Abstract
We present a one-step hydrothermal synthesis of hybrids consisting of nickel sulfides in the form of Ni3S4–NiS (NN) and Ni3S4–NiS-rGO (NNR), i.e. with the addition of reduced graphene oxide (rGO), for application as catalysts. After accurate physical characterization and confirmation of successful synthesis, we evaluate the ability of these catalysts in the processes of methanol and ethanol oxidation. The precise electrochemical analyses show relatively good potential and excellent cyclic stability in methanol oxidation reaction (MOR) and ethanol oxidation reaction (EOR) processes. The comparison of the two catalysts shows the superiority of NNR over NN, confirming that rGO introduces a higher specific surface area and a higher electrical conductivity in the NNR structure. In the process of MOR, NNR has an oxidation peak at a current density of 55 mA cm−2 and a peak potential of 0.54 V. In EOR, this peak is located at a current density of 11 mA cm−2 and at a peak potential of 0.59 V. NNR has 97% and 94% stability in MOR and EOR after 1000 consecutive cycles, respectively, which are acceptable values.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The huge fossil fuel consumption and the consequent increase of CO2 in the atmosphere [1] is concentrating the attention of researchers toward renewable energy sources for the conversion and production of energy [2]. Among the modern and advanced devices for energy production and storage, we can mention all types of fuel cells, supercapacitors, and types of electrochemical batteries. Among the most important components of these devices are the catalysts and electrode materials used in their structure. A fuel cell converts chemical energy into electrical energy [3], and consists of three parts; the anode, the cathode, and the polymer membrane that is placed between the anode and cathode and is responsible for proton exchange [4]. One of the concerns of catalyst science, especially in the field of designing and manufacturing high-efficiency catalysts for use in the anode and cathode of fuel cells, is the synthesis of affordable and sustainable materials for the industrialization of these modern and attractive devices. In addition to having high electrocatalytic activity, catalysts should also have a suitable electrochemical active surface area and acceptable electrical conductivity [5].
Direct alcohol fuel cells with high efficiency, low operating temperature, environmental friendliness, and green energy sources are promising candidates to replace fossil fuel sources [6]. Despite several positive features, the slow kinetics of the methanol oxidation reaction (MOR), ethanol oxidation reaction (EOR), oxygen evolution reaction (OER), and catalyst poisoning are barriers to the commercialization and industrialization of alcohol fuel cells [7–10]. So far, platinum and its compounds are the best catalysts for use in methanol and ethanol oxidation for application in the anode of alcohol fuel cells [11]. However, the high price and toxicity at moderate temperature do not allow Pt and its compounds to be used as easy and cost-effective materials [12, 13]. These drawbacks have led researchers to find alternative materials. Recently, it has been shown that transition metal oxides [14–16] and transition metal sulfides [17, 18] are promising for use in the anodic structure of catalysts due to their low cost and high cyclic stability performance in MOR and EOR [19, 20]. Specifically, nickel sulfide is a cost-effective catalyst [21] that has shown excellent performance in the processes of hydrogen evolution reaction [22], OER [23], supercapacitors [24], water oxidation [25], electrochemical sensors [26], and electrochemical batteries [27–29]. Despite these encouraging results, there are still few research efforts that have investigated nickel sulfide-based material in the MOR process.
The use of different types of carbon in the structure of catalysts, considering the abundance of this element in nature and also improving the electrocatalytic performance of catalysts, can be an attractive option for the synthesis of inexpensive, stable catalysts with excellent mechanical and electrochemical properties. Among the types of these carbon structures, we can mention reduced graphene oxide (rGO), multi-walled carbon nanotubes (MWCNTs), single-walled carbon nanotubes, carbon structures obtained from biomass, activated carbon, hollow carbon nanospheres, and recently graphdiyne [30, 31].
As the morphology, structure, conductivity, and specific surface area are the important and effective factors in the catalytic performance [32], in this study, we succeeded in synthesizing Ni3S4–NiS (NN) catalysts with starfish morphology using an easy and low-cost hydrothermal method. In the next step, we used rGO to produce Ni3S4–NiS-rGO (NNR) hybrids that provide a higher surface area. The rGO nanosheets, as a high-strength structure with a large surface area and high electrical conductivity, increase the catalyst stability in the alcohol process [33]. In this work, we show that both NN and NNR possess adequate catalytic performance for alcohol oxidation to be promising for use in the anode of alcohol fuel cells. It is noteworthy that in a previous study we investigated the potential of these catalysts as supercapacitor electrodes, demonstrating their great potential in electrochemical energy storage devices [34].
2. Experiment
2.1. Materials and instruments
All chemical precursors, such as deionized water, pristine graphite, potassium permanganate, hydrochloric acid, sulfuric acid, hydrogen peroxide, phosphoric acid, nickel nitrate hexahydrate (Ni(NO3)2.6H2O), polyvinylidene fluoride, N-methyl pyrrolidone, and thiourea (CH4N2S), were purchased with high purification from MERK Company. The crystalline structure of the prepared samples was analyzed using an x-ray diffraction (XRD, PHILIPS PW1730) model with monochromatized Cu-Kα radiation (λ = 1.541874 Å) at room temperature within the range of two thetas of 20°–70°. Raman spectra were analyzed with a micro Raman spectrometer at 532 nm wavelengths (Takram N1-541). The morphology and structure of the synthesized samples were characterized by field emission scanning electron microscope (Mira3 XMU from TESCAN Company), transmission electron microscope (TEM, JEOL 2010), and an energy-dispersive x-ray analyzer.
2.2. Synthesis and characterization of NN and NNR
The synthesis of NN was performed using the hydrothermal method. To prepare NN, 5.4 mmol of Ni(NO3)2.6H2O was dissolved in a 35 ml mix of ethanol and distilled water. Afterwards, 2.4 mmol of CH4N2S was added dropwise into the solution until the final color turned green and then stirred for 30 min. Simultaneously, a 1 × 1 cm2 piece of cleaned nickel foam (NF) was immersed in the prepared solution. Finally, suspensions were sealed into a Teflon-lined autoclave and maintained at 180 °C for 21 h. Subsequently, the autoclave was cooled to room temperature naturally. The resulting samples were washed several times with distilled water and finally with ethanol to remove the impurities and dried at 50 °C in a vacuum oven. In this research, four samples were synthesized, including NN (S1) (sample without rGO) and three samples of NNR with 0.03 (S2), 0.05 (S3), and 0.07 (S4) grams of GO added to the precursors.
The synthesis method of NNR with the optimal amount of GO was almost the same as that of NN, with the difference that we added 0.05 grams of GO to the precursors at the beginning of the synthesis [34]. It should be mentioned that the optimal sample had the best electrochemical results in the process of methanol and ethanol oxidation.
2.3. Electrochemical test on NN and NNR catalysts
A three-electrode system was used for electrochemical analysis. A platinum wire with a diameter of 0.5 mm was used as an auxiliary electrode and the Ag/AgCl electrode was our reference electrode. The modified NN and NNR on NF (1 × 1 cm) was our working electrode to investigate the capability of NN and NNR in the methanol and ethanol oxidation process that takes place in the anode of the fuel cell.
3. Results and discussion
The XRD patterns of NN and NN with an optimized amount of rGO (NNR) are shown in figure 1(a). According to the patterns, the significant peaks of NiS can be observed at 2θ equal to 18.44, 32.2, 35.7, 40.45, 48.84, and 57.41°, which matches with the (110), (300), (021), (211), (131), and (330) crystal planes. All the XRD peaks for the sample were indexed by the rhombohedral structure with the reported data (JCPDS No. 000120041). Also, for Ni3S4, we have significant peaks at 2θ equal to 26.6, 31.34, 38.03, and 54.87°, related to (220), (311), (400), and (440) indexed by the cubic phase with fd3m structure, consistent with the reported data (JCPDS No. 010761813). Using the Debye–Scherrer equation (D = 0.9λ/βcos θ), the average crystal size of NN was 68.6 nm. In addition, in the NNR XRD pattern, we almost see the characteristic peaks of NN. The addition of rGO reduced the intensity of the peaks, and a weak peak at around 26 degrees appeared that is attributed to the presence of rGO [34].
Figure 1. XRD patterns (a) and Raman analysis (b) of NN and NNR (Figure (b) is adapted from [34], copyright (2021), with permission from Elsevier). Reprinted from [34]. © 2021 Published by Elsevier Ltd.
Download figure:
Standard image High-resolution imageRaman analysis can reveal the structure of carbon-based materials and was employed to indicate the presence and quality of carbon in the structure of the catalyst. Figure 1(b) shows the Raman spectra of NN and NNR. The NNR spectra show significant peaks of graphene at 1300 and 1570 cm−1, which are related to the D and G bands of graphene. The D band refers to an asymmetric state called A1g that corresponds to phonons near the K region, and the G band is related to the spinel structure of the graphite plate, which appears as E2g in active Raman modes. Peaks also appeared at around 411, 313, 228, and 73 cm−1 in both samples due to NN [34].
The surface morphologies of all the synthesized samples (S1–S4), as well as an SEM image of the rGO nanosheets, are shown in figures 2(a)–(o), respectively. Three SEM images of each sample are shown in the figure. The SEM images of 2(a)–(c) are related to the NN sample, which has a uniform starfish-like morphology.
Figure 2. SEM images of NN (S1) (a)–(c), NNR (S2) (d)–(f), NNR (S3) (g)–(i), NNR (S4) (j)–(l), and rGO (m)–(o).
Download figure:
Standard image High-resolution imageThe SEM images of S2 are shown in figures 2d–f. Figures 2g–i and figures 2j–l are the SEM images of S3 and S4, respectively.
SEM images of graphene nanosheets are also shown in figures 2(m)–(o). The sheet and two-dimensional morphology of these materials are also clearly visible in the images.
EDS mapping analysis was performed to examine the presence of elements. Figure 3 clearly shows the presence of nickel, sulfur, and carbon in the synthesized nano-hybrids, and also confirms the almost uniform distribution of NN nanoparticles on the graphene surface [34].
Figure 3. EDS mapping analysis of NNR (adapted from [34], copyright (2021), with permission from Elsevier). Reprinted from [34]. © 2021 Published by Elsevier Ltd.
Download figure:
Standard image High-resolution imageTEM images of rGO, NN (at two scales), and NNR are shown in figure 4. Figure 4(a) shows the very transparent plate morphology of the rGO nanosheets at the scale of 100 nm. In figures 4(b) and (c), the NN with its special morphology is visible at the scale of 2 μm. Due to the large size of NN, its unique morphology is difficult to observe in the TEM image at a very low scale. Figure 4(d) shows a corner of the NN placed on the transparent rGO surface.
Figure 4. TEM images of rGO (a), NN (b), (c), and NNR (d) .
Download figure:
Standard image High-resolution image3.1. Electrochemical investigation on NN and NNR catalysts for MOR process
For this purpose, first, 1 M KOH solution was prepared, and cyclic voltammetry (CV) analysis of the modified electrodes was performed. As figure 5(a) shows, the addition of rGO to the NN structure increased the current density of the hybrid catalyst. rGO as a conductive material with a high active surface area has a positive effect on the electrochemical process. The CV analysis of the NN catalyst shows a redox peak that is due to the conversion of Ni+3
 Ni+2 [35]. We can see that adding rGO to the catalyst increases the peak intensity in the CV curve and improves the pseudocapacitive behavior of the catalyst in alkaline media.
Ni+2 [35]. We can see that adding rGO to the catalyst increases the peak intensity in the CV curve and improves the pseudocapacitive behavior of the catalyst in alkaline media.
Figure 5. CV curves of NN and NNR (a), and EIS plots of NN and NNR in 1 M KOH (b) .
Download figure:
Standard image High-resolution imageThe electrochemical properties of the catalysts were also investigated through electrochemical impedance spectroscopy (EIS) analysis. Measurements were made in the frequency range from 0.01 Hz to 100 kHz. Figure 5(b) compares the EIS spectra for NNR and NN. As can be seen, all the Nyquist plots of the EIS spectra consist of a rough semicircle in the high-frequency region followed by a linear component in the low-frequency region. The diameter of the semicircle indicates the surface charge transfer resistance (Rct) caused by Faraday reactions, which is lower in NNR than NN, at 12 and 16 ohms, respectively. According to the slope of the Warburg line, it can be concluded that the ion diffusion rate in NNR is higher than in NN, indicating higher electrochemically active sites in the catalyst.
According to figures 5(a) and (b), we can conclude that the NNR catalyst, having lower Rct and higher current density, is a better performing catalyst than NN. However, the potential of both catalysts in the oxidation process of methanol and ethanol alcohols was evaluated.
To investigate the behavior of NN and NNR catalysts in the methanol oxidation process, a solution containing 1 M KOH and 0.1 M methanol was prepared. According to figure 6, both NN and NNR catalysts have relatively good potential in the MOR process. The behavioral comparison of these two catalysts indicates the apparent superiority of NNR in the MOR process. This advantage can be inferred from comparing the overvoltage and the maximum current density oxidation peak of the two catalysts. The maximum current density for NN is 8 and for NNR it is 15 mA cm−2, and the overvoltage for these catalysts is 400 and 420 mV, respectively. In the following, we intend to obtain the optimal concentration of methanol and the optimal scan rate in the MOR process for the two catalysts. However, a solution containing 1 M KOH and different concentrations of methanol (0.1, 0.3, 0.5, 0.7, 0.9, and 1 molar of methanol) was prepared.
Figure 6. CV curves of NN and NNR in 1 M KOH and 0.1 M methanol.
Download figure:
Standard image High-resolution imageFigures 7(a) and (b) show the behavior of these two catalysts at different concentrations of methanol at a scan rate of 10 mV s−1. In the MOR process with NN, the current density of methanol oxidation increases in the concentration of 0.5 M methanol, and from this concentration onwards, the current density decreases. Also, the current density variation trend for NNR increases up to a concentration of 0.7 M, and from this concentration onwards it decreases. This behavior is probably due to blocking the penetration of methanol into the catalyst core and preventing the MOR process by-products [36]. It can be concluded that rGO increases the active surface area of NNR and simultaneously causes delays in the saturation of the NNR surface by methanol oxidation by-products.
Figure 7. CV curves of NN and NNR at different concentrations of methanol (a), (b) and different scan rates (c), (d).
Download figure:
Standard image High-resolution imageTo obtain the optimal scan rate, the CV analysis of NN and NNR catalysts was performed at different scan rates and given concentrations. According to figure 7(c), by increasing the scan rate from 10 to 60 mV s−1, the current density in the MOR process increases, but from 60 mV s−1 onwards, we see a decreasing trend in the current density. For NNR (figure 7(d)), we see an increase in the oxidation peak current density up to 80 mV s−1, followed by a decrease in the 100 and 120 mV s−1 range. It seems that at higher scan rates than the optimal scan rate, the electrolyte and methanol do not have sufficient contact with the catalyst and do not penetrate deep into the catalyst core. The optimal scan rate in the methanol oxidation process for NNR is 80 mV/s, which is higher than NN (60 mV/s). rGO provides a more active surface area in the face of the electrolyte and methanol by enhancing more active electrochemical sites in the catalyst.
In many studies, the mechanism of methanol oxidation has been mentioned as a six-electron mechanism, and based on this, the MOR mechanism in the research is proposed as follows [37]:
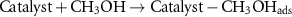
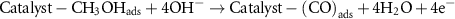
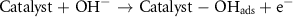

To investigate the effect of temperature on the MOR process, CV analysis was performed at the optimal concentration and optimal scan rate at different temperatures (ambient temperature of 50 °C). As shown in figures 8(a) and (b), the oxidation peak for both catalysts increases with the increasing temperature. Methanol oxidation and the absorption of OH− seem to be facilitated by the increasing temperature.
Figure 8. CV curves of NN and NNR at different temperatures (a), (b) and cyclic stability of NN and NNR in MOR process (c), (d) .
Download figure:
Standard image High-resolution imageThe stability of the catalysts in the MOR process was evaluated by performing 1000 CV scans at the optimal concentration and optimal scan rate. As shown in figure 8(c), NN has a good cyclic stability of 92%, and this stability for NNR is 97% (figure 8(d)); both stability values are relatively acceptable. rGO increases the electrical conductivity and provides a high surface area for NNR, thus facilitating the MOR process at NNR in comparison with NN. In addition, in order to check the stability of the NNR catalyst in the methanol oxidation process, chronoamperometry analysis was performed at peak potential. As shown in the inset of figure 8(d), the catalyst maintains about 70% of the initial current density after 2000 s, which is a relatively acceptable amount.
3.2. Electrochemical investigation of NN and NNR catalysts for the ethanol oxidation process
The ethanol oxidation capability of NN and NNR catalysts was evaluated by performing a CV test in an alkaline medium (figure 9). For this purpose, a solution consisting of 1 M KOH and 0.1 M ethanol was prepared. As shown in figure 8, the two catalysts are relatively capable of ethanol oxidation. Due to the stronger carbon bonds of ethanol than methanol, the oxidation of this alcohol by catalysts naturallyoccurs at higher overvoltages and lower current densities [38]. A comparison of NN and NNR behavior at different concentrations of ethanol was evaluated to obtain the optimal concentration by performing a CV test.
Figure 9. CV curves of NN and NNR in 1 M KOH and 0.1 M ethanol.
Download figure:
Standard image High-resolution imageAs shown in figures 10(a) and (b), the oxidation current density in NN and NNR catalysts increases to 0.3 and 0.5 methanol concentrations, respectively, and after these critical concentrations, we see a decrease in the peak current density with the saturation of the surface of the electrodes by the by-products of ethanol oxidation. In addition, the presence of rGO in the catalyst structure increases the surface area of the catalyst and delays the creation of contamination-affected by-products [39].
Figure 10. CV curves of NN and NNR at different concentrations of ethanol (a), (b) and different scan rates (c), (d) .
Download figure:
Standard image High-resolution imageA CV test was performed using the optimal concentrations of ethanol at different scan rates, to obtain the optimal scan rate in the process of ethanol oxidation by two catalysts.
Figures 10(c) and (d) show that by changing the scan rate to 40 and 60 mV s−1, the peak oxidation current density has an upward trend, and from this scan rate onwards, it was decreased in NN and NNR, respectively.
In general, at scan rates higher than the optimal scan rate, the electrolyte and alcohol do not have enough opportunity to contact and penetrate deep into the catalyst, so the peak current density will be decreased.
The mechanism of the ethanol oxidation process by catalysts can be presented as follows [40]:
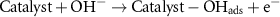



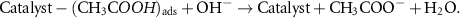
In the EOR process, we also see an increase in the current density with the increasing temperature. Figures 11(a) and (b) show that by increasing the temperature in the range of environments to 50 °C, the current density for both NN and NNR catalysts increases, and the ethanol oxidation process is facilitated by increasing the temperature.
Figure 11. CV curves of NN and NNR at different temperatures (a), (b) and cyclic stability of NN and NNR in the EOR process (c), (d) .
Download figure:
Standard image High-resolution imageThe stability measurements of the two catalysts were also investigated by performing 1000 consecutive CV cycles at the optimal scan rate and concentration for NN and NNR in the EOR process.
As figure 11(c) indicates, after this number of consecutive cycles, NN still has a relatively good stability of 90% and NNR shows a stability of 94% (figure 11(d)) due to the role of rGO in its structure as a superior component for the creation of higher electrical conductivity and active surface area than NN. The stability of the NNR catalyst in the EOR process was determined by chronoamperometry analysis at the oxidation peak potential. As shown in the inset of figure 11(d), the catalyst maintains about 65% of the initial current density after 2000 s.
Table 1 compares the performance of the NNR catalyst in the methanol and ethanol oxidation process with other research.
Table 1. Comparison of MOR and EOR performances for some reported electrocatalysts and the NNR in this work.
| Electrocatalyst | Electrolyte composition | Peak potential (V) | Current density (mA cm−2) | References |
|---|---|---|---|---|
| Ni3S4–NiS-rGO | 0.7 M MeOH/1 M KOH | 0.54 | 55 | This work |
| Ni3S4–NiS-rGO | 0.5 M EtOH/1 M KOH | 0.59 | 11 | This work |
| Ni-BTC/NiS2 | 1 M MeOH/0.1 M KOH | 0.7 | 34.36 | [41] |
| NiO/Ni–P | 1 M MeOH/0.5 M KOH | 0.5 | 28.56 | [42] |
| NiMoO4 | 2 M MeOH/1 M KOH | 0.45 | 49 | [43] |
| NiMoS2/MXene | 1 M MeOH/1 M KOH | 0.75 | 7.7 | [44] |
| NiCo2O4 | 0.5 M MeOH/1 M KOH | 0.7 | 129 | [45] |
| NF/Co3O4/NiCo2O4 | 0.5 M MeOH/1 M KOH | 0.6 | 140 | [46] |
| MoS2/Ni3S2/rGO | 1 M MeOH/1 M KOH | 0.4 | 80.04 | [47] |
| Co3O4-Ni3S4-rGO | 0.3 M MeOH/1 M KOH | 0.4 | 52.9 | [48] |
| Pd@NixB/ rGO | 1 M EtOH/1 M KOH | −0.31 | 19.7 | [49] |
| ZnO-MWCNT@Fe3O4 | 0.5 M EtOH/1 M KOH | 0.6 | 10 | [50] |
| MnO2–NiO–MWCNTs | 0.5 M EtOH/1 M KOH | 0.55 | 148 μA cm−2 | [51] |
| Mn3O4-CeO2-rGO | 0.8 M MeOH/1 M KOH | 0.51 | 17.7 | [52] |
4. Conclusion
In this study, we succeeded in synthesizing NN and NNR on the surface of NF using a one-step hydrothermal method. The electrochemical application of these catalysts with star-shaped morphology was performed through the alcohol oxidation process. The results show the relatively good ability of these catalysts in the oxidation of methanol and ethanol alcohols. NNR was introduced as a better and more effective catalyst than NN in the oxidation process of alcohols due to its higher active surface area and higher electrical conductivity. The presence of rGO in the metallic structure affected the surface of the NNR catalyst and created better stability in the oxidation of alcohols. The stability of NNR in MOR and EOR was measured as 97% and 94% respectively after 1000 consecutive cycles. The degree of stability in MOR and EOR was also evaluated by chronoamperometry analysis for NNR after 2000 s and at the oxidation peak potential. The amount of stability in the current density and in the methanol and ethanol oxidation processes was obtained as 70% and 65%, respectively. Based on the recent studies by researchers aimed at introducing inexpensive and stable catalysts for use in alcoholic fuel cells, NN and NNR are very promising for use in this field of study.
Conflict of interest
The authors declare that there is no conflict of interest.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.











