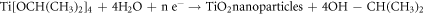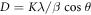Abstract
The characteristics and biological qualities of the nanomaterial rely heavily on how it was made. Green nanoparticle production has been developed to minimize pollution, cut expenses, and enhance safety for both human health and the environment by reducing metal ions using plant extracts as opposed to industrial chemical agents. The goal of the current work is to synthesize titanium dioxide nanoparticles in an environmentally friendly manner by using an extract from the combined shells of Stone apples (Aegle marmelos) and Wood apples (Limonia acidissima). Titanium dioxide nanoparticle formation was verified using various characterization techniques. Well diffusion was used to measure the antimicrobial activity. The fungal strains that were employed were Aspergillus Niger, Candida Albicans, and Aspergillus Flavus. All fungul strains were successfully inhibited by both the crudely prepared extract and the biosynthesized titanium dioxide nanoparticles; however, the biosynthesized titanium dioxide nanoparticles exhibited a high zone of inhibition ranging from 25 to 30 mm, while the crudely prepared extract had a low zone of inhibition ranging from 13 to 19 mm. A moderately sized zone of inhibition was observed in both the crude produced extract and the biosynthesized Titanium dioxide nanoparticles at a dilution of 100 μg ml−1. Lower dilutions demonstrated less noticeable inhibition. Overall, these results showed that treatment with biosynthesized titanium dioxide nanoparticles significantly slowed the growth of many microorganisms.

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In order to successfully produce nanoparticles (NPs) from plants that belong to various taxonomic groups, nanoscience depends on 12 fundamental principles that are taken from green chemistry [1]. Thermal, optical, surface, and electrical characteristics are just a few of the physicochemical characteristics of metal oxide nanoparticles made through biosynthesis. Plants, bacteria, fungi, and algae are among the natural environmental resources used in green-mediated nanoparticle synthesis [2]. Plants are used to create NPs in a variety of forms and sizes using their different parts, including as the stem, root, leaves, flowers, seeds, and latex. The majority of studies have concentrated on the leaves, seeds, and flowers of plants. Their superior capacity to reduce metal ions is the main reason they are preferred. Safe handling and quick access to plants are two other advantages. Bioactive substances derived from plants include salicylic acids, sugars, alkaloids, diterpenoids, lactones, glycosides, steroids, polysaccharides, terpenoids, flavonoids, phenolic acids, vitamins, enzymes, minerals, and sterols, which have reducing and stabilizing properties [3, 4]. The green chemistry method has been effectively employed to synthesise a variety of nanoparticles [5]. Due to its many benefits, including lower toxicity, greater uses, cost-effectiveness, and environmental friendliness, green synthesis is recommended over chemical methods [6]. When making nanoparticles, plant extracts can function as stabilizing and reducing agents. It is well known that the plant extract's source can change the characteristics of nanoparticles. This is due to the fact that different extracts have variable ratios and mixes of organic reducing agents [7].
Because of its exceptional physical stability and lack of toxicity, titanium dioxide (TiO2) is a versatile metallic oxide that has grown in popularity. Numerous techniques, such as template, sol–gel, hydrothermal, thermal hydrolysis, electrochemical anodic oxidation, flame synthesis, and photochemical reduction procedures, can be used to synthesize titanium dioxide nanoparticles [8–10]. The most often used materials to synthesize TiO2 nanoparticles were titanium (IV) alkoxide, titanium butoxide, titanium tetra isopropoxide (TTIP), and titanium tetrachloride (TiCl4). While titanium (IV) alkoxide is costly and soluble in organic solvents, titanium tetrachloride is the most dangerous and corrosive of the precursors.
Physical and chemical processes are equally viable for the synthesis of nanoparticles, but the green road is preferable because it is more ecologically friendly and sustainable [11]. Because of its many applications, TiO2 NPs have become more and more popular in recent years. TiO2 nanoparticles' excellent optical quality, chemical stability, and lack of toxicity have piqued interest worldwide.
A range of phytochemicals and bioactive compounds found in common aqueous extracts from medicinal plants function as non-toxic capping and reducing agents, as well as shape and size control agents for synthesized nanoparticles. Although edible, the fruit of the Limonia acidissima (Wood apple) is utilized in very few recipes. This fruit, along with other components of the L. acidissima plant, is believed to have antihyperglycemic and antihyperlipidemic characteristics and is used in folk medicine to treat a wide range of ailments. L. acidissima is a fruit rich in nutrients, containing a surprisingly high percentage of protein (10%) and phenolic content, suggesting that its dry powder is a potent source of antioxidants [12]. The sample's moisture level, at 6.4%, is comparatively low, which could potentially extend its shelf life. Carbohydrate content in L. acidissima pulp was found to be high (70.14%). It was a good source of protein since it included a substantial amount of protein (13.8%) and a reasonable level of fiber [13].
In India, the Bael tree, Aegle marmelos (L.) Correa, is also referred to as the begal-quince, golden apple, or stone apple. It is revered in Hindu communities. Devotees frequently worship bael trees, which are typically grown near Lord Shiva temples [14]. Ancient Indian and South Asian populations considered bael to be one of the most valuable plants for use in ayurvedic medicine [15]. Perfectly ripe Bael fruits are well-liked for their delicious pulp, which may be used to create pudding, syrup, and jam. Bael is used as an ingredient in Ayurvedic herbal medicine treatments because of its many therapeutic qualities. Among the bioactive materials found in Bael's fruits, bark, leaves, seeds, and roots are coumarin, xanthotoxol, imperatorin, aegeline, and marmeline. These substances may be immunogenic, antidiabetic, anticancer, antifertility, antibacterial, and insecticidal [16]. In this work, the antibacterial activity of TiO2 NPs was evaluated after they were synthesized utilizing a straightforward aqueous reduction procedure by mixing the extracts of Limonia acidissima and Aegle marmelos.
2. Material and methods
2.1. Preparation of extract
The fruits of the wood apple and stone apple were collected from the local market in Bhopal, Madhya Pradesh, India. The shells of both fruits are then separated, washed thoroughly, and dried at room temperature. The dried shells were then ground and blended in equal parts, 25 g each. 50 g of this produced combination was steeped in 500 ml of distilled water for 48 h. After that, the extract was filtered using filter paper, and the solution was stored in the refrigerator for future use.
2.2. Green synthesis of TiO2 nanoparticles
TiO2 NPs were created by mixing 70 ml of extracted extract with 30 ml of a 5 mM Titanium isopropoxide (TTIP) solution using the green method. The mixture was then stirred for eight hours at room temperature. Their development was caused by the hydrolysis of TTIP, which is the primary step that produces TiO2 NPs [17]. To stop agglomeration and help the TiO2 NPs take on the appropriate size and shape, the prepared extract that was added to the solution combination served as a stabilizing agent. To separate the nanoparticles, the liquid was centrifuged for 10 min at 9000 rpm after stirring. Obtained wet powdered TiO2 NPs were dried at 100 °C for one night and then subjected to calcination at 570 °C in a muffle furnace for 3 h. Pellets of TiO2 NPs enriched with natural extract were recovered and used for further study (figure 1). The whole reaction for the creation of TiO2 nanoparticles utilising a plant extract as a reducing agent is summarised as follows:
Figure 1. Biosynthesis of TiO2 nanoparticles.
Download figure:
Standard image High-resolution image2.3. Characterization of green synthesized TiO2 nanoparticles
The properties of green synthesised TiO2 NPs were investigated using a variety of techniques, including Scanning electron-energy dispersive x-ray spectroscopy (SEMEDS), x-ray powder diffraction (XRD), and Fourier transform infrared spectroscopy. An FTIR-6600 spectrophotometer was used to record FTIR spectra in the 4000 – 400 cm−1 range. After mixing the sample with potassium bromide (KBr) at a 1:100 ratio, it was compacted to a 2 mm semi-transparent disc with a specially designed screw knot. The presence of several functional groups in the produced extract that are involved in the synthesis and stability of TiO2 NPs was then investigated utilising different vibration modes. The x-ray diffraction (XRD) investigation was carried out using a Shimadzu 7000 x-ray diffractometer (Japan). It was equipped with a Cu Kα1 radiation source (λ = 1.5406 Å) with an accelerating voltage of 40 kV and a source current of 30 mA. The scanning rate was 4° min−1 in a 2θ range of 10 to 80°. An SEM was used to examine the surface morphology of TiO2 NPs. The powdered sample was wrapped in carbon tape and placed on top of the sample holder for SEM analysis. Platinum was then sputter coated onto it for 120 s in an ion coater. From an expanded SEM image, the size distribution of TiO2 NPs was determined by counting roughly 100 particles according to the Ali et al 2023 [18].
2.4. Antimicrobial assay
2.4.1. Test microorganism
Aspergillus Niger, Candida Albicans, and Aspergillus Flavus were selected due to their clinical and pharmacological significance. All strains were bought from the Institute of Microbial Technology in Chandigarh and utilised to assess antibacterial activity.
2.4.2. Growth media preparation
Potato Dextrose Agar was prepared according to the following standard procedure.
Prepare the powder by suspending it in 1 litre of clean water. Mix thoroughly. Heat with constant stirring, then boil for 1 min to completely dissolve the powder. Autoclave at 121 °C for 15 min. To adjust the agar medium's reaction to pH 3.5, cool the base to 45 °C–50 °C and add an appropriate amount of sterile 10% tartaric acid to each litre of medium. Mix well. Don't reheat the medium. The potato dextrose agar media used had the following composition:
potato starch: 4.0 g, dextrose: 20.0 g, agar: 15.0 g and distilled water: 1000 ml. The fungal strains were grown in Potato Dextrose Agar media at 28 °C. The stock cultures were maintained at 4 °C.
2.4.3. Determination of antimicrobial activity
The Potato Dextrose Agar media was autoclaved at 121 °C and 15 pounds pressure for 15 min. The sterilised medium was poured into Petri dishes. The hardened plates were drilled with a 5 mm diameter cork holder. The plates with wells were employed in the antifungal study. Before testing, the stock solution was diluted to provide a working solution with varying concentration. The crude extract and biosynthesized TiO2 NPs were dissolved in DMSO and evaluated for antifungal activity against Aspergillus Niger, Candida Albicans, and Aspergillus Flavus at doses of 100, 75, 50, and 25 μg ml−1, respectively. It was shown using the well diffusion approach.
The prepared culture plates were infected with various fungus strains using the streak plate method. A 6 mm cork borer was used to create wells on the surface of the media. The various samples were poured into the well with a sterile syringe. The plates were incubated at 37 °C for 24 h to test for antibacterial activity. Each concentration of the several samples was tested against a distinct bacterium. The zone of inhibition was determined by measuring the diameter of the inhibition zone surrounding the well (in millimetres), including the good diameter.
3. Result and discussion
3.1. X-ray diffraction
X-ray diffraction was used to examine the crystalline phase, crystal structure, purity, and average crystalline size of TiO2 NPs. Figure 2, shows the XRD pattern of bio-mediated synthesised TiO2 nanoparticles. The diffraction angle (2ϴ) at 27.45°, 36.75°, 41.27°, 54.27°, 56.54°, 62.78°, and 69.85° correspond to the Braggs reflection plane of (110), (101), (111), (211), and (301). These Miller indices values confirm the anatase phase. The peak at 36.75° was observed due to the orthorhombic crystalline structure. The observed angle of 27.45° (101) indicates the high crystalline structure of TiO2 NPs. The average crystalline size of TiO2 NPs was determined from the XRD pattern using the Debye-Scherer formula.
where D is an average crystalline size, K is a dimension-less form factor with a value close to unity, λ is the wave length of the x-ray, β is the whole width of the maximum intensity, and θ is the Bragg angle [19, 20]. The typical crystalline size of TiO2 NPs was between 35 and 40 nm. The observed average crystalline size values are consistent with prior reports [21]. These results was also justified by other research [22]. The presence of polyphenolic chemicals in the plant extract caused the green TiO2 nanoparticles to exhibit greater intensity TiO2 peaks [23].
Figure 2. XRD-spectra of green synthesized nanoparticles.
Download figure:
Standard image High-resolution image3.2. Fourier transform infrared spectroscopy
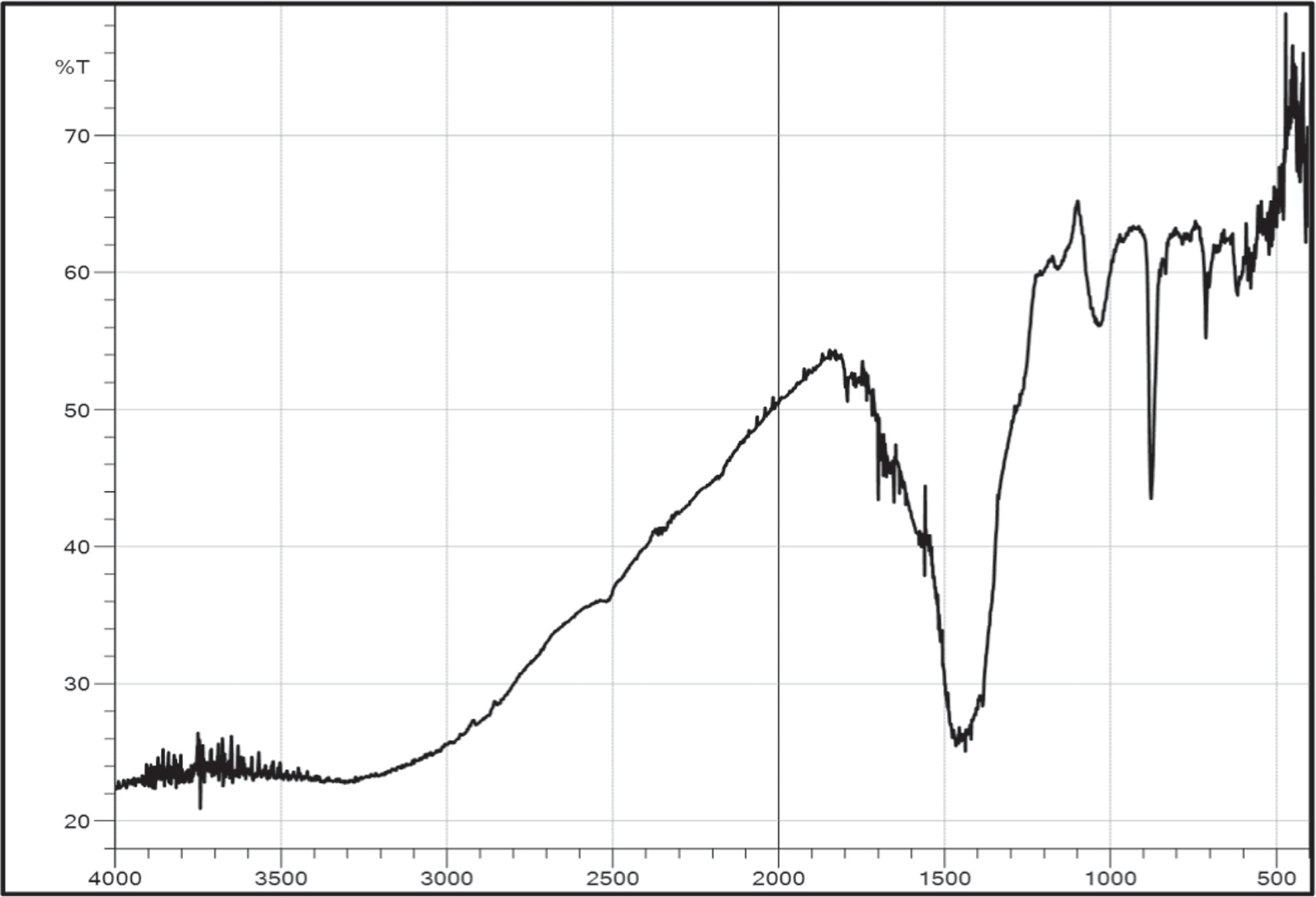
Alcohol functional groups can be seen in the narrow band at 1269 – 1278 cm−1. The 1057 – 1100 cm−1 band represents C-O groups in aromatic stretching vibration. The strong bands at 490 cm−1 and 850 cm−1 indicate the creation of Ti-O and Ti-O-Ti bending vibrations, figure 3. Metal oxide linkages such as Ti-O-Ti and Ti-O demonstrate the presence of TiO2 in the produced TiO2 NPs. The Ti-O-Ti connection exists because biomolecules engage strongly (capped) with TiO2 NPs, resulting in the presence of different phytochemicals such as alkaloids, flavonoids, tannins, and terpenoids [24]. These phytochemicals are responsible for converting the majority of titanium dioxide into stable TiO2 during green synthesis [25].
Figure 3. FTIR-spectra of green synthesized nanoparticles.
Download figure:
Standard image High-resolution image3.3. Scanning electron microscope
The figure 4(a), depicts SEM pictures of produced TiO2 NPs. The SEM image of bio-mediated TiO2 nanoparticles shows a spherical shape, while chemically synthesised TiO2 nanoparticles have a sphere-like surface morphology. The average particle size of spherical TiO2 NPs ranged from 35 to 40 nm. Figure illustrates the material's elemental analysis using an energy-dispersive x-ray (EDAX) picture and spectrum. In biosynthesized TiO2 NPs, titanium predominates over oxygen content. Weight percentage of Titanium and Oxygen are 73.89 and 26.02 respectively, figure 4(b).
Figure 4. (a) SEM image and (b) EDAX image biosynthesized TiO2 NPs.
Download figure:
Standard image High-resolution image3.4. Antimicrobial study
Because they have better tolerance profiles than their synthetic counterparts, natural products are becoming more and more popular and are a valuable source for future treatments. These natural chemicals have the interesting ability to function as antibacterial agents, providing an efficient method of managing specific microorganisms. These amazing plants have many advantages, but their antibacterial properties are particularly valuable in fields like pharmacology, medicine, and agriculture. Investigating nature's pharmacy opens forth new possibilities for creative, long-lasting medical treatments. Consequently, in the present work, we have adopted the notion of green synthesis, which enables us to use the advantages of nanoparticles while simultaneously minimizing their environmental impact and encouraging sustainable practices for a more environmentally friendly future [26].
The antibacterial activity of titanium dioxide nanoparticles against a range of microbes has been studied in a number of academic papers. TiO2 NPs have been shown in certain studies to have the ability to effectively inhibit bacterial growth and kill germs, which is encouraging. The antibacterial properties of titanium dioxide nanoparticles have been well investigated [27, 28]. TiO2 nanoparticles' antibacterial efficacy against a pathogenic strain of Escherichia coli was studied by Haghi et al Their research showed that TiO2 NPs induce the formation of microscopic holes in bacterial cell walls, which raise permeability and ultimately result in cell death. TiO2 NPs exhibit strong antibacterial activity as a result of this approach, making them a viable option for addressing microbial issues in dental settings. This is in line with the positive results of the current study, which employed titanium oxide nanoparticles (NPs) as one of the main ingredients of the mixture to produce potent antibacterial action [29]. The main factor influencing TiO2 NPs' antibacterial effectiveness is their photocatalytic potential. TiO2 NPs have the ability to produce powerfully oxidative reactive oxygen species (ROS) such hydroxyl radicals when exposed to ultraviolet (UV) radiation. Furthermore, the optical density of TiO2 NPs is compared to other bacterial strains in a study conducted by another researcher. For every studied strain, there was a little drop in optical density as the concentration of TiO2 NPs increased. These results indicate that titanium oxide has bactericidal properties against a variety of bacterial species [30].
TiO2 NPs infused with ginger and cloves may be employed as an antibacterial agent against Lactobacillus species, according to a different study from 2023. This implies that TiO2 NPs against Lactobacillus may represent a viable antibacterial agent [31]. TiO2 NPs were taken into account for antibacterial research after Senapati et al 's study from the early days of NP synthesis on gold and silver NPs demonstrated their structural properties for biomedical applications. Most of the investigations that showed the antibacterial activity of the NPs involved floating TiO2 in a solution and observing the inhibition and activity of bacteria [32]. In a study, Rezaei et al investigated the antibacterial activity of cadmium oxide NPs and TiO2 NPs against Escherichia coli. When tested on E. coli, 0.1% of TiO2 NPs inhibited bacterial growth, demonstrating more antibacterial efficacy than cadmium oxide NP. This data demonstrates the superior advantages of titanium oxide NPs, which makes them a more desirable choice when examining metallic oxide NPs for the creation of novel dental varnishes that combine NPs and herbal ingredients [33, 34].
We evaluated the effects on the growth inhibition of several fungal strains at various concentrations of produced crude extract and biosynthesized TiO2 NPs, namely at 100 μgml−1, 75 μg ml−1, 50 μg ml−1, and 25 μg ml−1 concentrations. Both the crude extract and the biosynthesized TiO2 NPs shown less inhibition for fungal strains table 1 at a dose of 25 μg ml−1. This indicates that TiO2 NPs may have a slight inhibitory effect on fungal growth at lower concentrations. A comparable result was seen with a less noticeable zone of inhibition for fungal strains when the test solution was made with a 50 μg ml−1 dilution of TiO2 NPs. More encouraging outcomes were obtained when TiO2 NPs were diluted to 100 μg ml−1. TiO2 NPs had a more inhibitory effect on fungal growth at this concentration than at lower dilutions, as evidenced by the much larger zone of inhibition that fungal strains displayed. Furthermore, in all dilutions, the zone of inhibition for biosynthesized TiO2 NPs is noticeably bigger than that of crude extract dilutions, figures 5 and 6.
Table 1. Showing zone of inhibition of prepared crude extract and biosynthesized TiO2 NPs against different fungal strains.
| Name of bacterial strains | Zone of inhibition of prepared crude extract in mm | Zone of inhibition of biosynthesized TiO2 nanoparticles in mm | ||||||
|---|---|---|---|---|---|---|---|---|
| 25 μg ml−1 | 50 μg ml−1 | 75 μg ml−1 | 100 μg ml−1 | 25 μg ml−1 | 50 μg ml−1 | 75 μg ml−1 | 100 μg ml−1 | |
| Aspergillus Niger | 13.46 ± 0.78 | 15.71 ± 0.60 | 17.04 ± 1.34 | 18.76 ± 2.45 | 25.12 ± 0.67 | 26.98 ± 1.50 | 28.03 ± 0.46 | 29.85 ± 0.90 |
| Candida Albicans | 13.98 ± 1.65 | 16.12 ± 0.28 | 17.87 ± 1.55 | 19.12 ± 1.43 | 26.17 ± 0.19 | 27.74 ± 0.26 | 28.49 ± 0.59 | 30.23 ± 1.08 |
| Aspergillus Flavus | 14.42 ± 0.29 | 15.43 ± 1.02 | 16.63 ± 0.52 | 18.45 ± 1.30 | 25.29 ± 0.68 | 27.36 ± 0.74 | 29.45 ± 0.19 | 30.02 ± 0.75 |
Figure 5. Disc showing zone of inhibition of different concentration of prepared crude extract against (a) Aspergillus niger (b) Candida albicans (c) Aspergillus flavus.
Download figure:
Standard image High-resolution imageFigure 6. Disc showing zone of inhibition of different concentration of biosynthesized TiO2 NPs against (a) Aspergillus niger (b) Candida albicans (c) Aspergillus flavus.
Download figure:
Standard image High-resolution image4. Conclusion
The study determines the antifungal efficacy of a mixture of Stone Apple and Wood Apple infused titanium dioxide nanoparticles, which results in a reduction in fungal counts when tested at various concentrations against different fungal strains. The results shown that the efficiency of biosynthesized TiO2 NPs in suppressing the growth of various fungal strains is concentration dependant. Lower dilutions showed less inhibition, although a 100 μg ml−1 dilution of TiO2 NPs showed a moderate zone of inhibition, suggesting possible antifungal effects comparable to the pure extract. This preliminary research establishes the groundwork for future medical applications, necessitating additional investigations for practical usage in the field of medicine.
Acknowledgments
This research was supported by Researchers Supporting Project number (RSP2024R27), King Saud University, Riyadh, Saudi Arabia.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Declaration of conflict of interests
All author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.