Abstract
Preclinical imaging and irradiation yields valuable insights into clinically relevant research topics. While complementary imaging methods such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) can be combined within single devices, this is technically demanding and cost-intensive. Similarly, bedding and setup solutions are often specific to certain devices and research questions. We present a bedding platform for mice that is compatible with various preclinical imaging modalities (combined PET/MRI, cone beam CT) and irradiation with photons and protons. It consists of a 3D-printed bedding unit (acrylonitrile butadiene styrene, ABS) holding the animal and features an inhalation anesthesia mask, jaw fixation, ear pins, and immobilization for the hind leg. It can be embedded on mounting adaptors for multi-modal imaging and into a transport box (polymethyl methacrylate, PMMA) for experiments outside dedicated animal facilities while maintaining the animal's hygiene status. A vital support unit provides heating, inhalation anesthesia, and a respiration monitor. We dosimetrically evaluated used materials in order to assess their interaction with incident irradiation. Proof-of-concept multi-modal imaging protocols were used on phantoms and mice. The measured attenuation of the bedding unit for 40/60/80/200 kV X-rays was less than 3%. The measured stopping-power-ratio of ABS was 0.951, the combined water-equivalent thickness of bedding unit and transport box was 4.2 mm for proton energies of 150 MeV and 200 MeV. Proof-of-concept imaging showed no loss of image quality. Imaging data of individual mice from different imaging modalities could be aligned rigidly. The presented bed aims to provide a platform for experiments related to both multi-modal imaging and irradiation, thus offering the possibility for image-guided irradiation which relies on precise imaging and positioning. The usage as a self-contained, stand-alone unit outside dedicated animal facilities represents an advantage over setups designed for specific devices.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In recent years, data from preclinical imaging studies have proven to be of high translational value for a number of clinically relevant topics. Especially in the context of radiation oncology, in vivo imaging studies contribute to improved detection of different tumor entities and characteristics [1, 2] as well as monitoring of treatment outcome [3, 4]. In vivo preclinical imaging studies have also demonstrated their value in the translation of clinically relevant endpoints [5, 6]. Current areas of research include the determination of parameters with relevance for application in radiotherapy, such as tumor hypoxia [7, 8], or the impact of different types of radiation (e.g. photons or protons) on tissue [9, 10]. The usage of different medical imaging methods allows obtaining complementary datasets, the most common imaging modalities being X-ray-based (cone beam) computed tomography (CBCT), magnetic resonance imaging (MRI), and positron emission tomography (PET).
The application of multi-modality imaging in preclinical experiments can be performed with integrated devices or spatially separated dedicated devices. While integrated devices (e.g. PET/CT or PET/MRI) provide advantages such as simultaneous acquisition of PET and MRI images [11], they are costly and superimpose technical challenges on the used hardware [12, 13]. On the other hand, the use of spatially separated devices demand for the repositioning of the animal for each scan. This imposes a key limitation on multi-modality imaging studies: Image data cannot be overlaid without elaborated co-registration methods, which hinders accurate evaluation of the sometimes submillimeter-sized substructure of the object of interest.
In this manuscript, we report on the development of a bedding system for mice, which is usable with different imaging and irradiation devices, thus eliminating the need for animal repositioning. It provides self-contained utilities for transportation of laboratory animals to experimental facilities outside designated animal housing facilities—a property, which is of special importance for animal models with a defined hygiene status. The bedding unit can readily be mounted in the tested imaging systems; adaptor elements for further devices can easily be fabricated with 3D-printers. We designed the platform to be compatible with multi-modal irradiation and measured its physical characteristics with respect to irradiation with photons and protons. The platform's usability for multi-modal imaging was demonstrated in our facility's open-source small animal image-guided radiation therapy (SAIGRT) system [14] as well as in a combined nanoScan® PET/MRI scanner (1 T, Mediso medical imaging Systems, Hungary, Budapest) for small animals.
2. Materials & methods
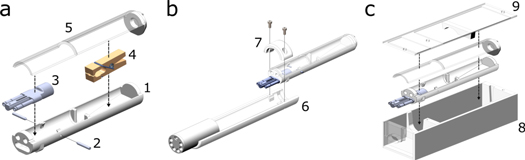
The devised platform consists of three main components as shown conceptually in figure 1: (a) The central bedding unit for holding the animal. It includes a breathing mask for inhalation anesthesia, a moveable jaw fixation ('bite block'), ear pins for cranial fixation, and an optional fixation clamp that locks the ankle joint in place for irradiation of subcutaneous tumors on the hind leg. The clamp can be fixed inside the bed via hook-and-loop tape. A gate-like structure in the middle of the bed restricts the animal's abdominal region from movement to prevent the irradiation of off-target areas. (b) Adaptor elements to mount the bedding unit onto various imaging devices. The adaptor elements ensure compatibility with the tested devices so that existing infrastructure (e.g. heating, anesthesia) may directly be rerouted into the bedding unit. (c) The transport box containing the central bedding for usage outside pathogen-free facilities, which can be connected to the auxiliary vital monitor.
Figure 1. (a) Concept drawing of central bedding unit featuring the bedding unit (1), ear pins (2), inhalation anesthesia mask (3), hind leg immobilization clamp (4), and the bed's cover (5). (b) Bedding unit mounted on adaptor element (6) held in place by a screw-on cap (7) for small animal nanoScan PET/MRI. (c) Transport box (8) with top piece (9) tightly holding the bedding unit in place for transit.
Download figure:
Standard image High-resolution imageThe design of our system's components was subjected to a set of specifications to ensure compatibility with the envisioned irradiation (photons/protons) and imaging (CBCT/PET/MRI) modalities: Objects in the beam paths were designed as thin as possible; cavities were avoided to reduce scattering and maintain beam quality. The materials had to be of low atomic number to reduce absorption of X-rays and proton scattering while ensuring mechanical stability. We used polymethyl methacrylate (PMMA) slabs for the transport box and 3D-printed acrylonitrile butadiene styrene (ABS) for the bedding unit and the adaptor elements. The walls of the bedding unit and the transport box consist of 1.6 mm ABS and 2.3 mm PMMA, respectively. We calculated the spectrum-weighted effective atomic number Zeff of ABS and PMMA for typical X-ray potentials of 40 kV, 60 kV, 80 kV and 200 kV [14] with the Auto-Zeff software [15] to validate their suitability.
High-Z and conductive materials were avoided to ensure compatibility with MRI, and to prevent imaging artifacts for PET, MRI, and CBCT. Electronic elements for environmental and vital monitoring were not used inside the bedding unit due to possible radiofrequency (RF) interference with the MRI. Dimensions of the bedding unit (33 mm outer diameter, 180 mm length) allow integration into various small animal imaging and irradiation devices (i.e. SAIGRT rat bed and MRI body coil).
2.1. Irradiation
To assess the bedding unit's characteristics for photon irradiation, beam attenuation was measured with a Semiflex ionization chamber type 31010 (PTW Freiburg, Freiburg, Germany) at an X-ray tube type Y.TU 320-D03 (Yxlon International GmbH, Hamburg, Germany) with an inherent filtration of 3 mm Al, 3 mm Be, and 0.5 mm Cu. The attenuation for 200 kV X-ray irradiation was calculated as the ratio of measured absolute dose with and without the bedding unit's cover in the beam path. The dose was administered for 60 s with a dose rate of approximately 1 Gy/min and measured with a Unidos dosemeter (PTW).
For proton irradiation, the stopping power ratio (SPR) describes a material's stopping power normalized to water. Multiplication with an object's physical thickness yields its water-equivalent thickness (WET). The SPR of PMMA was previously reported by Beyreuther et al [16]. We measured the SPR of ABS with a Giraffe multilayer ionization chamber detector (IBA Dosimetry, Schwarzenbruck, Germany) at proton energies of 150 MeV and 200 MeV as well as with 3D-printed samples of different thicknesses and printing parameters. We used massive ABS samples of 3.1 mm and 6.25 mm thickness to determine the accurate SPR of ABS. For this, the range of the beam was measured with and without the ABS samples placed in front of the Giraffe detector. A Bragg-curve model [17] was fitted to the depth-dose distribution to calculate the location of the distal 80% (R80) dose fall-off. SPR was calculated as the ratio of the measured range shift in R80 and the thickness of the material traversed. Due to inherently low SPR variation in the relevant energy range, the result is averaged over both proton beam energies and sample thicknesses.
We repeated the measurement for ABS samples of 1.6 mm thickness and different 3D printing parameters (layer height of 0.127 mm, 0.178 mm and 0.254 mm and infill values of 62% and 100%) to assess the impact of material and print parameters for practical irradiation experiments.
2.2. Mounting
The adaptor element to fit the bedding unit into the rat bed of the SAIGRT was designed to ensure spatially reproducible mounting. For testing the reproducibility, five consecutive pairs of orthogonal radiographs were taken whereas the bedding was removed and repositioned inside the adaptor element between each scan. The spatial translation of the bedding unit was measured through an image cross-section perpendicular to the bedding unit's walls in the X-ray radiographs. The positional shift induced by the repositioning of the bed manifests itself as a measurable lateral shift of the cross-section around the bed's edges. The overall shift was calculated as the standard deviation of the position of a point with identical image intensity in the cross-section. This procedure was repeated along every spatial dimension.
Due to the lack of a detectable external position reference on the bedding unit for MRI and PET imaging, this procedure could not be conducted accordingly for the nanoScan PET/MRI. However, since all adaptor elements are subjected to the same design and manufacturing precision, we assume the reproducibility of the MRI's mounting system to be of similar quality as measured for the CBCT's mounting.
The design of the mounting adaptor for the PET/MRI (figure 1(b)) allows for easy attachment of the device's existing infrastructure (breath monitor, heating and anesthesia).
2.3. Imaging
To quantify the interaction between the bedding unit and X-ray irradiation at typical CBCT imaging potentials, we measured the beam attenuation induced by the bedding unit's walls at potentials of 40 kV, 60 kV and 80 kV. We tested image resolution at 40 kV with a PMMA-made phantom (QRM GmbH, Möhrendorf, Germany) containing hole patterns of six different diameters (0.3–1.0 mm). The scan was repeated with and without the bedding unit. Image noise levels were tested by measuring the standard deviations of CBCT image intensity within a ROI inside a water-filled 3 ml plastic syringe. Lastly, the impact of possible beam-hardening due to the bed's presence was evaluated by comparing the CT numbers of materials inside or in the absence of the bed. This was tested with small cuboids of tissue equivalents (e.g. adipose, muscle and bone from Gammex-RMI GmbH, Gießen, Germany) as well as liquid water in a 3 ml plastic syringe and surrounding air with 40 kV, 60 kV and 80 kV X-rays. The increase of the noise level due the bed's presence was measured as the standard deviations inside the liquid water.
We determined the spatial relationship between each modality's frame of reference with a multi-modal imaging protocol using a 5 ml syringe filled with 17.6 MBq [18F] fluorodeoxyglucose (FDG) dissolved in water. The syringe was scanned with a T1-weighted sequence (spoiled 3D gradient echo (GRE), TR = 25 ms, TE = 3.36 ms, in-plane resolution 256 × 256, field of view (FOV) = 60 mm, slice thickness = 0.5 mm, Naverages = 4), and a static [18F] FDG PET scan with a duration of 10 min. For CBCT, a medium dose scan protocol [14] with 40 kV potential was chosen. The spatial shifts between the respective frames of reference were determined manually with the μ-RayStation 5 treatment planning software (RaySearch Laboratories, Stockholm, Sweden). The translational shifts between the coordinate systems of different imaging modalities were corrected with Python (Python Software Foundation, www.anaconda.org, version 3.6) by adjusting the image metadata (frame of reference coordinate origin), accordingly. The coordinate shift was visually verified with the medical imaging interaction toolkit (MITK 4.13, www.mitk.org). To quantify the variance of the user-based registration procedure, the image alignment was carried out by three observers. The standard deviation of the determined translations denoted the procedure's variance.
The measured shifts were then validated by applying multi-modal imaging protocols to one deceased and one living tumor-bearing NMRI (nu/nu) mouse (head and neck squamous cell carcinoma xenograft model SAS). The dead animal was scanned with a medium dose 40 kV CBCT protocol and a fast T1-weighted multi-FOV 3D GRE overview sequence (TR = 5.8 ms, TE = 1.9 ms, in-plane resolution = 192 × 192, FOV = 60 mm, slice thickness = 0.5 mm, Naverages = 4). The multi-modal protocol for the tumor-bearing animal consisted of the 40 kV CBCT scan, a T1-weighted sequence (spoiled GRE, TR = 25 ms, TE = 3.36 ms, in-plane resolution 256 × 256, field of view (FOV) = 60 mm, slice thickness = 0.5 mm, Naverages = 4) with 80 μl Magnevist® (Bayer Vital GmbH, Leverkusen, Germany) contrast agent (CA) injected intravenously (i.v.) 5 min prior to scan and a 30 min static [18F] fluoromisonidazole (FMISO) PET scan (i.v. injection of 18.9 MBq 3.5 h prior to acquisition). We chose the high-resolution T1w sequence for the tumor region over the faster T1w sequence used for the overview of the dead mouse. Tumor contours were delineated based on the CA-enhanced T1-weighted MRI dataset and then projected onto the other image modalities. The animal facility and the experiments were approved according to the European Parliament and Council (EU Directive 2010/63/EU) on the protection of animals used for scientific purposes, the German animal welfare regulations, and to the local ethics committee (approval 24.1-5131/449/52, Dresden, Germany).
2.4. Auxiliary components
To access experimental facilities outside the animal facility (i.e. University Proton Therapy Dresden (UPTD)), the bedding unit can be inserted into the transport box (figure 1(c)) to maintain the defined hygiene status of animals. The transport box can be attached to a vital support unit on a trolley. A control unit was designed which regulates the supply with warm air into the transport box and allows the monitoring of the animal's respiration with a breathing sensor. It is based on a Python 2.7 program running on an open-source single-board Raspberry Pi computer (Raspberry Pi Foundation, Cambridge, UK, www.raspberrypi.org). The sensor function was verified and recorded during a time period of 20 s during the wake-up phase of an exemplary animal after inhalation anesthesia. The air-based warming has a heat supply of up to 39 W. The necessary air intake from the surrounding is cleaned with a HEPA-grade filter, heated in a separate chamber and propelled towards the transport box via an 80 cm long tube. The temperature is measured with a DS1820 1-wire digital thermometer (accuracy ± 0.5 °C). To verify stable animal body temperature during anesthesia with air-based heating, we used an Optocon FOTEMP-1 (Weidmann Technologies Germany GmbH, Dresden, Germany) fiber optic rectal probe to measure the core temperature of a mouse under inhalation anesthesia (2% isoflurane, 98% oxygen) for a duration of 60 min.
Depending on the scenario of usage, inhalation anesthesia may be attached to the optional breathing mask that can be inserted into the bed. The vital monitor consists of an air-filled pad (Graseby respiration sensor, Medicare Health & Living, Dublin, Ireland), which is attached to a pressure sensor to measure the breathing motion-induced compression of the pad.
As an additional visual control for positioning accuracy and animal vitality, the top of the bedding unit was covered with a translucent, red film. The latter is transparent for the human eye, but optically opaque for mice to enable visual vitality check, e.g. when the mouse position slightly changed during transport and the contact to the sensor gets lost or when the measurement signal is distorted due to movement.
3. Results
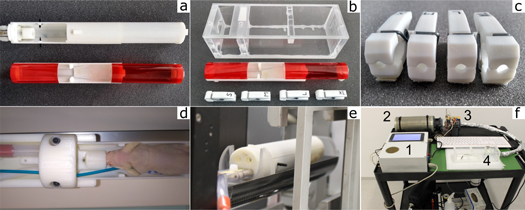
The devised bedding unit was tested for its suitability for the integration into various irradiation and imaging devices. The mean spectrum-weighted effective atomic numbers Zeff of the used materials were 3.9–4.1 for ABS and 4.2–4.4 for PMMA for all X-ray potentials. Figure 2 exemplary shows the bedding unit's use for small animal PET/MRI imaging (figure 2(a)), proton irradiation (figure 2(b)) with differently-sized hind-leg immobilization clamps (figures 2(b), (c)), as well as its usage with adaptor element for MRI imaging (figure 2(d)) and CBCT image-guided photon irradiation (figure 2(e)). The trolley, holding the auxiliary components, is shown on figure 2(f).
Figure 2. (a) Different outfits for the central bedding unit. The top bed outfit is used for brain irradiation and features a breathing mask and ear pins for cranial fixation. The bottom bed outfit is used for subcutaneous tumor irradiation of awake mice and features a transparent cover. (b) Transport box and bedding unit for subcutaneous tumor irradiation using immunocompromised mice outside the pathogen-free facility including (c) Immobilization clamps for the hind leg of various sizes. (d) NMRI (nu/nu) mouse inside bedding unit prior to MRI scan. The bed is attached to the MRI's positioning table with an adaptor element. The blue cable connected the breathing sensor under the mouse belly. (e) Bedding unit mounted on adaptor element inside the SAIGRT rat bed for X-ray imaging and photon irradiation. (f) Trolley for auxiliary vital support featuring the control unit (1), HEPA-grade air filter (2), heating chamber (3) and the connection to the transport box (4).
Download figure:
Standard image High-resolution image3.1. Irradiation
For irradiation with 200 kV X-rays, the 3D-printed ABS-made bedding unit reduced the administered dose by less than 1.5%.
For proton irradiation, the measured mean SPR of the massive ABS-made elements for 150 MeV and 200 MeV was 0.951 ± 0.001, which yields a WET of the bedding unit's walls of 1.5 ± 0.0 mm. SPR deviations for 3D-printed samples of 1.6 mm thickness and all printer settings (± 0.016) yielded WET deviations of ± 0.03 mm.
Using the reported SPR of 1.163 ± 0.012 for PMMA, we derived the WET of the walls of the transport box (2.3 mm PMMA) to be 2.7 ± 0.0 mm.
3.2. Mounting
The spatial variation of the bedding unit's position (i.e. the reproducibility of the mounting position) along suitable image cross-sections was ± 0.01 mm, ± 0.13 mm and ± 0.10 mm in x-, y- and z-direction in the course of five consecutive pairs of orthogonal radiographs, respectively.
3.3. Imaging
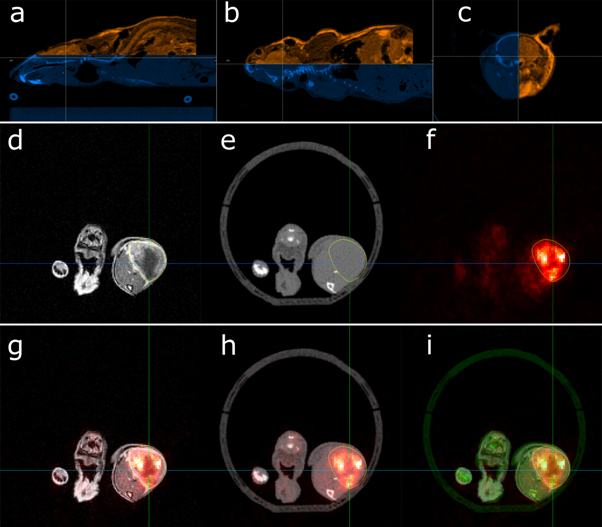
The CBCT scans of the resolution phantom showed no loss of quality; all substructures could be visibly separated from each other (see Supplementary Materials, Fig S1 is available online at stacks.iop.org/BPEX/6/037003/mmedia). While the beam's attenuation with all tested X-ray potentials was smaller than 3%, the CT numbers of phantom objects inside the bed appeared lowered by an average of 15.6 HU (40 kV), 22.1 HU (60 kV) and 25.5 HU (80 kV). The noise levels within the water-filled syringe were elevated by factors of 1.5 (40 kV), 1.2 (60 kV) and 1.2 (80 kV) when imaged within the bedding unit. CBCT scans using the designed bedding unit with small animals showed no loss of image quality. Complex structures such as the rodent's skeleton could be resolved in detail (see figures 3(a)–(c)); The MRI/CBCT/PET imaging data showed no artefacts (figures 3(d)–(f)) and internal organs and anatomy could be identified.
Figure 3. Multi-modal image overlay of mouse body sites. (a) Sagittal MRI (orange)/CBCT (blue) overview. The crosshair indicates the location of the (b) coronal and (c) axial image overlay. (d) Axial MRI, (e) CBCT and (f) PET images of a subcutaneous tumor on the hind leg of a NMRI (nu/nu) mouse. The tumor contour is indicated in yellow. The bottom row shows axial image overlays of (g) PET (red)/MRI (gray), (h) PET (red)/CBCT (gray), and (i) PET (red)/MRI (gray)/CBCT (green). The image contrast of the overlay images (g - i) was increased for better visibility.
Download figure:
Standard image High-resolution imageFollowing the integration of the bedding unit into the SAIGRT and the nanoScan PET/MRI, the spatial relation of both coordinate systems was determined by scanning the 5 ml water-filled syringe. The remaining translational shifts between the respective frames of reference were further aligned by matching the finer internal and external structure of a representative laboratory mouse (see figure 3). After this procedure, substructures like the mouse brain (figure 3(a)–(c)) show a good match between the bone structure of the skull and the rim of the brain as depicted by the MRI data. The co-alignment of CBCT and MRI data in figure 3(a)–(c) that was repeated by three independent observers yielded translation vectors with standard deviations of ± 0.1 mm, ± 0.0 mm and ± 0.1 mm (n = 3) in left/right, inferior/superior and anterior/posterior direction, respectively. The voxel size of the acquired image data is 0.1 mm. We overlaid the imaging data (MRI/CBCT/PET) as demonstrated in figure 3(g)–(h). The layered imaging data show that the tumor contour largely comprises the PET signal. The latter shows an accumulation of PET tracer around the low-signal central region as depicted by the contrast-enhanced MRI, which we attribute to necrosis in the tumor core.
3.4. Auxiliary components
Air-based heating attached to the transport box delivered a stable temperature of (31.6 ± 0.4)°C inside the transport box throughout a period of 2 h; see figure 4(a). This is slightly lower than the set temperature of 32.0°C. Core temperature measurements of a mouse during 60 min under inhalation anesthesia yielded stable overall temperatures (figure 4(b)) with the use of the PET/MRI's integrated air-based heating. The designed vital monitor visualized the breathing of the mouse as exemplarily shown in figure 4(c).
Figure 4. (a) Measured air temperature curve inside the transport box over a time span of more than 2 h. (b) Core temperature curve of an anesthetized mouse. The black arrow points towards the rising temperature slope due to an initially low body temperature after the introduction of the anesthesia. The grey arrow marks an intermittent decrease in body temperature after the hatch of the bedding unit was opened for a brief amount of time. (c) Breathing curve of an anesthetized mouse in a stable position over a period of 20 s.
Download figure:
Standard image High-resolution image4. Discussion
We successfully integrated the developed multi-modality setup for small animals into all available imaging and irradiation devices with maintained image quality and only minor interference with incident proton or photon beams. Although different 3D printer parameters yield otherwise considerable WET deviations, these translate into small absolute range shifts due to the thin walls of the bedding unit. The transport box enables access to a large variety of spatially separated experimental facilities (e.g. animal facility and UPTD), while ensuring a stable vital and hygiene status of the animal.
A viable approach to foster the combination of several imaging modalities as previously proposed by several groups [18–20], is the development of cross-platform bedding units as presented in this manuscript. Importantly, the advantages of different imaging modalities (MRI/CT/PET) can be maintained this way without repositioning and co-registration efforts. The application of this setup is, in principle, possible in a large variety of preclinical imaging devices. The central bedding unit's radial dimensions (inner diameter approximately 30 mm) are suitable for many mouse strains and purposes and can be used for MRI, CT, PET and SPECT imaging. Depending on the application, the bedding unit provides enough space for additional equipment such as MRI surface coils or further immobilization devices like grooved inlays corresponding to the animal's anatomy. Mounting elements can be readily fabricated with 3D printers in a cost-effective way and allow for a high mounting reproducibility. Therefore, our setup helps to overcome technical limitations for an advanced integration of multi-modal imaging. This could only be quantitatively demonstrated for the CBCT mounting with positioning variations in the range of ± 0.1 mm. Similar measurements could be performed with MRI subsystem by introducing detectable water-filled beads into the bed, but these may impair the criterion of geometrical simplicity and may be outside the desired field of view. However, since all adaptor elements are subjected to the same fabrication methods, the achievable positioning reproducibility can be expected to be of similar order of magnitude.
Additionally, the presented platform offers a suitable means to perform radiation therapy-related experiments that require the application of X-ray or particle irradiation. Similar platforms have been proposed by several groups [14, 21–23] for specific experimental purposes. The presented setup, however, focuses on high versatility to allow its usage for both photon and particle irradiation experiments and realize MRI- or CT-guidance through the integration into the various imaging devices. This is of particular importance for the treatment planning of target volumes inside single organs: Precise irradiation requires accurate contouring and treatment planning, which demands both soft tissue contrast as provided by MRI imaging and calculation of the dose distribution in the beam path based on CT data. The measured CT number reduction of objects inside the bedding unit has to be taken into consideration for the utilization of acquired CBCT data for treatment planning and dose calculation. We consider the occurrence of beam hardening (absorption of low energy photons in thick materials) as the primary source of this effect, especially since a suitable correction method for this effect has currently not been implemented on the SAIGRT platform. Therefore, this should be regarded as a limitation of the used CBCT reconstruction protocol rather than of the bedding unit itself. Thus, the presented setup nonetheless poses a method for high-precision irradiation based on MRI- or CT-guidance while allowing for the computation of the spatial dose distribution. The geometrical simplicity of the setup and the altogether thin bedding unit and transport box (combined WET about 4.2 mm, X-ray attenuation less than 3%) cause minimal interactions with the beam and can be accounted for during treatment planning. At the same time, the setup offers enough structural information to be of use for image-guided positioning with the bedding unit used as a landmark [23]. The system could also be used for experiments with heavier particles (e.g. helium or carbon ions), provided the WET for the respective beam modalities are known. The integrated fixation devices allow the irradiation of various target regions.
Unlike other setups [19, 24], the attached transport box and auxiliary vital support enable the bedding unit to be used outside designated animal facilities. While this is of minor concern for irradiation and imaging devices that are installed inside large, dedicated small animal research laboratories [18, 25], it provides a suitable platform for experiments relying on clinical devices to circumvent conflicts with hygiene regulations [26]. Typically used clinical equipment includes MRI scanners [27] and linear accelerators [28, 29]. Where clinical MRI scanners are concerned, the applicability of the attached trolley carrying the vital support is limited by the typical magnetic fringe fields that occur around such platforms. Particle irradiation facilities are becoming increasingly relevant in preclinical research [23]. Therefore, solutions to overcome the inevitable bottleneck of removal and reintroduction of animals from keeping facilities [30] are required.
5. Conclusion
We present a bedding platform for mice that is suitable for multi-modal irradiation (photons or protons) and imaging (CT/MRI/PET). It can be mounted onto the frameworks of different imaging devices via 3D-printed adaptors and can be used outside pathogen-free areas (e.g. clinical imaging or irradiation facilities) while preserving animal- and clinic-specific hygiene regulations if embedded in a dedicated transport box. Vital support (breathing monitor, heating) can either be used as provided by the respective imaging devices or from an auxiliary transport trolley if animals are transported outside the keeping facility. The setup provides a versatile, unified platform for imaging and irradiation, and is suitable for both multi-modal, high-precision image-guided irradiation experiments of small subvolumes as well as irradiation of larger entities (e.g. subcutaneous tumors) with homogeneous fields.
Future developments will focus on the integration into further imaging devices (e.g. Bruker 3T MRI) and modalities (e.g. optical imaging, ultrasound) as well as the simultaneous monitoring of several mice via the transportation unit's vital support to increase throughput.
Acknowledgments
We would like to acknowledge the support of Liane Heinrich of the Institute of Radiation Physics of the Helmholtz-Zentrum Dresden-Rossendorf for assistance in the production of 3D-printed items. Furthermore, Michael Reiche and Gert Rothe from the mechanical workshops at OncoRay, Dresden contributed to a great extent through their expertise in the design and fabrication of the presented platform. We further want to acknowledge the technical support of Julia Severin, Katja Schumann and Dorothee Pfitzmann in the animal facility. This project has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No. 730983 (INSPIRE).