Abstract
Nanoparticulate silver inhibits bacterial growth. Here we evaluated the antimicrobial properties of a novel form of silver nanoparticles, synthesized by a sonochemical method with chitosan as reducing and stabilizing agent (chitosan-silver nanoparticles). The chitosan-silver nanoparticles were positively charged and stable in the long-term, as determined by the high Z-potential. The minimum inhibitory concentration for vegetative bacteria and spores was measured with a resazurin microtiter assay. The interaction between nanoparticles and the bacterial surface was observed by atomic force microscopy and transmission electron microscopy. We found that chitosan-silver nanoparticles inhibited vegetative bacteria and bacterial spores. Furthermore, chitosan-silver nanoparticles enhanced the performance of ampicillin against bacteria and were also effective against an ampicillin-resistant strain. Atomic force microscopy and transmission electron microscopy images revealed a close interaction between the nanoparticles and bacterial extracellular structures, such as flagella. We suggest that chitosan-silver nanoparticles could be used as a topical antimicrobial that can enhance antibiotic performance.
Export citation and abstract BibTeX RIS
Introduction
Nanoparticles are currently one of the most used nanomaterials [1–5]. This form of nanoparticulated matter presents, in some cases, the interesting property of inhibiting the growth of microorganisms including bacteria, yeast and protozoa [6]. This phenomenon has been proposed as a potential new strategy to deal with emergent clinical threats, such as antibiotic-resistant bacteria [7]. Silver nanoparticles are remarkable in this regard due to their antimicrobial properties [8], which involve the induction of oxidative stress to microorganisms [9]. Toxicological and pharmacological studies on silver nanoparticles are an active area of research [10]. Several reports show negligible toxic effects [11–13]. However, other studies show that silver nanoparticles could present cytotoxicity depending on their size, stability and method of synthesis [11, 14–16]. Therefore, to fully unlock the potential of silver nanoparticles in biomedicine, it is still necessary to develop new methods to produce highly bioactive and biocompatible nanoparticles.
The decision to use silver nanoparticles in biomedicine could be restricted based on whether their synthesis required toxic compounds to humans [17]. Most protocols for nanoparticle synthesis are based on the reduction of silver ions, which cluster as nanoparticles by the addition of an ionic or polymeric stabilizing agent [18]. The chemical synthesis of nanoparticles is the best characterized method of production and allows precise control of the particle size; however, in many cases the process requires the use of toxic reducing compounds such as NaBH4, hydrazine or dimethylformamide (DMF) which could limit clinical applications [19, and references therein]. To overcome this limitation, synthesis methods combining physical reducing agents and naturally occurring organic polymers have been established [20]. Silver ions can readily interact with functional groups present in organic polymers such as the carboxylic, hydroxyl or amine groups.
Among the organic polymers used to produce nanoparticles, chitosan is especially interesting for biomedicine due to its intrinsic antimicrobial properties and its hemostatic effects [21–23]. Although the latter property prevents the use of chitosan in applications that require intravenous delivery or direct contact with internal organs, it makes it an optimal material for wound dressings [24], to treat external infections topically [25, 26] and to enhance the biocompatibility and stability of different materials [27]. The mechanism of action for the antibacterial effect of chitosan it not well understood, but it seems to involve electrostatic interactions between the positively charged chitosan and the negatively charged bacteria, which leads to altered cell permeability, leakage of the intracellular content and cell death [28].
Chitosan is obtained by the deacetylation of chitin, the major constituent of the exoskeleton of invertebrates. This polymer is composed of D-glucosamine and N-acetyl-D-glucosamine subunits. Importantly, the final residue in a chitosan chain, and the oligomer derived from it, is a reducing sugar. Due to the presence of amine groups, chitosan is positively charged in acidic conditions and its solubility is reduced at high pH [29, 30]. Several groups have taken advantage of these properties to develop both chemical and physical methods for the production of chitosan-AgNPs [31, 32] (supplementary table 1 is available online at stacks.iop.org/BPEX/4/035011/mmedia).
Recently, researchers have successfully synthesized chitosan nanoparticles using sonication as a physical green method to trigger the reduction of the metallic ions by ultrasound-generated cavitations [33–36]. Chitosan silver nanoparticles showed remarkable antimicrobial properties against vegetative bacteria [37, 38]. However, their effect on dormant, persistent or latent bacterial forms and antibiotic strains is just starting to be explored [39–42].
There are two main bacterial strategies to overcome antibiotics: resistance factors and spore formation. Both can be influenced by the architecture of extracellular structures. Bacteria can be classified as negative or positive for the gram staining depending on the structure and composition of extracellular structures [43]. Upon constant exposure to antibiotics, bacterial populations become resistant by acquiring plasmids that contain resistance factors [44]. For instance, the wide use of penicillin after World War II promoted the appearance of bacteria harboring plasmids that produce β-lactamase enzymes, which render penicillin inactive by cleaving its β-lactam ring [45]. Another strategy that enhances the survival of bacteria in the presence of antibiotics is the formation of dormant bodies, called spores. These are multilayered structures that present a physical barrier to antibiotics [46]. Spores are also almost metabolically inactive and, therefore, antibiotics that take advantage of processes such as protein synthesis are ineffective. Spore-forming bacteria are Gram-positive, whereas resistance factors against antibiotics are a general survival strategy for all bacteria. Antibiotic resistant bacteria have become a widespread problem in our health system, responsible for millions of infections and thousands of deaths [47]. This figure is predicted to rise to 10 million deaths if effective countermeasures are not enacted promptly [48]. Therefore, the search for new methods to control bacteria proliferation has been recognized as a priority for public health [49].
In this work we analyze the antibacterial properties of chitosan-coated, positively charged, silver nanoparticles synthesized using a sonochemical method. Our approach is an original protocol for the synthesis of very stable chitosan-silver nanoparticles (chitosan-AgNPs) by applying sonication cycles to a mixture of a low concentrated chitosan solution (0.4%) and a silver salt (AgNO3) at high pH (11.7). Under these conditions, the formation of chitosan hydrogels was avoided during the synthesis at high pH. The resulting nanoparticles were very stable (>8 months) and had a wide spectrum of antimicrobial activity, being effective against gram positive and negative vegetative bacterial cells, bacterial spores, and antibiotic resistant bacteria. We also observed an improvement in the antibacterial effect of the chitosan-AgNPs when used in combination with the β-lactam antibiotic ampicillin. We suggest that chitosan-AgNPs must be explored as an approach to control bacterial proliferation, especially for bacterial spores and antibiotic-resistant strains.
Materials and methods
Synthesis of chitosan-AgNPs
A chitosan (Acros Organics, MW: 100.000–300.000) solution of 0.4% (w/v) was prepared in 1% (v/v) acetic acid. 10 ml of the solution was adjusted with NaOH 1 M to a pH of 11.6. Then, 1 ml of a 10 mM solution of AgNO3 was added dropwise, while the solution was shaken with a magnetic stirrer at room temperature. Distilled water was used to reach a final volume of 20 ml.
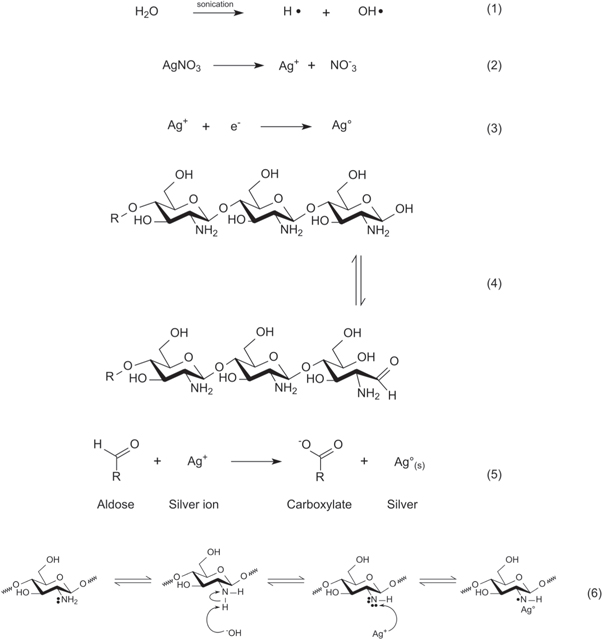
Silver reduction was triggered by sonication at 20 kHz for 30 min with a 6 mm titanium ultrasonic probe (GEX130 Cole-Palmer). The solution was sonicated for 45 s pulse on, 15 s pulse off, at 35% amplitude. Afterwards, 2 drops of acetic acid (99%) were added and sonication for an additional minute was repeated under the same conditions. Finally, the solution containing nanoparticles was diluted to 250 ml with distilled water. A proposed set of equation for the reduction of silver under our experimental conditions is given in figure 1.
Figure 1. Proposed mechanisms for the reduction of silver ions facilitated by ultrasound in alkaline media. When ultrasound is applied to an aqueous media, the water molecules dissociate in H· and OH· radicals (1). Silver nitrate, when in aqueous solution, dissociates in silver ions (Ag+) and nitrate ions  (2). The hemiacetal group present in the last residue of a chitosan chain (R stands for a chitosan chain or an oligomer derived from its breakdown) acts as a reducing agent (3). In this reaction, the hemiacetal group is oxidized, yielding a carboxylate ion, and silver ions are reduced to metallic silver (4). Another possible reaction, although not as favorable as the previous one, involves the reduction of silver ions mediated by the amine groups of the chitosan. In this case, R' stands for the amine group. The OH
(2). The hemiacetal group present in the last residue of a chitosan chain (R stands for a chitosan chain or an oligomer derived from its breakdown) acts as a reducing agent (3). In this reaction, the hemiacetal group is oxidized, yielding a carboxylate ion, and silver ions are reduced to metallic silver (4). Another possible reaction, although not as favorable as the previous one, involves the reduction of silver ions mediated by the amine groups of the chitosan. In this case, R' stands for the amine group. The OH radicals produced by water sonolysis in (1) reacts with a H from the amine, forming water (5) and in turn providing the electrons necessary for the reduction of silver, as seen in (6).
radicals produced by water sonolysis in (1) reacts with a H from the amine, forming water (5) and in turn providing the electrons necessary for the reduction of silver, as seen in (6).
Download figure:
Standard image High-resolution imageAnalysis of the size distribution and Z-potential were made with a Z-sizer Nano-ZS90, after synthesis or after eight months of storage, to evaluate the stability of the chitosan-AgNPs over time. Both analyses were made at a scattering angle of 90° at 25 °C.
Evaluation of antimicrobial and sporicidal activity of chitosan-AgNPs by the resazurin microtiter assay (REMA)
The REMA was performed using Gram-positive (Staphylococcus aureus ATCC 25923 and Bacillus subtilis ATCC 11774) and Gram-negative (Escherichia coli ATCC 25922) bacteria. Briefly, 30 μl of 3.3× Müller Hinton broth and 10 μl of resazurin were pipetted in each well of the 96-well microtiter plate. Chitosan-AgNPs were linearly diluted within a range of 6.99 to 1.21 μg ml−1, and added to the plate with a multichannel pipette. This range of chitosan-AgNPs was identified from our previous observations as the best range for evaluating the action of the nanoparticles (Data not shown). Approximately 5 × 104 bacteria were inoculated in each well. Sterile water was added to reach a final volume of 100 μl. As a negative control for growth, the first and last rows of each plate were not inoculated. As a positive control for growth, the last two columns were inoculated but left untreated with chitosan-AgNPs. The plates were incubated at 30 °C and the REMA assay was scored for growth after 24 h. Wells that turned pink were considered positive for bacterial growth since the resazurin was reduced, whereas wells that remained blue were considered negative. Furthermore, REMA control experiments performed by using all the reagents and conditions from the synthesis, except the silver nitrate, showed that the reaction mixture has negligible antimicrobial activity when silver nitrate is omitted (supplementary figure 1).
Sporicidal activity of chitosan-AgNPs using a modified resazurin microtiter assay for spore viability and germination (REMA)
B. subtilis spores were obtained by incubating a loopful of the bacteria in 15 ml of Müller Hinton Broth for 2 h. Afterwards, 1 ml of the culture was transferred to 15 ml of Difco Sporulation Medium [50] with some modifications: KCl was replaced by NaCl and Ca(NO3)2 was not added to the medium. The bacteria were incubated for 1 week at 30 °C with constant shaking at 120 rpm. Spores were isolated by sonicating the sporulation culture at 55 °C for 2 h in a sonic bath at 40 kHz and centrifuging the medium at 4900 × g for 25 min. The spore pellet was resuspended in sterile saline, centrifuged again, resuspended again and used immediately. In order to test the sporicidal capacity of chitosan-AgNPs, we developed a two-step modified REMA. First, approximately 5 × 105 B. subtilis spores were inoculated in sterile water in a 96-well microtiter plate with increasing amounts of chitosan-AgNPs (21.42 to 2.14 μl ml−1), for 24 h at 30 °C. Second, the spores were taken from each well and transferred to the corresponding well on the second plate, which lacked chitosan-AgNPs, but contained 3.3× concentrated Müller-Hinton medium, resazurin and sterile water. After incubation under the same conditions as the previous plate, germination depended on whether the spores were inactivated by the chitosan-AgNPs or not.
Effect of silver nanoparticles on antibiotic resistant bacteria and on antibiotic performance
The original REMA was modified in order to explore the effect of chitosan-AgNPs on antibiotic resistant bacteria. A strain of ampicillin-resistant S-17 E. coli was exposed to increasing concentrations of chitosan-AgNPs or ampicillin and the viability was compared. As controls wild-type E. coli was exposed to chitosan-AgNPs or ampicillin. To evaluate whether chitosan-AgNPs might influence the performance of the antibiotic, we also analyzed the response of wild-type E. coli exposed to both the antibiotic and chitosan-AgNPs. The nanoparticle concentration was the same as in the previous tests, and the ampicillin concentration ranged between 32 and 0.06 μg ml−1.
Statistical analysis
A simple Wilcoxon rank sum test was applied to determine whether there was a significant difference between the MIC values in each experiment. A Kruskall-Wallis rank sum test was used to evaluate whether the cell wall composition and the bacterial morphology influenced the resistance to the chitosan-AgNPs.
Atomic force and transmission electron microscopy of bacteria and chitosan-AgNPs.
The MFP-3D system (Asylum Research) was used to obtain the AFM images of wild-type E. coli after exposure to nanoparticles. Images were acquired in AC mode at room temperature, with 256 × 256 pixels of resolution. Scan speed was 1 Hz. The cantilever was rectangular, with a force constant of aprox. 5 N m−1 and a resonance frequency of 150 kHz (Tap150Al-G). Amplitude Modulated–AFM was selected for scanning because lateral forces are reduced and the cantilever's tip is less likely to disrupt surface features [51]. For AFM, 50 μl of E. coli cells (OD600 of 0.500) were mixed with 50 μl of chitosan-AgNPs (100 mM) and allowed to interact for 3.5 h in sterile water. Whenever we needed to observe bacterial flagella, the samples were gently centrifuged. To confirm that the nanostructures observed in contact with the bacterial surface were chitosan-AgNPs, the following controls were observed: (1) untreated bacteria, (2) bacteria treated with chitosan and (3) bacteria treated with chitosan and AgNO3. For AFM, 5 μl of bacterial suspension were adsorbed on a mica surface and dried for 10 min before being imaged. For TEM, after exposure to chitosan-AgNPs, 2–4 μl volume of bacterial suspension were mounted onto a copper/palladium 400 mesh formvar coated grid and allowed to dry. Bacteria were not fixed or stained. TEM images were obtained on a JEOL JEM2010 Transmission Electron Microscope set at 120 kV using magnifications of between 5.000× and 10.000×. GatanMultiScan 794 was used for image capture. Energy dispersive spectroscopy was done with the built-in EDS (Oxford, Model INCA).
Results
Chitosan-AgNPs were stable on the long term
Long-term stability is a critical property of nanoparticles. The values registered by the Z-sizer showed that the chitosan-AgNPs are positively charged with values of 50.7 ± 2.77 mV, a polydispersity index (PdI) of 0.55 ± 0.064 (figure 2(A)), and a radius of 72.0 ± 23.56 nm. Even after nine months the chitosan-AgNPs remained stable as judged by their Z-potential of aprox. 31.5 mV, a PdI of 0.477 ± 0.016 and a radius of 133.7 ± 27.56 nm (figure 2(B)). AFM measurements confirmed these dimensions, showing an average radius of 73.91 ± 15.33 nm (figures 2(C) and 5(E) and (F)). Our measurements were consistent with chitosan-AgNPs having a have a core of silver and an outer layer of chitosan (figure 2(D)).
Figure 2. Chitosan AgNPs are stable on the long term. Characterization of chitosan-AgNPs synthesized by the sonochemical method. DLS measurements for Z-potential and size distribution of the chitosan-AgNPs (A) newly synthesized and (B) 8 months later. (C) AFM height image of the chitosan-AgNPs. (D) Model of the distribution of the chitosan and the silver in the synthesized nanoparticles.
Download figure:
Standard image High-resolution imageChitosan-AgNPs showed a wide spectrum of antibacterial activity against vegetative cells and spores
In order to test the effect of chitosan-AgNPs on bacteria presenting different types of cell wall and metabolic states, we exposed both vegetative cells and spores to increasing concentrations of chitosan-AgNPs, and determined the minimum inhibitory concentration (MIC) by REMA. The susceptibility of spores to chitosan-AgNPs has not been extensively analyzed [40]. When exposed to chitosan-AgNPs, Gram-positive bacteria were more resistant than Gram-negative bacteria (K = 26.2079, p-value = 3.06 565 × 10−7). MIC values for E. coli were 2.26 ± 0.17 μg ml−1, while values for S. aureus were 5.83 ± 0.19 μg ml−1 (figures 3(A) and (B)). In the case of B. subtilis, spores were more resistant than the vegetative forms (figures 3(C) and (D)), with MIC values of 10.35 ± 1.24 μg ml−1 and 2.93 ± 0.12 μg ml−1, respectively (W = 18, p = 1.532 × 10−6). Although E. coli and B. subtilis showed a similar degree of sensitivity towards the chitosan-AgNPs, E. coli was statistically more sensitive than B. subtilis (W = 276.0, p-value = 1.0 × 10−4), whereas S. aureus showed more resistance than the bacilli (K = 36.1164, p-value = 1.858 78 × 10−9) (figure 3(E)).
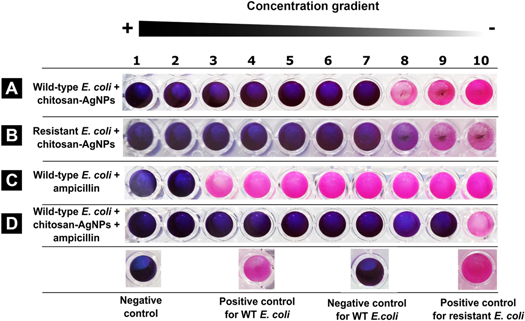
Figure 3. Chitosan-AgNPs have a wide rage antibacterial activity. Evaluation of the antibacterial and sporicidal effect of chitosan-AgNPs using the REMA. A change from blue to pink after 24 h of incubation was considered as positive for bacterial growth. (A), (B), (C) The corresponding bacteria were inoculated in medium containing the resazurin dye and decreasing concentrations of chitosan-AgNPs. (D) B. subtilis spores were first exposed to chitosan-AgNPs, then washed and inoculated on fresh medium. (E) Quantification of the effect of chitosan-AgNP on different vegetative bacteria and spores (n = 100). Positive control, no chitosan-AgNPs. Negative control, no bacteria.
Download figure:
Standard image High-resolution imageChitosan-AgNPs enhanced the antibiotic performance and were effective against β-lactams resistant strains
In order to test whether nanoparticles could control the proliferation of antibiotic resistant bacteria, we exposed ampicillin-resistant E. coli to increasing concentrations of chitosan-AgNPs. The resistant strain was able to grow at concentrations of ampicillin as high as 8.25 μg μl−1, that is, 500 times the value that inhibits the wild-type [52]. However, when exposed to nanoparticles, the antibiotic resistant bacteria and the wild-type strain showed no difference in the MIC values (figures 4(A) and (B), W = 49.5, p-value = 0.7769). Thus, the resistance to the antibiotic did not confer protection from the chitosan-AgNPs. Given that antibiotics could interact directly with nanoparticles [53] we tested the combined effect of adding chitosan-AgNPs and ampicillin on wild-type E. coli. Treating the bacteria with ampicillin only resulted in MIC values of 16 μg ml−1, which are in agreement with previous reports for wild-type E. coli [51]. Remarkably, the combination of chitosan-AgNPs with ampicillin produced a synergistic effect that enhanced by 100-fold the antibacterial activity (figures 4(C) and (D)), resulting in MIC values as low as 0.163 ± 0.025 μg ml−1.
Figure 4. Chitosan-AgNPs are effective against antibiotic resistant bacteria and enhance ampicillin effect. Evaluation of the effect of chitosan-AgNPs on ampicillin performance and against antibiotic resistant bacteria using the REMA. A change from blue to pink after 24 h of incubation was considered as positive for bacterial growth. (A) Wild type E. coli plus chitosan-AgNPs (B) ampicillin-resistant E. coli plus chitosan-AgNPs. (C) Wild type E. coli exposed to ampicillin (D) ampicillin-resistant E. coli exposed to both chitosan-AgNP and ampicillin. Positive control, no chitosan-AgNPs. Negative control, no bacteria. Wild-type E. coli was killed by ampicillin, but the resistant strain survived.
Download figure:
Standard image High-resolution imageChitosan-AgNPs interacted directly with the bacterial surface
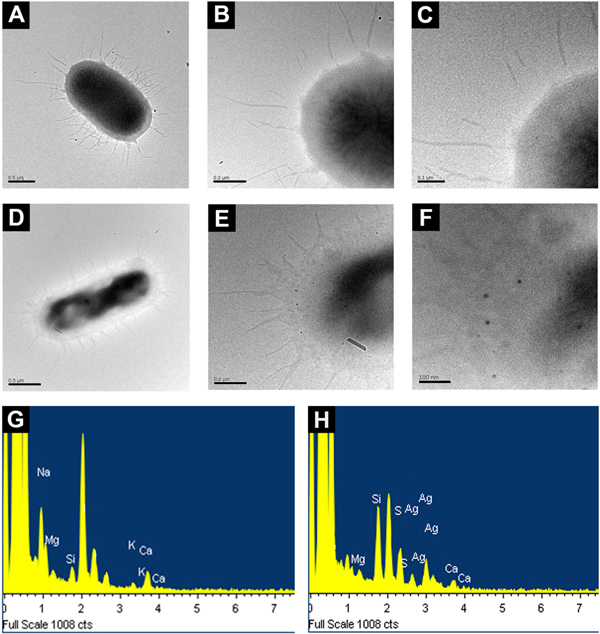
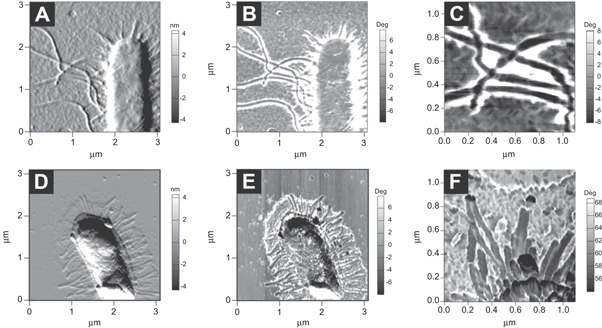
In order to gain a better understanding of the interaction between chitosan-AgNPs and E. coli, we decided to analyze with high resolution techniques the surface of the bacteria after being exposed to chitosan-AgNPs. AFM images showed that untreated bacteria presented the typical rod-shape morphology without spherical particles on their surface (figure 5(A)). In contrast, upon exposure of E. coli to chitosan-AgNPs, spherical structures were observed in close contact with the bacterial surface (compare figures 5(B) and (C) versus (E) and (F)). Interestingly, bacteria exposed only to chitosan had a coating of irregularly-shaped nanostructures (figures 5(B) and (D)), probably due to chitosan aggregation on the bacterial surface; however no spheroid structures could be distinguished upon close inspection with phase contrast AFM (figure 5(C)). In agreement, TEM images showed that untreated bacteria did not have nanoparticles on their surface (figures 6(A)–(C)) whereas those treated with chitosan-AgNPs presented irregular morphologies and were in close contact with electron-dense particles of a radius of 10.87 ± 4.51 nm (figures 6(D)–(F)). These spherical structures were only observed in the samples exposed to silver nanoparticles. EDS measurements confirmed that silver was present in the nanoparticles, but absent in the chitosan control (figures 6(K) and (J)). Bacteria exposed only to chitosan also presented a layer of aggregated chitosan on their surface as seen by TEM (supplementary figure 2). After careful isolation by mild centrifugation of a fresh inoculum of E. coli, we were able to observe by AFM the long intact flagella of untreated E. coli cells (figures 7(A)–(C)). Upon exposure to chitosan-AgNPs, we observed nanoparticles directly interacting with extracellular structures such as the flagella (figures 7(E)–(G)), which appear as black dots in the phase contrast images. Interestingly, the flagella from cells treated with nanoparticles seemed shorter and more distorted than the same structures in the wild-type (compare figures 7(C) versus (F)).
Figure 5. Chitosan-AgNPs accumulate on the bacterial surface. Analysis of the interaction of chitosan-AgNPs by AFM. AFM amplitude images of (A) E. coli untreated, (B) exposed to chitosan and AgNO3, (D) treated with chitosan and (E) exposed to chitosan-AgNPs. AFM phase contrast images showing (C) the bacterial edge surface after treatment with chitosan or (F) exposure to chitosan-AgNPs.
Download figure:
Standard image High-resolution imageFigure 6. Chitosan-AgNPs interact closely with the bacterial surface. Analysis of the interaction of chitosan-AgNPs by TEM. TEM micrographs of (A) E. coli untreated, with (B), (C) increasing magnifications of A and (D) E. coli exposed to chitosan-AgNPs with (E), (F) increasing magnifications of D. Energy dispersive scattered composition analysis (EDS) of the bacterial surface from control cells (G) or treated with chitosan-AgNPs (H).
Download figure:
Standard image High-resolution imageFigure 7. Chitosan-AgNPs interact closely with the bacterial extracellular structures. Analysis of the interaction of chitosan-AgNPs with extracellular structures by AFM. AFM amplitude images of the flagella of (A) untreated E. coli and (D) E. coli treated with chitosan-AgNPs. AFM phase images of the flagella of (B) E. coli untreated, (E) E. coli treated with chitosan-AgNPs. Magnification of the flagella of (C) E. coli untreated and (F) E. coli exposed to chitosan-AgNPs.
Download figure:
Standard image High-resolution imageDiscussion
In this work we synthesized very stable chitosan-AgNPs by a sonochemical method using chitosan as a reducing/stabilizing agent. Three lines of evidence suggest that the nanoparticles are composed of a metallic core surrounded by a positively charged chitosan layer. First, the Z-potential of the chitosan-AgNPs is consistent with the idea that the positively charged chitosan covers a silver core (figures 2(A) and (B)). Second, in phase contrast AFM images, the particles showed a coating that is distinct from the core (figure 5(F)). Third, an electrodense metallic core explains why the nanoparticles observed in TEM looked smaller (figure 6(F)). The amines and hemiacetals (aldehydes) present in chitosan are capable of acting as reducing agents (figure 1, reaction 4) favoring the interaction with silver ions that facilitates the nucleation of a metallic core [54].
Chitosan-AgNPs show a wide spectrum of antibacterial activity against vegetative cells and spores
The antimicrobial effectiveness of positively charged chitosan-AgNPs has not been assessed previously [19]. Our results show that these nanostructures are able to inhibit bacterial spores as well as both Gram-negative and Gram-positive bacteria. For proliferating cells, the differences in MIC values were not related to the cell wall composition; for instance, the Gram-negative E. coli and the Gram-positive B. subtilis had similar MIC results. Interestingly, S. aureus was more resistant to nanoparticles than other bacteria. The increased of resistance of cocci could arise from their tendency to form complex spatial arrays in clusters [55]. In a similar manner to biofilms, the cells on the outer boundaries of a cluster could act as a physical barrier that protects the inner cells from the nanoparticles [56]. The spores of B. subtilis were the most resistant bacterial form to chitosan-AgNPs in our experiments. Such resilience can be explained by the different layers present in the spore wall. For example, the spore coat, the outermost layer, has a fraction of insoluble proteins with large quantities of thiol groups, which are responsible for the resistance to oxidizing agents, such as the Ag+ ions released from the chitosan-AgNPs [57].
Chitosan-AgNPs enhance the antibiotic performance and are effective against β-lactam resistant strains
Alternatives to deal with antibiotic resistance in bacteria usually involve the costly development of new antibiotics; however, in the last decade only one new antibiotic has been approved [58] and, as of 2016, only 40 were in clinical development in the United States [48]. In this study, we found that ampicillin-resistant E. coli showed the same susceptibility to silver nanoparticles as the wild-type E. coli. Thus, the mechanism used by bacteria to gain resistance to β-lactams does not confer resistance to silver nanoparticles and, importantly, the chitosan-AgNPs did not interfere with the mechanism of action of β-lactams. In fact, we observed that ampicillin and chitosan-AgNPs act synergistically on wild-type E. coli. Ampicillin interferes with the synthesis of the cell wall by inhibiting the peptidoglycan transpeptidase (EC 3.4.16.4), which controls the essential peptidoglycan crosslinking step required for cell wall integrity. The synergy could be the result of a more permeable cell wall that facilitates the action of the chitosan-AgNPs. It would be necessary to test whether this synergistic effect is also possible with other antibiotics that target, for instance, protein synthesis. It is also important to consider whether bacteria can develop resistance to chitosan-AgNPs. It has been observed that when E. coli is cultured on agar plates supplemented with silver nanoparticles, some resistance appears in the course of various generations [59].
Chitosan-AgNPs interact directly with the bacterial surface
AFM and TEM analysis showed that chitosan-AgNPs established a direct interaction with the bacterial surface. Nanoparticles decorated the outline of the treated bacteria, being in contact with flagella or structures that would be in the range of the glycocalyx or the bacterial capsule. A common feature of all these structures is the presence of negatively charged groups under physiological conditions. The positively charged chitosan-AgNPs could then interact by virtue of electrostatic forces. It has been previously observed that this kind of mechanism mediates the interaction of cell wall components, such as teichoic acids and LPS, with cationic particles [60]. For instance, LPS presents negatively charged phosphate groups which could enhance the antimicrobial effect by attracting the positively charged nanoparticles to the cell wall [61]. Furthermore, biological barriers such as the cell membrane can be crossed more efficiently by positively charged particles [62]. Once in the vicinity of the bacteria, the nanoparticles release silver ions that can trigger oxidative damage [63, 64]. However, it is still necessary to determine the specific mechanism of interaction between chitosan-AgNPs and their targets on the bacterial surface.
Conclusions
Our results suggest that chitosan-AgNPs produced by the sonochemical method are stable over time and can act in combination with β-lactam antibiotics. This information can be used to develop alternative strategies to control antibiotic resistant bacteria, bacterial spores and could potentially be used for treating topical infections.
Acknowledgments
This work was supported by the National Center for Advanced Technologies and the National Laboratory of Nanotechnology. We thank Elizabeth Argüello for image editing and Rocío Zamora for fruitful discussions regarding the chitosan chemistry in alkaline media.