Abstract
Platinum-binding (Pt-binding) peptides have been used for fabrication of complex platinum nanomaterials such as catalysts, metallopharmaceuticals, and electrodes. In this review, we present an understanding of the mechanisms behind Pt-binding peptides and their applications as multifunctional biomaterials. We discuss how the surface recognition, roles of individual amino acids, and arrangement of amino acid sequences interplay. Our summary on the current state of understanding of Pt-binding peptides highlights opportunities for interdisciplinary research which will expand the applicability of these multifunctional peptides.
Export citation and abstract BibTeX RIS
1. Introduction
Peptides are sequences of 2–50 amino acids linked together by covalent peptide bonds. With careful selection of sequence and amino acid content, a peptide can be designed to self-assemble and bind to specific materials. Peptides with selective binding can facilitate the purification of proteins [1, 2], targeted drug delivery [3, 4], and development of new biosensors [5]. Selective binding and self-assembly can also be used to direct the fabrication and functionalization of nanomaterials [6–8].
The importance of directing the fabrication and functionalization of platinum (Pt) nanomaterials is highlighted in many studies. The development of sensitive biosensors has utilized creative functionalization of Pt with biomolecules [9]. Furthermore, Pt is still a leading catalyst choice for important industrial reactions such as methanol oxidation [10], hydrogen oxidation [10, 11], oxygen reduction [12], and hydrogenation [13–15], as well as for experimental enzyme mimicking reactions [16]. Modifications to improve stability and catalyst utilization have been demonstrated via functionalization of Pt materials with polymers [17], aromatic macrocycle molecules [18], thiol ligands [19], and chiral surface modifiers [20]. Overall, the motivation to find suitable methods that can improve the functionality of Pt materials opens new research opportunities and encourages the emergence of interdisciplinary fields to fundamentally study and develop multifunctional, selective, Pt modifiers.
Tuning catalytic Pt materials with peptides has recently been of great interest [10, 16, 21, 22]. Pt-binding peptides have gained attention over the past 20 years due to their main advantage of being highly tunable, allowing for multifunctionality [10, 11, 23]. There are excellent studies on the molecular interactions between Pt materials and Pt-binding peptides that facilitate understanding of peptide-assisted Pt nanomaterial fabrication. However, complete understanding of Pt-binding peptide mechanisms is still under development. This mini-review covers the discovery of Pt-binding peptides, the theoretical and experimental approaches in probing the molecular interactions responsible for Pt-binding, the applications of Pt-binding peptides, and strategies for Pt-binding peptide design. To the best of our knowledge, this is the first time a list of Pt-binding peptides, their origin, and their binding properties have been synthesized. Further, we discuss the gaps in the fundamental understanding of Pt-binding peptides as well as opportunities for future studies.
2. Origin, history, and fundamental understanding of Pt-binding peptides
Biopanning using phage display is a primary method in the discovery of affinity peptides [24–26], including most Pt-binding peptides. First, a library of randomly generated peptides is produced by bacteriophages. Each phage contains genetic information to express a specific peptide sequence and display the sequence on its surface. The bacteriophages with peptides on their surface are suspended in a buffer solution where a Pt material is incubated for binding. The Pt material is then washed several times with a peptide-free buffer. Phages that are bound to the Pt surface are then eluted and cloned. The DNA corresponding to the Pt-binding peptide is extracted from the clones to identify Pt-binding peptide sequences. The process of target-binding, washing off the non-selective peptides, and elution of the selective peptides is called biopanning. The process is often repeated to achieve the desired selectivity and affinity strength.
One of the first studies on a Pt-binding peptide however was about a sequence not discovered via biopanning. The study described an interaction between Pt(II) ions and sequences with histidine residues at both the C- and N-terminus [27]. It was discovered that the carboxylate oxygen and imidazole nitrogen of the histidine residues aided in the Pt(II) ion binding. In addition, the selectivity of the binding depended on the pH and position of histidine residues [27]. This inspired early studies on the application of histidine-rich peptides for the synthesis of advanced Pt nanostructures [28, 29]. Eventually, more Pt-binding peptides were discovered via biopanning for uses in different environmental conditions as summarized in table 1.
Table 1. A summary of specific platinum-binding peptides reported in the literature. No peptides that were proven to bind as strong or stronger to other metals were included.
| Year | Reference | Peptide sequence | Phage display method | Platinum material used for peptide selection | Reported conditions for incubation of peptide library with platinum material |
|---|---|---|---|---|---|
| 2007 | [63] | SRLTHSNYATPT EHTNPILSHTHN QSFSTNVLHTHH | Phage library: Ph.D.-12 Rounds of biopanning: 1 | Material: slurry of finely divided solid platinum substrate Method of production: n/a Purity: n/a | Solvent: tris buffer saline (TBS) Duration: 1 h pH: n/a Temperature: n/a |
| 2005 | [34] | Strong binders: PTSTGQA CPTSTGQAC QSVTSTK CQSVTSTKC Moderate binders: LGPSGPK CLGPSGPKC Weak binders: APPLGQA CAPPLGQAC LNDGHNY CLNDGHNYC | Phage library: Ph.D.-C7C (NEB) Rounds of biopanning: 1 Notes: Different strengths of affinity were determined by immunofluorescence microscopy | Material: polycrystalline platinum powder Method of production: n/a Purity: n/a | Solvent: potassium carbonate (PC) buffer containing 0.1% detergent (50:50 (v/v) Tween 20 and Tween 80) Duration: 30 min pH: n/a Temperature: room temperature |
| 2009 | [35] | Linear peptides: PTSTGQA QSVTSTK Three repeating units of linear peptides: PTSTGQAPTSTGQAPTSTGQA QSVTSTKQSVTSTKQSVTSTK Cyclic peptides: CPTSTGQAC CQSVTSTKC | Phage library: Ph.D.-C7C (NEB) Rounds of biopanning: 1 | Material: polycrystalline platinum powder Method of production: n/a Purity: n/a | Solvent: potassium carbonate (PC) buffer containing 0.1% detergent (50:50 (v/v) Tween 20 and Tween 80) Duration: 30 min pH: n/a Temperature: room temperature |
| 2009 2010 2011 2011 2013 2013 2014 2016 | [53] [12] [39] [41] [49] [10] [13] [52] | TLHVSSY | Phage library: Ph.D.-7 (NEB) M13 Rounds of biopanning: 3 | Material: 1 cm long and 0.25 mm diameter platinum wire Method of production: obtained from Sigma Aldrich Purity: 99.99% | Solvent: tris-Buffered Saline containing 0.1% TWEEN-20 Duration: 1 h pH: n/a Temperature: n/a |
| 2010 | [51] | PWxxQRELSV (x is random amino acid) | Phage library: Ph.D.-12 Rounds of biopanning: 3 | Material: platinum nanocubes bound with Pt{100} planes Method of production: Homemade following a published protocol [64] Purity: n/a | Solvent: n/a Duration: 2 h pH: n/a Temperature: n/a Other reported conditions: humid environment |
| 2011 2011 2013 2015 2019 2019 | [39] [41] [42] [32] [44] [21] | TLTTLTN | Phage library: Ph.D.-7 (NEB) M13 Rounds of biopanning: 3 | Material: platinum nanocubes bound by six Pt{100} facets deposited on silicon substrates Method of production: homemade following the variant of published protocols [65, 66] Purity: n/a | Solvent: TBS containing 0.1% TWEEN-20 Duration: 1 h pH: 7.5 Temperature: room temperature |
| 2011 2011 2013 2013 2015 2019 | [39] [41] [30] [42] [32] [44] | SSFPQPN | Phage library: Ph.D.-7 (NEB) M13 Rounds of biopanning: 3 | Material: platinum nanocubes bound by six Pt{100} facets deposited on silicon substrates Method of production: homemade following the variant of published protocols [65, 66] Purity: n/a | Solvent: TBS containing 0.1% TWEEN-20 Duration: 1 h pH: 7.5 Temperature: room temperature |
| 2017 | [33] | YKRGYK | Phage library: LARFH library constructed by the protocol reported [33] Rounds of biopanning: 3 | Material: 0.1 mg of 0.5–1.2 µm diameter platinum beads Method of production: obtained from Sigma Aldrich Purity: n/a | Solvent: T7 elution buffer (Merck Millipore) containing 2 M urea Duration: 10 min incubation followed by 10 min sonication pH: n/a Temperature: room temperature |
From 1999 to 2021, studies on applications of Pt-binding peptides have grown consistently, yet studies of fundamental understanding of selective binding are in the minority. Most studies (71%) focus on applications such as biotemplating, Pt carriers for cell-targeting, Pt nanocrystal fabrication, electrode modification, and peptide selection development—all of which demonstrate ease of utilizing Pt-binding peptides in multifunctional materials. The rest of the studies (29%) cover fundamental understanding of Pt binding and include studies on the chemistry of interaction and adsorption kinetics. Results from application-based studies can also be useful for developing a fundamental understanding of the interfacial interaction between peptides and Pt. For example, rearranging the amino acid order in peptide sequences yielded Pt nanocrystals with a different morphology compared to that of original Pt-binding peptides. This result implies the importance of amino acid position for Pt-binding affinity [13, 30]. However, as we will discuss in this review, there are more opportunities to complete the understanding of the Pt-binding mechanisms and encourage more advanced applications of these multifunctional biological tools.
The information gained from studies detailing the chemistry of interaction [27, 30–34], binding kinetics [22, 35–37], and binding affinity [22, 35, 36, 38, 39] can be useful in describing the binding mechanisms of Pt-binding peptides [40]. The rest of this section summarizes the findings, techniques used, and implications for each category of study (surface recognition chemistry, binding kinetics and binding affinity).
2.1. Probing the chemistry of Pt surface recognition
The chemistry of the interaction between peptides and Pt is dictated by the functional groups in the peptide chain. These interactions are responsible for affinity, molecular orientation of peptides on the Pt surface, and facet specificity. Understanding the chemistry of binding interactions has led to precise control of Pt morphology. One experimental approach to understand peptide interactions with Pt has been to alter the Pt-binding peptide sequences and include them as precursors during Pt nanocrystal growth. Sequence alterations can be made by randomizing the peptide sequence (scrambling), removing fractions of the sequence (truncating), and substituting amino acids (substitution) with those that do not introduce considerable effect from their functional groups (e.g. alanine and glycine). The final shapes and sizes of the Pt nanocrystals directed by the altered peptide sequences can reveal the important amino acids responsible for Pt affinity. Ruan et al reported studies of a Pt-binding peptide with the sequence TLHVSSY (BP7A, sometimes also referred to as P7A) discovered via biopanning with a polycrystalline Pt target [13, 30, 39]. BP7A peptide was synthesized with an acetylated N-terminus and an amidated C-terminus, removing electrostatic end groups of the peptide. The BP7A was found to promote single-twinned seed formation at the nucleation stage of the Pt nanocrystal synthesis [41]. Using high-resolution transmission electron microscopy (HR-TEM), a difference in anisotropic growth of Pt crystals was observed between tests with and without BP7A. The single-twinned seeds gave rise to mono-, bi-, and tri-pod formations at the nucleation state of Pt crystals facilitated with BP7A. On the other hand, single near-spherical cuboctahedrons crystal seeds were generated in synthesis of Pt crystal without BP7A (figure 1) [41].
Figure 1. BP7A (TLHVSSY) peptide encourages single-twinned crystal seed formation at the nucleation state and promotes multipod formation in Pt nanocrystal synthesis. Reprinted with permission from [41]. © 2011, American Chemical Society.
Download figure:
Standard image High-resolution imageRuan et al truncated BP7A into a VSSY peptide and TLH peptide [13]. The experiment revealed that VSSY did not yield multipod-shaped Pt nanocrystals [13]. In addition, switching the placement of H, S, and L amino acids in the BP7A sequence, still produced multipod-shaped Pt nanocrystals [13]. From these results, it is the presence of L and H in BP7A, not the arrangement of these amino acids, that directs Pt crystal growth into the multipod shape. To better understand the single twinned-seed formation seen in the experiment, the research group utilized a molecular dynamics (MD) simulationusing the CHARMM-METAL force field. The simulation revealed that BP7A's preferential stabilization of the Pt-{111} facet encouraged the twinned seed formation [13]. The fragment of BP7A peptide, TLHV, as well as the variant, TLGV (where the H of TLHV is replaced by G), were analyzed. The simulation results showed that the TLGV sequence has a higher adsorption energy toward cuboctahedrons (representing initially formed single-crystal cluster) as opposed to TLHV, which has a higher adsorption energy toward single-twinned seeds. The simulation results agreed with the experimental HR-TEM images [13]. It was concluded that the binding of BP7A peptide (TLHVSSY) on single-twinned seed Pt is partially attributed to the preferential adsorption toward the Pt-{111} facet and a strong attraction of histidine toward Pt ions [13]. Figure 2 summarizes the understanding of the BP7A peptide binding chemistry. Overall, it was demonstrated that coupling experiments and MD simulation can reveal chemistry important to Pt-binding in peptides. The information about the chemical interactions can be used to develop new materials that can direct the shape of Pt nanostructures.
Figure 2. Conclusion drawn from MD simulation showing that the first four amino acids (TLHV) of BP7A peptide are responsible for initiating single-twinned crystal seed formation and the last three amino acids (SSY) are responsible for promoting multipod formation. Reprinted with permission from [41]. © 2011, American Chemical Society.
Download figure:
Standard image High-resolution imageFacet specificity of peptides is an essential aspect in directing the growth of Pt nanostructures. With an MD simulation, Ramezani-Dakhel et al reported that facet preferences in previously discovered Pt-binding peptides [39] are driven by the difference in how water molecules lie on Pt facets and how the conformation of the peptides matches epitaxial sites on Pt surfaces [32]. In theory, facet specificity of the peptides could be set by the type of Pt materials used in the biopanning process. If the Pt target in the biopanning process is composed of specific facets such as Pt-{111} and Pt-{100}, intentionally or unintentionally, the peptides would have more affinity for Pt with Pt-{111} and Pt-{100} facets. In addition to facet choice for biopanning, sequence manipulation can help determine the chemistry responsible for the specificity. For example, a study on S7 peptide (SSFPQPN) with Pt-{111} facet specificity reported that by replacing phenylalanine (F) with glycine (G) or tyrosine (Y), the peptide lost the ability to recognize and bind to the Pt-{111} facet [30]. The loss of Pt-{111} facet specificity when F was replaced by Y implies that the –OH in the phenyl ring functional group of Y interrupts the overall binding [30]. To complement the experimental results, the research group used CHARMM-METAL force field parameters in an MD simulation to study the S7 peptide-Pt interaction [30]. The difference in binding constants toward different Pt facets determined facet specificity of the peptide. The computational analysis of competitive binding between S7 peptide and water molecules on Pt-{111} and Pt-{100} surfaces showed that the phenyl ring of F in S7 peptide tends to lie flat on the Pt-{111} facet. In addition, the phenyl ring has epitaxial coordination with the facet, strongly contributing to its facet specificity [30]. Ramakrishnan et al further elucidated the binding contributions from other amino acids in the S7 peptide using constant valence force field parameters [42]. It was further verified that the presence of water and buffering ions is important for the peptide adsorption process [42]. For the S7 peptide, the F residue has the highest affinity toward the Pt-{111} facet. In addition, glutamine (Q), asparagine (N), and proline (P) have higher adsorption energy toward Pt-{111} as opposed to Pt-{100} [42]. The hydrophilic nature of Q, the polar nature of N, the hydroxyl group in serine (S), and the added structural constraint from P contribute to the specific adhesion of the S7 peptide to the Pt-{111} surface [42]. Based on the MD simulation, the group also modified a previously reported Pt-binding peptide, PTSTGQA, that was selected against a polycrystalline Pt substrate [36]. The glycine residue was replaced with phenylalanine to make PTSTGQA Pt-{111} specific [30]. In a different peptide, T7 (TLTTLTN), leucine (L) and threonine (T) were found to have higher adsorption energies toward the Pt-{100} facet via methyl groups and their relative position on the peptide [42]. Therefore, the peptide's functional groups and molecular orientation on Pt collectively contribute to its chemistry of selective binding. Overall, Pt synthesis experiments and MD simulations have proven to be excellent approaches in understanding Pt-binding and facet specificity. These combined with studies [20, 43, 44] featuring facet specificity of single amino acids set the stage for the future development of Pt-binding peptides.
2.2. Probing the binding kinetics of Pt-binding peptides
Binding kinetics can elucidate the mechanisms of Pt-binding peptides. Time-resolved mass monitoring techniques such as quartz-crystal micro-balance (QCM) and surface plasmon resonance (SPR) spectroscopy have been utilized to study peptides at solid/liquid interfaces. Figure 3 illustrates the measurement of resonant frequency, which changes with mass adsorption of peptides on a Pt surface over time. QCM and SPR can be used to study both the kinetics and equilibria (discussed in the next section) of solid-binding peptides. The binding mechanism can be revealed by fitting an appropriate kinetic model to the measurement obtained from QCM or SPR. Generally, kinetic studies of Pt-binding peptides involve a simplified version of the Langmuir adsorption model, widely known as the simple Langmuir model or the pseudo-first order adsorption model. It is derived from the original Langmuir adsorption equation by assuming that first-order kinetics dominates the overall adsorption process. The derivations of the adsorption equations can be found in the literature [45]. The simple Langmuir model is shown in equation (1) [45]. θt and θe represent the fractions of Pt surface occupied by peptides at time t and at equilibrium, respectively:

Figure 3. Shifts in resonant frequency measured using QCM to monitor the mass adsorption of peptides over time. The decrease in resonant frequency indicates mass adsorption. This approach is suitable for studying the binding kinetics of a Pt-binding peptide (shown in orange).
Download figure:
Standard image High-resolution imageTherefore, equation (1) describes the overall change of surface coverage on the target over time. k1 is the first order rate constant.
The kinetic studies of a peptide CPTSTGQAC, possessing a constrained configuration due to a cysteine loop connecting both ends, involved fitting a simple Langmuir model to the measured frequency from QCM-D [22] and data from SPR [36]. An MD simulation with consistent valence force field parameters was conducted on the peptide CPTSTGQAC and another peptide with similar affinity (CQSVTSTKC) [31]. The T-S-T sequence in the peptides appeared to be interacting with the Pt metal surface to a greater extent than the non-polar A and P amino acids. The hydroxyl groups of the T-S-T sequence in the peptides lie more closely to the Pt surface than those of the similar peptides with weaker affinities (CVRTSTWRC and CIMRDGPMC) [31]. However, it has not been proven whether the weaker Pt-binders have binding kinetics that are similar to the stronger Pt-binders. Şeker et al found that the time-resolved SPR data of the PTSTGQA peptide fit better with a bi-exponential Langmuir model, whereas the CPTSTGQAC peptide fits better with a simple Langmuir adsorption model [36]. This indicates more complex binding kinetics of the PTSTGQA peptide compared to that of the CPTSTGQAC peptide [36]. The bi-exponential Langmuir model is described as a series of two single exponential terms—each corresponding to a first-order process characterized by a different apparent rate constant [35], depicted in equation (2):
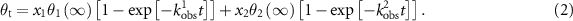
 and
and  are fractions of the sites on the Pt surface that correspond to the adsorption behaviors characterized by the observed binding rate constants
are fractions of the sites on the Pt surface that correspond to the adsorption behaviors characterized by the observed binding rate constants  and
and  , respectively [35, 46] Thus,
, respectively [35, 46] Thus,  +
+  is unity.
is unity.  and
and  are steady-state fractional coverages for the different first-order processes. For those interested in full details of how this modified Langmuir adsorption model was used, the full derivations and steps can be found in the publications from Sarikaya's research group [35, 46, 47].
are steady-state fractional coverages for the different first-order processes. For those interested in full details of how this modified Langmuir adsorption model was used, the full derivations and steps can be found in the publications from Sarikaya's research group [35, 46, 47].
Understanding binding kinetics is essential for understanding of overall binding mechanisms. More efforts in kinetic studies would greatly improve our understanding of selective binding of the peptides. Kinetic information can also facilitate selection of Pt-binding peptides for applications with different assembly needs.
2.3. Probing the affinity strength of Pt-binding peptides
SPR and QCM can also give an insight on binding equilibria. Equilibrium binding constants are essential inputs for MD simulation models in order to help understand affinity [40]. By allowing for the binding processes to reach the equilibrium, thermodynamic parameters can be extracted from the Langmuir isotherm as described in equation (3) [45]:

The parameters qe and qmax are the adsorption capacity of the Pt surface at equilibrium and its maximum value respectively. Ce is the concentration of peptides in the liquid solution at the binding equilibrium, and Keq is the equilibrium binding constant. By fitting the experimental equilibrium capacities at controlled peptide concentrations (from a set of experiments with different peptide concentrations introduced to a Pt metal surface), Keq can be obtained [45]. The Keq is an important parameter to describe the strength of the peptide-metal interactions and can be used to compare Pt-binding peptides that require different levels of affinity. Several metal-binding peptides reported in the literature have Keq values in the range of 105–106 M−1, where the majority are in magnitude of 106 M−1 [48]. Interestingly, the Keq values tabulated in table 2 for Pt-binding peptides exclusively, are mostly within the same magnitude of 106 M−1.
Table 2. Binding constants reported in the literature for platinum-binding peptides.
| Year | Reference | Sequence | Given name | End modifications | Binding constant, 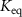
| Kinetic model | Equipment used in binding study |
|---|---|---|---|---|---|---|---|
| 2008 | [22] | CPTSTGQAC |

| Simple Langmuir | QCM | ||
| 2009 | [35] | CPTSTGQAC | c-PtBP1 | — |

| Simple Langmuir | SPR |
| ,, | ,, | PTSTGQA | l-PtBP1 | — |
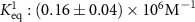
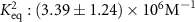
| Bi-exponential Langmuir | SPR |
| ,, | ,, | PTSTGQAPTSTGQAPTSTGQA | 3l-PtBP1 | — |

| Simple Langmuir | SPR |
| ,, | ,, | CQSVTSTKC | c-PtBP2 | — |

| Simple Langmuir | SPR |
| ,, | ,, | QSVTSTK | l-PtBP2 | — |

| Simple Langmuir | SPR |
| ,, | ,, | QSVTSTKQSVTSTKQSVTSTK | 3l-PtBP2 | — |

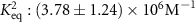
| Bi-exponential Langmuir | SPR |
| 2011 | [39] | TLTTLTN | T7 | Acetylated N-terminal and amidated C-terminal |
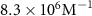
| — | HR-TEM, MD simulation |
| ,, | ,, | SSFPQPN | S7 | Acetylated N-terminal and amidated C-terminal |
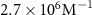
| — | HR-TEM, MD simulation |
| 2017 | [33] | YKRGYK | — | — |
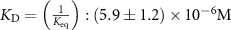 which corresponds to a which corresponds to a 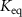 of of 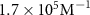
| Simple Langmuir | QCM, AFM |
3. Applications of Pt-binding peptides
We have categorized five main types of applications for Pt-binding peptides: Pt nanocrystal growth, catalytic Pt nanomaterials, electrode modification, biotemplates, and cell targeting. This section will present work in the literature describing the roles of Pt-binding peptides as modulators for each type of application.
3.1. Peptide-mediated Pt nanocrystal growth
A major portion of the application studies focuses on the modulation of Pt nanocrystal growth with Pt-binding peptides [12, 13, 21, 39, 41, 49–53]. The control of Pt nanocrystal morphology is of major interest due to a wide range of catalytic applications, including electrochemical [10, 12, 21], and the use of Pt in nanomedical devices [54]. The morphology of Pt nanoparticles dictates their properties [41]. Thus, using peptides to control particle shape and size provides a mechanism to optimize their performance for various applications. A widely adopted method for directing the formation of Pt nanocrystals consists of two steps: (a) the peptide is added to a precursor (usually K2PtCl4, H2PtCl6, or PtCl3) in an aqueous solution and (b) a reducing agent (usually NaBH4) is added to initiate the reduction reaction that converts Pt ions into Pt metal. TEM image analysis is a method of choice to visualize the morphology of the peptide-assisted Pt nanocrystals, while UV–vis is used for estimation of the Pt nanocrystal production and consumption of the Pt precursors.
Li et al discovered a P7A peptide (TLHVSSY) via phage display selection against a Pt nanowire [53]. They demonstrated the peptide's ability to enhance uniformity of the smaller Pt nanoparticles compared to synthesis without the P7A peptide. The group also conducted a study of Pt nanoparticle formation kinetics using time-resolved TEM with the P7A peptide [12]. The study showed that P7A slowed down the growth rate of Pt nanocrystals while also modulating the nanocrystal shape over the course of 10 min of the reaction. Without P7A, the nanocrystals formed irregular clusters with an average size of about 10 nm. A small concentration (25 µg ml−1) of P7A allowed for the formation of multi-pod Pt nanocrystals. This is the result of preferential binding of P7A to the Pt-{110} facet, as described earlier in the section about the chemistry of Pt surface recognition (section 2). At higher P7A concentrations, the pod length of Pt nanocrystals decreased, which is the result of a higher amount of peptide molecules covering other available Pt facets. Note that the TEM study was conducted with P7A peptide containing N-terminal acylation and C-terminal amidation. Later, a P7A peptide with N-terminal acetylation only (P7A-NH2) demonstrated the ability to activate a selective spreading property of Pt nanoparticles on a hydrophobic surface [52]. The additional roles that hydrophilic and hydrophobic surfaces bring to Pt nanocrystal synthesis demonstrate another creative pathway in using Pt-binding peptides for multifunctional nanomaterial fabrication.
Using facet specificity in different Pt-binding peptides, the Huang group created a protocol to generate more diverse shapes in Pt nanocrystals [39, 41]. They found two other Pt-binding peptides via phage display, TLTTLTN (T7) and SSFPQPN (S7), that bound specifically to Pt nanocubes with Pt-{100} facets and Pt octahedrons with Pt-{111} facets, respectively [39]. By using BP7A (TLHVSSY) to generate single-twinned seeds at the nucleation state, T7 and/or S7 peptides were used to regulate crystal growth and generate different final crystal shapes, including right bipyramid and {111}-bipyramid [41].
3.2. Catalysis of peptide-mediated Pt nanocrystals
Catalytic allyl alcohol hydrogenation was demonstrated with Pt nanospheres directed by self-assembling R5 peptide (SSKKSGSYSGSKGSKRRIL) during synthesis [15]. Turn over frequency (TOF) numbers (moles of product/mole of Pt metal × time) were used to quantify and compare the efficiency of the hydrogenation. It was reported that the TOFs of allyl alcohol hydrogenation by R5-assisted Pt nanoparticles were two orders of magnitude higher than those of Pt nanoparticles synthesized by organic molecules with similar size [15]. While the reason for higher reactivity was not reported, the type of capping agent and reaction environment could be important factors. Furthermore, the R5-assisted Pt nanospheres exerted higher recyclability (consistently 2 h to complete the reaction) for allyl hydrogenations over four cycles of reaction completion compared to R5-assisted palladium (Pd) nanoparticle networks [15].
Insights from the ability to direct morphology of Pt nanocrystals with Pt-binding peptides resulted in expansion of novel Pt nanomaterial synthesis. Studies [13, 14, 55] have shown the ability to tailor Pt and Pd nanocrystals using small molecules with analogous biomolecular functional groups responsible for directing the nanocrystal growth. Ruan et al prepared Pd twinned seed crystals with benzimidazole. The benzimidazole additive provided Pd{111} facet stabilization as predicted by the results of peptide-assisted Pd twinned seed crystals. Comparing catalytic efficiency of Pd twinned seed crystals prepared with benzimidazole and Pd single crystals prepared without benzimidazole, the TOF was nearly two times higher for the Pd twinned seed crystals [13]. The bio-inspired facet recognition strategy was expanded to other molecules such as 3-hydroxybutyric acid (3-HB) and tropic acid (TA) [14]. Pt nanocubes were dominant products of Pt synthesis with 3-HB as a result of epitaxial matching of three oxygen atoms on Pt{111}. Pt tetrahedrals with small spheres at the tips, called pseudo-tetrahedra, were dominant products of Pt synthesis with TA. Hydrogenation of 4-nitrophenol was used as a model reaction to probe the efficiency of the catalysts. The results suggested that TOF numbers of the 3-HB-assisted and TA-assisted Pt catalysts are comparable to the TOF numbers of conventional catalysts. This shows that the bio-inspired strategy is a competitive choice for Pt catalyst synthesis [14].
Another method commonly adopted to evaluate catalytic performance of peptide-assisted Pt nanoparticles is electrochemical characterization. Higher electrochemically active surface area (ECSA) of Pt nanoparticles is associated with high catalytic performance in electrocatalytic reactions [10–12]. The multipod Pt nanocrystals directed by BP7A peptides were tested in an electrochemical cell and were found to have approximately 1.5 times higher ECSA for the oxygen reduction reaction compared to a Pt nanosphere control [12]. The higher ECSA can be explained by a higher facet index for reactive species adsorption on the multipod morphology [12]. More examples of the use of ECSA in electrocatalytic performance assessment are described in the subsection Biotemplates to structure Pt materials.
3.3. Pt electrode modification with Pt-binding peptides
As previously mentioned, peptide-assisted Pt nanocrystals serve in the development of high performance electrocatalysts. Pt-binding peptides can also be used on electrode surfaces in electrochemical devices such as fuel cells, batteries, and electrolyzers. Studies demonstrated modification of electrode surfaces with Pt-binding peptides, which gives control over the reactions that occur at electrode-electrolyte interfaces [5, 53]. Viguier et al immobilized a peptide NCGAITIG onto gold and Pt electrodes to develop peptide-based copper-ion detectors [56]. The presence of a cysteine residue gives the peptide affinity toward gold and Pt [56, 57]. In addition to anchoring to the metal electrode surfaces, the peptide can also complex the copper ions in the solution near the electrode surface, allowing the copper to be detected via changes in the current response of the electrodes [56]. Another example from our group utilized the P7A peptide sequence, discovered via phage display [10, 12], as a Pt-binding tag to anchor elastin-like polypeptides onto a Pt electrode [58]. Our results show that the cationic, elastin-like polypeptides are capable of controlling ionomer arrangement on the Pt electrode surface (figure 4) [58]. Owing to the relationship between ionomer structure and electrochemical cell performance, the additional control given by the Pt-binding tagged elastin-like polypeptide has excellent potential for the optimization of electrochemical devices for hydrogen energy.
Figure 4. Pt-binding tagged elastin-like polypeptide provides an improved mass uptake of Nafion® ionomer on Pt electrode surfaces [58]. This results in an innovative path to improve utilization of Pt catalyst in fuel cell/electrolyzer devices.
Download figure:
Standard image High-resolution image3.4. Biotemplates to structure Pt materials
Pt-binding peptides can also serve in shaping Pt nanomaterials as templates. A Pt ion-attracting peptide, HPGAH, was immobilized on nanotubes (made out of self-assembled bolaamphiphile peptide monomers) [29]. Using Raman microscopy, Yu et al found that in acidic conditions (pH 4), the carboxylate oxygen of histidine binds to Pt2+, while the imidazole nitrogen of histidine does not bind to Pt2+ [29]. In basic conditions (pH 10), the opposite occurred, which allowed for a more uniform coating of Pt on nanotubes compared to that created in acidic conditions [29]. In another work, P7A peptide was used to functionalize a template for synthesis of a 1D Pt nanostructure shown in figure 5 [10]. The P7A was immobilized onto Ac-IIIK-CONH2 (I3K) peptide that self-assembles into nanofibrils. Pt precursors (PtCl4 2−) were first bound to the I3K surface via electrostatic attraction, while P7A acted as a capping agent for the Pt that nucleated on the I3K surface [10]. The 1D Pt nanostructure was made in the presence of P7A with an unmodified C-terminal. Yu et al reported that the deprotonated C-terminal of P7A peptide provides electrostatic repulsion between its adjacent P7A molecules on the I3K template, resulting in 1D Pt nano assemblies that were shown to have a higher ECSA compared to that of a commercial Pt black catalyst [10]. The reason for the higher ECSA was discussed in the previous section on catalysis. Another aspect that affects ECSA is uniformity of the Pt dispersion. The uniform dispersion of peptide-assisted Pt nanocatalysts potentially provided better diffusion of the reactants to Pt active sites [53]. For example, Pt nanoparticles assembled on amyloid-beta peptide (KLVFF) were shown to have three times higher ECSA compared to polycrystalline Pt nanoparticles due to nanochannels created by highly uniform size distribution and interparticle space [59]. Nevertheless, the balance between loading and distribution of Pt nanomaterials on biotemplates should be further studied. In contrast to the previous example, another study on 1D discrete Pt nanoparticles assembled with net negatively charged BP7A peptides showed lower ECSA compared to the 1D continuous Pt nanoparticles assembled on I3K without BP7A. The larger interparticle space of the BP7A-assisted Pt nanoparticles on I3K seemed to control the loading of the Pt nanoparticles on the I3K. This resulted in a lower amount of Pt particles compared to that of the continuous 1D Pt nanoparticle assemblies. While the ECSA of both 1D discrete and 1D continuous Pt particle assemblies were higher compared to commercial Pt black [10], the lower ECSA of the 1D discrete assembly could be investigated further to study the balance of Pt loading on the I3K biotemplate and interparticle spacing.
Figure 5. TEM image of 1D Pt nanostructure synthesized from P7A peptides functionalized on I3K nanofibrils. Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature, Scientific Reports [10], © 2013, The Author(s).
Download figure:
Standard image High-resolution imageAnother example of biotemplated electrocatalysts is a β-hairpin peptide Ac-VKVKVHVHVDPPTHVHVKVKV-NH2 (CBHH) that was used to synthesize Pt nanorods having a higher ECSA compared to that of commercial carbon-supported Pt catalysts [11]. Isothermal titration calorimetry (ITC) was used to verify the spontaneous attraction between the CBHH peptide and the Pt (IV) ions in a continuously stirred solution [11]. Atomic force microscopy revealed the ability of Pt ions to trigger self-assembly of the CBHH peptide [11]. These studies prove that Pt-binding peptides are promising tools for future development of Pt electrocatalyst assemblies.
3.5. Pt carriers for cell-targeting
Pt nanoparticles have been used to develop anti-cancer targeted drug delivery systems [54, 60]. After the discovery of a Pt-binding peptide from a peptide library and conducting a structure-activity relationship study, Shoshan et al optimized the sequence to stabilize monodisperse Pt nanoparticles. The dispersion with the peptide was stable for more than a year [54]. The peptide, LPGLPGL, was also found to help internalize Pt nanoparticles into cancer cells. The stabilized Pt nanoparticles had high toxicity towards hepatic cancer cells but were significantly less toxic to other cancer cells and non-cancerous cells [54]. An additional glucose moiety was added to the first proline in the sequence and was found to enhance the cell internalization of the peptide-functionalized Pt nanoparticles [54]. Another study aimed to stabilize Pt nanoparticles took inspiration from a CALNN peptide sequence that was shown to stabilize gold nanoparticles. The research group synthesized HALNN and HALNNE6 and showed that the peptides facilitated synthesis of Pt nanoparticles with monodisperse sizes of approximately 2.8 nm and 3.3 nm, respectively [60]. However, the peptide HALNNE6 provided a better electrostatic stabilization for Pt nanoparticles compared to HALNN due to its hexaglutamic acid tag at the end of the sequence [60]. Moreover, addition of poly(ethylene glycol) and cell-binding amino acids (RGD) to the peptide sequence enhanced nanoparticle solubility, drug circulation, and the binding of the Pt nanoparticle drugs to cancer cells [60]. An illustration of the two-step synthesis of cell-targeted Pt carriers using Pt-binding peptides can be found in the literature [60]. These studies suggested that Pt-binding peptides are convenient additives to multifunctional materials for cell-targeting and drug delivery.
4. Opportunities for future studies
4.1. Creative strategies for Pt material synthesis with pH changes
As the studies [27, 29, 52] mentioned in this review suggested, the selectivity of the Pt-binding can be heavily influenced by the pH of the solution. Typically, pH decreases during Pt nanocrystal synthesis due to a release of protons from the oxidation of a reducing agent [52] such as NaBH4. Therefore, pH is another parameter worth taking into consideration when designing Pt materials with Pt-binding peptides. Recently, pH was demonstrated to have an impact on morphology when making Pt nanoparticles in the presence of glycine [55]. Care should be taken with pH change as the strong bonds of certain Pt-binding peptides can be reversed by pH modulation [53]. The fundamental understanding of Pt-binding peptides can be strengthened by incorporating adjustment of pH into both experimental and computational studies. One can take inspiration from nonpeptide-based studies that involve capping agents with the ability to switch the surface charge of Pt nanoparticles [19]. In doing so, one can appreciate the potential for peptides to add multifunctional capability based on their pH responsiveness.
4.2. Stability that will lead to the practical use of peptide-assisted Pt materials
Pt-binding peptides that act as sacrificial templates or tools to direct Pt-based nanofabrication may not need long-term stability. However, some applications such as Pt nanowires that need biotemplates to sustain such ultra-thin and long structures [10, 23] could gain more attention with long-term stability testing and improvement. In a few thorough studies, the stability of Pt nanomaterials assembled with the aid of Pt-binding peptides was quantified by the absence of observable agglomeration over a year [54] and accelerated electrochemical aging tests [59, 61]. In a related study, human hemoglobin was shown to facilitate Pt nanoclusters that served as a stable oxygen reduction reaction catalyst [62]. The study suggested that an interaction between the amino groups in hemoglobin and the carbon support prevented the disassociation of the Pt nanoclusters from the electrode, increasing stability. In the future, researchers could utilize this approach to generate highly stable Pt nanoclusters for fuel cells and related devices. Future studies could also can take inspiration from other non-peptide studies that utilize zeta-potential measurements to reveal net charge of the synthesized Pt nanomaterials [19]. Obtaining the net charge can help tune aggregation and could enhance stability of the peptide-assisted Pt nanomaterials.
4.3. Search for high selectivity Pt-binding peptides
Many of the reported Pt-binding peptides still lack robust testing for selectivity. Many amino acids in the peptide undoubtedly have inherent affinity for other metals [15, 27, 28]. Nevertheless, more efforts to study the facet specificity of the Pt-binding peptides especially at single amino acid level can help unveil the keys to the design of peptides with high selectivity toward Pt. Characterization on different metal surfaces and different oxidation levels of Pt and other metals will help elucidate the Pt-binding behavior and the selectivity. Finally, more peptide design rules [43] may be established to help tune behavior of Pt-binding peptides for different applications as multifunctional tools.
4.4. Utilizing Pt-binding peptides for sensor applications
A small number of publications feature Pt-binding peptides as tools for sensor development. For example, in one system the Pt-binding peptide CPTSTGQAC was conjugated with photosensitive azobenzene [22]. The conjugation with the peptide did not affect the light sensitivity of azobenzene. Since the azobenzene did not inhibit adsorption of the peptide to Pt, the authors suggest a strong potential for the light-responsive surface to be used for biosensor development [22]. Nevertheless, there is still room for future studies on the development of biomolecular sensors and Pt electrodes that utilize Pt-binding peptides. Such development may take inspiration from other biomolecules such as DNA. Taking DNA-functionalized micromotor work as an example, one study shows that Pt nanoparticles can be used in a DNA-enabled motion-based sensor [9]. Gold-coated PEDOT microtubes were functionalized with a monolayer of DNA probe 1, which interacts with DNA probe 2 via DNA hybridization. The DNA probe 2 was attached to Pt nanoparticles via a thiol functional group. Once the DNA probe 2 found DNA probe 1 in hydrogen peroxide solution, the Pt nanoparticles near the gold surface decomposed hydrogen peroxide, forming oxygen gas that drove the micromotor. Additionally, Pt-binding peptides could be conjugated to other affinity peptides to detect entities similar to the system developed by Viguier et al which used a biorecognition element attached to a Pt electrode to detect copper ions [56]. Another interesting option is to use Pt-binding peptides to conjugate redox probes to Pt surfaces, where current systems exist using cysteine as an anchor [63]. For Pt electrodes in electrochemical devices and sensors, more studies on surface-immobilized peptides are encouraged for an increase in performance and functionality of these devices.
5. Conclusion
This review highlights Pt-binding peptides as biomolecular tools that enable new Pt structures and functionalities, thus providing many opportunities for multifunctional material development. A combination of experimental and computational results has been pioneered and has demonstrated greater understanding of the binding mechanisms and surface recognition of Pt-binding peptides. In combination with simulation techniques, metal-peptide interactions can be elucidated at the atomic level. The discussion presented in this review reveals opportunities for future studies involving Pt-binding peptides, which include understanding the impact of pH, characterizing stability and selectivity, and exploring sensing applications. Overall, tunable biomolecules such as Pt-binding peptides can be excellent tools to direct the shape, size, and multifunctionality of Pt materials used in catalysis, cell-targeting, electrode modification, and beyond.
Acknowledgement
This work was partially supported by the United States Department of Agriculture (Award No. 2018-68011-28691).
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.





