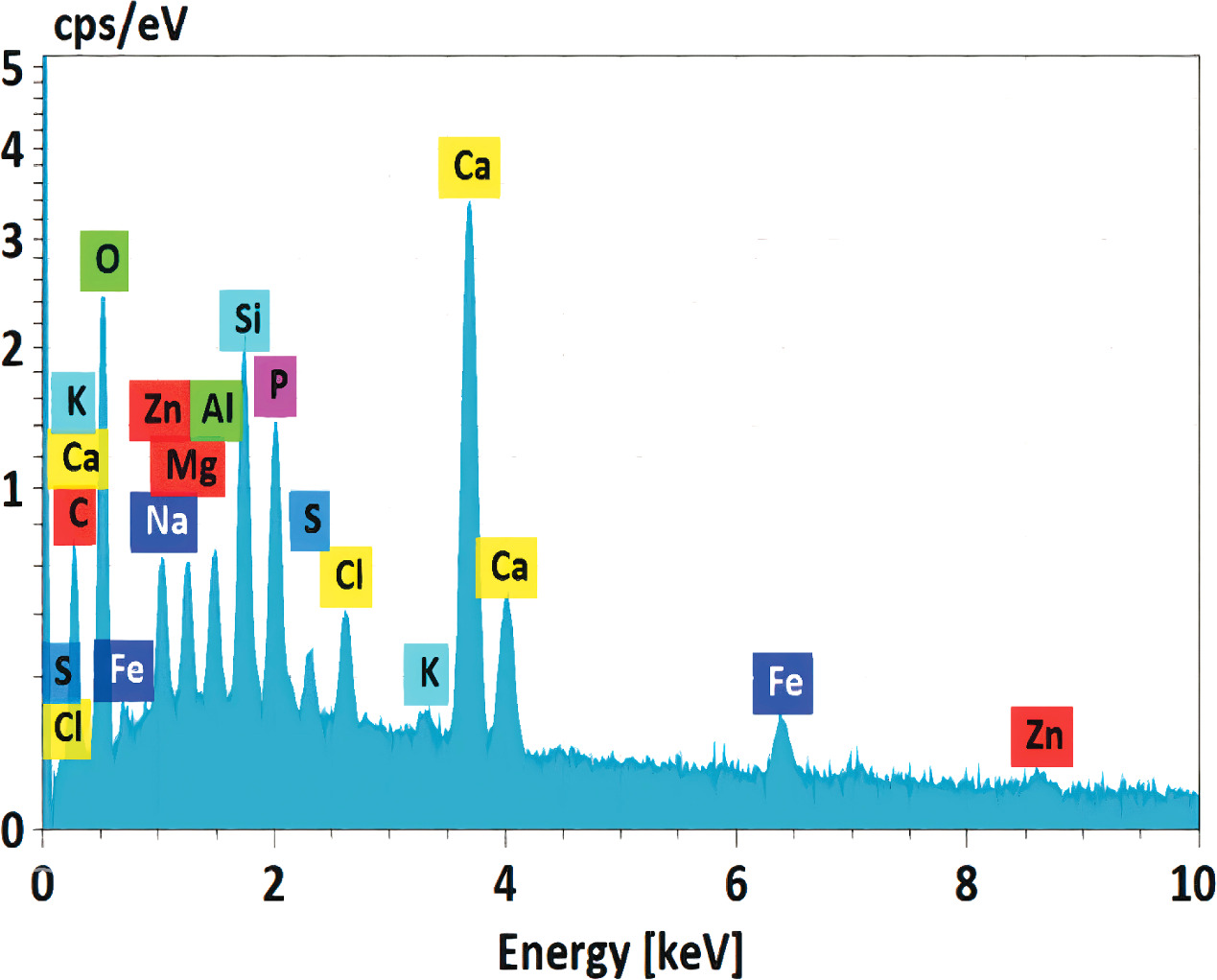
The elemental compositions of fly ash samples were assessed through EDX analysis, and the mapping data are presented in figures 3 and 4, as well as table 1. The SMPP's fly ash contains a variety of metal elements, and the identification of these heavy metals in fly ash may provide the foundation for further solid-phase chemical treatment and burial procedures. All of the results point to the EDX method's enormous potential for quick detection of heavy metals in incinerator fly ash. The elemental mapping data presented in figure 3 offers valuable insights into the distribution of various elements within the components of fly ash from the SMPP. This information represents elements' spatial distribution and potential interactions within the fly ash matrix. The elemental mapping data reveals the presence and distribution of elements such as Si, S, Mg, K, Ca, Zn, Al, Fe, P, Cr, Pb, Ag, Ti, Cd, and Cl within the fly ash components. The distribution of these elements provides important insights into the heterogeneity of the fly ash composition and the potential association of certain elements with specific phases or components. For example, Si, S and Al may indicate the abundance of aluminosilicate phases within the fly ash, common in coal combustion by-products. The distribution of heavy metals such as Cr, Pb, Ag and Cd, can also be visualized, providing information on their spatial association within the fly ash matrix. The results from figure 4 and table 1 showed that the metal element content in fly ash at the plant is arranged in descending order as follows: Ca > Si > Al > Mg > Zn > Fe > Pb > Cr > Ag > Cd. The ICP-MS approach was employed by Luan et al (2016) [17] to evaluate the heavy metal concentration in Shanghai incinerator fly ash, while Yao et al (2020) [18] used LIBs sensors to quantify the heavy metal components, with the following ranking: Zn > Cr > Cd > Pb > Cu. Fly ash contained the following concentrations of metal elements: 6,200 ppm, 2,400 ppm, 1,700 ppm, 546 ppm, 338 ppm, 104 ppm, and 91 ppm of Zn, Pb, Fe, Mg, Cu, Cr, and Cd, respectively.
Figure 3. Mapping image of fly ash from Soc Son waste power plant.
Download figure:
Standard image High-resolution imageFigure 4. EDX analysis of fly ash of SMPP.
Download figure:
Standard image High-resolution imageTable 1. Elemental composition of fly ash of the SMPP analyzed with EDX methods.
| Element | Concentration (ppm) | QCVN 07:2009/BTNMT |
|---|---|---|
| Mg | 16,800–28,900 | — |
| Al | 14,900–23,000 | — |
| Si | 9,120–19,100 | — |
| P | 11,100–17,600 | — |
| S | 4,600–8,950 | — |
| K | 5,460–9,040 | — |
| Ca | 201,600–266,000 | — |
| Ti | 6,650–9,765 | — |
| Fe | 20,400–32,900 | — |
| Zn | 9,800–12,900 | 5000 |
| Pb | 2,154–2,652 | 300 |
| Cr | 238–286 | 100 |
| Ag | 154–195 | 100 |
| Cd | 56–95 | 10 |
(−) Not specified.QCVN 07:2009/BTNMT: National technical regulation on hazardous waste.
The concentrations of all elements, except Zn, Pb, Cr, Ag, and Cd, are not defined in the QCVN 07:2009/BTNMT standards for the disposal of industrial waste, according to table 1. It is essential to note that the Pb, Cr, Ag, Cd and Zn concentrations in the fly ash sample exceed the QCVN limits of 300, 100, 100, 10 and 5,000 ppm, respectively. This suggests that the fly ash produced by the municipal solid waste power plant contains elevated levels of certain elements, which may pose a risk to the environment and human health if not managed properly. The power plant needs to implement effective waste management practices to minimize the release of these elements into the environment. Moreover, The combustion of municipal solid waste, including food waste, dough, coffee grounds, and fruit and vegetable peels, results in the presence of phosphorus (P) and sulfur (S) components in the fly ash of the SMPP. These substances comprise organic components that are a rich source of trace elements, such as P and S, including proteins, lipids, and carbohydrates [19]. The P and S are gases or vapors that are generated during the burning of these materials and interact with other compounds in the combustion system to form fly ash.
This aligns with the conclusion drawn by Zhang et al (2008) [19], who investigated the attributes of particulate carbon emissions originating from real-world Chinese coal combustion. Their study unveiled that the organic compounds within carbonaceous particulate matter emissions offer crucial insights into source identification. The constitution of particulate matter emissions from coal combustion systems can exhibit substantial variation contingent upon the coal type and combustion circumstances. For instance, industrial boilers operating at elevated temperatures and controlled air ratios tend to yield emissions featuring diminished levels of organic carbon (OC), elemental carbon (EC), and distinct organic compounds compared to household stoves. Oxygenated compounds, including organic acids, predominate in emissions from industrial boilers, while polycyclic aromatic hydrocarbons (PAHs) and n-alkanes dominate the emissions from residential stoves [19].
The identification of phosphorus (P) and sulfur (S) components within the fly ash generated via the SMPP process concurs with the observation that municipal solid waste encompasses minor proportions of nitrogen, sulfur, chlorine, and phosphorus [20]. These elements are found within both the organic and inorganic constituents of waste, spanning plastics, metals, solvents, paper, wood, and petroleum products [21].
According to the information given, the amount of Pb, Cr, Ag, Cd and Zn in the fly ash sample exceeds the acceptable limit established by the Vietnamese regulatory authority since it exceeds the QCVN level from 2 to 8 times. The nature of the waste, the kind of incineration method employed, or the origin of the waste could all be contributing causes to the fly ash sample's increased Pb, Cr, Ag, Cd and Zn concentrations. A complicated mixture of different elements, including plastics, organic material, paper and textile dyeing, makes up municipal garbage, also known as home waste [22]. The Pb, Cr, Ag, Cd and Zn may be generated into the fly ash during the burning of these materials [22]. This corresponds to the outcomes of a study conducted by Gupta et al (2005) [23], which explored the vermicomposting potential of Eisenia foetida using fly ash as a substrate. Their research revealed elevated levels of heavy metals within the fly ash, including zinc, chromium, lead, nickel, and copper. Hence, the presence of zinc-containing compounds in the municipal garbage may cause the high Zn content in the fly ash sample from the SMPP.
The response of trace elements during the incineration process of municipal solid waste has been the subject of investigation through thermodynamic equilibrium calculations. These calculations have unveiled that specific details, such as cesium, can undergo conversion into gaseous compounds or crystalline aluminosilicates under the conditions of incineration temperatures. The occurrence of aluminosilicate compounds within the fly ash can influence the leaching conduct of trace elements. This observation coincides with the conclusions of Yui et al (2018) [22], who employed thermodynamic equilibrium calculations to predict the feasible chemical forms of radioactive cesium within incineration residues. The investigation of enrichment in particular constituents, including heavy metals, within fly ash originating from biomass combustion facilities has also been addressed in prior research [24]. The enrichment ratios for heavy metals like cadmium, lead, and zinc within the fly ash exceeded the reported values in specific investigations. This implies that the composition of the waste and the combustion conditions can play a role in determining the concentration of heavy metals within the fly ash.
Additionally, several elements like O and C were found on the energy dispersive x-ray spectroscopy (EDX) map (figure 3) but were not included in table 2. This is so that the surrounding environment in the machine might taint the detection of O element. At the same time, the Ccould be impacted by the carbon tape used to mount the sample, since the EDX map displays the energy spectrum of each element in the measurement chamber. As a result, both the environment and the given data might impact the composition shown on the EDX analysis. The elements Cl and Na were found on the EDX map but were not measured in the data table, probably because of their low concentrations below the instrument's detection range.
Table 2. Analytical results for element composition in fly ash samples at SMPP utilizing XRF techniques.
| Elements | Concentration (ppm) | QCVN 07:2009/BTNMT |
|---|---|---|
| Al | 16.777 ± 0.15383 | — |
| Si | 23.308 ± 0.04243 | — |
| P | 7.465 ± 0.01217 | — |
| S | 15.018 ± 0.01876 | — |
| K | 24.643 ± 0.02298 | — |
| Ca | 57.520 ± 0.032 | — |
| Ti | 6.598 ± 0.00546 | — |
| Mn | 2.375 ± 0,00099 | — |
| Fe | 29.341 ± 0.00573 | — |
| Ni | 234 ± 0.0099 | 1400 |
| Cu | 6.311 ± 0.00212 | — |
| Zn | 35.189 ± 0.0478 | 5000 |
| Br | 7.892 ± 0,00157 | — |
| Sr | 1.910 ± 0.001 | — |
| Cd* | 386 ± 0.00196 | 10 |
| Sn | 2.936 ± 0.00242 | |
| Sb | 1.331 ± 0.00278 | 20 |
| Pb | 1.786 ± 0.00256 | 300 |
(−) Not specified.QCVN 087:2009/BTNMT: National technical regulation on hazardous waste. (*) Special hazardous ingredients (with extremely toxic properties or very high carcinogenic or mutagenic potential) with absolute concentration thresholds less than or equal to 100 ppm.
The Ca constituted over 60% of the entire elemental composition of the fly ash sample from the SMPP process, exhibiting the highest concentration among all elements within the range of 201,600 to 266,000 ppm. This is due to the facility using a semi-dry flue gas treatment method, which involves the addition of lime to absorb HCl and sulfur oxides to create CaSO4 [9]. Chemical reactions occur when sulfur oxides and hydrogen chloride are combined with lime (equations (1) and (2)):
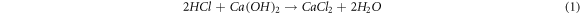
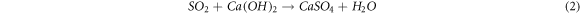
Some of the calcium sulfide formed is converted to calcium sulfate by reacting with excess oxygen in the exhaust gas (equation (3)):
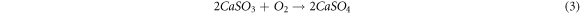
Due to the sluggish reaction between lime and acid gas and the poor mixing of chemicals, the lime-to-acid gas ratio must be 1.5 to 2.0 times the stoichiometric reaction, resulting in surplus lime [9]. According to the study by Zhu et al (2020) [15], the primary components of fly ash include calcium, chloride, sulfate, carbonate, sodium, and potassium, with a high concentration of calcium (almost 23 percent by weight of fly ash). The technology employed in the incineration process operates at temperatures between 800 °C and 1,000 °C, resulting in a substantial amount of non-combustible materials with a primarily mineral composition, such as Al2O3, Fe2O3, and CaO compounds, which constitute a significant component of the fly ash.
Specifically, compared to the national technical regulation on hazardous waste (QCVN 07:2009/BTNMT), the content of Zn, Pb, Cd, and Ag in the fly ash samples exceeded the permissible limit by two to eight times. According to QCVN 07:2009/BTNMT, if any element in a compound exceeds the allowable level, the compound is considered hazardous waste. Hence, it may be firstly asserted that the fly ash from the SMPP possesses dangerous properties. At the time of sampling, the SMPP was in its first operation phase, with a capacity of approximately 800 tons/day. As a result, the operating procedure needed to be changed, and the treatment efficiency still required to be 100%. Moreover, as most of the waste was unsorted domestic waste, the fly ash samples in the research area had high concentrations of organic matter, including P, S, K, and Ti.
The presence of elevated levels of Zn, Pb, Cd, and Ag in the fly ash samples, surpassing acceptable thresholds, stems from various factors. A study conducted by Tang et al (2021) [25] delved into the heavy metal content and species distribution within municipal solid waste incineration (MSWI) fly ash of varying particle sizes. The study revealed that volatile metals, including Zn, Pb, Cu, and Cd, exhibited a propensity to accumulate within finer particles, which posed a notable health risk. This implies that the particle size distribution of the fly ash plays a role in shaping the concentration of heavy metals. Moreover, a separate study emphasized that heavy metals, such as Zn, Pb, Cu, and Cd, could quickly amass in the finer particles of fly ash, thereby raising health concerns [26]. Additionally, the efficiency of processing and treatment at the incineration facility can impact the concentration of heavy metals in the fly ash. Another investigation explored the stabilization of heavy metals within the fly ash from municipal solid waste incineration. It concluded that the hydrothermal process could diminish the leaching of heavy metals, including the Pb, from the fly ash [27]. This underscores the idea that distinct treatment methods can influence the leaching behavior and the potential environmental repercussions of heavy metals present in fly ash. It is pivotal to acknowledge that the heightened concentrations of heavy metals within the fly ash designate it as possessing hazardous attributes, as surpassing the permissible limits set by the Vietnamese regulatory body classifies the substance as hazardous waste [28].
3.1.3. * Evaluation of metal composition of fly ash by x-ray fluorescence (XRF) method
Fourteen elements, including Mg, Al, Si, P, S, K, Ca, Ti, Fe, Zn, Pb, Cr, Ag, and Cd, have been identified by SEM/EDX analysis of fly ash. Ca has the largest concentration among these elements, accounting for 60%–70% of the overall composition. Furthermore, various heavy metals such as Zn, Pb, Cd, and Ag in the fly ash samples have surpassed the permitted limits established by the QCVN 07:2009/BTNMT - National technical regulation on hazardous waste by two to eight times. An entire table of fly ash's chemical composition and energy diagram was generated using the XRF approach to the SEM/EDX and mapping analysis data. The XRF technique utilizes x-ray energy with a broad measuring range to detect light constituents with low power. The measurement technique is therefore thorough and accurate.
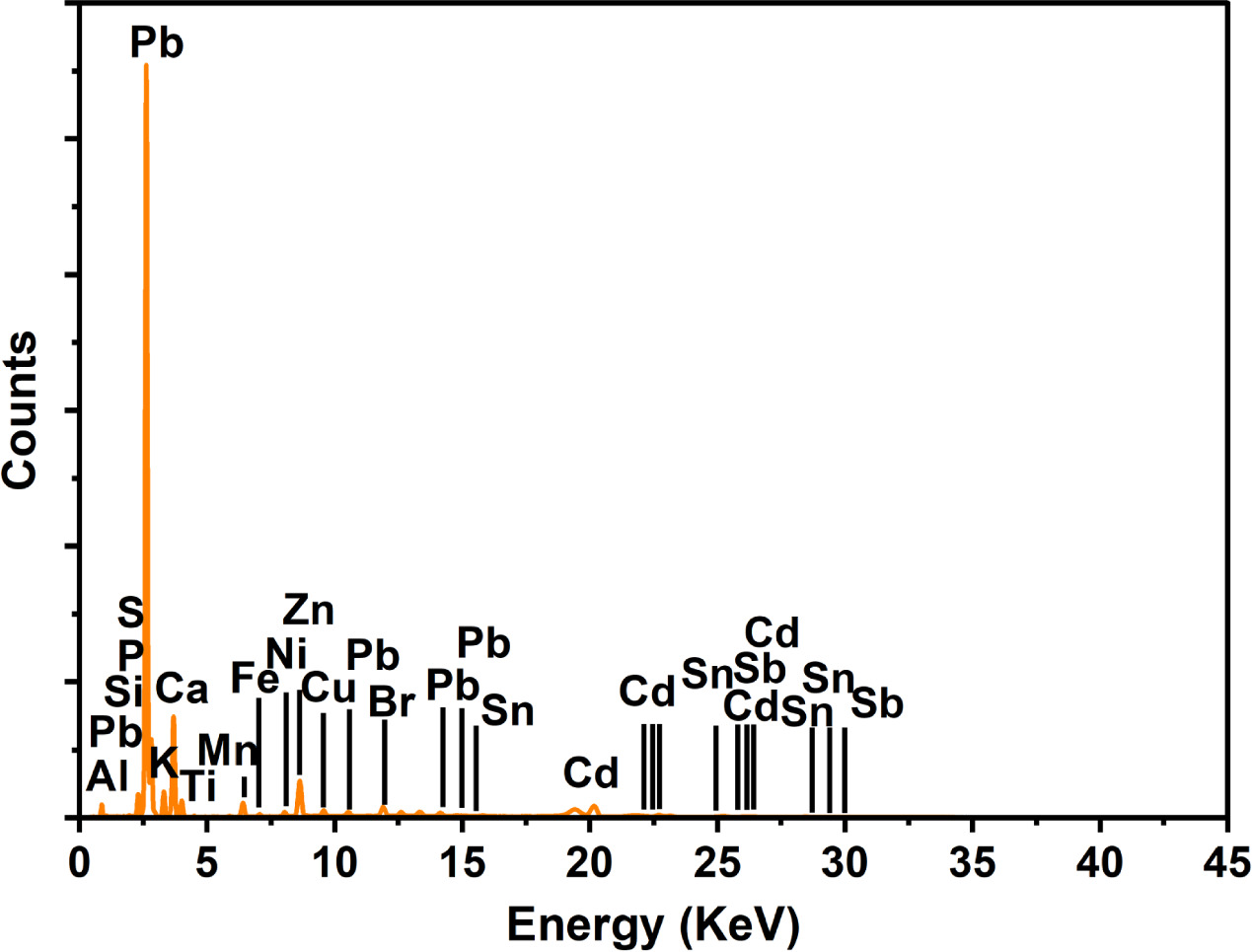
The fly ash of the SMPP comprises a variety of elements, including Al, Si, P, S, K, Ca, Ti, Mn, Fe, Ni, Cu, Zn, Br, Sr, Cd, Sn, Pb and Sb, as indicated by the XRF data table of the elemental composition of the fly ash (figure 5). Among these elements, the Ca has the most significant abundance among these elements, accounting for approximately 57,520 ppm. Besides, some heavy elements, including Cd, Sb, and Pb, exceeded the QCVN 07:2009/BTNMT permissible level. The excessive levels of Cd and Pb within the fly ash samples, surpassing established limits, carry potential implications for health and the environment. The Cd is recognized for its high toxicity, and its accumulation within plants and food chains can pose risks to human health. The study conducted by Arduini et al (2006) [29] investigated the response of miscanthus to toxic cadmium applications and found that the Cd concentrations in the above-ground parts of plants increased as the Cd supply augmented, eventually reaching levels associated with toxicity. The presence of Cd in the fly ash samples suggests the possibility of contamination and underscores the necessity for appropriate waste management to avert environmental and health hazards. Similarly, elevated Pb concentrations exceeding permissible limits in the fly ash samples signal potential contamination and the presence of a hazardous element. The Pb is a widely acknowledged toxic heavy metal capable of negatively affecting human health, particularly the nervous system and cognitive development [30].
Figure 5. Fly ash energy diagram of the SMPP analyzed by XRF.
Download figure:
Standard image High-resolution imageIt is crucial to acknowledge that the presence of Cd and Pb in the fly ash samples could be influenced by multiple factors, encompassing waste composition and the incineration process. Based on the comparative study of the chemical composition of the fly ash sample from the SMPP, it can be concluded that, according to the energy dispersive spectrum, the identified metals were found in varying ranges between 0 and 30 kV. The fly ash from the SMPP contains the elements of Al, Si, P, S, K, Ca, Ti, Mn, Fe, Ni, Cu, Zn, Br, Sr, Cd, Sn, Sb, and Pb. Al, S, Si, Fe, Ca, Ti, K, Sr, and Zr were detected in high amounts in fly ash using the XRF method in a study conducted by Wen et al (2023) [31]. The heavy metal composition found by XRF analysis is likewise compatible with the previously disclosed EDX analysis results.
According to statistics published by Vietnam's Ministry of Natural Resources and Environment, over 70% of the input waste components in various Vietnamese areas are municipal solid waste [32]. Furthermore, to clean the flue gas, the plant's gas treatment technology combines nitrogen removal in SNCR furnace + acid removal by semi-dry technique + dry lime injection + active coal adsorption + bag filter [33, 34]. Thus, as stated previously, the SMPP's fly ash from SMPP mainly consists of heavy metals. From a quantitative perspective, according to the XRF analysis results presented in the table, the fly ash sample from the SMPP had a high proportion of Ca, accounting for approximately 60% due to the presence of unburned Ca and excess lime from the flue gas desulfurization process. Additionally, heavy metals were detected in the fly ash, with their concentration levels in the following order: Zn > Fe > Al > Al > Cu > Cd > Mn > Pb > Ni. These results are consistent with studies conducted worldwide, which have found that the heavy metals in fly ash in China and Canada are mainly Zn, Sn, Pb, Mn, and Cu [17, 18].
Comparing the XRF analysis results with the Vietnamese standard QCVN 07:2009/BTNMT to assess the danger of fly ash revealed that certain constituents in the fly ash from the SMPP exceeded the allowable limits. The Zn concentrations were 6–7 times higher than the threshold, the Pb concentrations were 5–6 times higher, the Sb concentrations were 60–65 times higher, and the Cd concentrations were 28–39 times higher (table 2). Significantly, the Cd content exceeded 100 ppm, making it a very hazardous or carcinogenic element with a high threshold limit. These results are comparable with research on heavy metal concentrations in fly ash from waste power plant in Shanghai determined by ICP-MS [18] and by LIBs sensor indicating the composition of heavy metals in fly ash as Zn > Cr > Cd > Pb > Cu [12].
The heightened Zn, Pb, Sb, and Cd concentrations detected within the fly ash samples signify potential environmental and human health risks. While Zn is an essential micronutrient, overexposure can adversely impact the environment and human well-being [35]. The Pb, a widely acknowledged toxic heavy metal, can inflict harmful effects on human health, particularly concerning the nervous system and cognitive development [36]. The Sb, another element of concern, is also categorized as toxic and can contribute to respiratory and cardiovascular complications [28]. Being highly toxic and carcinogenic, the Cd boasts an exceedingly low threshold limit, thereby intensifying apprehensions about potential health hazards stemming from its presence within the fly ash samples [36].
The emergence of these heavy metals in fly ash can be attributed to various factors, including the waste input's composition, the combustion procedure, and the origin of the utilized coal. The exploration by Ohbuchi et al (2008) [37] scrutinized the characteristics of fly ash and unearthed elevated concentrations of heavy metals, including Zn, Pb, Ni, and Cu, within the fly ash samples. This underscores the likelihood that the composition of the waste input may contribute to the presence of heavy metals within the fly ash.
Prudent handling of the fly ash are crucial to forestall potential environmental contamination and health risks. The inquiry undertaken by Jahromy et al (2021) [38] probed into the plausible utilization of fly ash from the pulp and paper industry for thermochemical energy and CO2 storage. This study ascertained that the CaO content within fly ash was pivotal in dictating its physical properties. This underscores the significance of comprehending the composition and attributes of fly ash, both for its conceivable applications and for its safe disposal. The limitations and capabilities of each technique can be used to explain the presence or lack of particular constituents in the study of fly ash utilizing EDX and XRF methodologies. Due to their high x-ray emission and ability to be detected by the EDX spectra, elements like Mg, Ag, and Cr are present in the study of fly ash using EDX technology [39]. It is imperative to acknowledge that the absence of an element from the Energy Dispersive x-ray (EDX) spectrum does not definitively indicate its absence in the fly ash particles . In some circumstances, particles might have elemental map markers not visible in the EDX spectra. This might result from EDX basic mapping's limitations, in which some elements might not be recognized or adequately represented in the spectra.
On the other hand, because the XRF may be used to detect them, elements including Mn, Ni, Zn, Br, Sr, Sn, and Sb are present in the analysis of fly ash. Fly ash elemental composition can be determined using the effective method known as the XRF analysis. These elements can be found in the fly ash using an XRF analysis, which confirms their presence in the samples and reveals essential details about the physicochemical makeup of the particles.
The distinct guiding principles and capabilities of EDX and XRF analysis can be used to explain the variations in the elements identified. The foundation of EDX analysis is the detection of distinctive x-rays that the sample's constituent elements produce when subjected to an electron beam bombardment. The geographic distribution of the elements in the sample can be shown using this method, which is very helpful for elemental mapping. On the other hand, the XRF analysis relies on detecting fluorescent x-rays that the elements of the sample emit when exposed to high-energy x-rays [39].
The outcomes above result from Vietnam's failure to classify municipal solid waste at the source. The waste given to the waste power plant in the research area is urban waste with a metal content between 1.4% and 4.9%, plastic and nylon content between 3.4% and 10.6%, and inert material content between 14.9% and 28.2% [32]. Before incineration, the garbage is likewise not categorized at the SMPP. Hence, the secondary byproduct of combustion and fly ash, includes many metals. This establishes the harmful nature of fly ash and gives a basis for appropriately evaluating SEM/EDX and XRF measurements.
3.2. The possibility of reusing FA in Vietnam
Based on the findings presented in section 3.1, it is evident that fly ash comprises primarily inorganic components, predominantly metals. Several of these metal constituents surpass the permissible thresholds for hazardous waste, as stipulated by Vietnamese governmental regulations, including Pb, Cr, Ag, Cd, and Zn. Consequently, the discharge of such fly ash into the environment poses a significant risk of pollution. The paramount concern centres around devising effective strategies for properly handling and reutilization of this waste. This study advocates for implementing reuse as a pivotal measure to facilitate efficient waste circulation. Given that the characteristics of fly ash from the SMPP are conducive to environmental pollution when released, redirecting its use towards purposes associated with metal separation is deemed impractical. Instead, a more fitting approach involves repurposing the fly ash to produce construction materials, as outlined in the study's recommendations. However, it is essential to emphasize thorough pretreatment of the fly ash from the Soc Son waste power plant before any reuse endeavours. This precautionary measure is paramount to ensure that the material undergoes adequate processing, preventing any potential for secondary pollution to the environment. This comprehensive approach seeks to address the immediate issue of hazardous waste and mitigate the risk of unintended environmental repercussions during the subsequent reuse stages.
To ensure the safe use of fly ash in construction, it is essential to pretreat it to convert it into a less or non-hazardous form. One proposed method is the use of a chelator to achieve this transformation and enable the reuse of fly ash. When treated with a chelating agent, fly ash can immobilize metals and reduce toxicity. Moreover, chelate treatment can significantly alter the surface of fly ash particles, leading to the formation of secondary minerals like ettringite, which may reduce the hazardous nature of fly ash. The primary components of the fly ash treatment system include silos, weighing hoppers, discharge valves, and measurement hoppers. Chelate agents are utilized in the facility's stabilization process of fly ash. A conveyor belt transports shaken fly ash from the baghouse through the silo and into the blending silo. During this operation of the SMPP, the weight of the fly ash is determined using electronic scales. Before the fly ash is reused, 2% chelating agents are mixed at the blending silo to stabilize it.
3.2.1. * Reuse of fly ash in the production of building materials
The reuse of fly ash from domestic garbage incineration is supported by data from worldwide studies and the practical experience of the Dan Phuong waste treatment plant in Hanoi. Using the atomic absorption spectroscopy method, the study of fly ash samples from the Dan Phuong plant reveals that the amount of CaO and MgO in Vietnamese fly ash is more than in China and the United States. Moreover, the concentration of harmful chemicals in the fly ash is below the QCVN 07:2009/BTNMT level. Thus, fly ash from waste incineration can supplement cement, concrete, and concrete blocks in the building industry.
Pham et al (2020) used municipal solid waste incinerator bottom ash and the dust collected after incineration in a laboratory-scale incinerator with a 5 kg h−1 capacity. The experimental results of bottom ash and dust samples from a municipal solid waste incinerator revealed the absence of harmful heavy metals such as Hg, As, Pb, and Cd. Besides, the SiO2 and Al2O3 concentrations were relatively high. Hence, the research team utilized alkalis such as NaOH and lime to combine the bottom ash from municipal solid waste incinerator with minerals (kaolin, claystone) in various proportions. Furthermore, the results suggested that this solidification procedure was adequate for manufacturing unfired construction bricks. Vietnam Water and Environment Joint Stock Corporation (BIWASE) has adopted a solidification method for incinerator bottom ash from industrial waste incineration processes by mixing it with cement, sand, and crushed stone in compliance with TCVN 6776:1999. Other research is being conducted in Vietnam, such as by the Hanoi University of Transport and Communication, Can Tho University, and the Materials Institute of the Vietnam Academy of Science and Technology, which focuses on using municipal solid waste incinerator bottom ash to produce non-fired bricks. According to the findings of these domestic investigations, it is recommended to use stabilized fly ash from the SMPP as follows:
3.2.1.1. Using fly ash to make unburnt bricks
The SEM/EDX particle size examination reveals that the fly ash particles from the SMPP are small and relatively homogeneous. According to TCVN 8827:2011, which stipulates fly ash as a mineral additive, the particle size of fly ash from the burning of domestic waste satisfies the standards. Regrettably, the chemical composition of fly ash encompasses heavy metals, which has the potential to impact the integrity of concrete and mortar compositions detrimentally. Also, the fly ash is composed of a blend of lime powder and primarily calcium- and mineral-rich activated carbon. Hence, pre-treatment is required to lower the amount of Cl- ions before material mixing to not alter material's properties.
Chemical analysis of the fly ash from the SMPP reveals that fly ash contains a high amount of alkali and Cl- ions and a large proportion of amorphous phases, such as glass, which are valuable components of building materials. To lower the heavy metal and Cl- content, the first concept is to combine the fly ash with the bottom ash (inorganic materials that do not burn during incineration) already present at the plant. These constituents' presence within fly ash makes incorporation in construction applications amenable. Nonetheless, it is imperative to mitigate the concentration of heavy metals and Cl- ions to uphold environmental integrity. The levels of heavy metals and Cl- ions can be attenuated through the amalgamation of fly ash with bottom ash, yielding a construction material endowed with enhanced safety considerations. While comprehensive experimental samples are yet to be devised, employing a blending proportion of less than 30% fly ash as a binding agent is advised to safeguard environmental well-being [40]. This proportion ensures that the ultimate amalgam's heavy metal and Cl- ion content remains within permissible thresholds.
In conjunction with its chemical composition, the physico-chemical attributes of fly ash are subject to the influence of combustion conditions (Pacewska et al 2006). Disparate fly ash variants may manifest disparities in their properties, potentially affecting their appropriateness for distinct applications. Consequently, it becomes imperative to account for the specific traits inherent to the employed fly ash, thereby facilitating the optimization of its efficacy within processes like cement hydration and other construction methodologies. Moreover, the augmentation of alkali-activated slag-fly ash concrete with steel fibres has in markedly enhanced its mechanical characteristics [41]. Investigations have elucidated that incorporating steel fibres, encompassing both unswerving micro steel fibres and contorted macro steel fibres, amplifies the robustness and longevity of the concrete matrix. The utilization of hybrid steel fibres, amalgamating both fiber types, has demonstrated the most substantial enhancement in the mechanical attributes of alkali-activated slag-fly ash concrete.
3.2.1.2. Using fly ash to make cement production materials
Cement's essential ingredients consist principally of limestone, clay, and sand, as well as iron ore, bauxite, silica stone, and several additional additives. These ingredients undergo multiple procedures before being cooled and packaged as cement, including crushing, calcining, and mixing. After being calcined and powdered, limestone, the primary component of the raw materials, is also used as a powder in the flue gas treatment process at the SMPP.
The fineness of cement is a fundamental and crucial physical characteristic. According to Vietnamese rules, several worldwide cement fineness requirements have been published. The following criteria can be mentioned briefly: AASHTO T 98 and ASTM C 115: Fineness of Portland cement by the turbidimeter. AASHTO T 128 and ASTM C 184: Fineness of hydraulic cement by the 150 μm (No. 100) and 75 μm (No. 200) sieves. AASHTO T 153 and ASTM C 204: Fineness of hydraulic cement by air permeability equipment.
Concurrently, several related standards and regulations, such as TCVN 10306–2014 and TCVN 2682:2009, describe the fineness or particle size of cement, indicating that the smallest particle size is 1.5 μm and the average particle size is between 10 and 20 μm. The SEM/EDX study of fly ash particle size from the SMPP suggests that it is suitable for cement manufacture as a blending material. Hence, fly ash from the SMPP can be repurposed as a cement additive. To increase cement quality by using fly ash, however, more testing is required to find the ideal mixing ratio. The mixing ratio pertains to the quantity of fly ash incorporated into the cement to attain the targeted properties and performance criteria. This evaluation guarantees that the resultant cement aligns with the stipulated standards and specifications. Beyond particle size, it is imperative to account for additional factors, including chemical composition, pozzolanic activity, and reactivity of the fly ash, when establishing the suitable blending proportion [42]. The variability of these attributes can be contingent upon the origin and distinctive features of the fly ash. Consequently, a comprehensive testing regimen and assessment become indispensable to ascertain the optimal incorporation of fly ash as a cementitious additive.
4. Conclusion
The examination of fly ash from the Soc Son municipal waste power plant (SMPP) reveals that over 70% of the fly ash is in the form of spherical, porous particles with a size range of 5–103 μm. In comparison, the remaining 30% is either more significant than 65 μm or in the form of asymmetric crystals. The EDX analysis of fly ash from SMPP showed that the composition of heavy metals in fly ash is Ca > Si > Al > Mg > Zn > Fe > Pb > Cr > Ag > Cd, with the Ca level of around 60%. According to QCVN 07:2009/BNTMT on hazardous waste, the local fly ash contains a Zn concentration over 6–7 times, a Pb content exceeding 5–6 times, the Sb content exceeding 60–65 times, and a Cd content exceeding 28–39 times, suggesting that it is hazardous waste. The Al, Si, P, S, K, Ca, Ti, Mn, Fe, Ni, Cu, Zn, Br, Sr, Cd, Sn, Sb, and Pb are among the chemical components discovered in the fly ash sample, as determined by XRF. Based on these findings, we propose a potential application for the reuse of fly ash as a building material. However, caution should be exercised when contemplating the mixing ratio to ensure safety, as this study is preliminary and requires more research.
Acknowledgments
This article is part of the results of the Project 'Research on the composition and properties of fly ash generated from domestic solid waste incinerators', code KHCBVL.06/22–23 under Physics Development Program at Vietnam Academy of Science and Technology (VAST), implemented in the period 2022–2023.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Author contribution
Tra Mai Ngo, Hang Nguyen Thi Thuy, Hong Khuat Thi, Nghiem Thi Ha Lien designed the research idea; Nguyen Trong Nghia, Phan Thi Thanh Hang took sampling; Vu Duc Toan, Trinh Thi Tham, Nguyen Thi Hoa prepared statistical analyses; Hang Nguyen Thi Thuy; Tra Mai Ngo, Van Hung Hoang and Huu Tap Van wrote the main manuscript text and revised the finished version. All authors reviewed the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.