Abstract
Physical breast phantoms can be used to evaluate x-ray imaging systems such as mammography, digital breast tomosynthesis and dedicated breast computed tomography (bCT). These phantoms typically attempt to mimic x-ray attenuation properties of adipose and fibroglandular tissues within the breast. In order to use these phantoms for task-based objective assessment of image quality, relevant diagnostic features should be modeled within the phantom, such as mass lesions and/or microcalcifications. Evaluating imaging system performance in detecting microcalcifications is of particular interest due to its' clinical significance. Many previously-developed phantoms have used materials that model microcalcifications using unrealistic chemical composition, which do not accurately portray their desired x-ray attenuation and scatter properties. We report here on a new method for developing real microcalcification simulants that can be embedded in breast phantoms. This was achieved in several steps, including cross-linking hydroxyapatite and calcium oxalate powders with a binder called polyvinylpyrrolidone (PVP), and mechanical compression. The fabricated microcalcifications were evaluated by measuring their x-ray attenuation and scatter properties using x-ray spectroscopy and x-ray diffraction systems, respectively, and were demonstrated with x-ray mammography and bCT images. Results suggest that using these microcalcification models will make breast phantoms more realistic for use in evaluating task-based detection performance of the abovementioned breast imaging techniques, and bode well for extending their use to spectral imaging and x-ray coherent scatter computed tomography.
Export citation and abstract BibTeX RIS
1. Introduction
Approximately 50% of nonpalpable carcinomas in the breast are detected solely by the presence of microcalcifications on mammograms, and about 93% of women with ductal carcinoma in situ (DCIS) also develop microcalcifications. Breast calcifications are very common, appearing on about half of all mammograms taken for women ages 50 and older. Some studies have highlighted differences in the chemical composition between benign and malignant microcalcifications (Cheriyedath 2020). Based on their chemical composition, two major types of microcalcifications are found in breast tissue. Type I calcifications consist of calcium oxalate crystals, mainly calcium oxalate dihydrate (weddellite); whereas type II deposits are composed of calcium phosphates, mainly calcium hydroxyapatite (hydroxylapatite) (HA) (Morgan et al 2005, Radi 1989, Frappart et al 1986). Full-field digital mammography (FFDM), and digital breast tomosynthesis (DBT) are proven modalities for microcalcification detection. In addition, new breast imaging applications such as spectral mammography, phase contrast and x-ray coherent scatter imaging also offer great promise for increasing diagnostic performance through more in-depth analysis of microcalcifications. (Wang et al 2014, Ghammraoui and Glick 2017, Martini et al 2017) These emerging breast imaging modalities are capable not only of detecting calcifications, but also of classifying them non-invasively based on their chemical compositions and molecular structures.
Commonly-used breast phantoms contain simulated microcalcifications made from aluminum (Warren et al 2013) and gold (Perry et al 2013). Such simulated microcalcifications lack the x-ray scatter and absorption properties of microcalcifications in the breast and may only approximate their contrast in a mammography image. More realistic calcium-based simulated microcalcifications made from calcium carbonate (Cho et al 2015, Petropoulos et al 2020), calcium phosphate (hydroxyapatite) (Calderón-García et al 2017, Martini et al 2020, Ikejimba et al 2019), crushed eggshells (Kiarashi et al 2015) and calcium oxalate (Warren et al 2013) also were used to simulate calcifications. Warren et al (2013) indicated that special attention should be given to the thicknesses and densities of these stimulants to avoid any excessive or insufficient contrast. For example, using the hydroxyapatite crystals in their natural forms (ρ = 3.08 g cm−3) at the same size of real breast microcalcifications would lead to excessive contrast in the mammographic image.
Therefore, to optimize and evaluate these imaging modalities, there is a need for breast phantoms with realistic microcalcifications with adjustable densities and sizes. In this paper, we report on the development of a technique for producing microcalcification models using materials that mimic real calcifications in their chemical compositions and molecular structures. We describe their fabrication and show results demonstrating their x-ray attenuation and scatter properties.
2. Experimental methods
2.1. Preparation of microcalcifications materials
Commercially available hydroxyapatite (HA) and calcium oxalate (CO) monohydrate powders, purchased from Sigma-Aldrich (St. Louis, MO, USA) and Millipore Sigma (Burlington, MA, USA), respectively, were used in this study. Due to availability, the monohydrate solvate of calcium oxalate was used instead of the dihydrate solvate that naturally formed in breast tissue. It is noteworthy that both solvate forms of calcium oxalate have similar chemical compositions and x-ray attenuation properties, described in more details in subsequent sections. The kneading, solvent evaporation followed by powder compaction techniques were used to prepare the phantom materials (figure 1). In this technique, the powder mixture of both materials was dispersed in a kneading agent and processed into a paste. Low molecular weight Polyvinyl pyrrolidone solution was used as a granulating agent to collect the particles of both materials and to create bonds between them. This kneading process were critical to ensure that homogeneous distribution of both materials was achieved in their mixture and to avoid powder segregation during the compaction process. The kneaded mixture was then dried in vacuum oven to remove the solvent and to form dried granules. The solvent evaporation process that occurred during the drying step was critical to control the extent of densification of the granulates by the compaction step. In compaction, a closer packing of the granules occurred as a result of rearrangement as the main mechanism for initial volume reduction and powder densification. As the force of compaction was further increased, bonds between the granules were formed to form more dense disks. It is worth noting that the parameters for the kneading and compaction steps are the most critical for increasing the mechanical strength and density of the phantoms, when subjected to rising compaction forces.
Figure 1. Schematic presentation of the method used in preparation of the phantom materials.
Download figure:
Standard image High-resolution imageThe abovementioned technique can be summarized in the following working procedure. A specified amount of either hydroxyapatite or calcium oxalate was mixed with 1% weight per weight (w/w) polyvinyl pyrrolidine (PVP) K30 in a dip mixer (W.A. Bachofen, Basel, Switzerland) for approximately 10 to 15 minutes at 50 rpm. The mixture then was granulated at 1:0.2 powder to binder solution weight ratio with 3% weight per volume (w/v) PVP alcoholic solution. The paste obtained was then dried under vacuum at 30 °C for more than 24 hours. The dried granules then were compressed into 8 mm diameter disks using a rotary tableting machine (PICCOLA, Riva S.A., Buenos Aires, Argentina) at a rotation speed of 20 rpm. The weights and thicknesses of the disks can be changed based on the desired densities. For example, using the 20 kN target compression load, disks of hydroxyapatite and calcium oxalate with densities of 1.85 and 1.7 g/cm3 were obtained, respectively. Finally and as explained in a previous study (Ikejimba et al 2019), the compressed disks were crushed using a mortar and pestle and then sieved to separate them into the desired size ranges. The created specks are then ready to be embedded in any phantom for studying.
2.2. Material characterization
2.2.1. x-ray attenuation measurements
For each microcalcification type, we created disks with two different densities. For CO disks, the densities were 1.7 and 1.8 g cm−3. For the HA disks, the densities were 1.85 and 2.1 g cm−3. Figure 2 shows the experimental setup for spectroscopic measurements with disks 0.8 mm thick used to measure the samples' linear x-ray attenuation coefficients across energy. The operating voltage the tungsten x-ray tube was 50 kVp with a load of 2 mA, and the beam was collimated with a 0.7 mm lead aperture (Dahal et al 2018). A single pixel spectroscopic CdTe detector (Amptek, Inc. Bedford, MA USA) size of 3 × 3 × 1 mm3 was used. The energy calibration of the detector was performed using the 109Cd and 57Co radionuclide sources. The raw measured spectra were first corrected for spectral distortion using an inverse problem approach (Dahal et al 2018). Linear coefficients of attenuation μ were estimated using the following equation:
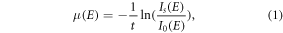
where Is and I0 indicate the recorded spectra with and without the sample present, respectively, and t is the thickness of the sample. Readings with and without were taken for 600 seconds each. The measured μ(E) were compared with reference values obtained from the National Institute of Standards and Technology (NIST) XCOM online physics database (BBerger et al 2010).
Figure 2. Diagram of the experimental setup in this study.
Download figure:
Standard image High-resolution image2.2.2. x-ray diffraction analysis
The processed powder samples (before compression) were analyzed using x-ray diffraction (XRD); 1.5 g of each specimen was placed in the sample holder and packed with a sterile glass slide to make an even and uniform surface. The measured scattering angles (θ) ranged from 3 to  with angular speed of
with angular speed of  per minute. Output spectra from the XRD machine represented the scatter intensity versus the scattering angle. For a better illustration, the scattering angle values
per minute. Output spectra from the XRD machine represented the scatter intensity versus the scattering angle. For a better illustration, the scattering angle values  were converted to momentum transfer using equation (2).
were converted to momentum transfer using equation (2).
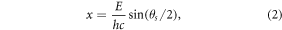
where E is the photon energy and hc is the product of Planck's constant and the speed of light in a vacuum. Finally, the peaks of each sample's diffraction profile were matched with those of the standard data in the powder diffraction files obtained from the RUFF database(rruff.info) (Downs 2006).
2.2.3. Uniformity and reproducibility studies
The SCANCO Medical MicroCT-100, a high-resolution micro-CT with a tungsten at 90 kVp, 88 μA, was used to evaluate the uniformity and reproducibility of the prepared disk. Three samples were independently prepared for each microcalcification type and density. All reconstructed slices were generated from 250 projections collected under 304 ms exposure time per projection. Cross-sectional images were reconstructed at various depths of each tube at a voxel size of 73.6 μm. For each calcification type and density, a cylindrical region of interest (ROI) was defined in the center of each disk to eliminate the edge artifacts. The mean pixel value and the standard deviation for each ROI were calculated per sample. Next, the global mean was calculated for all three disks, with error bars representing the standard deviation among the disks.
2.3. Imaging
The fabricated microcalcification specks were arranged into clusters and embedded into breast phantoms for mammography and CT imaging. Figures 3(a) and (b) show custom templates made with HA and CO microcalcification specks grouped together to form an array of clusters. Fabricated microcalcifications were carefully placed onto sticky tape and covered with parchment paper. Fiducial markers were added to the edges of each template for ease in extracting ROIs that contain microcalcification clusters. A template containing an array of clusters was embedded in the center of two 3D-printed anthropomorphic breast phantoms developed using inkjet printing with doped ink printed onto specific paper for imaging (Ikejimba et al 2019). In addition, images also were collected for the same microcalcification templates embedded in the BR3D background phantom (4cm thick) with a swirl pattern. The phantom and template inserts were imaged using a clinical FFDM machine (Selenia Dimensions, Hologic, Bedford, MA) system with 700 mm SID and a direct a-Se detector with 70-μm pixel pitch. The exposure technique used was 31 kVp (W/Al) and 80 mAs.
Figure 3. Phantoms used in this study.
Download figure:
Standard image High-resolution imageFor CT imaging, the clusters were embedded in the middle of a cylindrical uniform gelatin-based breast phantom (figure 3(c)) (Dahal et al 2018). In this case, the phantom and inserts were imaged using the MARS spectral CT scanner with CdTe Medipix2-MXR detector (MARS Bioimaging Ltd, (MBI) Christchurch, New Zealand). A total of 720 projections over 360° were acquired were acquired with enabled charge summing mode at the detector and using four low energy thresholds (15, 20, 25, 30 keV). The x-ray tube was operated in continuous mode at 50 kVp with a load of 120 μA.
3. Results and discussions
Figures 4(a) and (b) show the measured linear coefficients of attenuation for hydroxyapatite and calcium oxalate monohydrate at two different densities. These graphs show good agreement between the experimental measurements and the theoretical data obtained from the NIST XCOM database; and most importantly, good agreement between the calcium oxalate monohydrate used in model fabrication and the calcium oxalate dihydrate that naturally occurs in the breast. Thus it is concluded that calcium oxalate monohydrate powder has similar attenuation properties as the calcium oxalate dihydrate; and can therefore can be used for studying and evaluating spectral imaging modalities. Figure 5 shows the measured calcium oxalate monohydrate spectra peaks as compared with the diffraction peaks of the weddellite obtained from the RRUFF database. The chemical composition of CO-monohydrate and CO-dihydrate are somewhat similar, but noticeable differences in their XRD spectra were observed. Figure 5(b) indicates that both materials show a sharp major peak intensity near the momentum transfer value of x = 0.8 nm−1. Figure 5(a) shows a sharp major peak in the diffraction profile of hydroxyapatite for a momentum transfer value of x = 1.8 nm−1, whereas the diffraction profiles of CO-dihydrate have two major peaks at x = 0.8 nm−1 and x = 1.8 nm−1. Therefore, the spectral peak at x = 0.8 nm−1 that is present in both CO-monohydrate and CO-dihydrate is probably the most important parameter to use for differentiation of hydroxyapatite and CO-dihydrate typically found within the breast. Because of this observation, we conclude that CO-monohydrate may be used as a substitute for CO-dihydrate (Wd) when studying coherent scatter properties of the CO-dihydrate calcifications typically found in the breast.
Figure 4. Solid lines: Measured linear coefficients of attenuation for hydroxyapatite and calcium oxalate at two different densities (left: HA at 1.85 g cm−3 and CO at 1.7 g cm−3, right: HA at 2.1 g cm−3 and CO at 1.8 g cm−3). Dotted lines: linear coefficients of attenuation for hydroxyapatite and calcium oxalate obtained theoretically from their chemical structure by using the NIST XCOM database.
Download figure:
Standard image High-resolution imageFigure 5. (a) Measured XRD spectra for hydroxyapatite and calcium oxalate monohydrate. The measured spectra present the theoretical peaks provided by the RRUFF crystallographic database. (b) Measured XRD spectra for calcium oxalate monohydrate compared to the theoretical peaks of weddellite. Both materials show a sharp major peak intensity near the momentum transfer value of x = 0.8 nm−1.
Download figure:
Standard image High-resolution imageMeasurements of the uniformity for a given disk were determined by calculating the relative deviation among voxels within a 3D ROI inside the CT image of the disk (figure 6(a)). Results showed a maximum of 8% standard deviation over all the voxels within the disk CT reconstruction (figure 6(b)). In addition, the standard deviation shown between the mean of the samples of each type and density in figure 6(c) was always less than 1% and values were very similar from one sample to another independently prepared in triplicates. These results confirm that the method is reproducible and that prepared specks from one disk will have the same density after being crushed.
Figure 6. Micro-CT images showing the calcification disks, which were prepared to be uniform and reproducible. Representative cross-section images of HA and CO disks are shown at two different densities. Three samples for each category were prepared independently for comparison. Cross-section images covering all sections of the tube were analyzed to report the average values and their corresponding standard deviations.
Download figure:
Standard image High-resolution imageImages of the different breast phantoms (figure 3) containing the fabricated microcalcifications are provided in figure 7 and 8. Figure 7(a) is an FFDM image acquired from the 6 cm anthropomorphic breast phantom with an embedded template consisting of an array of clusters each containing microcalcifications of size 150–180 μm as shown in figure 3(a). Figures 7(b) and (c) show the images collected with the clusters of the random arrangement phantom of the 3D-printed anthropomorphic breast phantom and the BR3D background phantom with swirl pattern. These examples demonstrated how fabricated microcalcification clusters can be introduced into the phantom. The aquired images of the inserted clusters showed varying visibility depending on their location in the high, medium, and low glandular density regions. This template and similar ones have already been used in several studies for assessing task performance in detecting clustered microcalcifications (Ghammraoui et al 2021). An FFDM image of the same phantom, but with the larger embedded specks 750–1000μm shown in figure 3(b), is shown in figure 7(d). Here, the two types of microcalcification clusters (HA and CO) are imaged with the breast phantom. Figure 8 shows a reconstructed CT slice of the phantom shown in figure 3(c), where two types of microcalcification were inserted. Phantoms imaged with the two types of microcalcifications presented in this study are now being used for assessing task performance of microcalcification classification in several ongoing studies (Ghammraoui and Glick 2017, Ghammraoui et al 2021).
Figure 7. (a) FFDM images of breast phantoms with the embedded microcalcification specks shown in figure 3(a). (b) FFDM image of the same phantom, but with the larger embedded specks 750–1000 μm shown in figure 3(b). (c) FFDM image of another 3D-printed anthropomorphic breast phantom with the embedded microcalcification specks of random locations. (d) FFDM image of the BR3D CIRS background phantom with swirl pattern with the embedded microcalcification specks shown in figure 3(a). At left we show small ROIs with improved brightness/contrast, window/level for better visualization.
Download figure:
Standard image High-resolution imageFigure 8. CT image showing the gelatin-based breast phantom with microcalcifcations embedded.
Download figure:
Standard image High-resolution imageThis approach for fabricating microcalcification specks could be used for insertion into other breast phantoms as well. The proposed method is flexible and can be extended to preparing microcalcifications for other imaging modalities, including ultrasound, by investigating other binders or dopants with the powder to adjust their mechanical and acoustic properties.
4. Conclusion
We presented a method for modeling microcalcifications in a breast phantom for x-ray imaging with realistic chemical composition and x-ray attenuation and scatter properties. By adding binders, we turned commercially available hydroxyapatite and calcium oxalate powders into compressible powders. Different compression strengths were used to create solid pellet calcifications of a desired density, which were crushed into small microcalcifications. We evaluated the fabricated microcalcifications by measuring their x-ray attenuation and scatter properties using x-ray spectroscopy and x-ray diffraction systems, respectively. Furthermore, we used micro-CT to demonstrate the uniformity and reproducibility of the formulated tablets. X-ray mammography and CT images also were shown for demonstration. Results suggest that the use of these developed microcalcifications makes the breast phantoms attractive for evaluation of breast imaging techniques such as mammography, digital breast tomosynthesis and CT, and for extending their use to spectral mammography and x-ray coherent scatter mammography.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).










